Summary
Anatomical changes in the gastrointestinal tract and subsequent weight loss may influence drug disposition and thus drug dosing following bariatric surgery. This review systematically examines the effects of bariatric surgery on drug pharmacokinetics, focusing especially on the mechanisms involved in restricting oral bioavailability. Studies with a longitudinal before‐after design investigating the pharmacokinetics of at least one drug were reviewed. The need for dose adjustment following bariatric surgery was examined, as well as the potential for extrapolation to other drugs subjected to coinciding pharmacokinetic mechanisms. A total of 22 original articles and 32 different drugs were assessed. The majority of available data is based on Roux‐en‐Y gastric bypass (RYGBP) (18 of 22 studies), and hence, the overall interpretation is more or less limited to RYGBP. In the case of the majority of studied drugs, an increased absorption rate was observed early after RYGBP. The effect on systemic exposure allows for a low degree of extrapolation, including between drugs subjected to the same major metabolic and transporter pathways. On the basis of current understanding, predicting the pharmacokinetic change for a specific drug following RYGBP is challenging. Close monitoring of each individual drug is therefore recommended in the early postsurgical phase. Future studies should focus on the long‐term effects of bariatric surgery on drug disposition, and they should also aim to disentangle the effects of the surgery itself and the subsequent weight loss.
Keywords: bariatric surgery, pharmacokinetics
Abbreviations
- AUC
area under the curve
- BCS
biopharmaceutics classification system
- BPD
biliopancreatic diversion
- CAR
constitutively activated receptor
- DS
duodenal switch
- C0
through concentration
- Cmax
maximum concentration
- CL
clearance
- CYP
cytochrome P450
- F
oral bioavailability
- GI
gastrointestinal
- HNF4α
hepatocyte nuclear factor 4α
- ka
absorption coefficient
- MeSH
medical subject headings
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OATP
organic anion transporting peptide
- PBPK
population‐based pharmacokinetics
- P‐gp
P‐glycoprotein
- PXR
pregnane X‐receptor
- RYGBP
Roux‐en‐Y gastric bypass
- SSRI
selective serotonin reuptake inhibitor
- SNRI
serotonin‐norepinephrine reuptake inhibitor
- SG
sleeve gastrectomy
- T1/2
terminal elimination half‐life
- Tmax
time to maximum concentration
- UGT
UDP‐glucuronosyltransferase
- Vd
volume of distribution
1. INTRODUCTION
Obesity is a well‐known global health problem. The prevalence of obesity in the industrialized world is high,1, 2 with obesity‐related comorbidities contributing significantly to the burden of disease in these patients. Pharmacological therapy is commonly needed, often including polypharmacy.3 Weight loss is associated with improvement of obesity‐related comorbidities and reduces the need for pharmacological intervention.4, 5, 6, 7 Bariatric surgery is the treatment modality that gives the best results for long‐lasting weight loss and associated improvement in comorbidities.8, 9 There are several techniques in use, including sleeve gastrectomy (SG), adjustable gastric band, biliopancreatic diversion (BPD), biliopancreatic diversion with duodenal switch (BPD‐DS), and Roux‐en‐Y gastric bypass (RYGBP). Most of the surgical techniques reduce the volume of the stomach, and many of them also reduce the intestinal absorptive surface area by bypassing different parts of the proximal intestine. In 2016, SG was the most performed primary surgical procedure worldwide (54%), followed by RYGBP (30%).10
The anatomical and physiological alterations in the gastrointestinal (GI) tract following bariatric surgery may change a wide variety of factors involved in restricting the oral bioavailability of drugs.11, 12, 13, 14, 15 The overall process of restricting oral drug bioavailability (F) may be divided into three main processes: the fraction of drug that is absorbed into the intestinal gut wall (F A), the fraction that escapes gut wall metabolism (F G), and the fraction that escapes hepatic metabolism (F H). The observed bioavailability is obtained by multiplying these three fractions (F = F A × F G × F H). Bariatric surgery will potentially affect both the F A and F G, while the subsequent weight loss may affect F G and F H.
Drug absorption is highly dependent on the physiochemical properties of a drug; ie, solubility, lipophilicity, molecular size and polarity, and changes in the GI tract following bariatric surgery will have different effects on different drugs. For example, a changed gastric pH may affect drug dissolution and solubility, as well as preabsorptive drug stability. Gastric emptying time may be the rate‐limiting step of systemic drug absorption for some drugs,16 and this factor seems to change after SG.17 On the other hand, the available mucosal absorption area in the small intestine is extensive and facilitates drug absorption.18 Even though RYGBP and other procedures bypass a significant length (50 to 150 cm) of the proximal intestine, this reduction in available intestinal surface area does not necessarily limit the absorption of drugs. These procedures, however, bypass a part of the intestine rich in metabolizing enzymes,18 which may be hypothesized to change the oral bioavailability of some drugs. Changes in intestinal motility can affect the transit time and hence affect, for example, the oral bioavailability of slow‐release formulations.19
Following absorption, drugs are subjected to presystemic intestinal and hepatic metabolism, also known as first pass metabolism, and this is an important factor limiting the oral bioavailability of many drugs. In RYGBP, the proximal part of the intestine, which is rich in metabolizing enzymes, is bypassed. This bypass places drugs directly into the more distal part of the intestine that expresses less metabolic capacity, which in turn results in a higher oral bioavailability. The predominant enzymes for metabolizing drugs are the cytochrome P450 (CYP) enzymes, with the most abundant being the CYP3A subfamily. Apart from its extensive hepatic expression, the CYP3A subfamily is widely expressed in the duodenum and proximal jejunum.20 At least 50% of drugs available on the market are metabolized via CYP3A.21 The CYP3A enzymes are reported to account for 80% of total P450 content in the proximal small intestine.22 Consequently, the rearrangement of the GI tract following bariatric surgery, especially RYGBP, will influence the oral bioavailability of CYP3A substrates to a large extent. Other enzymes expressed in the intestine are CYP2C9, CYP2C19, and CYP2D6,18 as well as UDP‐glucuronosyltransferases (UGTs), and to a lesser extent other phase II enzymes.
Drugs cross the intestinal mucosa by passive diffusion or active transport, depending on solubility and lipophilicity. Transport proteins located in the GI tract facilitate the active transport and may thus also influence both the absorption and the intestinal metabolism of substrate drugs. Numerous different drug transporters are expressed in the GI tract, including P‐glycoprotein (P‐gp), which functions as a drug efflux pump for a wide number of substrates. P‐gp is expressed on the apical side of the intestinal mucosa, showing an increased abundance in the more distal parts of the midgut.
Besides bariatric surgery–induced anatomical alterations to the GI tract, the resulting weight loss may also influence the activity of drug‐metabolizing enzymes and transporters itself. It is known that obesity is associated with low‐grade inflammation in the adipose tissue, which secretes inflammatory cytokines, chemokines, and adipokines.23 This in turn may lead to the reduced activity of CYP enzymes.24 Additionally, the prevalence of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) is higher among people with overweight and obesity than people with normal weight.25 The current literature indicates that there is an altered activity of CYP enzymes and transport proteins in the livers of these patients.26, 27
According to the outline above, it is intuitive that the rearrangement of the GI tract following bariatric surgery may influence the pharmacokinetics of drugs. The biopharmaceutics classification system (BCS) distinguishes between four classes of drugs according to drug permeability and solubility and could potentially be helpful when predicting some of the effects of bariatric surgery on different drugs based on their physiochemical properties.28 However, as outlined in this review, this is not the case, probably because of the considerable changes in the GI tract following this kind of surgery. A potential contributing factor that needs to be taken into consideration is the weight loss and the physical adaption of the GI tract following bariatric surgery.29 The complicated and time‐dependent interplay of several factors in restricting oral drug bioavailability needs to be deconstructed into their components in order to gain reliable predictions as to how different drugs will be affected. Limited preexisting knowledge in this field and the lack of guidelines make drug prescription and dosing following bariatric surgery difficult. We aimed to systematically review studies examining the effects of bariatric surgery on drug pharmacokinetics by focusing on the mechanisms involved in restricting oral bioavailability and thereafter to evaluate the potential of using extrapolations for dosing recommendations following bariatric surgery.
2. METHOD
2.1. Protocol and registration
This systematic review has been registered at PROSPERO (CRD42016049348). The protocol can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=49348. We based our methods on the principles outlined in the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement.30
2.2. Information sources and search strategy
Literature search strategies were developed in cooperation with two health science librarians, using medical subject headings (MeSH) and text words related to pharmacokinetics, bioavailability, bariatric surgery, and weight loss. We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. The search strategy in the Ovid interface is attached as Supporting information Appendix S1. The literature search was restricted to English language articles and human subjects. There was no restriction on publication dates, and as such, this review includes articles published as of November 2018.
2.3. Eligibility criteria
Longitudinal short‐ and long‐term studies of adults (18 y or older) with obesity including a pharmacokinetic examination of at least one drug before and after bariatric surgery were included. Protocols and conference abstracts not leading to publication were excluded, as were studies involving inhaled drugs were excluded.
2.4. Data collection
Literature search results were uploaded to http://www.covidence.org, an internet‐based review programme that facilitates review literature screening and collaboration among reviewers. Test exercises were made prior to the formal screening process in order to ensure a uniform inclusion. Two members of the review team (P.C.A. and I.R.) independently screened titles and abstracts yielded by the search. Full reports for titles meeting our criteria were subsequently obtained. A senior member of the review team (J.H.) resolved possible conflicts during the inclusion process.
2.5. Outcomes
The main focus of the analysis was to evaluate the acute changes in pharmacokinetic parameters before surgery and early postsurgery and how these potential changes developed over time. Pharmacokinetic parameters used to assess the effects of surgery were clearance (CL), volume of distribution (V d), terminal elimination half‐life (T 1/2), and oral bioavailability (F), in addition to the absorption coefficient (k a). The following pharmacokinetic variables were primarily evaluated to assess changes in relevant pharmacokinetic parameters: trough concentrations (C 0), maximum concentration (C max), the time to reach C max (T max), and systemic exposure assessed by the area under the concentrations versus time curve (AUC).
3. RESULTS
The search yielded a total of 2108 records for title and abstract screening. After exclusion of 2029 abstracts not fulfilling the screening criteria, a total of 79 full‐text papers were assessed for eligibility. The majority of these (n = 57) were excluded on the basis of the eligibility criteria. Twenty‐two publications fulfilled the prespecified inclusion criteria and were included in our analysis. The study selection process is illustrated in Figure 1.
Figure 1.
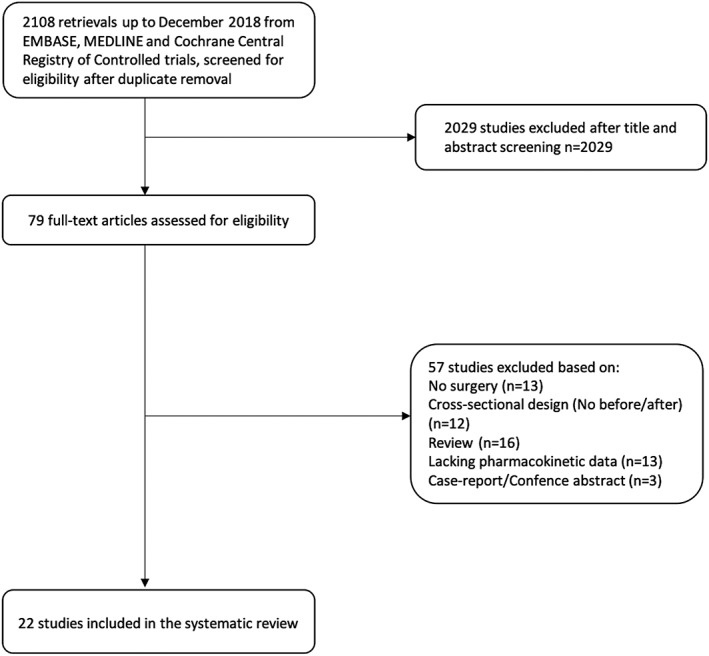
Flow chart of study selection
3.1. Population and design
Nineteen publications included 10 or more patients. The study populations were predominantly female. Mean or median age ranged from 37 to 52 years. All patients who underwent surgery fulfilled the standard criteria for bariatric surgery (body mass index [BMI] ≥ 40 kg/m2 or ≥35 kg/m2 and associated with at least one obesity‐related comorbidity). None of the patients had liver or renal disease.
All papers included prospective, longitudinal cohort studies, with bariatric surgery as the sole clinical intervention. In most studies, the patients undergoing surgery served as their own controls. Two studies also included control groups of healthy volunteers.31, 32
3.2. Intervention
RYGBP was the predominant surgical procedure (n = 18).32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 In one study, BPD was investigated as the surgical procedure,31 while two studies investigated BPD‐DS,41, 50 six studied SG,32, 33, 34, 43, 44, 51 and one included jejunoileostomy.52
3.3. Drugs
Among the 22 studies, 16 utilized a single‐dose design,31, 32, 33, 34, 35, 36, 37, 40, 42, 43, 44, 45, 47, 48, 52, 53 administering a given dose of the studied drug on the day of the pharmacokinetic study intervention. In the remaining six studies, patients were investigated in steady state.39, 41, 46, 49, 51 Several drugs were studied because of their status as probe drugs/substrates for different CYP enzymes and drug transporters, eg, midazolam for CYP3A33, 34, 35 as well as digoxin for P‐gp.35 A few of the studies utilized population pharmacokinetic methods in their analyses.33, 34, 36, 40
3.4. Follow‐up
All studies had at least one assessment point after surgery, and subjects were studied for up to 2 years.41 The majority were, nonetheless, limited to a 1‐year follow‐up or shorter.
3.5. Results according to pharmacokinetic mechanisms involved in restricting oral bioavailability
The results presented below are summarized in Table 1.
Table 1.
General characteristics of included studies
| Drugs | Population (n) | Surgery (n) | Control Group | Assessment Points | Outcomes | ||
|---|---|---|---|---|---|---|---|
| Absorption | |||||||
| Goday‐Arno et al32 | – | Paracetamol | 24, all female | RYGBP (14), SG (10) | Normal weight (n = 14) and overweight (n = 14) volunteers | Presurgery baseline, 4 wk and 6 mo after surgery | Increased rate and extent of absorption. No difference in surgical procedures. |
| Gesquiere et al37 | – | Fenofibrate and posaconazole | 23, gender distribution not available | RYGBP | Before‐after two groups: one group (n = 12) received fenofibrate and one group (n = 11) received posaconazole | Presurgery baseline, 6 mo after surgery | Unaltered pharmacokinetics for fenofibrate. Decreased C max and AUC for posaconazole. |
| Mitrov‐Winkelmolen et al47 | – | Acetylsalicylic acid | 40, 8 male and 32 female | RYGBP | Before‐after in one group | More than 2 wk presurgery, more than 6 wk after surgery | C max, AUC increased after surgery, T max decreased. |
| Metabolism | |||||||
| Goday‐Arno et al32 | CYP1A2 | Caffeine | 24, all female | RYGBP (14), SG (10) | Normal weight (n = 14) and overweight (n = 14) volunteers | Presurgery baseline, 4 wk and 6 mo after surgery | No change in caffeine pharmacokinetics. |
| Mitrov‐Winkelmolen et al47 | CYP2C19 | Omeprazole | 40, 8 male and 32 female | RYGBP | Before‐after in one group | More than 2 wk presurgery, more than 6 wk after surgery | Omeprazole: C max increased, T max decreased, AUC no change. |
| Gesquiere et al36 | CYP2D6 | Metoprolol, immediate and controlled release | 14, 4 male and 10 female | RYGBP | Before‐after in one group, pop‐PK | Presurgery, post‐op 6 mo | Nonsignificant change in AUC, C max, and T max, tendency to higher exposure. |
| Brill et al34 | CYP3A | Midazolam, iv and oral | 20, 8 male and 12 female | RYGBP, Sleeve (2) | None | Presurgery, post‐op 12 mo | F unchanged, systemic CL higher. |
| Brill et al33 | CYP3A | Midazolam, iv and oral | 20, 8 male and 12 female | RYGBP, Sleeve (2) | None | Semiphysiologically based pharmacokinetic model showed increased hepatic CYP3A metabolizing capacity after surgery. | |
| Chan et al35 | CYP3A | Midazolam | 12, 3 male and 9 female | RYGBP | Before‐after in one group | Presurgery, post‐op 3 and 12 mo | Midazolam: decreased T max, increased C max, AUC unchanged. AUC 1‐OH‐mdz/mdz: no change. |
| Jakobsen et al41 | CYP3A | Atorvastatin | 22, 9 male and 11 female | BPD/DS (10), RYGBP (12) | Before‐after in two groups; groups based on surgical technique | Presurgery, post‐op 4‐8 wk, 27 mo (21‐47 mo) | Long‐term decreases in AUC and C max. Long‐term increase in T max. |
| Kröll et al44 | CYP3A | Rivaroxiban | 12, 4 male and 8 female | RYGBP (6), SG (6) | Before‐after in two surgical groups | Presurgery baseline, 3 d after surgery | No significant change in AUC, in all patients. |
| Kröll et al43 | CYP3A | Rivaroxiban | 12, 4 male and 8 female | RYGBP (6), SG (6) | 6 mo postsurgery | No change, no difference between groups. | |
| Skottheim et al49 | CYP3A | Atorvastatin | 12, 4 male and 8 female | RYGBP | Before‐after in one group | Presurgery, post‐op 4‐6 wk | High interindividual variability. No significant change in T max, C max, and AUC. |
| Skottheim et al50 | CYP3A | Atorvastatin | 10, 5 male and 5 female | BPD/DS | Before‐after in one group | Presurgery, post‐op 4‐8 wk | Increased AUC, increased T max and C max. |
| Llloret‐Linares et al45 | UGT2B7 | Morphine | 30, 6 male and 24 female | RYGBP | Before‐after in one group | Presurgery, post‐op 2 wk and 6 mo | C max, T max, and AUC increase. BMI, surgery as covariates have influence on CL. |
| Transport | |||||||
| Chan et al35 | P‐gp | Digoxin | 12, 3 male and 9 female | RYGBP | Before‐after in one group | Presurgery, post‐op 3 and 12 mo | Digoxin: no change in T max, C max, and AUC. |
| Other | |||||||
| Amouyal et al51 | – | Abacavir, Atazanavir, Darunavir, Efavirenz, Emtricitabine, Lamivudine, Raltegravir, Ritonavir Tefonorvir | Totally 17. PK investigated only in a subgroup | SG | Before‐after a subgroup of patients | Presurgery baseline, 3 and 6 mo post‐op | Absorption of atazanavir and raltegravir decreased. Otherwise, small changes. n < 10 per drug. |
| Cossu et al31 | – | Ranitidine | 11, 7 male and 4 female | BPD | Compared with historical data on 10 subjects with normal weight. Before‐after in one group | Presurgery, post‐op 8 mo | No significant change in AUC and C max. |
| Ginstman et al38 | – | Desogestrel | 14, all female | RYGBP | Before‐after in one group | No significant change in AUC, C max, and T max. | |
| Hamad et al39 | – | Venlafaxine (n = 5), citalopram (n = 2), escitalopram (n = 2), sertraline (n = 2), duloxetine (N = 1) | 12, 1 male and 11 female | RYGBP | Before‐after in one group | Presurgery, post‐op 1, 6, and 12 mo | In 8 pts: AUC decreases after 1 mo, then tendency to normalization. |
| Hamilton et al40 | – | Linezolid, iv and oral | 5, all male | RYGBP | Historical data on normal subjects, pop‐PK | No change in F oral. C max increased, no change in T max. | |
| Kampmann et al52 | – | Ampicillin (n = 6), Propylthiouracil (N = 6). Both drugs given in iv and oral formulations | 15, 5 male and 10 female | Jejunoileostomy | Before‐after in two groups based on drug administered. Groups were not compared with each other | Presurgery, post‐op 1‐2 wk, 6 and 12 mo | Ampicillin: increased F. Propylthiouracil: no change in F. |
| Krieger et al42 | – | Venlafaxine | 10, 3 male and 7 female | RYGBP | Before‐after in one group | Presurgery baseline, 3‐4 mo post‐operative | No change in AUC, C max, T max. |
| Marzinke et al46 | – | Escitalopram | 4, all female | RYGBP | Before‐after design in case series | Presurgery, post‐op 2 and 6 wk | Decreased serum concentrations of escitalopram. |
| Rocha et al48 | – | Amoxicillin | 8, gender distribution not available | RYGBP | Before‐after in one group | Presurgery baseline, 2 mo after surgery | Increased AUC and C max. Unchanged T max. |
Note. Studies included in the review according to relevant pharmacokinetic mechanism. Some articles are listed twice when one or more probe drugs are investigated.
Abbreviations: AUC, area under the curve; BPD/DS, biliopancreatic diversion/duodenal switch; C max, maximum plasma concentration; CL, clearance; F, bioavailability; RYGBP, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy; T max, time to C max.
The drugs investigated in the studies discussed above were sorted according to either their status as probe drugs or according to their most important pharmacokinetic characteristics (ie, main disposition pathway) that may be involved in determination of oral bioavailability. The results were divided into three categories: “Drug Absorption,” “Drug Metabolism,” and “Drug Transport.” Small sample size studies and studies including drugs with pharmacokinetics characteristics not belonging to the sections above are described in the subsection “Other.”
3.5.1. Drug absorption
Paracetamol is a widely recognized probe for gastric emptying.54 The effects of SG and RYGBP on paracetamol pharmacokinetics were reported in a prospective single‐dose study before versus 4 weeks and 6 months after surgery.32 At both postoperative time points, AUC and C max increased, and T max decreased after 6 months. This increase in both the rate and extent of paracetamol absorption is suggestive of an accelerated gastric emptying time following SG and RYGBP. No differences were observed between the two surgical methods. Another interesting finding was that the patients with morbid obesity also had lower C max and AUC of paracetamol compared with normal weight individuals before surgery.32 Six months following surgery, the C max and AUC were, however, similar to that of normal weight individuals. This normalization of paracetamol's pharmacokinetic parameters after surgery is probably due to the subsequent weight loss although a surgery‐specific effect cannot be ruled out.
Fenofibrate and posaconazole, two model compounds belonging to the BCS class II (high permeability, low solubility), were studied on the basis of their absorption characteristics before and after RYGBP.37 To investigate the consequence of delayed contact with bile salts after surgery, fenofibrate can be used as a model drug because its solubility is highly dependent on bile salt concentrations,55 while posaconazole dissolution is pH dependent and can thus be used to indicate the effect of increased gastric pH following surgery.56 The results showed that the disposition of fenofibrate remained unaltered, whereas the exposure of posaconazole was reduced following surgery.37 A substrate less dependent on bile acids for its absorption would have been expected to show an increased absorption rate, but since the contact with bile acids is delayed after RYGBP, the accelerated gastric emptying time had a less visible effect on a substrate such as fenofibrate. The decreased oral exposure of posaconazole is most probably due to the lower solubility caused by an increased pH following surgery, also counteracting the expected increased absorption rate following an accelerated gastric emptying time postsurgery.
Acetylsalicylic acid is regularly absorbed by passive diffusion as an unionized drug in the stomach and partly as an ionized drug in the duodenum.47 After RYGBP, a significant increase in both the rate and extent of acetylsalicylic acid absorption has been reported.47 This may suggest that the absorption of ionized acetylsalicylic acid can also take place in the jejunum, replacing the stomach and duodenum as absorption site, and that this might be a result of an accelerated gastric emptying time.47, 57 Although the increases in C max and AUC were statistically significant, the changes were not considered to be clinically relevant, and dose adaption of acetylsalicylic acid following surgery was considered unwarranted.47
3.5.2. Drug metabolism
CYP3A
Midazolam undergoes hydroxylation via both intestinal and hepatic CYP3A and is the most commonly used probe drug to investigate in vivo CYP3A activity.58, 59, 60 The effects on midazolam pharmacokinetics were explored in three of the studies that make up this systematic review.33, 34, 35 In one of these studies, absolute bioavailability and clearance were assessable because of a combined oral and intravenous administration of midazolam.34 Midazolam clearance increased 1 year after surgery, whereas the absolute bioavailability (F total) remained unchanged. This is suggestive of augmented hepatic CYP3A activity because of weight loss, hence the decreasing F H, while the unaltered absolute bioavailability may be explained by a counteracting increase in fraction escaping intestinal drug metabolism (F G) from the bypass of the part of the small intestine rich in CYP3A enzymes. The same authors further developed a semiphysiologically based pharmacokinetic model of midazolam and its primary metabolite aiming to understand the influence of RYGBP on the intrinsic CYP3A activities in the intestine and liver.33 These sophisticated analyses suggested that hepatic intrinsic clearance increased 1.5‐fold following bariatric surgery, whereas the intrinsic metabolism of midazolam in the gut wall was low and highly variable.33 Following oral dosing of midazolam, the rate of absorption was faster both in the short term (3 mo) and long term (12 mo) after surgery.34, 35
Atorvastatin is subject to extensive first pass metabolism by CYP3A enzymes in the intestine as well as in the liver, resulting in a low oral bioavailability.61 In vitro experiments using human microsomes clearly demonstrate that CYP3A4 and CYP3A5 are the major enzymes involved in the metabolism of atorvastatin.62 However, since the disposition of atorvastatin is also affected both by uptake (organic anion transporting peptides, OATPs) and efflux (P‐gp) transporters in the liver,61, 63, 64 atorvastatin is a less selective probe drug than midazolam to study CYP3A activity. The impact of bariatric surgery on the pharmacokinetics of atorvastatin both in the short term (3‐8 wk) and long term (2 y) was investigated in three studies.41, 49, 50 RYGBP produced a variable effect on the individual systemic exposure of atorvastatin, ranging from a threefold decrease to a twofold increase 3 to 6 weeks following surgery.49 The study showed that presurgical first pass metabolic capacity may influence the effect of RYGBP after surgery, as the patients with the highest systemic exposure before surgery showed reduced exposure whereas those with lower systemic exposure before surgery showed an increase in the exposure of atorvastatin. Atorvastatin pharmacokinetics were also investigated both presurgery and postsurgery (4‐8 wk) in patients undergoing BPD‐DS.50 The oral bioavailability of atorvastatin was significantly increased with a mean twofold higher systemic exposure after BPD‐DS. The long‐term effects of these two surgical procedures were investigated over a 2‐year time period.41 Interestingly, the observed initial increase in atorvastatin systemic exposure49, 50 was reversed during long‐term follow‐up.41
Rivaroxaban, the first direct oral anticoagulant to be marketed, is metabolized by several CYP enzymes (CYP3A4 and CYP2J2) and CYP‐independent mechanisms.65 The contribution of the CYP3A4 and CYP2J2 clearance pathways has been quantified to account for approximately 18% and 14% of total rivaroxaban elimination, respectively.66 In contrast to atorvastatin and midazolam, rivaroxaban has a high bioavailability (80%).65 In a single‐dose study, the effects of both RYGBP and SG on rivaroxaban pharmacokinetics were investigated prior to and 3 days following surgery.44 The systemic exposure of rivaroxaban was similar both prior to and after surgery, with no difference between the two surgical procedures observed. In the recently published extension study, it was shown that rivaroxaban pharmacokinetics remained unchanged 6 to 8 months following both RYGBP and SG.43
CYP1A2
CYP1A2 is almost exclusively expressed in the liver, with caffeine as the most widely used probe to study CYP1A2 activity in vivo as approximately 90% of systemic caffeine clearance is mediated by CYP1A2.67, 68 Over a 6‐month period, no relevant changes in caffeine concentrations or the paraxanthine/caffeine ratio were observed following both SG and RYGBP.32 Moreover, there was no difference in caffeine pharmacokinetics between patients with morbid obesity and normal weight individuals.32 The activity of CYP1A2 does not therefore seem to be influenced by bariatric surgery.
CYP2C19
CYP2C19 is the primary enzyme responsible for omeprazole metabolism given that the amount of CYP2C19 in human liver microsomes has correlated highly with omeprazole hydroxylation.69, 70 The impact of RYGBP on omeprazole pharmacokinetics was assessed presurgery and at least 6 weeks after surgery.47 The mean systemic exposure of omeprazole was significantly lower following surgery. In addition, a faster absorption of omeprazole with a significantly shorter T max and a higher C max was shown after surgery.
CYP2D6
Approximately 70% to 80% of an oral metoprolol dose is metabolized by CYP2D6 in the liver,71 and this drug is thus considered a valid probe to investigate CYP2D6 activity in vivo.72 The effect of RYGBP on immediate and controlled release formulations was investigated both presurgery and 6 to 8 months after surgery.36 Although a tendency of higher exposure was observed following surgery, the difference in systemic metoprolol exposure before and after surgery was not significant for either of the formulations, suggesting unaltered CYP2D6 activity following RYGBP. Venlafaxine, a serotonin‐norepinephrine reuptake inhibitor (SNRI), is also predominantly metabolized by CYP2D6 into its major active metabolite, O‐desmethylvenlafaxine.73, 74 The effect of RYGBP on venlafaxine exposure was investigated in a prospective, single‐dose study presurgery and 3 to 4 months after surgery.42 There were no significant differences in venlafaxine exposure, C max and T max, neither prior to or after RYGBP, substantiating the metoprolol findings above of an unaltered CYP2D6 activity.
UDP‐glucuronosyltransferase
Morphine is primarily metabolized in the liver by the phase II enzyme UGT2B7 into pharmacologically active metabolites.75 The pharmacokinetics of oral morphine was investigated in RYGBP patients presurgery, as well as 2 weeks and 6 months after surgery.45 Compared with values before surgery, the morphine AUC increased at both postoperative time points. The population pharmacokinetic analysis performed by the authors showed that morphine clearance was associated with BMI, suggesting that the observed alteration in oral clearance might be partly explained by the surgery‐induced weight loss. In addition, the rate of absorption was significantly increased, reflected by the decreased T max and increased C max, at both 2 weeks and 6 months, supporting the paracetamol findings of a more rapid gastric emptying following bariatric surgery. The changes in overall morphine pharmacokinetics were more pronounced at 6 months, further substantiating the hypothesis of a major effect of weight loss.
3.5.3. Drug transport
P‐glycoprotein
Digoxin has been identified in vitro and in animal experiments as a substrate of renal and intestinal P‐gp.76, 77, 78 As P‐gp plays a pivotal role in the absorption and elimination of digoxin without the confounding influence of metabolism, this drug has become a well‐established probe to phenotype P‐gp in vivo.79 Single oral dose of digoxin was investigated before and 3 and 12 months after RYGBP.35 The systemic exposures and C max of digoxin were unchanged at both postoperative time points. However, the absorption of digoxin was faster, reflected by a shorter median T max at both 3 and 12 months following surgery, which is also an indication of a faster gastric emptying after bariatric surgery.
3.5.4. Other
Patients undergoing bariatric surgery may develop gastric ulcers, and prophylactic use of H2 receptor antagonist is common. The effect of BPD on the pharmacokinetics of ranitidine was studied both presurgery and 8 months after surgery, including control subjects with normal weight.31 The major route of elimination for ranitidine is renal, and the drug is not extensively metabolized.80 Postsurgical AUC and C max of ranitidine were only slightly higher than those before surgery in patients undergoing BPD. Conventional dosage regimens of ranitidine can thus be applied following this kind of surgery.31
Efficient contraception is important after bariatric surgery, and the effect of RYGBP on the pharmacokinetics of the hormonal contraceptive desogestrel was investigated in nine women.38, 53 Desogestrel is a prodrug, and the formation of the biologically active metabolite is crucial for its effect,81 but this bioactivation appears to be independent of CYP2C and CYP3A4 activities.82 There were no changes in the absorption rate or systemic exposure of desogestrel 4 and 12 months after RYGBP,38, 53 suggesting that oral desogestrel may be used in unchanged doses following surgery. The results should however be interpreted with caution because of the small sample size (n = 9).
The effect of bariatric surgery on the bioavailability of three antibiotics—linezolid, ampicillin, and amoxicillin—has been explored in different studies.40, 48, 52 As the kidneys are the major elimination route for these drugs, hepatic metabolism via CYP enzymes is considered to be of minor importance.83, 84 The bioavailability of linezolid was not affected 3 months following RYGBP.40 In contrast, the bioavailability of ampicillin decreased compared with presurgical values at three different time points following bariatric surgery: 2 weeks, 6 months, and 1 year.52 Furthermore, higher AUC and C max of amoxicillin were observed 2 months after RYGBP, possibly because of an increased absorption of amoxicillin.48 Despite this increase, amoxicillin exposure was lower than values reported for nonobese subjects.48
The influence of SG on the pharmacokinetics of nine different antiretroviral drugs was investigated in HIV patients 3 and 6 months following surgery.51 There were some potential large pharmacokinetic effects shown in this study. However, the study was significantly underpowered as each respective drug was only investigated in two to three patients. It is therefore not possible to draw conclusions regarding the influence of bariatric surgery on each individual drug. Close therapeutic drug monitoring following surgery is therefore warranted until more data are presented for these drugs.
Finally, the effects of RYGBP on different selective serotonin reuptake inhibitors (SSRIs) and SNRIs were investigated in two studies.39, 46 There was an indication of lower drug exposure following RYGBP. However, both the small number of patients39, 46 and the pharmacokinetic investigations46 limit the conclusions drawn from these two studies.
4. DISCUSSION
This review evaluated studies addressing the effects of bariatric surgery on drug pharmacokinetics, focusing on mechanisms involved in restricting oral bioavailability. Both the need for dose adjustment of the investigated drugs/probes following bariatric surgery and the potential to extrapolate the results to drugs subjected to coinciding pharmacokinetic mechanisms were reviewed.
4.1. Pharmacokinetic mechanisms involved in restricting oral bioavailability
A faster absorption of oral drugs, as shown by a shorter T max and a higher C max following surgery, was observed early after surgery in the majority of the included studies. This increase in the rate of drug absorption may in part be due to the reduced gastric volume in the newly created stomach, as this has been shown to increase gastric emptying rate,19 resulting in a faster transfer of drug to the intestine and hence an earlier absorption. The accelerated gastric emptying following bariatric surgery was confirmed using the gold standard probe for investigating this, paracetamol.32 The anatomical changes may explain the reduced T max after surgery whereas the increased C max may also in part result from weight loss and the potentially associated lower volume of distribution. The rapid absorption following surgery did not necessarily lead to an overall increased absorption as the systemic exposure remained unaltered after surgery in several of the included studies. Consequently, the clinical relevance of an increased absorption rate following surgery may depend on the drug in question, its mechanism of action, and the magnitude of the change. Most studies included in this review have been performed in patients undergoing RYGBP. In the study by Goday Arno et al, however, there were no differences in the absorption rate of paracetamol between RYGBP and SG, suggesting that the two surgical techniques had a similar effect on gastric emptying time.32
Hepatic CYP3A activity has been shown to be inversely correlated with body size measures.85 Following bariatric surgery, it is suggested that hepatic‐CYP3A activity increases to a new steady‐state level within 1 year.34 Studies have shown a reduction in inflammatory adipokines in patients following bariatric surgery,23 which may be partly explained by the accompanying weight loss. Furthermore, data from in vitro cell models, animal studies as well as in vivo studies in patients, show an association between a reduced CYP3A activity and obesity and NAFLD.26, 86, 87 It has therefore been hypothesized that the chronic low‐grade inflammation observed in patients with obesity decreases expression of pregnane X‐receptor (PXR) and constitutively activated receptor (CAR), resulting in reduced expression of certain CYP enzymes.88 The potential for extrapolation is, however, further complicated by many additional factors such as alteration in liver size and liver blood flow following surgery. It has in fact been shown that the liver size and intrahepatic fat content are reduced 6 months after bariatric surgery89 and depending on the extraction ratio of the drug, the reduction in liver size may have the opposite effect on drug clearance. Brill et al utilized their semi–population‐based pharmacokinetics (PBPK) model of midazolam to simulate the effects of different blood flow scenarios and increased hepatic CYP3A clearance.33 From the simulations, it was concluded that clearance of low extraction ratio CYP3A substrates is increased by at least 1.3 times after bariatric surgery. However, because of the lack of data on hepatic blood flow after surgery, no definite conclusion regarding high CYP3A extraction drugs could be drawn from the simulations.33 The hypothesis of an increased fraction escaping intestinal metabolism (F G) based on an unchanged absolute bioavailability of midazolam was also adequately predicted by the PBPK analysis of Darwich et al. In this study, the F G of the CYP3A substrate simvastatin increased with 13% following RYGBP.90 The increase in F G may be explained by the bypassing of a part of intestine rich in drug‐metabolizing enzymes. The important effect of the small intestine in restricting oral bioavailability of CYP3A drugs was also evident in the studies of atorvastatin.91 When compared with RYGBP, a longer bypass of the intestine with BPD‐DS resulted in increased bioavailability of atorvastatin.49, 50 Only one study investigated the effect of SG on the oral bioavailability of CYP3A substrate (rivaroxaban), with no difference between RYGBP and SG observed.44 However, rivaroxaban shows a relatively low degree of first pass metabolism (ie, high oral bioavailability), and the potential effect of bypassing the small intestine is thus expected to be low compared with CYP3A drugs with low bioavailability such as midazolam and atorvastatin. Additionally, since the elimination of rivaroxaban proceeds through a dual pathway (both renal elimination and metabolic degradation), and the fact that several CYP enzymes are involved in the metabolic degradation,65 this drug is expected to be less vulnerable to the physiological changes associated with bariatric surgery, compared with other drugs where only one elimination pathway dominates. Conversely, a preliminary clinical study investigating direct‐acting oral anticoagulant (DOAC) blood levels in post‐bariatric patients who underwent long‐term anticoagulation therapy, peak drug levels of rivaroxaban were below the expected range in four of six patients who underwent SG.92
Systemic exposure of omeprazole was shown to be decreased following bariatric surgery, potentially because of an increased activity of CYP2C19.47 The CYP2C19 content in the intestine is low relative to that in the liver, suggesting that the intestine contributes minimally to the overall first pass metabolism of CYP2C19 substrates.93 The impact of bypassing part of the intestine on the bioavailability of CYP2C19 drugs may thus be limited. It can be speculated that, similar to CYP3A, the surgery‐induced weight loss may lead to increased hepatic CYP2C19 activity. By contrast, the activity of both CYP2D6 and CYP1A2 seems to be unaltered following bariatric surgery. Similar to CYP2C19, the content of both CYP2D6 and CYP1A2 is low in the intestine, and the intestinal forms of these enzymes are expected to contribute minimally to the overall first pass metabolism of drugs.93 As the activities of CYP2D6 and CYP1A2 appear to be unaltered following surgery, the subsequent weight loss seems to have less impact on the activity of these CYP enzymes compared with CYP3A and CYP2C19. The isoforms within the CYP3A and CYP2C subfamilies are in fact known to be regulated by many common transcription factors, including CAR and PXR, and hepatocyte nuclear factor 4α (HNF4α) is identified as a key regulator of constitutive expression of CYP2D6 and CYP1A2.32, 94, 95 However, the complex interplay of several factors makes the interpretation of these results difficult, with more studies warranted in order to further clarify the impact of weight loss on the different CYP enzymes.
Limited data exist regarding the impact of bariatric surgery on drug transporters.35 Given the overlapping substrate specificity, especially with CYP3A, several of the drugs included in the different studies are also P‐gp substrates (ie, atorvastatin, venlafaxine, rivaroxaban, and morphine). Because of this, the contribution from P‐gp on the oral bioavailability of these drugs is difficult to differentiate from the effect on drug metabolism, and studies using selective probe drugs for single transporters and enzymes are thus needed.
Different modelling and simulation approaches have the potential to examine the impact of drug‐specific characteristics on bioavailability trends following bariatric surgery. In the absence of clinical data, these models may serve as useful tools when studying the effect of physiological alterations on drug pharmacokinetics. PBPK models predicting oral drug exposure and dose adjustment following bariatric surgery have been developed.33, 90 The observed data for immediate and controlled release metoprolol were compared with matched simulations utilizing a previously developed physiology‐based pharmacokinetic RYGBP model.90 The PBPK modelling and simulation predicted values were in agreement with the observed data,36 illustrating the validity of PBPK modelling and simulation in predicting the impact of bariatric surgery on drug exposure. The semi‐PBPK model developed for midazolam is valuable for predicting the effect of surgery of low extraction CYP3A4 drugs.33 However, more data regarding the physiological alterations following bariatric surgery, such liver size and blood flow to both the liver and intestines, need to be included in these models to allow for reliable prediction of potential pharmacokinetic changes for different drugs.
Data on the long‐term effects of bariatric surgery on the oral bioavailability of drugs are limited. Most of the included studies in this review, with a few exceptions,34, 41 have only examined the short‐term effects. In the 2‐year follow‐up study of atorvastatin, the initial increase in atorvastatin systemic exposure was to be reversed back to presurgery conditions.41 This long‐term normalization of atorvastatin pharmacokinetics may be due to an intestinal adaption over time.
Obesity itself may change the drug‐metabolizing properties of the liver. RYGBP is associated with changes in cardiometabolic risk factors that are induced by calorie restriction as well as weight loss–independent effects. It remains uncertain whether pharmacokinetic changes can be attributed to weight loss per se or the surgery‐induced anatomical alterations. To disentangle the effects of these changes after bariatric surgery, patients undergoing bariatric surgery should be compared with patients undergoing nonsurgical calorie restriction. Future studies should also evaluate the long‐term effect of bariatric surgery on drug disposition as well as the time perspective of potentially restored CYP activity.
4.2. Dosing recommendations following bariatric surgery
Several review papers have assessed the challenges related to changes in drug disposition following bariatric surgery and the consequences on drug dosing.11, 12, 13, 14, 15 The complex interplay of various factors potentially linked to drug pharmacokinetics such as solubility, permeability, drug‐metabolizing enzymes and transporters, changes in body weight and composition, inflammatory status, and gut microbiota should be considered in relation to pharmacodynamics and efficacy variables that may change after surgery. The combined effect of all these variables makes it challenging to propose clear recommendations for dose adaption strategies outside of the specifically investigated cases in clinical practice. Further research and models capable of considering all relevant factors linked to both the drug and the patients are warranted. For drugs where a biomarker of efficacy cannot be measured, eg, blood pressure and plasma glucose, therapeutic drug monitoring by drug concentration measurements should be encouraged, especially for drugs with a narrow therapeutic index, in order to avoid toxicity or therapeutic failure following bariatric surgery.
5. CONCLUSIONS
The multifactorial physiological changes after bariatric surgery make extrapolations of dosing recommendation on the basis of major pharmacokinetic profiles impossible at the time being even between drugs mainly metabolized by the same enzyme. The general recommendation for now must therefore be that drug monitoring should be applied in the early postsurgery phase, by the use of assessable efficacy variables, side effects, or drug concentrations. The effect of weight loss per se and the adaptive potential of the intestine over time should however also be considered. Most of the existing data regarding pharmacokinetic changes after bariatric surgery are derived from patients undergoing RYGBP. As conclusions from RYGBP cannot necessarily be extrapolated to patients subjected to other bariatric procedures, dosing consideration is warranted in these patient populations, especially in SG. Future research should have a longer follow‐up time and focus on identifying relevant mechanisms for changes in drug disposition.
CONFLICT OF INTEREST
Philip Carlo Angeles received PhD‐project funding from AstraZeneca. He also received a tuition fee grant from Johnson & Johnson/Ethicon to attend the European Obesity Academy 3.
Supporting information
Supporting info item.
Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: A systematic review. Obesity Reviews. 2019;20:1299‐1311. 10.1111/obr.12869
Philip Carlo Angeles and Ida Robertsen have contributed equally to this work for first authorship. Anders Åsberg and Jøran Hjelmesæth have contributed equally to last authorship.
REFERENCES
- 1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999‐2010. JAMA. 2012;307(5):491‐497. [DOI] [PubMed] [Google Scholar]
- 2. Midthjell K, Lee CM, Langhammer A, et al. Trends in overweight and obesity over 22 years in a large adult population: the HUNT study, Norway. Clin Obes. 2013;3(1‐2):12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523‐1529. [DOI] [PubMed] [Google Scholar]
- 4. Cremieux PY, Ledoux S, Clerici C, Cremieux F, Buessing M. The impact of bariatric surgery on comorbidities and medication use among obese patients. Obes Surg. 2010;20(7):861‐870. [DOI] [PubMed] [Google Scholar]
- 5. Kennedy AL, Nelson T, Pettine S, Miller BF, Hamilton KL, Donovan EL. Medication use following bariatric surgery: factors associated with early discontinuation. Obes Surg. 2014;24(5):696‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42(5):878‐884. [DOI] [PubMed] [Google Scholar]
- 8. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683‐2693. [DOI] [PubMed] [Google Scholar]
- 9. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta‐analysis. Am J Med. 2009;122(3):248‐256 e245. [DOI] [PubMed] [Google Scholar]
- 10. Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783‐3794. [DOI] [PubMed] [Google Scholar]
- 11. Greenblatt HK, Greenblatt DJ. Altered drug disposition following bariatric surgery: a research challenge. Clin Pharmacokinet. 2015;54(6):573‐579. [DOI] [PubMed] [Google Scholar]
- 12. Azran C, Wolk O, Zur M, et al. Oral drug therapy following bariatric surgery: an overview of fundamentals, literature and clinical recommendations. Obes Rev: Off J Int Assoc Study Obes. 2016;17(11):1050‐1066. [DOI] [PubMed] [Google Scholar]
- 13. Yska JP, van der Linde S, Tapper VV, et al. Influence of bariatric surgery on the use and pharmacokinetics of some major drug classes. Obes Surg. 2013;23(6):819‐825. [DOI] [PubMed] [Google Scholar]
- 14. Hachon L, Decleves X, Faucher P, Carette C, Lloret‐Linares C. RYGB and drug disposition: how to do better? Analysis of pharmacokinetic studies and recommendations for clinical practice. Obes Surg. 2017;27(4):1076‐1090. [DOI] [PubMed] [Google Scholar]
- 15. Edwards A, Ensom MH. Pharmacokinetic effects of bariatric surgery. Ann Pharmacother. 2012;46(1):130‐136. [DOI] [PubMed] [Google Scholar]
- 16. Wallden J, Thorn SE, Wattwil M. The delay of gastric emptying induced by remifentanil is not influenced by posture. Anesth Analg. 2004;99(2):429‐434. table of contents [DOI] [PubMed] [Google Scholar]
- 17. Sioka E, Tzovaras G, Perivoliotis K, et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol Res Pract. 2018;2018:4135813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thelen K, Dressman JB. Cytochrome P450‐mediated metabolism in the human gut wall. J Pharm Pharmacol. 2009;61(5):541‐558. [DOI] [PubMed] [Google Scholar]
- 19. Dirksen C, Damgaard M, Bojsen‐Moller KN, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux‐en‐Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346‐e255. [DOI] [PubMed] [Google Scholar]
- 20. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic‐pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38(1):41‐57. [DOI] [PubMed] [Google Scholar]
- 21. Guengerich FP. Cytochrome P‐450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1‐17. [DOI] [PubMed] [Google Scholar]
- 22. Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A‐dependent metabolism. J Pharmacol Exp Ther. 1997;283(3):1552‐1562. [PubMed] [Google Scholar]
- 23. Rao SR. Inflammatory markers and bariatric surgery: a meta‐analysis. Inflamm Res. 2012;61(8):789‐807. [DOI] [PubMed] [Google Scholar]
- 24. Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease‐drug‐drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89(5):735‐740. [DOI] [PubMed] [Google Scholar]
- 25. Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359‐404. [DOI] [PubMed] [Google Scholar]
- 26. Kolwankar D, Vuppalanchi R, Ethell B, et al. Association between nonalcoholic hepatic steatosis and hepatic cytochrome P‐450 3A activity. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2007;5(3):388‐393. [DOI] [PubMed] [Google Scholar]
- 27. Ferslew BC, Johnston CK, Tsakalozou E, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2015;97(4):419‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benet LZ. The role of BCS (biopharmaceutics classification system) and BDDCS (biopharmaceutics drug disposition classification system) in drug development. J Pharm Sci. 2013;102(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ribeiro‐Parenti L, Cavin JB, Le Gall M. Intestinal adaptations following bariatric surgery: towards the identification of new pharmacological targets for obesity‐related metabolic diseases. Curr Opin Pharmacol. 2017;37:29‐34. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cossu ML, Caccia S, Coppola M, et al. Orally administered ranitidine plasma concentrations before and after biliopancreatic diversion in morbidly obese patients. Obes Surg. 1999;9(1):36‐39. [DOI] [PubMed] [Google Scholar]
- 32. Goday Arno A, Farre M, Rodriguez‐Morato J, et al. Pharmacokinetics in morbid obesity: influence of two bariatric surgery techniques on paracetamol and caffeine metabolism. Obes Surg. 2017;27(12):3194‐3201. [DOI] [PubMed] [Google Scholar]
- 33. Brill MJ, Valitalo PA, Darwich AS, et al. Semiphysiologically based pharmacokinetic model for midazolam and CYP3A mediated metabolite 1‐OH‐midazolam in morbidly obese and weight loss surgery patients. CPT Pharmacometrics Syst Pharmacol. 2016;5(1):20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brill MJ, van Rongen A, van Dongen EP, et al. The pharmacokinetics of the CYP3A substrate midazolam in morbidly obese patients before and one year after bariatric surgery. Pharm Res. 2015;32(12):3927‐3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan LN, Lin YS, Tay‐Sontheimer JC, et al. Proximal Roux‐en‐Y gastric bypass alters drug absorption pattern but not systemic exposure of CYP3A4 and P‐glycoprotein substrates. Pharmacotherapy. 2015;35(4):361‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gesquiere I, Darwich AS, Van der Schueren B, et al. Drug disposition and modelling before and after gastric bypass: immediate and controlled‐release metoprolol formulations. Br J Clin Pharmacol. 2015;80(5):1021‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gesquiere I, Hens B, Van der Schueren B, et al. Drug disposition before and after gastric bypass: fenofibrate and posaconazole. Br J Clin Pharmacol. 2016;82(5):1325‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ginstman C, Frisk J, Carlsson B, Arlemalm A, Hagg S, Brynhildsen J. Plasma concentrations of etonogestrel in women using oral desogestrel before and after Roux‐en‐Y gastric bypass surgery: a pharmacokinetic study. BJOG: Int J Obstet Gynecol. 2019;126(4):486‐492. [DOI] [PubMed] [Google Scholar]
- 39. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamilton R, Thai XC, Ameri D, Pai MP. Oral bioavailability of linezolid before and after Roux‐en‐Y gastric bypass surgery: is dose modification necessary in obese subjects? J Antimicrob Chemother. 2013;68(3):666‐673. [DOI] [PubMed] [Google Scholar]
- 41. Jakobsen GS, Skottheim IB, Sandbu R, et al. Long‐term effects of gastric bypass and duodenal switch on systemic exposure of atorvastatin. Surg Endosc. 2013;27(6):2094‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krieger CA, Cunningham JL, Reid JM, et al. Comparison of bioavailability of single‐dose extended‐release venlafaxine capsules in obese patients before and after gastric bypass surgery. Pharmacotherapy. 2017;37(11):1374‐1382. [DOI] [PubMed] [Google Scholar]
- 43. Kroll D, Nett PC, Borbely YM, et al. The effect of bariatric surgery on the direct oral anticoagulant rivaroxaban: the extension study. Surg Obes Relat Dis. 2018;14(12):1890‐1896. [DOI] [PubMed] [Google Scholar]
- 44. Kroll D, Stirnimann G, Vogt A, et al. Pharmacokinetics and pharmacodynamics of single doses of rivaroxaban in obese patients prior to and after bariatric surgery. Br J Clin Pharmacol. 2017;83(7):1466‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lloret‐Linares C, Hirt D, Bardin C, et al. Effect of a Roux‐en‐Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet. 2014;53(10):919‐930. [DOI] [PubMed] [Google Scholar]
- 46. Marzinke MA, Petrides AK, Steele K, et al. Decreased escitalopram concentrations post‐Roux‐en‐Y gastric bypass surgery. Ther Drug Monit. 2015;37(3):408‐412. [DOI] [PubMed] [Google Scholar]
- 47. Mitrov‐Winkelmolen L, van Buul‐Gast MC, Swank DJ, Overdiek HW, van Schaik RH, Touw DJ. The effect of Roux‐en‐Y gastric bypass surgery in morbidly obese patients on pharmacokinetics of (acetyl)salicylic acid and omeprazole: the ERY‐PAO study. Obes Surg. 2016;26(9):2051‐2058. [DOI] [PubMed] [Google Scholar]
- 48. Rocha MBS, De Nucci G, Lemos FN, et al. Impact of bariatric surgery on the pharmacokinetics parameters of amoxicillin. Obes Surg. 2018;10:10. [DOI] [PubMed] [Google Scholar]
- 49. Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86(3):311‐318. [DOI] [PubMed] [Google Scholar]
- 50. Skottheim IB, Jakobsen GS, Stormark K, et al. Significant increase in systemic exposure of atorvastatin after biliopancreatic diversion with duodenal switch. Clin Pharmacol Ther. 2010;87(6):699‐705. [DOI] [PubMed] [Google Scholar]
- 51. Amouyal C, Buyse M, Lucas‐Martini L, et al. Sleeve gastrectomy in morbidly obese HIV patients: focus on anti‐retroviral treatment absorption after surgery. Obes Surg. 2018;28(9):2886‐2893. [DOI] [PubMed] [Google Scholar]
- 52. Kampmann JP, Klein H, Lumholtz B, Molholm Hansen JE. Ampicillin and propylthiouracil pharmacokinetics in intestinal bypass patients followed up to a year after operation. Clin Pharmacokinet. 1984;9(2):168‐176. [DOI] [PubMed] [Google Scholar]
- 53. Ginstman C, Frisk J, Carlsson B, Arlemalm A, Hagg S, Brynhildsen J. Plasma concentrations of etonogestrel in women using oral desogestrel before and after Roux‐en‐Y gastric bypass surgery: a pharmacokinetic study. BJOG: Int J Obstet Gynecol. 2018;126(4):486‐492. [DOI] [PubMed] [Google Scholar]
- 54. Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol. 1973;47(2):415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mohsin K. Design of lipid‐based formulations for oral administration of poorly water‐soluble drug fenofibrate: effects of digestion. AAPS PharmSciTech. 2012;13(2):637‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux‐en‐Y gastric bypass for morbid obesity. Ann Surg. 1993;218(1):91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Needs CJ, Brooks PM. Clinical pharmacokinetics of the salicylates. Clin Pharmacokinet. 1985;10(2):164‐177. [DOI] [PubMed] [Google Scholar]
- 58. Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36(1):89‐96. [PubMed] [Google Scholar]
- 59. Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro‐in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271(1):549‐556. [PubMed] [Google Scholar]
- 60. Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter‐ and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther. 1994;271(1):557‐566. [PubMed] [Google Scholar]
- 61. Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141‐1160. [DOI] [PubMed] [Google Scholar]
- 62. Jacobsen W, Kuhn B, Soldner A, et al. Lactonization is the critical first step in the disposition of the 3‐hydroxy‐3‐methylglutaryl‐CoA reductase inhibitor atorvastatin. Drug Metab Dispos. 2000;28(11):1369‐1378. [PubMed] [Google Scholar]
- 63. Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the Caco‐2 cell model: contributions of P‐glycoprotein and the proton‐monocarboxylic acid co‐transporter. Pharm Res. 2000;17(2):209‐215. [DOI] [PubMed] [Google Scholar]
- 64. Hsiang B, Zhu Y, Wang Z, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver‐specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl‐CoA reductase inhibitor transporters. J Biol Chem. 1999;274(52):37161‐37168. [DOI] [PubMed] [Google Scholar]
- 65. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mueck W, Kubitza D, Becka M. Co‐administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53(5):503‐514. [DOI] [PubMed] [Google Scholar]
- 68. Tang BK, Zhou Y, Kadar D, Kalow W. Caffeine as a probe for CYP1A2 activity: potential influence of renal factors on urinary phenotypic trait measurements. Pharmacogenetics. 1994;4(3):117‐124. [PubMed] [Google Scholar]
- 69. Lasker JM, Wester MR, Aramsombatdee E, Raucy JL. Characterization of CYP2C19 and CYP2C9 from human liver: respective roles in microsomal tolbutamide, S‐mephenytoin, and omeprazole hydroxylations. Arch Biochem Biophys. 1998;353(1):16‐28. [DOI] [PubMed] [Google Scholar]
- 70. Streetman DS, Bertino JS Jr, Nafziger AN. Phenotyping of drug‐metabolizing enzymes in adults: a review of in‐vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10(3):187‐216. [DOI] [PubMed] [Google Scholar]
- 71. Johnson JA, Burlew BS. Metoprolol metabolism via cytochrome P4502D6 in ethnic populations. Drug Metab Dispos. 1996;24(3):350‐355. [PubMed] [Google Scholar]
- 72. Turpault S, Brian W, Van Horn R, et al. Pharmacokinetic assessment of a five‐probe cocktail for CYPs 1A2, 2C9, 2C19, 2D6 and 3A. Br J Clin Pharmacol. 2009;68(6):928‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Otton SV, Ball SE, Cheung SW, Inaba T, Rudolph RL, Sellers EM. Venlafaxine oxidation in vitro is catalysed by CYP2D6. Br J Clin Pharmacol. 1996;41(2):149‐156. [DOI] [PubMed] [Google Scholar]
- 74. Fogelman SM, Schmider J, Venkatakrishnan K, et al. O‐ and N‐demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA‐transfected cells: effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacol: Off Publ Am College Neuropsychopharmacol. 1999;20(5):480‐490. [DOI] [PubMed] [Google Scholar]
- 75. Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab Dispos. 1998;26(1):73‐77. [PubMed] [Google Scholar]
- 76. Ito S, Koren G, Harper PA, Silverman M. Energy‐dependent transport of digoxin across renal tubular cell monolayers (LLC‐PK1). Can J Physiol Pharmacol. 1993;71(1):40‐47. [DOI] [PubMed] [Google Scholar]
- 77. de Lannoy IA, Silverman M. The MDR1 gene product, P‐glycoprotein, mediates the transport of the cardiac glycoside, digoxin. Biochem Biophys Res Commun. 1992;189(1):551‐557. [DOI] [PubMed] [Google Scholar]
- 78. Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P‐glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96(4):1698‐1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fenner KS, Troutman MD, Kempshall S, et al. Drug‐drug interactions mediated through P‐glycoprotein: clinical relevance and in vitro‐in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther. 2009;85(2):173‐181. [DOI] [PubMed] [Google Scholar]
- 80. Roberts CJ. Clinical pharmacokinetics of ranitidine. Clin Pharmacokinet. 1984;9(3):211‐221. [DOI] [PubMed] [Google Scholar]
- 81. Viinikka L, Ylikorkala O, Nummi S, et al. Biological effects of a new and potent progestagen. A clinical study. Acta Endocrinol. 1976;83(2):v429‐438. [DOI] [PubMed] [Google Scholar]
- 82. Korhonen T, Tolonen A, Uusitalo J, Lundgren S, Jalonen J, Laine K. The role of CYP2C and CYP3A in the disposition of 3‐keto‐desogestrel after administration of desogestrel. Br J Clin Pharmacol. 2005;60(1):69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129‐1140. [DOI] [PubMed] [Google Scholar]
- 84. Barza M, Weinstein L. Pharmacokinetics of the penicillins in man. Clin Pharmacokinet. 1976;1(4):297‐308. [DOI] [PubMed] [Google Scholar]
- 85. Ulvestad M, Skottheim IB, Jakobsen GS, et al. Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther. 2013;93(3):275‐282. [DOI] [PubMed] [Google Scholar]
- 86. Yoshinari K, Takagi S, Yoshimasa T, Sugatani J, Miwa M. Hepatic CYP3A expression is attenuated in obese mice fed a high‐fat diet. Pharm Res. 2006;23(6):1188‐1200. [DOI] [PubMed] [Google Scholar]
- 87. Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab Dispos. 2015;43(10):1484‐1490. [DOI] [PubMed] [Google Scholar]
- 88. Banerjee M, Robbins D, Chen T. Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discov Today. 2015;20(5):618‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Immonen H, Hannukainen JC, Iozzo P, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non‐diabetic patients. J Hepatol. 2014;60(2):377‐383. [DOI] [PubMed] [Google Scholar]
- 90. Darwich AS, Pade D, Ammori BJ, Jamei M, Ashcroft DM, Rostami‐Hodjegan A. A mechanistic pharmacokinetic model to assess modified oral drug bioavailability post bariatric surgery in morbidly obese patients: interplay between CYP3A gut wall metabolism, permeability and dissolution. J Pharm Pharmacol. 2012;64(7):1008‐1024. [DOI] [PubMed] [Google Scholar]
- 91. Darwich AS, Pade D, Rowland‐Yeo K, et al. Evaluation of an in silico PBPK post‐bariatric surgery model through simulating oral drug bioavailability of atorvastatin and cyclosporine. CPT Pharmacometrics Syst Pharmacol. 2013;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rottenstreich A, Barkai A, Arad A, Raccah BH, Kalish Y. The effect of bariatric surgery on direct‐acting oral anticoagulant drug levels. Thromb Res. 2018;163:190‐195. [DOI] [PubMed] [Google Scholar]
- 93. Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34(5):880‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wortham M, Czerwinski M, He L, Parkinson A, Wan YJ. Expression of constitutive androstane receptor, hepatic nuclear factor 4α, and P450 oxidoreductase genes determines interindividual variability in basal expression and activity of a broad scope of xenobiotic metabolism genes in the human liver. Drug Metab Dispos. 2007;35(9):1700‐1710. [DOI] [PubMed] [Google Scholar]
- 95. Martinez‐Jimenez CP, Castell JV, Gomez‐Lechon MJ, Jover R. Transcriptional activation of CYP2C9, CYP1A1, and CYP1A2 by hepatocyte nuclear factor 4α requires coactivators peroxisomal proliferator activated receptor‐γ coactivator 1α and steroid receptor coactivator 1. Mol Pharmacol. 2006;70(5):1681‐1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item.


