Abstract
Aim
To evaluate treatment data from DISCOVER (NCT02322762 and NCT02226822), a global, prospective, observational study programme of patients with type 2 diabetes initiating a second‐line glucose‐lowering therapy.
Materials and Methods
Data were collected using a standardized case report form. First‐ and second‐line treatments were assessed in 14 668 patients from 37 countries across six regions. Among patients prescribed first‐line metformin monotherapy, Firth logistic regression models were used to assess factors associated with second‐line treatment choices.
Results
The most common first‐line therapies were metformin monotherapy (57.9%) and combinations of metformin with a sulphonylurea (14.6%). The most common second‐line therapies were combinations of metformin with other agents (72.2%), including dipeptidyl peptidase‐4 (DPP‐4) inhibitors (25.1%) or sulphonylureas (21.3%). Among patients prescribed first‐line metformin monotherapy, the most common second‐line therapies were combinations of metformin with a DPP‐4 inhibitor [32.8%; across‐region range (ARR): 2.4%‐51.3%] or a sulphonylurea (30.0%; ARR: 18.3%‐63.6%); only a few patients received combinations of metformin with sodium‐glucose co‐transporter‐2 inhibitors (6.7%; ARR: 0.0%‐10.8%) or glucagon‐like peptide‐1 receptor agonists (1.9%; ARR: 0.1%‐4.5%). Both clinical and non‐medical factors were associated with choice of second‐line therapy after metformin monotherapy.
Conclusions
Fewer patients than expected received metformin monotherapy at first line, and the use of newer therapies at second line was uncommon in some regions of the world. Patients' socioeconomic status was associated with treatment patterns, suggesting that therapy choices are influenced by cost and access.
Keywords: antidiabetic drug, population study, type 2 diabetes
1. INTRODUCTION
Optimal glycaemic control, along with the management of co‐morbidities such as hypertension and hyperlipidaemia, and prevention of long‐term complications, remains the cornerstone of the management of patients with type 2 diabetes. Current clinical guidelines recommend an HbA1c target of less than 7.0% (53 mmol/mol) for most patients.1, 2, 3, 4, 5 To achieve this target, most guidelines recommend the use of metformin in conjunction with diet and lifestyle modifications as first‐line therapy. When metformin monotherapy fails to control glycaemic levels, a second glucose‐lowering drug should be added to metformin in a timely fashion (within 3‐6 months of an HbA1c value above target).1, 2, 3, 4, 5 The Japanese guidelines take a different approach and recommend monotherapy (metformin or other) in conjunction with medical nutrition therapy as first‐line pharmacological treatment; the choice of first‐line glucose‐lowering agent should be tailored to the patient's characteristics and metformin is not recommended over any other agent.6
Although guidelines suggest using a personalized approach to select second‐line therapy based on patient characteristics (e.g. duration of diabetes, presence of co‐morbidities, age and risk of hypoglycaemia), only the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes provides a clear treatment algorithm based on the presence of atherosclerotic cardiovascular disease or chronic kidney disease.5 The wide variety of glucose‐lowering drug classes available as possible second‐line therapies results in difficult treatment decisions for clinicians.
DISCOVER is a 3‐year, global programme of observational research conducted in 38 countries, assessing the treatment of patients with type 2 diabetes in routine clinical practice.7 The primary objective of the study is to describe global disease management patterns and associated clinical outcomes in people with type 2 diabetes initiating a second‐line glucose‐lowering therapy. In many of the included countries, data are currently scarce or non‐existent. Here, we report patterns of treatment changes from first to second line at the start of the study in the overall patient population, and assess factors associated with choice of second‐line therapy in the subset of patients who were prescribed metformin monotherapy as first‐line treatment.
2. MATERIALS AND METHODS
The methods for the DISCOVER study programme have been reported in detail elsewhere,7, 8 and are briefly summarized below.
2.1. Study design
The DISCOVER study programme comprises two similar, 3‐year, non‐interventional, prospective studies conducted in parallel in 38 countries, and including a total of 15 992 patients; DISCOVER (NCT02322762) in 37 countries and J‐DISCOVER (NCT02226822) in Japan. Countries are grouped into regions according to World Health Organization (WHO) categories: Africa (Algeria and South Africa); Americas (Argentina, Brazil, Canada, Colombia, Costa Rica, Mexico and Panama); South‐East Asia (India and Indonesia); Europe (Austria, Czech Republic, Denmark, France, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden and Turkey); Eastern Mediterranean (Bahrain, Egypt, Jordan, Kuwait, Lebanon, Oman, Saudi Arabia, Tunisia and the United Arab Emirates); and Western Pacific (Australia, China, Japan, Malaysia, South Korea and Taiwan). The study protocols were approved by the relevant clinical research ethics committees in each country and institutional review boards at each site, and complied with the Declaration of Helsinki, the International Conference on Harmonization of Good Clinical Practice, and the local regulations for clinical research.
2.2. Site and investigator selection
For each participating country, the characteristics of physicians and clinical centres involved in the management of people with type 2 diabetes were assessed before the start of the study by conducting literature searches and interviewing key local diabetes experts who acted as national coordinating investigators.9 Information on the proportions of different types of practices (primary care centres, specialized diabetes centres and different types of hospitals) and physicians (primary care physicians, diabetologists/endocrinologists, cardiologists and other specialist physicians) treating patients with type 2 diabetes were collated. A list of sites that would match these characteristics as closely as possible was established for each country, and these sites were invited to participate. Among the invited sites, approximately one‐third were able to take part and subsequently recruited patients into the study.
2.3. Patient enrolment
Inclusion and exclusion criteria were kept to a minimum to reflect the diversity of patients treated in routine clinical practice. Briefly, patients older than 18 years of age who had type 2 diabetes and who were initiating a second‐line glucose‐lowering therapy (add‐on or switch, including switching between two agents in the same class) were eligible for inclusion if: they were not pregnant; they were not undergoing dialysis; they did not have a history of renal transplant; and their first‐line therapy was not an injectable agent, a herbal remedy alone or natural medicine alone. Full inclusion and exclusion criteria can be found in Table S1. Of note, J‐DISCOVER enrolled only patients who were receiving oral monotherapy as first‐line treatment, whereas DISCOVER enrolled patients who were receiving any type of oral therapy (one or more oral agents, or a fixed dose combination). The study protocol stated that participating physicians should invite consecutive eligible patients to enrol in the study. All participating patients provided signed informed consent. DISCOVER recruited patients from December 2014 to June 2016; J‐DISCOVER recruited patients from September 2014 to December 2015.
2.4. Data collection
Data were collected using a standardized electronic case report form and were transferred to a central database via a web‐based data capture system. Some data were extracted from existing electronic health records in Canada, Denmark, France, Norway and Sweden; in these countries, an abbreviated case report form was used. All data not automatically extracted from electronic medical records were manually transferred from existing medical records (electronic or otherwise) to the web‐based data capture system by the participating physicians. To ensure data quality, a risk‐based monitoring model was implemented. On‐site visits were organized at each data collection time point, and key variables entered into the web‐based data capture system were checked against the original medical records for a random sample of 10% of patients at each site. Variables collected at baseline included: physician and site characteristics; patient socioeconomic demographics; physiological variables [including body mass index (BMI) and blood pressure]; laboratory test results (including HbA1c, fasting plasma glucose and cholesterol levels); change in glucose‐lowering therapies and reason(s) for change; co‐morbidities including microvascular (nephropathy, retinopathy or neuropathy) and macrovascular (coronary heart disease, cerebrovascular disease, peripheral artery disease, heart failure or implantable cardioverter defibrillator use) diseases; and co‐medications. In line with the observational nature of the study, clinical variables, such as HbA1c levels, were measured and recorded in accordance with routine clinical practice; data collection was not mandatory for any of the clinical variables. Similarly, the presence and severity of co‐morbidities such as microvascular and macrovascular complications were not adjudicated and relied on the judgement of the participating physicians. Reasons for changing first‐line therapy and reasons for choosing second‐line therapy were entered in the data capture system by the participating physicians, who selected one or several reasons from predefined lists.
2.5. Statistical analysis
For the present analysis, patients from China (n = 1293) were excluded because complete data were not available at the time of publication. Patients were also excluded if they had treatment information missing in the database (n = 4), or if they had metformin monotherapy recorded as both first‐ and second‐line therapy (n = 27). The total number of patients included in the analysis was therefore 14 668 (91.7% of the total DISCOVER population). Descriptive data are presented as numbers and percentages for categorical variables; mean (standard deviation) values are reported for continuous variables.
Factors associated with choice of second‐line therapy were exclusively assessed in participants who were receiving metformin monotherapy at first line, to increase homogeneity and eliminate differences in choice of second‐line therapy because of differences in first‐line therapy. Conventional logistic regression was deemed inappropriate to assess these associations because some second‐line therapies were prescribed to small numbers of patients. In such cases, the maximum likelihood estimates used in the logistic regression model may be biased. Firth logistic regression models were used instead because they are based on penalized likelihood, which corrects the small‐sample bias.10, 11 Based on the five most prescribed second‐line dual therapies after first‐line metformin monotherapy, four models were run testing: (a) likelihood of prescribing a dipeptidyl peptidase‐4 (DPP‐4) inhibitor relative to a sulphonylurea (SU), in combination with metformin, (b) likelihood of prescribing a sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitor relative to an SU, in combination with metformin, (c) likelihood of prescribing insulin relative to an SU, in combination with metformin, and (d) likelihood of prescribing a glucagon‐like peptide‐1 (GLP‐1) receptor agonist relative to an SU, in combination with metformin. Combinations of metformin and an SU were chosen as the comparator because SUs are well established and readily available in most countries, including the lower‐income countries that participate in DISCOVER.
Multiple imputation was used to account for unreported data in the Firth logistic regression models. At least one covariate was missing for 34.5% of patients, with HbA1c having the highest proportion of patients with missing data (18.2%); numbers of patients with missing data are shown in Table S2. The imputation model included all independent variables from the regression models [sex, age, BMI, education duration, time since diabetes diagnosis, HbA1c level, health insurance coverage status, employment status, medical history (microvascular and macrovascular complications, chronic kidney disease, minor and major hypoglycaemic events), physician's specialty and region] as well as other variables that may be informative for the imputation of the variables included in the regression model [country, ethnicity, smoking status, systolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol and triglyceride levels, and use of co‐medications (diuretics, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blockers, beta blockers, statins and acetylsalicylic acid)]. Iterative sequential regression was used to sample missing values from the predictive distribution of each variable conditional on all other variables. Ten randomly imputed datasets were generated in this way; analyses were replicated on each imputed dataset and the model estimates were pooled across imputations. IVEware (Imputation and Variance Estimation Software; University of Michigan's Survey Research Center, Institute for Social Research, Ann Arbor, Michigan) was used to conduct the imputation. SAS Proc MIANALYZE (SAS Institute Inc., Cary, North Carolina) was used to pool the results. All other statistical analyses were carried out using the SAS statistical software system (SAS Institute Inc.).
3. RESULTS
3.1. Baseline patient characteristics
Table 1 shows the characteristics of the 14 668 patients included in the present analysis. Overall, 53.9% of patients were male [across region range (ARR): 37.6%‐58.7%], the mean age was 57.5 years (ARR: 53.1‐61.9 years), and patients were mostly Asian (45.0%; ARR: 0.6%‐99.5%) or Caucasian (27.9%; ARR: 0.0%‐94.8%).
Table 1.
Patient baseline characteristics, overall and by region
| Total | Africa | Americas | South‐East Asia | Europe | Eastern Mediterranean | Western Pacific | |
|---|---|---|---|---|---|---|---|
| (N = 14 668) | (n = 811) | (n = 2002) | (n = 3351) | (n = 3470) | (n = 2179) | (n = 2855) | |
| Proportion of patients, % | 100.0 | 5.5 | 13.6 | 22.8 | 23.7 | 14.9 | 19.5 |
| Sex, male | 7909 (53.9) | 305 (37.6) | 963 (48.1) | 1846 (55.1) | 1853 (53.5) | 1278 (58.7) | 1664 (58.3) |
| Missing | 4 | 0 | 0 | 0 | 4 | 0 | 0 |
| Age, years, mean (SD) | 57.5 (12.0) | 54.9 (11.2) | 58.3 (11.8) | 53.1 (11.3) | 61.9 (10.9) | 53.8 (10.8) | 60.1 (12.7) |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Self‐reported ethnicity | |||||||
| Caucasian | 3911 (27.9) | 105 (13.0) | 480 (29.4) | 1 (0.0) | 3014 (94.8) | 165 (7.6) | 146 (5.1) |
| Black | 310 (2.2) | 235 (29.0) | 61 (3.7) | 0 (0.0) | 13 (0.4) | 0 (0.0) | 1 (0.0) |
| Asian | 6299 (45.0) | 177 (21.9) | 9 (0.6) | 3330 (99.5) | 20 (0.6) | 72 (3.3) | 2691 (94.3) |
| Hispanic | 942 (6.7) | 1 (0.1) | 928 (56.8) | 0 (0.0) | 11 (0.3) | 0 (0.0) | 2 (0.1) |
| Arabic | 2147 (15.3) | 199 (24.6) | 4 (0.2) | 2 (0.1) | 12 (0.4) | 1930 (88.9) | 0 (0.0) |
| Mixed | 213 (1.5) | 91 (11.2) | 115 (7.0) | 0 (0.0) | 4 (0.1) | 0 (0.0) | 3 (0.1) |
| Other | 174 (1.2) | 2 (0.2) | 36 (2.2) | 15 (0.4) | 104 (3.3) | 5 (0.2) | 12 (0.4) |
| Missing | 672 | 1 | 369 | 3 | 292 | 7 | 0 |
| Education | |||||||
| No formal education | 405 (3.0) | 57 (7.3) | 50 (3.2) | 26 (0.8) | 78 (2.5) | 158 (7.7) | 36 (1.4) |
| Primary (1‐6 years) | 2059 (15.5) | 182 (23.2) | 442 (28.7) | 342 (10.3) | 586 (19.1) | 360 (17.6) | 147 (5.7) |
| Secondary (7‐13 years) | 6600 (49.5) | 420 (53.6) | 587 (38.1) | 1427 (43.2) | 1778 (58.0) | 765 (37.5) | 1623 (62.8) |
| Higher education (>13 years) | 4258 (32.0) | 125 (15.9) | 463 (30.0) | 1510 (45.7) | 623 (20.3) | 758 (37.1) | 779 (30.1) |
| Missing | 1346 | 27 | 460 | 46 | 405 | 138 | 270 |
| Main working status | |||||||
| Employed | 5046 (36.7) | 262 (32.7) | 473 (29.9) | 875 (26.2) | 1094 (34.1) | 952 (45.5) | 1408 (50.8) |
| Self‐employed | 1679 (12.2) | 59 (7.4) | 343 (21.7) | 751 (22.5) | 170 (5.3) | 209 (10.0) | 147 (5.3) |
| Disabled | 82 (0.6) | 6 (0.7) | 6 (0.4) | 0 (0.0) | 59 (1.8) | 2 (0.1) | 9 (0.3) |
| Not working | 4009 (29.1) | 336 (41.9) | 428 (27.1) | 1385 (41.4) | 505 (15.8) | 683 (32.6) | 672 (24.3) |
| Retired | 2892 (21.0) | 139 (17.3) | 330 (20.9) | 332 (9.9) | 1378 (43.0) | 247 (11.8) | 466 (16.8) |
| Other | 67 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 67 (2.4) |
| Missing | 875 | 9 | 422 | 8 | 264 | 86 | 86 |
| Health insurance coverage | |||||||
| Private | 2021 (14.7) | 165 (20.7) | 517 (32.3) | 592 (18.8) | 130 (3.9) | 466 (22.3) | 151 (5.4) |
| Public/government | 8249 (59.8) | 465 (58.2) | 783 (48.8) | 371 (11.8) | 3038 (91.3) | 1226 (58.7) | 2366 (84.0) |
| Mixed | 354 (2.6) | 16 (2.0) | 137 (8.5) | 51 (1.6) | 26 (0.8) | 69 (3.3) | 55 (2.0) |
| No insurance | 3163 (22.9) | 153 (19.1) | 166 (10.4) | 2140 (67.9) | 134 (4.0) | 327 (15.7) | 243 (8.6) |
| Missing | 881 | 12 | 399 | 197 | 142 | 91 | 40 |
| Years since type 2 diabetes diagnosis, mean (SD) | 5.7 (5.3) | 6.9 (5.7) | 6.2 (6.1) | 4.6 (4.1) | 6.6 (5.4) | 5.8 (5.1) | 5.4 (5.6) |
| Missing | 394 | 0 | 63 | 1 | 151 | 3 | 176 |
| HbA1c, %, mean (SD) | 8.3 (1.7) | 8.6 (1.9) | 8.5 (1.9) | 8.6 (1.7) | 8.1 (1.6) | 8.7 (1.6) | 7.9 (1.6) |
| Missing | 2799 | 345 | 471 | 1307 | 470 | 135 | 71 |
| BMI, kg/m2, mean (SD) | 29.4 (6.0) | 30.6 (6.2) | 30.6 (6.1) | 27.3 (4.5) | 31.9 (6.1) | 31.1 (5.7) | 26.5 (5.4) |
| Missing | 1150 | 13 | 202 | 207 | 258 | 324 | 146 |
| Microvascular complicationsa | 2843 (19.4) | 118 (14.5) | 302 (15.1) | 556 (16.6) | 808 (23.4) | 398 (18.3) | 661 (23.2) |
| Missing | 18 | 0 | 0 | 0 | 18 | 0 | 0 |
| Macrovascular complicationsb | 2147 (14.7) | 78 (9.6) | 276 (13.8) | 140 (4.2) | 1045 (30.5) | 236 (10.8) | 372 (13.0) |
| Missing | 42 | 0 | 0 | 0 | 42 | 0 | 0 |
| History of major hypoglycaemiac | 133 (1.0) | 11 (1.4) | 26 (1.6) | 15 (0.5) | 44 (1.3) | 22 (1.1) | 15 (0.5) |
| Missing | 951 | 34 | 415 | 102 | 130 | 225 | 45 |
| History of minor hypoglycaemiad | 479 (3.5) | 43 (5.5) | 41 (2.6) | 83 (2.6) | 116 (3.5) | 147 (7.4) | 49 (1.7) |
| Missing | 903 | 26 | 410 | 104 | 119 | 205 | 39 |
Data are n (%) unless otherwise indicated. Percentages were calculated for all patients with data available; patients with missing data were excluded.
Abbreviations: BMI, body mass index; SD, standard deviation.
Composite of nephropathy, retinopathy and neuropathy.
Composite of coronary heart disease, cerebrovascular disease, peripheral artery disease, heart failure and implantable cardioverter defibrillator use.
Hypoglycaemic events requiring external/third party assistance in the previous year.
Hypoglycaemic events that were self‐reported, and which occurred in the previous 4 weeks.
The mean HbA1c was 8.3% (ARR: 8.1%‐8.7%) and the mean BMI was 29.4 kg/m2 (ARR: 26.5‐31.9 kg/m2). The majority of patients (81.5%; ARR: 68.1%‐92.9%) had secondary or higher education, and 48.9% (ARR: 39.4%‐56.2%) were employed or self‐employed. The mean time from diagnosis of type 2 diabetes to initiation of second‐line therapy was 5.7 years (ARR: 4.6‐6.9 years). A total of 19.4% (ARR: 14.5%‐23.4%) and 14.7% (ARR: 4.2%‐30.5%) of patients had established microvascular and macrovascular complications, respectively.
3.2. Treatment patterns
Patients' first‐line therapies and changes to second‐line therapy for the overall population are presented in Table 2; first‐ and second‐line therapies by region have been presented elsewhere.12 Metformin monotherapy was the most commonly prescribed first‐line treatment [n = 8488 (57.9%)], followed by combinations of metformin and an SU [n = 2139 (14.6%)], DPP‐4 inhibitor monotherapies [n = 1172 (8.0%)] and SU monotherapies [n = 1061 (7.2%)]. Overall, 11 837 participants (80.7%) received metformin either alone or in combination at first line.
Table 2.
Choice of second‐line glucose‐lowering therapy, overall and according to first‐line therapy
| Second‐line therapy | First‐line therapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | MET mono. | SU mono. | DPP‐4i mono. | Other mono. | MET + SUa | MET + DPP‐4ia | MET + SU + DPP‐4ib | MET + other(s) | Other comb. | |
| N = 14 668 | n = 8488 | n = 1061 | n = 1172 | n = 474 | n = 2139 | n = 489 | n = 217 | n = 504 | n = 124 | |
| MET monotherapy | 238 (1.6) | – | 129 (12.2) | 41 (3.5) | 30 (6.3) | 22 (1.0) | 9 (1.8) | 2 (0.9) | 2 (0.4) | 3 (2.4) |
| SU monotherapy | 406 (2.8) | 322 (3.8) | 19 (1.8) | 18 (1.5) | 5 (1.1) | 13 (0.6) | 22 (4.5) | 1 (0.5) | 3 (0.6) | 3 (2.4) |
| DPP‐4i monotherapy | 635 (4.3) | 376 (4.4) | 58 (5.5) | 9 (0.8) | 85 (17.9) | 93 (4.3) | 6 (1.2) | 0 (0.0) | 7 (1.4) | 1 (0.8) |
| Other monotherapyc | 534 (3.6) | 224 (2.6) | 14 (1.3) | 37 (3.2) | 11 (2.3) | 183 (8.6) | 20 (4.1) | 14 (6.5) | 27 (5.4) | 4 (3.2) |
| MET + SUa | 3117 (21.3) | 2549 (30.0) | 349 (32.9) | 2 (0.2) | 7 (1.5) | 137 (6.4) | 23 (4.7) | 2 (0.9) | 31 (6.2) | 17 (13.7) |
| MET + DPP‐4ia | 3678 (25.1) | 2780 (32.8) | 61 (5.7) | 462 (39.4) | 11 (2.3) | 243 (11.4) | 58 (11.9) | 3 (1.4) | 56 (11.1) | 4 (3.2) |
| MET + SGLT‐2ia | 634 (4.3) | 571 (6.7) | 2 (0.2) | 0 (0.0) | 25 (5.3) | 15 (0.7) | 15 (3.1) | 2 (0.9) | 3 (0.6) | 1 (0.8) |
| MET + GLP‐1 RAa | 187 (1.3) | 159 (1.9) | 0 (0.0) | 1 (0.1) | 1 (0.2) | 13 (0.6) | 5 (1.0) | 2 (0.9) | 5 (1.0) | 1 (0.8) |
| MET + SU + DPP‐4ib | 1040 (7.1) | 135 (1.6) | 88 (8.3) | 10 (0.9) | 0 (0.0) | 588 (27.5) | 131 (26.8) | 29 (13.4) | 37 (7.3) | 22 (17.7) |
| MET + other(s)d | 1936 (13.2) | 812 (9.6) | 67 (6.3) | 13 (1.1) | 70 (14.8) | 507 (23.7) | 132 (27.0) | 85 (39.2) | 219 (43.5) | 31 (25.0) |
| Insuline | 914 (6.2) | 349 (4.1) | 50 (4.7) | 15 (1.3) | 10 (2.1) | 266 (12.4) | 46 (9.4) | 68 (31.3) | 92 (18.3) | 18 (14.5) |
| Other combinations | 1349 (9.2) | 211 (2.5) | 224 (21.1) | 564 (48.1) | 219 (46.2) | 59 (2.8) | 22 (4.5) | 9 (4.1) | 22 (4.4) | 19 (15.3) |
Data are presented as n (%).
Abbreviations: comb., combination; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; MET, metformin; mono., monotherapy; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor; SU, sulphonylurea.
Dual therapy.
Triple therapy.
Excluding insulin monotherapy.
Excluding combinations containing insulin.
Alone or in combination with other agents.
Combinations of metformin with either a DPP‐4 inhibitor [n = 3678 (25.1%)] or an SU [n = 3117 (21.3%)] were the most commonly prescribed second‐line therapies. Only 634 patients (4.3%) received an SGLT‐2 inhibitor in combination with metformin at second line; 10 patients (0.1%) received this combination at first line. A small number of patients [n = 187 (1.3%)] received a GLP‐1 receptor agonist in combination with metformin at second line. Overall, 914 participants (6.2%) were prescribed insulin at second line, either alone or in combination with other therapies.
Of the 11 195 participants who were receiving a monotherapy as first‐line treatment, 1378 (12.3%) switched to another monotherapy (including changes within a drug class) as second‐line treatment. Among patients who were receiving metformin either as monotherapy or as part of a combination therapy at first line (n = 11 016; after excluding patients receiving insulin on its own or as part of a combination at second line), a total of 1634 (14.8%) were not prescribed metformin at second line.
Among participants who were prescribed metformin monotherapy at first line (n = 8488), the most common second‐line therapies were combinations of metformin with either a DPP‐4 inhibitor [n = 2780 (32.8%)] or an SU [n = 2549 (30.0%)]. Among patients prescribed SU monotherapy at first line (n = 1061), the most common second‐line therapy was a combination of an SU with metformin [n = 349 (32.9%)], whereas among patients prescribed a DPP‐4 inhibitor monotherapy at first line (n = 1172), the most common second‐line therapy was a combination of a DPP‐4 inhibitor with metformin [n = 462 (39.4%)].
Among participants prescribed a combination of metformin with either an SU (n = 2139) or a DPP‐4 inhibitor (n = 489) at first line, the most common second‐line therapy was triple therapy combining metformin with both an SU and a DPP‐4 inhibitor [n = 588 (27.5%) and n = 131 (26.8%), respectively]. For a minority of patients [n = 252 (1.7%)], drug classes were recorded as being the same at both first and second line; for these patients the change in treatment consisted of a switch between agents in the same drug class.
3.3. Factors associated with second‐line treatment choices in patients prescribed metformin monotherapy as first‐line treatment
The baseline characteristics of the subset of participants who received metformin monotherapy at first line (n = 8488), and in which the factors associated with second‐line treatment choices were assessed, are shown in Table S2. Of note, mean HbA1c level was considerably higher in patients prescribed insulin in combination with metformin at second line than in other groups of patients (10.2% vs. 7.9%‐8.5%; Table S2). Lack of efficacy was the most commonly stated reason for changing therapy in all treatment groups (72.8%‐93.7%), but adverse effects were more commonly reported as the reason for changing treatment in the group of patients who discontinued metformin at second line (n = 1194) than among other patient groups (20.0% vs. 2.2%‐3.9%; Table S3). Overall, expected efficacy was the most commonly stated reason for choosing second‐line therapies (63.2%), and weight was often stated as a reason for choosing combinations of metformin with an SGLT‐2 inhibitor or a GLP‐1 receptor agonist (59.9% and 85.2%, respectively; Table S3).
Variations in the choice of second‐line therapy according to region are shown in Table S4. The most common second‐line therapies overall were DPP‐4 inhibitors (32.8%) or SUs (30.0%) in combination with metformin; SUs in combination with metformin were the most common choice in Africa (63.6%), the Americas (34.2%) and South‐East Asia (41.2%), whereas a DPP‐4 inhibitor in combination with metformin was the most common choice in Europe (34.9%), the Eastern Mediterranean region (51.3%) and the Western Pacific region (41.3%). Expected efficacy was the most commonly stated reason for choosing second‐line therapies in all regions (43.4%‐88.6%; Table S5). In Africa, access, convenience and cost were also commonly selected reasons for choosing a second‐line therapy (17.4%, 14.0% and 11.8%, respectively), whereas tolerability, weight and hypoglycaemia were common factors in Europe (28.9%, 28.9% and 26.9%, respectively).
The results of the Firth logistic regression models assessing the factors associated with second‐line treatment choices are shown in Figure 1 for: (A) the likelihood of prescribing a DPP‐4 inhibitor relative to an SU, in combination with metformin; (B) the likelihood of prescribing an SGLT‐2 inhibitor relative to an SU, in combination with metformin; (C) the likelihood of prescribing insulin relative to an SU, in combination with metformin; and (D) the likelihood of prescribing a GLP‐1 receptor agonist relative to an SU, in combination with metformin.
Figure 1.
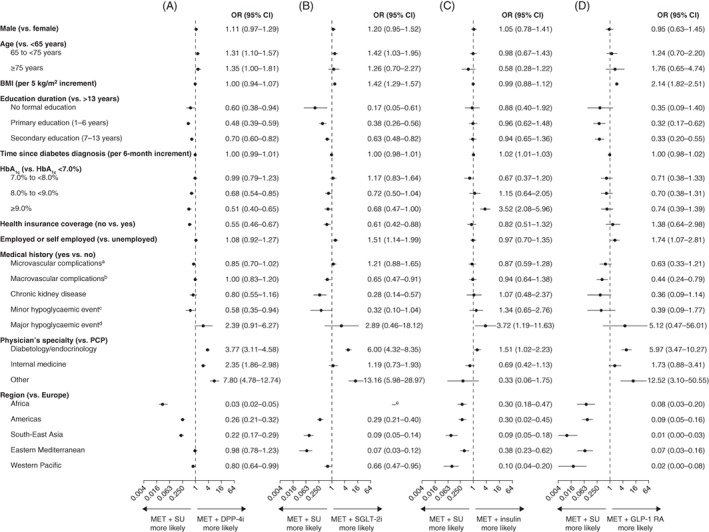
Likelihood of receiving (A) MET and a DPP‐4i, (B) MET and an SGLT‐2i, (C) MET and insulin, and (D) MET and a GLP‐1 RA, compared with receiving MET and an SU in DISCOVER patients who received MET monotherapy as first‐line treatment. Data are presented as odds ratios (95% CI), estimated using Firth multinomial logistic regression models adjusted for all variables in the table.10 BMI, body mass index; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; MET, metformin; OR, odds ratio; PCP, primary care physician; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor; SU, sulphonylurea. aComposite of nephropathy, retinopathy and neuropathy. bComposite of coronary heart disease, cerebrovascular disease, peripheral artery disease, heart failure and implantable cardioverter defibrillator use. cHypoglycaemic events requiring external/third‐party assistance in the previous year. dHypoglycaemic events that were self‐reported and non‐confirmed, and which occurred in the previous 4 weeks. eOdds ratio was not estimated because none of the patients in Africa received an SGLT‐2i
3.3.1. Likelihood of prescribing a DPP‐4 inhibitor relative to an SU, in combination with metformin
Patients were more likely to receive a combination of a DPP‐4 inhibitor with metformin than a combination of an SU with metformin if: they were older than 65 years of age (relative to <65 years of age); they had a longer duration of education (>13 years relative to none, 1‐6 years or 7‐13 years); they had an HbA1c level less than 7.0% (relative to 8.0% to <9.0% and ≥ 9.0%); they had health insurance; they had not experienced a minor hypoglycaemic event in the previous 4 weeks; and the second‐line therapy was prescribed by a specialist physician (relative to a primary care physician). Patients in Europe were more likely than patients in Africa, the Americas, South‐East Asia or the Western Pacific region to be prescribed a DPP‐4 inhibitor relative to an SU, in combination with metformin.
3.3.2. Likelihood of prescribing an SGLT‐2 inhibitor relative to an SU, in combination with metformin
Factors significantly associated with having an increased likelihood of receiving an SGLT‐2 inhibitor relative to an SU in combination with metformin included: being 65‐74 years of age (relative to being <65 years of age); increasing BMI (per 5 kg/m2 increment); being employed or self‐employed (relative to not being employed); having health insurance coverage; and being prescribed a second‐line therapy by a diabetologist/endocrinologist (relative to a primary care physician). Conversely, factors significantly associated with having a lower likelihood of receiving an SGLT‐2 inhibitor relative to an SU in combination with metformin included: having less education (none, 1‐6 years or 7‐13 years, relative to >13 years); having an HbA1c level of at least 9.0% (relative to <7.0%); and having a medical history of macrovascular complications or chronic kidney disease (relative to having no such medical history). In all regions, compared with Europe, treatment with an SU in combination with metformin was more likely than treatment with an SGLT‐2 inhibitor in combination with metformin (odds ratios could not be calculated for the likelihood of receiving an SGLT‐2 inhibitor relative to an SU in Africa because no patients in this region received an SGLT‐2 inhibitor) (Table S4).
3.3.3. Likelihood of prescribing insulin relative to an SU, in combination with metformin
Patients were more likely to be prescribed insulin than an SU in combination with metformin if: they had a longer time since diagnosis of diabetes (per 6‐month increment); they had an HbA1c level of at least 9.0% (relative to <7.0%); they had experienced a major hypoglycaemic event in the previous year; and the second‐line therapy was prescribed by a diabetologist or endocrinologist (relative to a primary care physician). In all regions, compared with Europe, treatment with an SU was more likely than treatment with insulin in combination with metformin.
3.3.4. Likelihood of prescribing a GLP‐1 receptor agonist relative to an SU, in combination with metformin
Factors significantly associated with having an increased likelihood of receiving a GLP‐1 receptor agonist relative to an SU in combination with metformin included: increasing BMI (per 5 kg/m2 increment); being employed or self‐employed (relative to not being employed); and being prescribed a second‐line therapy by a diabetologist/endocrinologist (relative to a primary care physician). Conversely, factors significantly associated with having a lower likelihood of receiving a GLP‐1 receptor agonist relative to an SU in combination with metformin included: having less education (1‐6 years or 7‐13 years, relative to >13 years). In all regions, compared with Europe, treatment with an SU in combination with metformin was more likely than treatment with a GLP‐1 receptor agonist in combination with metformin.
4. DISCUSSION
This study provides important new global data on the treatment of people with type 2 diabetes initiating a second‐line glucose‐lowering therapy. Despite guideline recommendations that metformin monotherapy should be used as first‐line therapy in most patients,1, 2, 3, 4, 5 approximately a fifth of participants were not recorded as having received metformin at first line, either alone or in combination with another agent. SUs were widely used at both first and second line, as were DPP‐4 inhibitors, whereas, at second line, only 4.3% of patients received an SGLT‐2 inhibitor in combination with metformin, and only 1.3% received a GLP‐1 receptor agonist in combination with metformin. For patients who received metformin monotherapy at first line, the most commonly prescribed second‐line therapies were an SU or a DPP‐4 inhibitor in addition to metformin. Just under 15% of these patients discontinued metformin at second line. These results are in line with findings from a population‐based, observational study conducted in five European countries,13 which showed that metformin monotherapy was the most commonly prescribed first‐line treatment (65%‐88% of patients). In these patients, the most common second‐line therapies were combinations of metformin with either an SU or a DPP‐4 inhibitor.
The relatively high proportion of DISCOVER patients who received first‐line treatment with a combination therapy rather than metformin monotherapy could reflect country‐specific treatment practices, or could be a result of late diagnosis of diabetes following the recommendation to use combination therapies in patients with an HbA1c level of at least 7.5% when the diagnosis of type 2 diabetes is established.1 Among patients who were not prescribed metformin, either alone or in combination at first line, explanations could include contraindications, such as chronic kidney disease, or physician preference for treatment with SUs or DPP‐4 inhibitors. A survey conducted in 2016 by the International Diabetes Federation and a recent epidemiological study also suggest that the availability of metformin is suboptimal in some low‐ to medium‐income countries.14, 15 Of note, more than 1 in 10 patients who received a monotherapy as first‐line treatment switched to another monotherapy when initiating second‐line treatment. This appears to be counter to clinical guidelines, which recommend the addition of a glucose‐lowering agent when monotherapy fails to control glycaemia.1, 2, 3, 4, 5 It should be noted, however, that some of these cases might reflect patients changing treatment because of poor tolerability or affordability reasons rather than a lack of efficacy. Among patients who received metformin monotherapy at first line and who subsequently discontinued metformin at second line, the reason stated for changing first‐line therapy was lack of efficacy in approximately 75%, and adverse effects in 20%. The use of SGLT‐2 inhibitors and GLP‐1 receptor agonists may become more common given recent evidence from several cardiovascular outcomes trials and a large international observational study that these classes of drugs reduce cardiovascular event rates in patients at high cardiovascular risk,16, 17, 18, 19, 20, 21 and the recommendation in recent guidelines to use these drugs in patients with established cardiovascular disease or chronic kidney disease.5
In the multivariate analyses of factors associated with second‐line treatment choices among patients receiving metformin monotherapy at first line, lower education increased the likelihood of SU prescription relative to DPP‐4 inhibitors, SGLT‐2 inhibitors and GLP‐1 receptor agonists. This may reflect lower socioeconomic status and fewer economic resources of these people, leading to the prescription of more affordable drugs. These findings are in line with recent guidelines from WHO, which recommends the use of SUs as second‐line treatment in settings with limited health system resources.22 Furthermore, being employed or self‐employed, relative to being disabled, not working or retired, was also associated with an increased likelihood of being prescribed an SGLT‐2 inhibitor or a GLP‐1 receptor agonist relative to an SU; again, likely to be related to the cost of treatment. Having an HbA1c level of at least 9.0% was a predictor of SU prescription in addition to metformin, relative to DPP‐4 or SGLT‐2 inhibitors. This may reflect the high 6‐month efficacy of SUs,23, 24 leading to their use for rapid glycaemic control in patients with the most severely elevated blood glucose levels. Our analysis also showed a significant association between physician's specialty and second‐line therapy prescription after first‐line metformin monotherapy. Endocrinologists and diabetologists were more likely to prescribe a DPP‐4 inhibitor, an SGLT‐2 inhibitor, insulin or a GLP‐1 receptor agonist than an SU, relative to primary care physicians. These findings suggest that prescription preferences may differ in primary care physicians and specialist physicians. Indeed, a recent survey in Japan highlighted that specialists and non‐specialists considered different factors when selecting first‐line therapy,25 which may also apply to second‐line treatment choices. However, such findings may not be generalizable to other countries, and our results may reflect the fact that patients with more severe disease, for whom these drugs would be recommended,1, 5 are more likely to be treated by specialists than primary care physicians. These results may also be confounded by the fact that people with a lower socioeconomic status, who are more likely to receive more affordable drugs such as SUs, may have limited access to specialist care.26 In addition, having a history of either macrovascular complications or chronic kidney disease was associated with a decreased likelihood of receiving an SGLT‐2 inhibitor relative to an SU. Similarly, having a history of macrovascular complications was associated with a decreased likelihood of receiving a GLP‐1 receptor agonist relative to an SU, and having a history of minor hypoglycaemic events was associated with a decreased likelihood of receiving a DPP‐4 inhibitor relative to an SU. Again, these findings contradict the recommendations from recent clinical guidelines for individualized treatment based on patients' characteristics,1, 2, 3, 4, 5 suggesting that non‐medical reasons may play a significant part in treatment choices.
An increased likelihood of receiving an insulin prescription relative to SUs was strongly associated with very high HbA1c levels (≥9.0%) and prescription by specialists (vs. primary care physicians). This is in line with the recommendations to initiate insulin in patients with very high HbA1c levels, and with the fact that these patients are more likely to be managed in secondary rather than primary care.
In the regional component of the multivariate analyses, regions were compared with Europe. With only one exception (DPP‐4 inhibitor prescription in the Eastern Mediterranean region), prescription of SUs was more likely in every region than prescription of DPP‐4 inhibitors, SGLT‐2 inhibitors or insulin. This is likely to reflect the relatively high cost and low availability of these agents in some low‐ and medium‐income countries compared with SUs.14, 15 In Africa, prescription of SUs was much more likely than prescription of DPP‐4 inhibitors (compared with Europe), again reflecting the relative costs of the two treatments. No patients in Africa received an SGLT‐2 inhibitor. These regional results are reflected in the descriptive assessment of treatment options shown in Table S4; the proportion of patients receiving an SU in combination with metformin was lower in Europe (18.3%) than in any other region, whereas the rate of DPP‐4 inhibitor prescription with metformin was highest in the Eastern Mediterranean region (51.3%).
Together, these results suggest that many patients in large parts of the world are prescribed SUs rather than newer agents, such as DPP‐4 inhibitors, SGLT‐2 inhibitors or GLP‐1 receptor agonists, when initiating second‐line therapy, for economic and other non‐medical reasons. This a real concern because these newer drugs likely to have a better cardiovascular safety profile than SUs.27 Indeed, the use of SUs as second‐line treatment (alone or in combination with metformin after first‐line metformin monotherapy relative to continuing metformin monotherapy) has been shown to be associated with an increased risk of myocardial infarction and all‐cause mortality.28 Conversely, cardiovascular outcomes trials have shown that the use of SGLT‐2 inhibitors and GLP‐1 receptor agonists was associated with improved cardiovascular outcomes relative to placebo, particularly in patients at high cardiovascular risk.18 Similarly, a large observational study identified a decreased likelihood of cardiovascular events in patients using SGLT‐2 inhibitors compared with other glucose‐lowering drugs.29
4.1. Strengths and limitations
DISCOVER is a large and global study, which provides valuable and unique insights into treatment patterns in patients with type 2 diabetes globally, and also reflects variation in local practice because of differences in healthcare systems, physicians' demographics and training, site organization and recommendations from local clinical guidelines across included countries. However, only a limited number of countries could be included for practical reasons, and the focus was placed on lower‐middle and upper‐middle income countries for which no alternative data sources are currently available. The USA and Western European countries, such as Germany and the UK, were not included because established and comprehensive databases already exist (for example, the Diabetes Collaborative Registry in the USA, The Health Improvement Network and Clinical Practice Research Datalink databases in the UK, and the Disease Analyzer database in Germany)30, 31, 32, 33 and have been used to assess treatment patterns in patients with type 2 diabetes in these countries.13, 34, 35 The substantial efforts made before the start of the study to survey the types and locations of medical practices treating patients with type 2 diabetes have resulted in a heterogeneous patient population from diverse clinical settings. However, as for any observational study of this type, a fully representative patient sample was not possible to achieve.9 Factors limiting the ability of the study to be completely representative of the treatment of patients with type 2 diabetes in each country include infrastructure and other practical constraints, which prevented the participation of some sites in rural locations and some primary care sites. Consequently, there is overrepresentation of urban locations and secondary care centres in the study. The likely result of this is an overestimate of the quality of care in some countries, because sites in urban locations may serve a well‐educated and well‐employed patient population. In addition, secondary care centres employing specialist physicians may provide a better quality of care than primary care centres. Finally, the limited number of included countries in each study region limits the generalizability of the findings to the whole region.
In conclusion, results from this study provide a global picture of treatment patterns in people with type 2 diabetes at initiation of second‐line glucose‐lowering therapy in countries and regions with few or no previous data. The findings showed that most patients received metformin as first‐line therapy, in line with clinical guidelines, either as monotherapy or in combination with another agent. SUs and DPP‐4 inhibitors were also commonly prescribed at both first and second line, with SGLT‐2 inhibitors and GLP‐1 receptor agonists prescribed to only a minority of patients. Second‐line therapy choices were diverse and varied among regions; the most common reason for changing therapy was lack of efficacy of first‐line treatment. Factors associated with the choice of second‐line therapy included patient characteristics, such as BMI and HbA1c level at the time of treatment change, and medical history. Non‐medical factors, such as access, affordability and availability, also appear to play an important role in the choice of SUs versus newer agents, such as SGLT‐2 and DPP‐4 inhibitors.
CONFLICT OF INTEREST
A. N., B. C., M. B. G., K. K., M. K., M. V. S., I. S., H. W. and S. P. are members of the DISCOVER Scientific Committee, and received financial support from AstraZeneca to attend DISCOVER planning and update meetings. H. C., P. F. and F. S. are employees of AstraZeneca. N. H. is a former employee of AstraZeneca. J. C.‐R. is an employee of Evidera. In addition, A. N. has received honoraria from Novo Nordisk, Medtronic, AstraZeneca and Eli Lilly, and research support from Novo Nordisk, Sanofi‐Aventis, Artsana and Dexcom; B. C. has received payment from AstraZeneca, Boehringer Ingelheim, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Takeda; M. B. G. has received payment from Merck‐Serono; K. K. has received payment from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, Takeda, Servier and Pfizer, research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Pfizer, and also acknowledges support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care – East Midlands (NIHR CLAHRC – EM) and the National Institute of Health Research (NIHR) Leicester–Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Centre; M. K. has received payment from Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Intarcia, Janssen, Novartis, Novo Nordisk, Glytec Systems, Merck (Diabetes) and Sanofi, and research support from AstraZeneca and Boehringer Ingelheim; M. V. S. has received payment from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharpe & Dohme, Novo Nordisk, Sanofi and Servier, and research support from Novo Nordisk and Sanofi; I. S. has received payment from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Kowa, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho and Takeda Pharmaceutical, and research support from Astellas Pharma, AstraZeneca, Daiichi Sankyo, Eli Lilly, Japan Foundation for Applied Enzymology, Japan Science and Technology Agency, Kowa, Kyowa Hakko Kirin, Midori Health Management Center, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Suzuken Memorial Foundation and Takeda Pharmaceutical; F. T. is an employee of the Mid America Heart Institute and has received research support from AstraZeneca; and H. W. has received payment from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Novartis, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho and Takeda, and research support from Abbott, Astellas Pharma, AstraZeneca, Bayer, Benefit One Health Care, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Johnson & Johnson, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nitto Boseki, Novartis, Novo Nordisk, Ono Pharmaceutical, Pfizer, Sanofi, Sanwakagaku Kenkyusho, Taisho Toyama Pharmaceutical, Takeda and Terumo Corp.
AUTHOR CONTRIBUTIONS
The general content of the manuscript was agreed upon by all authors. The first draft of the manuscript was developed by A. N., and all authors contributed to its development. All authors approved the final version of the manuscript before its submission. An AstraZeneca team reviewed the manuscript during its development and was allowed to make suggestions. However, the final content was determined by the authors. A. N. is the guarantor of this work.
DATA ACCESSIBILITY
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supporting information
Table S1. Study inclusion and exclusion criteria
Table S2. Baseline characteristics of DISCOVER patients receiving metformin monotherapy as first‐line treatment, overall and by second‐line therapy
Table S3. Reasons for therapy change for DISCOVER patients receiving metformin monotherapy as first‐line treatment, overall and by second‐line therapy
Table S4. Second‐line glucose‐lowering therapies in DISCOVER patients who received metformin monotherapy as first‐line treatment, overall and by region
Table S5. Reasons for choosing second‐line glucose‐lowering therapies in DISCOVER patients receiving metformin monotherapy as first‐line treatment, overall and by region
ACKNOWLEDGMENTS
The authors would like to thank all the investigators and patients participating in the DISCOVER study. Medical writing support was provided by Stéphane Pintat, PhD, of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
The DISCOVER study programme is funded by AstraZeneca. DISCOVER is a non‐interventional study programme and no drugs were supplied or funded. A team of AstraZeneca employees contributed to the design of the study. All authors had full access to the data. H. C., P. F. and F. S. are employees of AstraZeneca; they contributed to the analysis and interpretation of the data, and to the development of the manuscript. The corresponding author had final responsibility for the decision to submit the manuscript. All statistical analyses were funded by AstraZeneca and conducted independently of the study sponsor, by the statistical group at Saint Luke's Mid America Heart Institute, Kansas City, Missouri.
Nicolucci A, Charbonnel B, Gomes MB, et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second‐line therapy: Results from the global DISCOVER study programme. Diabetes Obes Metab. 2019;21:2474–2485. 10.1111/dom.13830
Funding information The DISCOVER study programme is funded by AstraZeneca.
REFERENCES
- 1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2018 executive summary. Endocr Pract. 2018;24:91‐120. [DOI] [PubMed] [Google Scholar]
- 2. Chinese Diabetes Society . Chinese guidelines for type 2 diabetes prevention (2013). Chin J Diabetes. 2014;22:2‐42. [Google Scholar]
- 3. International Diabetes Federation . Global guideline for type 2 diabetes. 2012. https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes.html. Accessed June 13, 2019.
- 4. Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:279‐290. [DOI] [PubMed] [Google Scholar]
- 5. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9:657‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31:1188‐1196. [DOI] [PubMed] [Google Scholar]
- 8. Katakami N, Mita T, Takahara M, et al. Rationale and design for the J‐DISCOVER study: DISCOVERing the treatment reality of type 2 diabetes in a real‐world setting in Japan ‐ a protocol. Diabetes Ther. 2018;9:165‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rathmann W, Medina J, Kosiborod M, et al. The DISCOVER study: diversity of sites, physicians, and patients. Pharmacoepidemiol Drug Saf. 2018;27:228. [Google Scholar]
- 10. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27‐38. [Google Scholar]
- 11. Allison P. Logistic Regression for Rare Events. 2012. http://statisticalhorizons.com/logistic-regression-for-rare-events. Accessed June 13, 2019.
- 12. Gomes MB, Rathmann W, Charbonnel B, et al. Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Diabetes Res Clin Pract. 2019;151:20‐32. [DOI] [PubMed] [Google Scholar]
- 13. Overbeek JA, Heintjes EM, Prieto‐Alhambra D, et al. Type 2 diabetes mellitus treatment patterns across Europe: a population‐based multi‐database study. Clin Ther. 2017;39:759‐770. [DOI] [PubMed] [Google Scholar]
- 14. International Diabetes Federation . Access to medicines and supplies for people with diabetes. 2016. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/106-global-survey-on-access-to-medicines-and-supplies-for-people-with-diabetes.html. Accessed June 13, 2019.
- 15. Chow CK, Ramasundarahettige C, Hu W, et al. Availability and affordability of essential medicines for diabetes across high‐income, middle‐income, and low‐income countries: a prospective epidemiological study. Lancet Diabetes Endocrinol. 2018;6:798‐808. [DOI] [PubMed] [Google Scholar]
- 16. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 17. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 18. Marso SP, Daniels GH, Brown‐Frandsen K, et al. LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 20. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study. Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:105‐113. [DOI] [PubMed] [Google Scholar]
- 22. Roglic G, Norris SL. Medicines for treatment intensification in type 2 diabetes and type of insulin in type 1 and type 2 diabetes in low‐resource settings: synopsis of the World Health Organization guidelines on second‐ and third‐line medicines and type of insulin for the control of blood glucose levels in nonpregnant adults with diabetes mellitus. Ann Intern Med. 2018;169:394‐397. [DOI] [PubMed] [Google Scholar]
- 23. Hirst JA, Farmer AJ, Dyar A, Lung TW, Stevens RJ. Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta‐analysis. Diabetologia. 2013;56:973‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427‐2443. [DOI] [PubMed] [Google Scholar]
- 25. Murayama H, Imai K, Odawara M. Factors influencing the prescribing preferences of physicians for drug‐naive patients with type 2 diabetes mellitus in the real‐world setting in Japan: insight from a web survey. Diabetes Ther. 2018;9:1185‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zgibor JC, Songer TJ. External barriers to diabetes care: addressing personal and health systems issues. Diabetes Spectr. 2001;14:23‐28. [Google Scholar]
- 27. Zhuang XD, He X, Yang DY, et al. Comparative cardiovascular outcomes in the era of novel anti‐diabetic agents: a comprehensive network meta‐analysis of 166,371 participants from 170 randomized controlled trials. Cardiovasc Diabetol. 2018;17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Douros A, Dell'Aniello S, Yu OHY, Filion KB, Azoulay L, Suissa S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population based cohort study. BMJ. 2018;362:k2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kosiborod M, Birkeland KI, Cavender MA, et al. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT‐2 inhibitors versus other glucose‐lowering agents in real‐world clinical practice: results from the CVD‐REAL study. Diabetes Obes Metab. 2018;20:1983‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnold SV, Inzucchi SE, McGuire DK, et al. Evaluating the quality of comprehensive cardiometabolic care for patients with type 2 diabetes in the U.S.: the Diabetes Collaborative Registry. Diabetes Care. 2016;39:e99‐e101. [DOI] [PubMed] [Google Scholar]
- 31. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251‐255. [DOI] [PubMed] [Google Scholar]
- 32. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69:4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Becher H, Kostev K, Schroder‐Bernhardi D. Validity and representativeness of the "Disease Analyzer" patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47:617‐626. [DOI] [PubMed] [Google Scholar]
- 34. Arnold SV, Inzucchi SE, Echouffo‐Tcheugui JB, et al. Understanding contemporary use of thiazolidinediones. Circ Heart Fail. 2019;12:e005855. [DOI] [PubMed] [Google Scholar]
- 35. Arnold SV, McGuire DK, Inzucchi SE, et al. Assessing use of patient‐focused pharmacotherapy in glycemic management through the Diabetes Collaborative Registry (DCR). J Diabetes Complications. 2018;32:1035‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study inclusion and exclusion criteria
Table S2. Baseline characteristics of DISCOVER patients receiving metformin monotherapy as first‐line treatment, overall and by second‐line therapy
Table S3. Reasons for therapy change for DISCOVER patients receiving metformin monotherapy as first‐line treatment, overall and by second‐line therapy
Table S4. Second‐line glucose‐lowering therapies in DISCOVER patients who received metformin monotherapy as first‐line treatment, overall and by region
Table S5. Reasons for choosing second‐line glucose‐lowering therapies in DISCOVER patients receiving metformin monotherapy as first‐line treatment, overall and by region
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


