Abstract
Recent studies suggest that a single bout of exercise can lead to transient performance improvements in specific cognitive domains in children. However, more knowledge is needed to determine the key exercise characteristics for obtaining these effects and how they translate into real‐world settings. In the present study, we investigate how small‐sided football games of either high‐ or moderate‐intensity affect measures of inhibitory control in a school setting. Eighty‐one children (mean age 11.8, 48 boys) were randomly allocated to three groups performing 20‐minute of high‐intensity small‐sided real football games (SRF), moderate‐intensity small‐sided walking football games (SWF) or resting (RF). Behavioral measures of inhibitory control and neurophysiological measures of attention (P300 latency and amplitude) were obtained during a flanker task performed at baseline and 20 minutes following the intervention. Retention of declarative memory was assessed in a visual memory task 7 days after the intervention. Measures of inhibitory control improved more in children performing SRF compared to SWF 19 ms, 95% CI [7, 31 ms] (P = 0.041). This was paralleled by larger increases in P300 amplitudes at Fz in children performing SRF compared both to RF in congruent (3.54 μV, 95% CI [0.85, 6.23 μV], P = 0.039) and incongruent trials (5.56 μV, 95% CI [2.87, 8.25 μV], P < 0.001) and compared to SWF in incongruent trials (4.10 μV, 95% CI [1.41, 6.68 μV], P = 0.010). No effects were found in measures of declarative memory. Together this indicates that acute high‐intensity small‐sided football games can transiently improve measures of inhibitory control and neurophysiological correlates of attention. Intense small‐sided football games are easily implementable and can be employed by practitioners, for example, during breaks throughout the school day.
Keywords: acute exercise, declarative memory, electroencephalography, inhibitory control, physical activity, school, small‐sided football games
1. INTRODUCTION
Recent trends in exercise‐cognition research suggest that a single bout of exercise could serve as a potent strategy for transiently improving performance in specific tests of different cognitive domains in children. In particular, performance in tests assessing inhibitory control and memory functions has been found to be malleable through a single bout of physical activity (for reviews, see Refs 1, 2, 3).These cognitive domains are critically important for everyday functioning. For example, academic aptitude is positively associated with performance in tests assessing inhibitory control and declarative memory.4 Hence, improving inhibitory control and declarative memory functions could ultimately lead to greater learning in children during the school day.
While an endogenous facilitation of cognitive processes through physical activity is interesting, current evidence on the behavioral effects and physiological underpinnings is still sparse. In relation to behavioral effects, recent studies have attempted to disentangle key influencing factors associated with effective cognitive enhancement, including the timing, intensity, and type of the physical exercise. Potentially, by manipulating these factors it would be possible to tailor exercise interventions for promotion of cognitive performance. For example, performing an exercise bout before tasks requiring inhibitory control could improve on‐task inhibitory control,5 while scheduling the exercise bout after a memory task might strengthen the offline processing of a newly encoded memory trace, leading to improved memory retention.6 Thus, the placement, that is, timing, of the exercise bout is essential to consider when aiming at improving cognitive performance. Interestingly, the timetable of the school day consists of academic classes interspersed with breaks. These naturally occurring breaks provide possible windows of opportunities throughout the school day that could be capitalized to promote cognitive functions by implementing physical activities. However, to harvest these potential effects, the exercise bouts must be optimally designed in terms of the exercise characteristics, for example, the intensity and type.
In relation to exercise intensity, a meta‐analysis by Chang et al5 found that acute bouts of moderate‐intensity exercise (64%‐74% of HRmax) could transiently improve executive functions, including inhibitory control. However, the thorough evidence‐synthetization also revealed that, in general, less focus has been given to investigating the effects of higher‐intensity exercise bouts on behavioral indices of cognitive functions.5 Recently, however, some studies have focused on this shortcoming. Kao et al7 demonstrated that both high‐ and moderate‐intensity exercise performed prior to a flanker task decreased the flanker interference effect, reflecting improved inhibitory control. The evidence is not univocal, though, as other studies have found a selective effect for moderate‐ but not high‐intensity exercise.8 There is, therefore, still some ambiguity surrounding the effects of high‐intensity exercise on tests assessing inhibitory control. In relation to memory functions, a large body of studies has demonstrated that memory processes are malleable through acute exercise interventions (reviewed in Ref. 1). Acute exercise might foster memory encoding,9 that is, the process where information is encoded as a memory trace, and memory consolidation,10 a multifaceted process in which a memory trace is stabilized and strengthened. Exercise intensity may play an essential moderating role in the exercise‐memory relationship. Indeed, a linear dose‐response relationship was found favouring higher‐intensity exercise performed immediately after practice in the long‐term retention (1‐7 days) of a motor memory task.11 On the other hand, a recently published study found similar effects between moderate‐intensity and high‐intensity exercise in a declarative word‐list memory task.12 However, the latter study did not assess long‐term memory performance, warranting the need for additional studies of the effects of acute exercise on long‐term declarative memory.
Until recently, most studies assessing the effects of acute exercise bouts on cognitive functions have focused on simple physical activities such as walking or running,12, 13 cycling8, 14, 15 or certain singled‐out aspects of sports, for example, practising decontextualised sports‐related skills.16 Less is known about the effects of participating in sports, but a few studies have emerged with positive preliminary findings.16, 17 Sports in general and team sports specifically require decision‐making and complex motor control in a dynamic environment, which additionally involve collaborative social interactions. At the same time, they can be motivating and emotionally stimulating.18 As argued by Diamond and Ling,19, 20 these could be key factors influencing whether cognitive improvements are observed or absent. Worldwide, football is one of the most commonly practiced team sports measured by number of registered players. Small‐sided football games (eg, 3v3) are a feasible alternative that is well suited for school settings, with variations naturally occurring in school playgrounds during break times. Small‐sided football games are easily implemented in school settings, and active participation is generally high for both boys and girls irrespective of prior football experience and body mass index (BMI).21 Moreover, the number of successful actions is higher in small‐sided football games (3v3 and 5v5) than in larger‐sided games (eg, 8v8 or 11v11).22 Thus, small‐sided football games could be a relevant type of activity facilitating both positive experiences and active participation. The effects of small‐sided football games on inhibitory control and memory processes are sparsely elucidated and have only been assessed through longer exercise interventions involving more than one session.23 Furthermore, the intervention adopted in this study was multifaceted, comprising high‐intensity small‐sided games, coordinative demanding low‐intensity football drills and health education, thus limiting the ability to pinpoint exactly what caused the changes in cognitive test performance. This warrants more well‐controlled studies of the effects of ballgames in general, and football in particular, on neurocognitive performance and brain functions to disentangle contributions from different potential moderators. Here, we specifically aim to evaluate the effects of the exercise intensity of small‐sided football games on inhibitory control and declarative memory performance.
While some studies have manipulated exercise characteristics and directly probed the behavioral manifestations, others have focused on uncovering potential underlying neurophysiological correlates. The findings have revealed that single bouts of physical activity may modulate brain functions, indicated by transient changes in the latency and amplitude of the P300—an event‐related potential (ERP) in the electroencephalogram (EEG). The P300 is a centrally evoked positive potential most commonly observed approximately 300‐700 ms post‐stimulus (for review, see Ref. 24). The P300 reflects the effective task‐related allocation of attention, with amplitudes reflecting the amount of neural resources allocated and latencies reflecting the neural processing speed.24 Results accumulated over the past decade suggest that the P300 component is molded by acute exercise. Modifications of the P300 have been sparsely explored in relation to ballgames, and more effort is warranted to investigate such activities. Moreover, effects on neurophysiological underpinnings are yet to be investigated in real‐world settings, as previous endeavors have largely been laboratory based.7, 12, 13 Thus, it remains uncertain whether early findings generalize to ecologically valid settings, for example, schools. Probing if this translational link can be established is the crucial next step for researchers in order to develop evidence‐based guidelines for the implementation of physical activity to promote physical and mental functioning in schools. Collectively, this warrants further research to clarify the role of physical activity in general, and ballgames in particular, as a strategy for improving neural underpinnings of cognitive processing in children in natural settings.
The aim of this study was therefore to investigate the effects of a single bout of small‐sided football games on inhibitory control and neurophysiological measures of attention and declarative memory in young children in a school setting. The hypotheses were that (I) moderate‐intensity small‐sided ball games result in improved immediate performance in tests assessing inhibitory control and neurophysiological underpinnings of attention compared to high‐intensity small‐sided ballgames and a resting control group. (II) high‐intensity small‐sided ballgames result in improved performance in tests assessing declarative memory compared to a resting control group and moderate‐intensity small‐sided ballgames.
2. METHODS
2.1. Participants
Based on previous studies investigating the effects of acute exercise on performance in cognitive tests,25 we invited four fifth‐grade classes (100 children, aged 11‐12) from a Danish public located in the greater Copenhagen area to participate in this study. Teachers, parents, and children were provided with comprehensive written and oral information about the study, and 81 children (48 boys) with a mean age of 11.8 years were included in the study after obtaining written consent from their legal guardians (see Table 1 for demographic characteristics). The study was approved by the Ethical Committee of Copenhagen and Southern Denmark (H‐16026885, appendix 59349) and carried out in accordance with the Helsinki Declaration II. The study was designed as a randomized controlled trial stratified on gender and prior football experience. Randomization was done by using an online randomizer (randomizer.org) ensuring that an equal number of children from each school class were placed randomly in either a small‐sided real football group (SRF), small‐sided walking football group (SWF), or a resting football group (RF) (Figure 1).
Table 1.
Characteristics of all children combined and by resting football (RF), small‐sided walking football (SWF), and small‐sided real football (SRF)
| All | SRF | SWF | RF | |
|---|---|---|---|---|
| Number of participants (n) | 81 (48 boys) | 27 (16 boys) | 27 (16 boys) | 27 (16 boys) |
| Age (years) | 11.8 (11.7, 11.9) | 11.7 (11.6, 11.9) | 11.8 (11.7, 12) | 11.9 (11.8, 12.1) |
| Body height (cm) | 152.5 (150.9, 154.1) | 152.0 (148.7, 155.4) | 153.1 (150.4, 155.9) | 152.3 (150.2, 154.5) |
| Boy weight (kg) | 44.2 (42.1, 46.4) | 43.4 (39.7, 47.2) | 44.3 (40.6, 48.1) | 44.8 (41.1, 48.6) |
| Body fat percentage (%) | 23.1 (21.2, 25.1) | 24.0 (20.5, 27.6) | 21.7 (18.8, 24.7) | 23.66 (19.9, 27.4) |
| Fitness status (m) | 1023 (904, 1143) | 1018 (846, 1191) | 1048 (798, 1299) | 1007 (792, 1223) |
| Prior football experience (arbitrary score) | 2.4 (1.7, 3.2) | 2.3 (1.2, 3.5) | 2.2 (1.1, 3.4) | 2.4 (1.3, 3.6) |
| Socioeconomic status (social class) | 2.8 (2.4, 3.2) | 2.5 (2.0, 3.2) | 2.8 (2.3, 3.5) | 3.0 (2.5, 3.6) |
Data reported as raw mean values and 95% CI. No significant between‐group differences were observed for any of the measures (P < 0.05).
Figure 1.
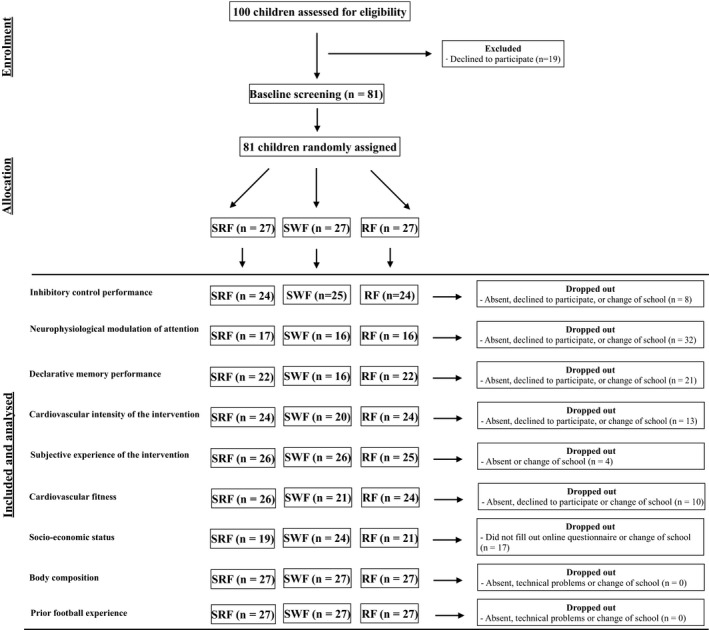
Flow diagram of the study. RF, resting football; SRF, small‐sided real football; SWF, small‐sided walking football
2.2. Design
The study was carried out between February 2018 and April 2018 over four individual test days: two baseline screening days, a main experiment day, and a delayed follow‐up test day (for details, see Figure 2). The baseline screening consisted of a test battery addressing the children's performance in tests assessing inhibitory control and declarative memory (set 1), as well as body composition, cardiovascular fitness, socioeconomic status (SES), and prior football experience. The main experiment included acquisition of pictures (used to assess declarative memory [set 2]), an acute intervention consisting of 2 × 10 minutes of activity separated by a 5 minutes break. The main experiment day was counterbalanced for weekday and time of day for all three intervention groups (Figure 3). Shortly after the acute intervention, an immediate follow‐up test assessed the children's performance in tests of inhibitory functions. During this assessment, the children also participated in supplemental physiological measurements of the modulation of attention, but due to technical issues, only data from 2/3 of the children were available for analysis. Measurements to quantify individual exercise intensity and the children's subjective experience of the acute intervention were performed during and immediately after the acute intervention. The delayed follow‐up test addressed the children's declarative memory (set 2).
Figure 2.
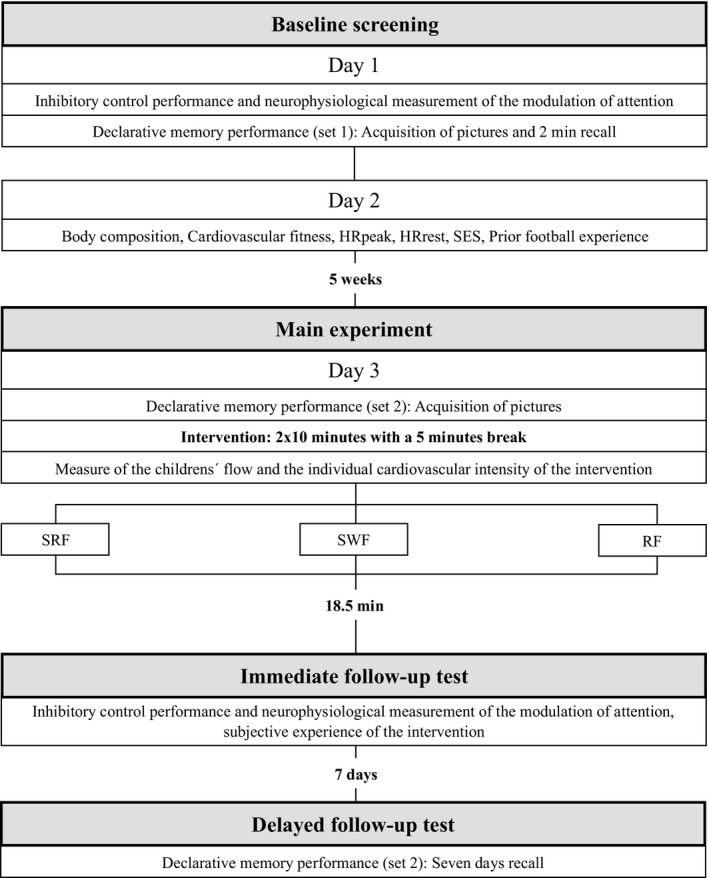
Schematic illustration of the study design. The children reported to the experimenters on four separate days. Days 1 and 2 comprised baseline screening. Day 3 comprised the main experiment, including the intervention, and immediate follow‐up testing. Day 4, 7 d after the main experiment, comprised delayed follow‐up testing. HRpeak, highest heart rate value registered during YYIR1C; HRrest, average heart rate in a 30‐s interval split by the lowest value registered during a 10‐min supine rest period; RF, resting football; SES, socioeconomic status; SRF, small‐sided real football; SWF, small‐sided walking football
Figure 3.
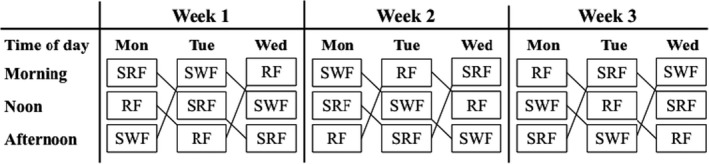
Schematic illustration of the intervention test design. The intervention test design was counterbalanced by weekday and time of day. RF, resting football; SRF, small‐sided real football; SWF, small‐sided walking football
2.3. Interventions
The interventions consisted of 2 × 10 minutes activity periods separated by a 5‐minute break. Four trained staff members administered the interventions. The SRF and SWF consisted of a 3v3 football session on a 6 × 16 m asphalt pitch with surrounding boards. All 3v3 sessions were controlled for an equal number of girls and boys on each team (1 girl and 2 boys or 2 girls and 1 boy). As our equipment only allowed three children to be tested per session, one team acted as opponents during the 3v3 football session without any testing. Each child therefore had to participate in two sessions (test session and opponent session), but to avoid that fatigue could interfere with test data, the first session for the children was the testing session. In addition, the sessions were separated by at least one day.
2.3.1. Small‐sided real football
The SRF intervention was designed to elicit on moderate to high (70%‐100% of HRreserve) cardiovascular exercise intensity. For a goal to count, all members of the team in possession of the ball had to be on the opponents' half of the pitch. This rule was based on pilot studies and aimed at avoiding a stationary goalkeeper (low cardiovascular exercise intensity) and eliciting higher activity levels. In addition, previous studies in Danish schoolchildren have also shown that 3v3 elicits high cardiovascular exercise intensity.21 Furthermore, having boards around the pitch has also been shown to further elicit high cardiovascular exercise intensity in young males.26
2.3.2. Small‐sided walking football
The SWF intervention was identical to SRF except for the cardiovascular demands. SWF focused on a low to moderate (60%‐80% of HRreserve) cardiovascular exercise intensity. Restricting children only to walk aimed to achieve the desired exercise intensity. This rule was based on both pilot studies and previous literature documenting that football with continuous walking can elicit low to moderate cardiovascular intensity in young children.27
2.3.3. Resting football group
The RF watched a taped recording of a SRF football session (taped during the present study) on a 13‐inch laptop in groups of three children (1 girl and 2 boys or 2 girls and 1 boy). The children were placed on a row of three chairs in front of the laptop in a temporary laboratory in a separated, isolated, and quiet room at the school facility. The children were encouraged to interact verbally with each other throughout the intervention about the content shown on the laptop.
2.4. Test procedures
A comprehensive test battery was administered assessing the interventions effects (effect measures), measurements to quantify the interventions (quantification of the interventions) and measurements of the participant characteristics.
2.4.1. Outcome measures
Cognitive test performance and neurophysiological modulation of attention
Cognitive testing was conducted on three children (one intervention group) at a time in an isolated and quiet room at the school facility. Each child was placed at one of three experimental setups separated with room dividers to ensure minimal contact between the children during the session. Each child was assigned an individual experimenter, who administered the tests. To ensure correct timing of all test events between the three experimental setups, a fourth experimenter supervised and time managed each test session. It was ensured that no scheduled physical activity was carried out before the children completed the cognitive tests.
Inhibitory control
To address the children's inhibitory control, a computer‐based version of the modified Eriksen flanker task was used.28 This modified version has previously been used in preadolescent children.29 The children were comfortably placed 40 cm in front of a 15.6‐inch laptop with their elbows resting on the table and their index fingers placed on the two response bottoms (ctrl and enter) and with the individually assigned experimenter on a chair to their left side. The laptop presented the stimuli using Eprime (Psychology Software Tools, Inc). The stimuli presented were either congruent (compatible) or incongruent (incompatible) trials. Congruent and incongruent trials require the children to press the response button corresponding to the direction of a centrally presented target arrow. Congruent trials consisted of an array of five arrows facing in the same direction (eg, < < < < < or > > > > >), and incongruent trials consisted of the flanking arrows facing in the opposite direction than the target arrow (eg, < < > < < or > > < > >). After detailed instructions had been given, 20 practice trials were performed prior to the start of testing. The experimenter supervised the children to ensure correct understanding of the task, and if the children performed below 60% correct trials another 20 practice trials were performed. After completing the practice trials, the children were presented with two blocks of 96 trials, separated by a 2.5 minutes break. The two blocs of 96 trials were presented in a pseudo‐randomised sequence with an equiprobable frequency and directionality of congruent and incongruent stimuli. The stimuli were five 2.5 cm tall white arrows presented focally for 120 ms on a black background with a 1000 ms response window and a variable inter‐stimulus interval of 1100, 1300, or 1500 ms The total duration of the task was 7.5 minutes. The children's accuracy and response latency for correct trials were logged for both congruent and incongruent trials and used as outcome measures. In addition, interference effect (ie, flanker‐effect: estimates of inhibitory control performance) for accuracy (congruent trials − incongruent trials) and response time (incongruent trials − congruent trials) was computed and used as outcome measures. For data analysis purposes, responses faster than 200 ms were considered as anticipatory and excluded. Furthermore, children with an accuracy below 60% for all trials were also excluded from analysis.
Neurophysiological modulation of attention
To assess the neurophysiological modulation of attention underlying inhibitory control performance, electroencephalographic (EEG) activity was recorded at nine electrode sites (POz, P3, P4, Cz, C3, C4, Fz, F3 and F4) labeled according to the International 10‐20 System using the B‐alert x10 wireless system (Advance Brain Monitoring, Inc) during the flanker task. Conductive gel was placed on each foam electrode and prior to conducting the testing, and the impedance of all electrodes was <40 kΩ. Continuous data were referenced online to the mastoids and digitised at a sampling rate of 256 Hz. Offline processing was carried out in Matlab (R2017a; Mathworks Inc), using the EEGlab (version 14.1.1b) and ERPLAB toolboxes (version 7.0.0). Continuous data were filtered using a basic FIR filter with a lower edge of the frequency passband at 1 Hz and a higher edge of the frequency passband at 30 Hz. Stimulus‐locked EEG epochs during the flanker task were created from −100 to 1000 ms around the stimulus presentation and baseline‐corrected using a −100 to 0 ms pre‐stimulus period. Independent component analysis (ICA) was performed on the epochs and prototypical ICA components representing eye movement and blinks were removed.30 To control for DC drifts in the epochs, linear‐detrended across the whole epoch was applied.12 Furthermore, epochs were rejected if either a response error occurred, or an artifact was identified by a simple voltage filter with a ±75 μV threshold. Correct response epochs without artifacts were averaged for trial type and group (SRF congruent/incongruent: 86.2 ± 1.3/75.7 ± 1.7; SWF congruent/incongruent: 86.4 ± 1.7/75.7 ± 2.3; RF congruent/incongruent: 87.4 ± 1.4/77.6 ± 2.1). The amplitude and latency for P3 for sites Fz, Cz and POz were quantified and logged using the peak latency and peak amplitude within a 50 ms interval surrounding the largest ongoing positive peak within a fixed 300‐700‐ms post‐stimulus presentation latency window. Due to technical problems, one of the three EEG systems did not record the timing of stimuli making subsequent data analyses impossible for 1/3 of the children.
Declarative memory
Declarative memory test performance was assessed using a custom‐made, tablet‐based visual memory task (VMT). The children were seated on a chair with a 10.1‐inch tablet (Lenovo®) in their hands. The experimenter carefully explained the task to the children before initiating the task. In the first part of the task, children were presented with 40 gray‐scaled acquisition pictures (AP) for 3 seconds each and instructed to remember as many of the APs as possible. The second part consisted of a recall task. The children were presented with the same 40 APs as before but mixed with 80 new gray‐scaled mixed pictures (MP), giving 120 total pictures (TP) presented. The children then had to indicate, with no time limit, by pressing either yes or no on the tablet screen whether the picture presented was one of the 40 APs. The VMT consisted of two sets of 120 comparable pictures. One set (set 1) was used at the baseline screening with a retention interval of 2 minutes between exposure to the acquisition pictures and the recall test to estimate the children's immediate declarative memory. The other set (set 2) was applied (40 APs) just before the beginning of the acute intervention (Figure 2) and then recalled 7 days after the intervention at the delayed follow‐up test. This was used to estimate the children's delayed declarative memory. All pictures used in the VMT were selected from the International Affective Picture System Database (IAPS) (Center for the Study of Emotion & Attention, Gainesville, USA), which has been validated and used in previous studies in children.31 IAPS pictures contain motive‐groups of animals, objects, humans, and scenes and are evaluated by valence and arousal. Previous studies have shown that strongly evoked emotion can affect memory performance.32 Thus, only pictures with neutral valence and low arousal were used in the VMT. To avoid priming by a certain motive group, the 40 AP and the 80 MP were counterbalanced with the same amount of pictures from each motive group.33 Furthermore, based on previous pilot and actual studies, the pictures were gray‐scaled from their original color, because colored pictures can elicit a larger positive effect on declarative memory than gray‐scaled pictures.34 From the VMT, the percentage of correct answers for TP, AP and MP were logged for both the baseline screening and the 7‐day follow‐up test.
Quantification of exercise intensity and subjective experience of the intervention
Cardiovascular exercise intensity during the intervention
To assess cardiovascular exercise intensity during the intervention, all children were equipped with heart rate monitors (Polar Team 2 System, Polar) that recorded their heart rate at 1 second intervals during the intervention. Individual heart rate during the interventions was compared to their heart rate reserve (HRreserve) calculated as peak heart rate (HRpeak) − resting heart rate (HRrest). Times spent during the intervention at 0%‐60% >60%‐70%, >70%‐80%, >80%‐90%, and >90%‐100% of HRreserve were used as outcome measures. HRpeak was determined as the highest value registered during the Yo‐Yo intermittent recovery level 1 children's test (YYIR1C). HRrest was determined as the average heart rate in a 30‐second interval split by the lowest value registered during a 10‐minute supine rest period.
Subjective experience of the intervention
Various questionnaires were used to assess the children's subjective experience of the intervention. Their psychological state (flow) during the intervention was assessed using a Danish translation of a flow questionnaire35 during the 5‐minute break between the two 2 × 10 activity periods. Measures of total flow and subscales of smoothness, absorption and worry were used as outcome measures. To assess the children's subjective experience of the intervention, the Intrinsic Motivation Inventory (IMI)36 was applied immediately after the intervention. Subscales of enjoyment perceived competence, effort, pressure and perceived choice were computed and used as outcome measures. To further explore the children's enjoyment of the intervention, the Physical Activity Enjoyment Scale (PACES)37 questionnaire was administered immediately after the intervention. A measure of total enjoyment was computed and used as an outcome measure.
Participant characteristics
Body composition
Measurements of body composition were collected and administered by trained staff members. Body weight (kg), lean body mass (kg), and body fat percentage (% fat) were measured using an InBody 230 multifrequency body composition analyzer (Biospace). Body height (cm) was assessed with 0.5 cm precision using a Tanita Leicester portable altimeter (Tanita).
Cardiovascular fitness
The children's cardiovascular fitness was estimated using the YYIR1C, which have previously been validated and used to estimate cardiovascular fitness in preadolescent children.38 The YYIR1C was conducted indoors on one‐half of a wooden‐floor handball court. The YYIR1C consisted of running bouts of two shuttle runs back and forth between cones placed 16 m apart (start/finish cone and turning cone) at a progressively increasing speed interspersed by 10 seconds of jogging after each running bout around a cone placed 4 m behind the start/finish cone. Total running distance (m) was recorded as a measure of cardiovascular fitness.
Socioeconomic status
The SES of the children was estimated using an online modified version of the Danish Occupational Social Class Measurement (DOSC) questionnaire, which was completed by the children's parents. The DOSC measure is based on assessments of the occupational skills and competencies necessary for the job as well as the power and control associated with the position.
Football experience
The children's football experience was assessed using a two‐question questionnaire. First, the children were asked if they were or had been a member of a football club. Depending on the child's answer (yes or no), the child was then asked how many years they had played for. A combined prior football experience‐score was calculated as member of a football club (yes = 1 and no = 0) plus number of years played and used as outcome measure.
2.5. Statistical analysis
All statistical analyses were carried out in the open access software R Studio (R Core Team). Data are reported as raw means with SEM, except for the measures of neurophysiological modulation of attention that are presented as baseline‐corrected values from the ERP plots (Figure 4) with SEM. Linear mixed models were used to assess the statistical significance of the hypotheses under investigations using the lme4 package.39 The effect measure of inhibitory control performance was analyzed with a 3 (group: SRF, SWF, RF) × 2 (time: baseline, follow‐up) × 2 (congruency: congruent, incongruent) linear mixed model for accuracy and reaction time, respectively. The interference effect for accuracy and reaction time was analyzed with a 3 (group: SRF, SWF, RF) × 2 (time: baseline, follow‐up) linear mixed model. Furthermore, to investigate the modulation of attention underlying inhibitory control performance 3 (group: SRF, SWF, RF) × 2 (time: baseline, follow‐up) × 2 (congruency: congruent, incongruent) linear mixed models were fitted for the P3 amplitude and P3 latency, respectively, at site; Fz, Cz, and POz. Analysis of VMT was conducted with a 3 (group: SRF, SWF, RF) × 2 (time: immediate recall, delayed recall) × 3 (type: AP, MP, TP) linear mixed model for accuracy.
Figure 4.
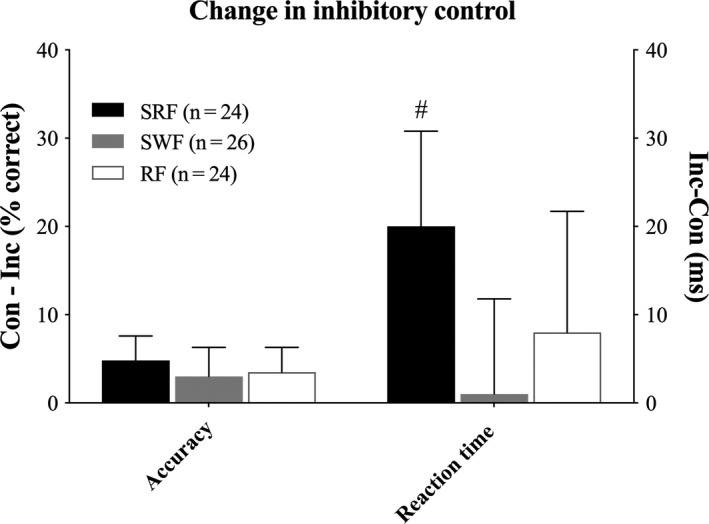
Change in inhibitory control after the intervention. Change from baseline to follow‐up in the interference scores for accuracy and reaction time for small‐sided real football (SRF), small‐sided walking football (SWF), and resting football (RF). #Significant difference from SWF (P < 0.05). Data are presented as means and 95% CI
Quantification of the interventions' cardiovascular exercise intensity was analyzed using a 3 (group: RF, SWF, SRF) × 5 (zone: 0%‐60%, >60%‐70%, >70%‐80%, >80%‐90%, >90%) linear mixed model for percentage of time spent in heart rate zones. Furthermore, to quantify the subjective experience of the intervention a 3 (group: SRF, SWF, RF) × 3 (questionnaire: Flow, IMI, PACES) × 10 (category: smoothness, absorption, worry, total flow, enjoyment, competence, effort, pressure, choice, PACES) linear mixed model for the children's subjective score was used. Between‐group differences in age, body height, body weight, body fat percentage, fitness status, football experience and SES were analyzed using model‐based t tests; the distribution of gender was analyzed using a chi‐squared test. All linear mixed models had age as an additional fixed effect. Furthermore, to account for dependencies between measurements on the same subject and sex, these were added as random intercepts. Model validation was based on visual inspection of residual plots and normal probability plots. Specific contrast between interventions groups across time‐points was considered and analyzed using global F tests to investigate the hypotheses of this study. Furthermore, linear mixed model‐based t tests were used for pairwise comparisons for between‐group differences and within‐group differences at and between specific time‐points. The pairwise comparisons were adjusted for multiplicity using the “single‐step” adjustment, which utilizes the correlations between test.40 A significance level of 0.05 was applied.
3. RESULTS
3.1. Outcome measures
The raw means from the baseline and follow‐up tests for performance in the test of inhibitory control and declarative memory as well as the neurophysiological measurement are presented in Table 2 and Figures 4 and 5.
Table 2.
Data from tests assessing inhibitory control, declarative memory, and neuroelectric modulation of attention for resting football (RF), small‐sided walking football (SWF), and small‐sided real football (SRF)
| Measure | SRF | SWF | RF | |||
|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | Baseline | Follow‐up | |
| Inhibitory control performance | ||||||
| Accuracy (% correct): congruent trials | 93.6 (78.9, 108.4) | 94.8 (85.4, 104.2) | 94.3 (80.1, 108.7) | 96.2 (85.6, 107) | 92.0 (74.3, 109.9) | 95.5 (81.2, 110) |
| Accuracy (% correct): Incongruent trials | 80.6 (59, 102.3) | 86.2* (68.3, 104.2) | 81.1 (58.2, 104) | 85.8 (63.1, 108.6) | 82.2 (61.8, 102.7) | 89.5* (71.1, 108) |
| Response latency (ms): congruent trials | 484 (351, 618) | 498 (365, 632) | 478 (362, 595) | 477 (357, 597) | 496 (361, 632) | 524* (399, 650) |
| Response latency (ms): incongruent trials | 561 (403, 720) | 555 (406, 704) | 543 (391, 696) | 543 (425, 662) | 563 (401, 726) | 584 (440, 728) |
| Interference effect for accuracy | 13.4 (9.9, 17) | 8.6* (6.1, 11.2) | 13.3 (10.2, 16.5) | 10.3 (6.8, 13.9) | 9.5 (6.4, 12.7) | 6.0 (3.5, 8.6) |
| Interference effect for reaction time | 77 (66, 89) | 57* (48, 67) | 65 (52, 79) | 66 (59, 74) | 67 (54, 81) | 59 (46, 73) |
| Declarative memory performance | ||||||
| Acquisition pictures (% correct) | 76.4 (47.1, 105.8) | 51.5* (21.3, 81.8) | 69.4 (38.2, 100.8) | 50.3* (18.3, 82.4) | 71.6 (40.7, 102.6) | 49.1* (18.7, 79.6) |
| Neutral pictures (% correct) | 98.2 (93.8, 102.7) | 84.5* (64.1, 105) | 94.5 (73.2, 116) | 85.4* (58.6, 112.3) | 98.1 (92.7, 103.7) | 87.5* (83.6, 91.5) |
| Total pictures (% correct) | 90.9 (80.4, 101.6) | 68* (53.2, 82.9) | 85.6 (66, 105.4) | 67.8* (52.6, 83.1) | 89.3 (78.7, 100.0) | 68.5* (54.8, 82.3) |
| Neuroelectric modulation of attention | ||||||
| Amplitude Fz congruent trials (μV) | 1.5 (−2.1, 5.1) | 4.4*, # (−0.4, 9.2) | 1.0 (−3.4, 5.4) | 1.6 (−2.6, 5.8) | 2.7 (−0.3, 5.7) | 3.5 (−0.3, 7.3) |
| Amplitude Cz congruent trials (μV) | 8.1 (1.1, 15.2) | 9.5* (2.1, 17) | 7.8 (1.2, 14.5) | 7.7 (1.3, 14.2) | 8.4 (2.4, 14.5) | 8.1 (2.5, 13.8) |
| Amplitude Poz congruent trials (μV) | 17.3 (7, 27.7) | 17.5 (7.9, 27.2) | 14.1 (4.7, 23.6) | 15.0 (6.2, 23.9) | 12.6 (4.4, 20.9) | 13.2 (5.8, 20.7) |
| Amplitude Fz incongruent trials (μV) | 2.1 (−1.9, 6.1) | 4.7*, # , § (−0.4, 9.8) | 1.5 (−4.2, 7.2) | 2.0 (−2.6, 6.6) | 4.0 (−0.4, 8.4) | 3.3 (−1.3, 7.9) |
| Amplitude Cz incongruent trials (μV) | 11.1 (2.1, 20.2) | 11.7 (1.9, 21.5) | 10.1 (1.5, 18.8) | 9.9 (9.4, 10.5) | 11.6 (5, 18.3) | 10.5 (2.7, 18.4) |
| Amplitude Poz incongruent trials (μV) | 16.3 (5.6, 27.1) | 15.3 (5.4, 25.3) | 14.1 (4.2, 24.1) | 14.6 (5.4, 23.9) | 12.9 (3.5, 22.4) | 12.4 (4.8, 20.1) |
| Latency Fz congruent trials (ms) | 499 (297, 701) | 462* (324, 601) | 503 (310, 697) | 508 (335, 682) | 446 (286, 607) | 480 (336, 625) |
| Latency Cz congruent trials (ms) | 494 (319, 669) | 467 (346, 590) | 508# (350, 667) | 495 (331, 660) | 446 (299, 593) | 483 (318, 649) |
| Latency POz congruent trials (ms) | 404 (292, 516) | 396 (296, 497) | 418 (299, 538) | 406 (311, 502) | 416 (312, 522) | 403 (318, 490) |
| Latency Fz incongruent trials (ms) | 508 (340, 677) | 536 (403, 671) | 514 (376, 653) | 526 (361, 692) | 517 (365, 671) | 551 (437, 666) |
| Latency Cz incongruent trials (ms) | 506 (428, 586) | 489 (366, 612) | 511 (403, 620) | 500 (343, 658) | 531 (416, 647) | 528 (389, 668) |
| Latency POz incongruent trials (ms) | 457 (327, 587) | 439 (325, 554) | 504 (359, 649) | 438* (329, 548) | 487 (372, 603) | 400 (320, 561) |
Data for inhibitory control and declarative memory are reported as raw mean values and 95% CI. Data for neuroelectric modulation of attention are reported as baseline‐corrected values from Figure 5.
Significant difference from baseline value.
Significant difference from RF.
Significant difference from SWF (P < 0.05).
Figure 5.
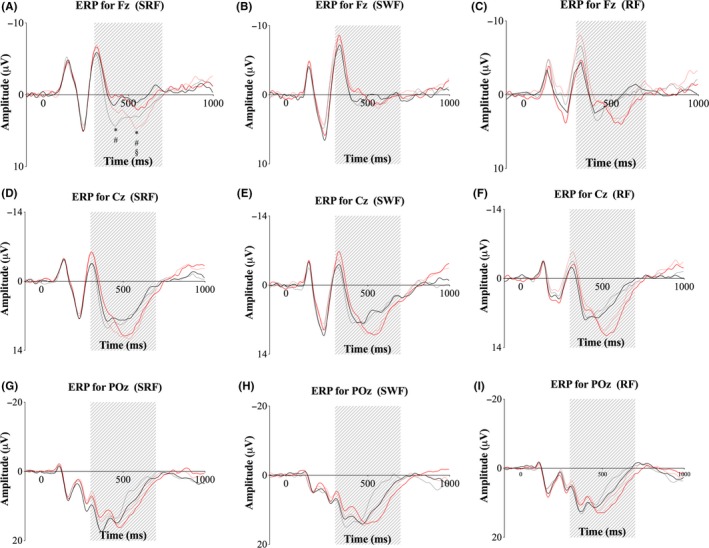
Neurophysiological modulation of attention. ERPs at site Fz, Cz, and POz for small‐sided real football (SRF) (A, D, G), small‐sided walking football (SWF) (B, E, H), and resting football (RF) (C, F, I). Black solid line = congruent trials for baseline. Black dotted line = congruent trials for follow‐up. Red solid line = incongruent trials for baseline. Red Dotted line = incongruent trials for follow‐up. All plots are baseline corrected from −100 to 0 ms pre‐stimulus presentation. The gray shaded area shows the latency window (300‐700 ms) used for analysis. *Significant difference from baseline value. #Significant difference in changes from baseline to follow‐up from RF. §Significant difference in changes from baseline to follow‐up from SWF (P < 0.05)
3.1.1. Inhibitory control
Analysis of flanker accuracy showed an interaction of group × time × congruency [F (6,277) = 6.28, P < 0.001]. Model‐based t tests revealed improvements from baseline to follow‐up for incongruent trials for SRF 5.39%, 95% CI [2.16, 8.62] (P = 0.006) and for RF 6.57%, 95% CI [3.34, 9.80] (P < 0.001) and a general higher accuracy for congruent trials compared to incongruent trials 9.56%, 95% CI [8.15, 10.97] (P < 0.001). However, no between‐group differences in the change in accuracy were observed. Analysis of the interference effect of accuracy showed an interaction of group × time [F (3,139) = 4.83, P = 0.003]. Model‐based t tests revealed an improvement from baseline to follow‐up for SRF 4.7%, 95% CI [1.56, 7.84] (P = 0.003), but no between‐group differences from baseline to follow‐up were observed.
Analysis of flanker reaction time showed an interaction between congruency × time × group [F (6,277) = 2.37, P = 0.030]. Model‐based t tests revealed a significantly slower reaction time from baseline to follow‐up for congruent trials for RF 26 ms, 95% CI [11, 47] (P = 0.023), and overall faster reaction times for congruent trials compared to incongruent trials −68 ms, 95% CI [−76, −60] (P < 0.001). No between‐group differences from baseline to follow‐up were observed. Analysis of the flanker interference effect of reaction time showed an interaction of group × time [F (2,139) = 3.13, P = 0.047]. Additionally, model‐based t tests showed a significant improvement for SRF from baseline to follow‐up in the flanker interference effect for reaction time 19 ms, 95% CI [7, 31] (P = 0.002). As summarized in Figure 3, the change in interference effect of the reaction time from baseline to follow‐up was significantly greater for SRF than for SWF 19 ms, 95% CI [3, 35] (P = 0.041).
3.1.2. Neurophysiological modulation of attention
Pooled ERPs for each group at each are presented in Figure 5. Analysis of the P3 amplitude at site Fz revealed an interaction of group × time × congruency [F (6,180) = 6.71, P < 0.001]. Model‐based t tests showed a significant increase from baseline to follow‐up for SRF for congruent trials 4.23 μV, 95% CI [2.43, 6.03] (P < 0.001) and incongruent trials 4.75 μV, 95% CI [2.95, 6.55] (P < 0.001). Furthermore, the change from baseline to follow‐up for SRF was significant larger compared to RF for congruent trials 3.54 μV, 95% CI [0.95, 6.13] (P = 0.039) and incongruent trials 5.56 μV, 95% CI [2.97, 8.15] (P < 0.001) and compared to SWF for incongruent trials 4.10 μV, 95% CI [1.51, 6.69] (P = 0.010). Analysis of P3 amplitude at Cz showed an interaction of group × time × congruency [F (6,180) = 2.47, P = 0.047]. Model‐based t tests revealed a non‐adjusted significant improvement from baseline to follow‐up for incongruent trials for SRF 2.95 μV, 95% CI [0.60, 5.30] (P = 0.014), but the effect disappeared after adjustment (P = 0.083). Furthermore, the non‐adjusted change from baseline to follow‐up was significantly greater for SRF compared to SWF for incongruent trials 4.48 μV, 95% CI [1.09, 7.87] (P = 0.009), but this disappeared after adjustment (P = 0.050). Additionally, a non‐adjusted tendency toward an effect was observed for SRF compared to SWF for incongruent trials 3.10 μV, 95% CI [−0.29, 6.49] (P = 0.073), but this disappeared after adjustment (P = 0.314). Analysis of P3 amplitude at POz did not show an interaction of group × time × congruency [F (6,180) = 0.46, P = 0.762] or group × time [F (3,180) = 0.40, P = 0.880]. Analyses of P3 latency did not show a main effect of group × time × congruency at Fz [F (6,180) = 1.65, P = 0.163], Cz [F (6,180) = 1.69, P = 0.154], or POz [F (6,180) = 1.20, P = 0.315]. Furthermore, no main effect of group × time was observed for Fz [F (3,186) = 1.46, P = 0.235], Cz [F (3,186) = 2.05, P = 0.132], or POz [F (6,186) = 0.96, P = 0.384].
3.1.3. Declarative memory
Analysis of VMT showed an interaction of group × time × type [F (9,434) = 53.25, P < 0.001]. Model‐based t tests revealed a significant difference between immediate and delayed memory performance for AP for SRF 26.6%, 95% CI [21.1, 32.1] (P < 0.001), SWF 25.5%, 95% CI [19.8, 31.2] (P < 0.001) and RF 25.2%, 95% CI [19.7, 30.7] (P < 0.001), and for MP for SRF 12.5%, 95% CI [7.0, 18.0] (P < 0.001), SWF 10.8%, 95% CI [5.1, 16.5] (P < 0.001) and RF 11.6%, 95% CI [6.1, 17.1] (P < 0.001), and for TP for SRF 23.4%, 95% CI [17.9, 28.9] (P < 0.001), SWF 21.5%, 95% CI [15.8, 27.2] (P < 0.001) and RF 22.6%, 95% CI [17.1, 28.1] (P < 0.001). However, no significant differences in change between immediate and delayed declarative memory performance were observed between the groups.
3.2. Quantification of the interventions
3.2.1. Cardiovascular exercise intensity during the intervention
Analyses of time spent in heart rate zones during the intervention revealed an interaction of group × zone [F (10,322) = 73.20, P < 0.001]. Model‐based t tests showed larger percentage of time spent in: (a) heart rate zone 0%‐60% of HRreserve for SRF compared to RF 89.4%, 95% CI [81.8, 97.0] (P < 0.001), SWF 48.6%, 95% CI [40.6, 56.6] (P < 0.001) and SWF compared to SRF 40.8%, 95% CI [32.8, 48.8] (P < 0.001) (b) heart rate zone 60%‐70% of HRreserve for SWF compared to RF 27.8%, 95% CI [19.8, 35.8] (P < 0.001), SRF 13.0%, 95% CI [5.0, 21.0] (P = 0.020), and SRF compared to RF 14.8%, 95% CI [7.1, 22.4] (P = 0.002) (c) heart rate zone >70%‐80% of HRreserve for SRF compared to RF 23.8%, 95% CI [16.2, 31.4] (P < 0.001) and SWF compared to RF 12.9%, 95% CI [4.9, 20.9] (P = 0.022) (d) heart rate zone 80%‐90% of HRreserve for SRF compared to RF 35.7%, 95% CI [28.1, 43.3] (P < 0.001) and SWF 29.4%, 95% CI [21.4, 37.4] (P < 0.001) (e) heart rate zone 80%‐90% of HRreserve for SRF compared to RF 15.1%, 95% CI [7.5, 22.7] (P = 0.001) and SWF 13.6%, 95% CI [5.6, 21.6] (P = 0.012) (Figure 6).
Figure 6.
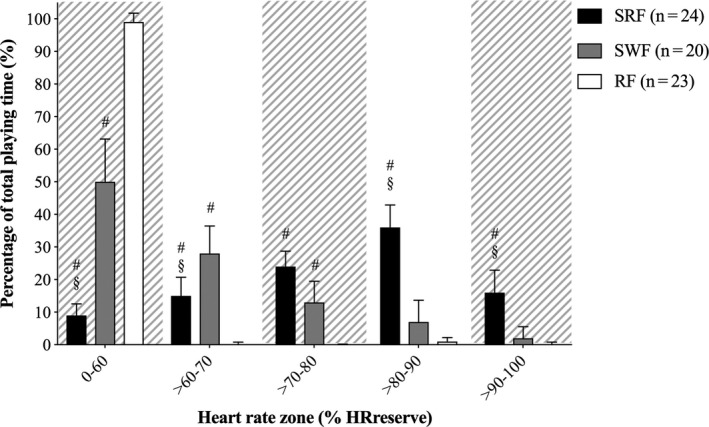
Cardiovascular exercise intensity during the intervention. Time spent in heart rate zone 0%‐60% of HRreserve, >60%‐70% of HRreserve, >70%‐80% of HRreserve, >80%‐90% of HRreserve, >90%‐100% of HRreserve, for small‐sided real football (SRF), small‐sided walking football (SWF) and resting football (RF). #Significant difference from RF. §Significance different from SWF (P < 0.05). Data are presented as means and 95% CI
3.2.2. Subjective experience of the interventions
Analysis of the children's subjective experience of the intervention shows an interaction effect of group × questionnaire × category [F (20,735) = 3.90, P < 0.001]. Model‐based t test revealed in the flow questionnaire a larger absorption for SRF compared to RF 0.96, 95% CI [0.37, 1.55] (P = 0.015) and a larger smoothness for RF compared to SWF 0.85, 95% CI [0.26, 1.44] (P = 0.049). In the IMI questionnaire model‐based t tests revealed larger enjoyment for SRF 1.58, 95% CI [0.99, 2.17] (P < 0.001) and SWF 0.91, 95% CI [0.32, 1.50] (P = 0.034) compared to RF. Furthermore, a non‐adjusted larger score in PACES was observed for SRF compared to RF 0.71, 95% CI [0.12, 1.30] (P = 0.020) but disappeared after adjustment (P = 0.051) (Figure 7).
Figure 7.
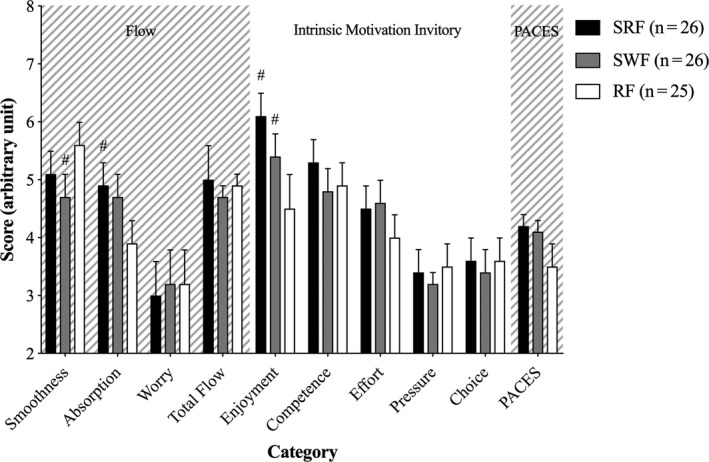
Subjective experience of the intervention. Subjective score for smoothness, absorption, worry, total flow, enjoyment, competence, effort, pressure, choice, and PACES for small‐sided real football (SRF), small‐sided walking football (SWF), and resting football (RF). #Significant difference from RF (P < 0.05). Data are presented as means and 95% CI
4. DISCUSSION
Three main findings emerged from this study. First, we found selective acute improvements in response time‐based indices of inhibitory control specifically in children playing high‐intensity small‐sided football games compared to moderate‐intensity walking football games. Second, following high‐intensity small‐sided football games children demonstrated greater increases in the amplitude of the P300 over frontal EEG electrode sites, reflecting a greater amount of task‐dependent attention allocated, compared to both moderate‐intensity small‐sided football games and rest. Third, neither high‐intensity nor moderate‐intensity small‐sided ballgames led to improved performance in tests assessing declarative memory consolidation. Additionally, our results demonstrated that small‐sided football games in general foster perceived enjoyment. The implications will be discussed in the forthcoming paragraphs.
4.1. Effects of acute small‐sided football on inhibitory control and neurophysiological indices of attentional allocation
The present study showed that performing a single bout of high‐intensity small‐sided football games improved inhibitory control test performance. This was selective for the response time‐based flanker interference score, but general response time and accuracy did not improve. Furthermore, a selective effect for the high‐intensity small‐sided football group was observed for neural allocation of attentional resources in response to stimuli, expressed as an increase in P300 amplitude over frontal regions irrespective of stimulus compatibility. This adds to the current body of research on the associations between acute exercise and executive functions and neurophysiological correlates, extending the positive effects to cover preadolescent children and adding small‐sided football games as a viable modality for promoting cognitive performance transiently. The present results are somewhat in line with earlier studies demonstrating a positive effect of acute exercise on inhibitory control and ERP amplitudes. However, some essential discrepancies were also found. Existing studies have supported a positive effect of acute exercise on performance in behavioral tests assessing inhibitory control and electroencephalographic event‐related components, with effect sizes traditionally favouring moderate‐intensity exercise.5 The current results, on the contrary, do not support a positive effect on measures of inhibitory control of moderate‐intensity small‐sided ball games, but rather tentatively suggest that higher exercise intensities are needed to elicit improvements in tests assessing inhibitory control and indices of cortical stimulus processing.
The lack of effects for the moderate‐intensity condition argues against the previously proposed inverted U‐shaped or J‐shaped hypothesis, which offers a putative psychophysiological framework for understanding the associations between exercise intensity and executive enhancement effects. The hypothesis suggests that higher‐order cognitive functions (eg, executive functions) mainly benefits from moderate intensity, while simple processing speed and memory processes show improvements as intensity increases in a linear fashion.41 The proposed physiological scaffold for these cognitive behavioral effects relates to alterations in the concentrations of plasma and brain monoamines (eg, norepinephrine, dopamine, and serotonin). These supposedly shift to an optimum for executive functioning following moderate‐ intensity exercise, while reaching detrimental levels following high‐intensity exercise.42 Evidence directly supporting this hypothesis is sparse.41 Our results, albeit strictly behavioral, also seem to contradict this view. Alternatively, the lack of positive effects of moderate‐intensity small‐sided football games could potentially be explained by the characteristics inherent in the applied interventions and the time lapsed from finishing the exercise bout until testing the effects. Meta‐evidence suggests that the robustness of cognitive performance improvements is linearly related to the intensity of the exercise performed.5 Specifically, moderate‐intensity exercise yields positive effects if testing is performed during or immediately after the cessation of exercise, whereas the carry‐over effects from higher exercise intensities persist until after a delay.5, 15 As the exercise‐induced cognitive improvements still may somehow result from the physiological response induced by the exercise bout, the transient physiological response from moderate‐intensity exercise may not suffice to influence cognitive control processes 20 minutes post‐exercise. This interpretation is supported by the lack of changes in P300 amplitudes following both rest and moderate‐intensity ballgames in the current study, suggesting that it is not sufficient to elicit changes in ERP components. However, it does not conform to a series of recent studies demonstrating positive effects of both moderate‐ and high‐intensity exercise tested after an identical delay as in the present study.7, 12 Importantly, these studies utilized different exercise modalities (eg, treadmill walking, running or cycling) to the present study. Tentatively, it suggests an interaction between exercise intensity and type in facilitating inhibitory control performance that warrants further exploration. Moreover, the aforementioned studies were largely laboratory‐based, whereas the present study was carried out in a school setting. Laboratory‐based studies offer several scientific advantages, one being strict control over the exercise characteristics. However, it also hampers the ability to determine whether potential effects also exist in the settings for which they are designed. Differences in contexts could partly explain the discrepant findings between the current and previous studies, highlighting that extrapolating findings from laboratories into practical settings must be done with caution. A field‐based approach as employed in the current study paves the road toward bridging together early laboratory findings with ecological settings.
Small‐sided real football demonstrated an improved performance in the reaction time‐based flanker interference score and changes in ERP components, but it is important to note that changes in brain state do not necessarily result in changes in performance.19 Indeed, the P300 reflects covert central nervous processing related to stimulus detection and processing. Hence, even though we measured changes in brain functions and behavioral performance and found paralleled improvements, this does not imply that the P300 amplitude changes led to the behavioral improvements. Previous studies have provided equivocal evidence on the interrelatedness of changes in P300 amplitude and behavior following exercise interventions as discussed in Ref. 19. This is not surprising given the various temporary physiological alterations occurring in the brain caused by exercise. For example, acute exercise shifts the balance between inhibitory and excitatory neurotransmitter availability43 and alters regional task‐related brain activation.44 The changes in P300 amplitude could therefore be epiphenomenal or might constitute just one of multiple physiological responses in the brain influencing cognitive aptitude. Further studies should seek to expand the framework for understanding the relationship between P300 amplitude changes and behavioral changes. In fact, it seems safe to say that the current evidence on the mechanisms governing cognitive benefits following acute exercise is premature at best, and more studies are needed to delineate the physiological underpinnings.
Although the exact mechanistic foundation is ambiguous, the practical implications of the present results are more straightforward. Variations of small‐sided games are often naturally occurring during break times at school. Our findings suggest that fostering intense participation in these activities could lead to subsequent transient improvements in performance and neural markers of attention when engaged in tasks, which depend upon executive functions. Indeed, in the context of a school day, a single 20‐minute bout of high‐intensity exercise structured to fit break time could be a feasible option for boosting inhibitory control in the upcoming lesson, whereas a moderate exercise bout would not carry with it additional positive after‐effects extending into that lesson. Importantly, however, moderate‐intensity exercise did not seem to compromise the ability to perform in tests assessing cognitive conflicts. This underlines that practitioners need not worry about potential detrimental effects of engaging in ballgames during break times.
4.2. Acute effects of small‐sided football games on visual memory consolidation
We hypothesized that performing a brief bout of ballgames following encoding of novel pictures would foster the memory consolidation process, resulting in an improved retention level. Surprisingly, declarative visual memory consolidation was not enhanced by exercise. Importantly, acute exercise was not detrimental to memory consolidation either. In contrast to the current study, earlier studies have demonstrated a performance‐enhancing effect of carrying out single bouts of exercise prior to or after memory tasks.10, 17 This was achieved through several exercise modalities. For example, both a single running session and a game of unihockey facilitated visuomotor memory consolidation in children, resulting in improved performance 7 days post‐training.45 The potential for modulation through acute exercise decays as time passes between encoding and the exercise bout. This is evident as reduced enhancements when exercise is performed after a 2‐hour delay compared to directly following memory encoding.46 Collectively, these behavioral studies indirectly suggest that the physiological stimulus triggered by the exercise bout supports memory consolidation processes, translating into better long‐term retention, that is, offline gains. Discrepancies between previous endeavors and the current study could perhaps reside in methodological features. For example, several of the aforementioned studies found greater retention levels using a novel visuomotor precision task,10, 11, 45, 46 whereas the current study did not find any additional benefits of performing a single bout of exercise following a task where visual information was encoded and probed. On the contrary, van Dongen et al47 demonstrated that acute high‐intensity exercise could improve long‐term retention in a visual memory task, albeit only when performed 4 hours post‐encoding. Other studies have revealed positive effects of both moderate‐ and high‐intensity exercise in a word‐list task when the bout was scheduled prior to the information to be remembered.12, 17 A parsimonious hypothesis could be that exercise demonstrates selective enhancement effects dependent on the memory subsystem mainly taxed and the stage of memory processing. For example, declarative memories might be susceptible to exercise‐induced modulation mainly during memory encoding or during late consolidation, whereas non‐declarative memories are mostly malleable through acute exercise during the early consolidation phase. Potential interactions between exercise characteristics and memory subsystems ought to be explored in future studies.
4.3. A call for multidisciplinary efforts
Performance in tests assessing the ability to process conflicting visual stimuli and suppress prepotent motor responses was improved by a brief bout of intense football and so was the neural allocation of attention. However, we also found that the subjective experience of the interventions differed between participants allocated to both football groups and the resting group. Children participating in the employed ballgames rated the activity more joyful compared to the resting group that watched a small‐sided football game. The greater enjoyment was experienced irrespective of the intensity at which it was performed. Recently, it was suggested that psychosocial factors could contribute to observed effects of exercise interventions.19, 20 Given the selective enhancement effect of high‐intensity small‐sided ball games on measures of inhibitory control and neural allocation of attention, it is, however, unlikely that differences in perceived enjoyment singlehandedly led to the observed improvements in executive functioning. Although perceived joy may not be a sole mediator of the observed effects, it is essential to note that the high‐intensity type of small‐sided football games found to transiently enhance flanker task performance and brain functions also resembled the original sport, whereas the walking football group was constrained by rules decontextualizing it from the traditional football paradigm. While this, as per intention, successfully reduced the cardiovascular demands, it might also have affected several other qualitative parameters influencing potential effects on cognitive and brain functions. Even though we did not find any clear differences in the included measures of motivation or flow between the two football groups, it does not exclude this possibility. Nonetheless, our results highlight the importance of embracing a multidisciplinary perspective to thoroughly understand the complex multifaceted relationship between exercise and cognitive functions.
5. METHODOLOGICAL CONSIDERATIONS
Our results demonstrate improved inhibitory control performance (evidenced by a smaller interference effect in the flanker task) for the high‐intensity football group compared to the moderate‐intensity football group, and this suggests that brief bouts of high‐intensity exercise can improve inhibitory control processes. It should however also be noted that these between‐group differences over time might also, to some extent, be influenced by natural variation around a common mean.20 For example, the individuals in the SRF group performed slightly worse, albeit not significantly different, in the computed response time interference effect compared to the other groups of children and improved their performance to the largest extent following exercise. Additionally, our results should be interpreted in light of the limited sample size and the between‐subject design that could affect the power of study, and hence, the ability to detect true differences,25 but also to overestimate the magnitude of detected significant differences.48, 49 Future studies with larger sample sizes are needed to confirm, and possibly extend, the findings of the present study.
6. CONCLUSION
The present study found that 20‐min of acute high‐intensity small‐sided football games can improve performance in tests assessing inhibitory control processes compared to moderate‐intensity small‐sided football games and task‐related allocation of attention evidenced by changes in the P300 amplitude compared to moderately intense small‐sided football games and seated rest in a school setting in 11‐ to 12‐year‐old children. Furthermore, the study found that neither high intensity nor moderate‐intensity small‐sided ball games improved declarative memory consolidation compared to rest.
7. PERSPECTIVES
Our findings imply that acute school‐based high‐intensity ballgames can be a viable strategy for transiently promoting neural function and inhibitory control performance as well as enjoyment in children, and this may be utilized strategically. Indeed, given the pivotal role of executive functioning and attention for academic learning, the effects presented in the present study could in turn translate into improved academic achievement in children. The present results can thus be used in conjunction with previous results to guide practitioners in implementing structured physical activities throughout the school day to improve inhibitory control, motivation and potentially learning in schoolchildren.
Lind RR, Beck MM, Wikman J, et al. Acute high‐intensity football games can improve children's inhibitory control and neurophysiological measures of attention. Scand J Med Sci Sports. 2019;29:1546–1562. 10.1111/sms.13485
REFERENCES
- 1. Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: a review with meta‐analysis. Neurosci Biobehav Rev. 2013;37:1645‐1666. [DOI] [PubMed] [Google Scholar]
- 2. Verburgh L, Königs M, Scherder E, Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta‐analysis. Br J Sports Med. 2014;48:973‐979. [DOI] [PubMed] [Google Scholar]
- 3. Ludyga S, Gerber M, Brand S, Holsboer‐Trachsler E, Pühse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta‐analysis. Psychophysiology. 2016;53:1611‐1626. [DOI] [PubMed] [Google Scholar]
- 4. Bull R, Scerif G. Executive functioning as a predictor of children’s mathematics ability: inhibition, switching, and working memory. Dev Neuropsychol. 2001;19:273‐293. [DOI] [PubMed] [Google Scholar]
- 5. Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta‐analysis. Brain Res. 2012;1453:87‐101. [DOI] [PubMed] [Google Scholar]
- 6. Roig M, Thomas R, Mang CS, et al. Time‐dependent effects of cardiovascular exercise on memory. Exerc Sport Sci Rev. 2016;44:81‐88. [DOI] [PubMed] [Google Scholar]
- 7. Kao SC, Westfall DR, Soneson J, Gurd B, Hillman CH. Comparison of the acute effects of high‐intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology. 2017;54:1335‐1345. [DOI] [PubMed] [Google Scholar]
- 8. Ligeza TS, Maciejczyk M, Kałamała P, Szygula Z, Wyczesany M. Moderate‐intensity exercise boosts the N2 neural inhibition marker: a randomized and counterbalanced ERP study with precisely controlled exercise intensity. Biol Psychol. 2018;135:170‐179. [DOI] [PubMed] [Google Scholar]
- 9. Winter B, Breitenstein C, Mooren FC, et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597‐609. [DOI] [PubMed] [Google Scholar]
- 10. Roig M, Skriver K, Lundbye‐Jensen J, Kiens B, Nielsen JB. A single bout of exercise improves motor memory. PLoS ONE. 2012;7:e44594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas R, Johnsen LK, Geertsen SS, et al. Acute exercise and motor memory consolidation: the role of exercise intensity. PLoS ONE. 2016;11:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kao SC, Drollette ES, Ritondale JP, Khan N, Hillman CH. The acute effects of high‐intensity interval training and moderate‐intensity continuous exercise on declarative memory and inhibitory control. Psychol Sport Exerc. 2018;38:90‐99. [Google Scholar]
- 13. Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gejl AK, Bugge A, Ernst MT, et al. The acute effects of short bouts of exercise on inhibitory control in adolescents. Ment Health Phys Act. 2018;15:34‐39. [Google Scholar]
- 15. Tsukamoto H, Suga T, Takenaka S, et al. Greater impact of acute high‐intensity interval exercise on post‐exercise executive function compared to moderate‐intensity continuous exercise. Physiol Behav. 2016;155:224‐230. [DOI] [PubMed] [Google Scholar]
- 16. Ishihara T, Sugasawa S, Matsuda Y, Mizuno M. The beneficial effects of game‐based exercise using age‐appropriate tennis lessons on the executive functions of 6–12‐year‐old children. Neurosci Lett. 2017;642:97‐101. [DOI] [PubMed] [Google Scholar]
- 17. Pesce C, Crova C, Cereatti L, Casella R, Bellucci M. Physical activity and mental performance in preadolescents: effects of acute exercise on free‐recall memory. Ment Health Phys Act. 2009;2:16‐22. [Google Scholar]
- 18. Best JR. Effects of physical activity on children’s executive function: contributions of experimental research on aerobic exercise. Dev Rev. 2010;30:331‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diamond A, Ling DS. Aerobic‐exercise and resistance‐training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev Cogn Neurosci. 2018;37:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond A, Ling DS. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev Cogn Neurosci. 2016;18:34‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bendiksen M, Williams CA, Hornstrup T, et al. Heart rate response and fitness effects of various types of physical education for 8‐ to 9‐year‐old schoolchildren. Eur J Sport Sci. 2014;14:861‐869. [DOI] [PubMed] [Google Scholar]
- 22. Randers MB, Andersen TB, Rasmussen LS, Larsen MN, Krustrup P. Effect of game format on heart rate, activity profile, and player involvement in elite and recreational youth players. Scand J Med Sci Sports. 2014;24:17‐26. [DOI] [PubMed] [Google Scholar]
- 23. Lind RR, Geertsen SS, Ørntoft C, et al. Improved cognitive performance in preadolescent Danish children after the school‐based physical activity programme “FIFA 11 for Health” for Europe – a cluster‐randomised controlled trial. Eur J Sport Sci. 2018;18:130‐139. [DOI] [PubMed] [Google Scholar]
- 24. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2009;118:2128‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pontifex MB, McGowan AL, Chandler MC, et al. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol Sport Exerc. 2019;40:1‐22. [Google Scholar]
- 26. Randers MB, Brix J, Hagman M, Nielsen JJ, Sciences H, Randers MB. Effect of boards in small‐sided street soccer games on movement pattern and physiological response in recreationally active young men. J Strength Cond Res. 2017. [DOI] [PubMed] [Google Scholar]
- 27. Chang YK, Tsai YJ, Chen TT, Hung TM. The impacts of coordinative exercise on executive function in kindergarten children: an ERP study. Exp Brain Res. 2013;225:187‐196. [DOI] [PubMed] [Google Scholar]
- 28. Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143‐149. [Google Scholar]
- 29. Hillman CH, Pontifex M, Themanson JR. Acute aerobic exercise effects on event‐related brain potentials In: McMorris T, Tomporowski P, Audiffren M, eds. Exercise and Cognitive Function. Indianapolis: Wiley; 2009:161‐178. [Google Scholar]
- 30. Chaumon M, Bishop D, Busch NA. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J Neurosci Methods. 2015;250:47‐63. [DOI] [PubMed] [Google Scholar]
- 31. McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38:222‐231. [PubMed] [Google Scholar]
- 32. Cahill L. Enhanced human memory consolidation with post‐learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003;10:270‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Viggiano MP, Marzi T, Forni M, Righi S, Franceschini R, Peru A. Semantic category effects modulate visual priming in neglect patients. Cortex. 2012;48:1128‐1137. [DOI] [PubMed] [Google Scholar]
- 34. Shakespeare TJ, Yong K, Frost C, Kim LG, Warrington EK, Crutch SJ. Scene perception in posterior cortical atrophy: categorization, description and fixation patterns. Front Hum Neurosci. 2013;7:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rheinberg F, Vollmeyer R, Engeser S. Die Erfassung des Flow‐Erlebens In: Stiensmeier-Pelster J, Rheinberg F, eds. Diagnostik von Motivation und Selstkonzept. Göttingen, Germany: Hogrefe; 2003:261‐279. [Google Scholar]
- 36. McAuley E, Duncan T, Tammen VV. Psychometric properties of the intrinsic motivation inventory in a competitive sport setting: a confirmatory factor analysis. Res Q Exerc Sport. 1989;60:48‐58. [DOI] [PubMed] [Google Scholar]
- 37. Kendzierski D, DeCarlo KJ. Physical activity enjoyment scale: two validation studies. J Sport Exerc Psychol. 1991;13:50‐64. [Google Scholar]
- 38. Ahler T, Bendiksen M, Krustrup P, Wedderkopp N, George KP. Aerobic fitness testing in 6‐ to 9‐year‐old children: reliability and validity of a modified Yo‐Yo IR1 test and the Andersen test. Eur J Appl Physiol. 2012;112:871‐876. [DOI] [PubMed] [Google Scholar]
- 39. Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed‐effects models using lme4. arXiv:14065823v1[statCO]23 2014;1‐51.
- 40. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346‐363. [DOI] [PubMed] [Google Scholar]
- 41. McMorris T, Turner A, Hale BJ, Sproule J. Beyond the Catecholamines Hypothesis for an Acute Exercise–Cognition Interaction: A Neurochemical Perspective. Amsterdam: Elsevier Inc.; 2016:65‐103. [Google Scholar]
- 42. McMorris T. Exercise and cognitive function: a neuroendocrinological explanation In: McMorris T, Tomporowski P, Audiffren M, eds. Exercise and Cognitive Function. Indianapolis: Wiley; 2009:41‐68. [Google Scholar]
- 43. Coxon JP, Cash R, Hendrikse JJ, et al. GABA concentration in sensorimotor cortex following high‐intensity exercise and relationship to lactate levels. J Physiol. 2018;596:691‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kujach S, Byun K, Hyodo K, et al. A transferable high‐intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. NeuroImage. 2018;169:117‐125. [DOI] [PubMed] [Google Scholar]
- 45. Lundbye‐jensen J, Skriver K, Nielsen JB, Roig M. Acute exercise improves motor memory consolidation in preadolescent children. Front Hum Neurosci. 2017;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomas R, Beck MM, Lind RR, et al. Acute exercise and motor memory consolidation: the role of exercise timing. Neural Plast. 2016;2016:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Dongen E, Kersten I, Wagner IC, Morris R, Fernández G. Physical exercise performed four hours after learning improves memory retention and increases hippocampal pattern similarity during retrieval report physical exercise performed four hours after learning improves memory retention and increases hippocampal. Curr Biol. 2016;26:1‐6. [DOI] [PubMed] [Google Scholar]
- 48. Gelman A, Carlin J. Beyond power calculations: assessing type S (sign) and type M (magnitude) errors. Pers Psychol Sci. 2014;9:641‐651. [DOI] [PubMed] [Google Scholar]
- 49. Button KS, Ioannidis J, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365‐376. [DOI] [PubMed] [Google Scholar]


