Abstract
Multicenter, phase‐4, randomized, comparative‐efficacy study in patients with VLUs or DFUs comparing for noninferiority the percentage change in target ulcer dimensions (area, depth, and volume) a single‐use negative pressure wound therapy (s‐NPWT) system versus traditional NPWT (t‐NPWT) over a 12‐week treatment period or up to confirmed healing. Baseline values were taken at the randomization visit. Randomized by wound type and size, 164 patients with non‐infected DFUs and VLUs were included. The ITT population was composed of 161 patients (101 with VLUs, 60 with DFUs) and 115 patients completed follow‐up (64 in the s‐NPWT group and 51 in the t‐NPWT group) (PP population). The average age for all patients was 61.5 years, 36.6% were women, and treatment groups were statistically similar at baseline. Primary endpoint analyses on wound area reduction demonstrated statistically significant reduction in favor of s‐NPWT (p = 0.003) for the PP population and for the ITT population (p < 0.001). Changes in wound depth (p = 0.018) and volume (p = 0.013) were also better with s‐NPWT. Faster wound closure was observed with s‐NPWT (Cox Proportional Hazards ratio (0.493 (0.273, 0.891); p = 0.019) in the ITT population. Wound closure occurred in 45% of patients in the s‐NPWT group vs. 22.2% of patients in the t‐NPWT group (p = 0.002). Median estimate of the time to wound closure was 77 days for s‐NPWT. No estimate could be provided for t‐NPWT due to the low number of patients achieving wound closure. Device‐related AEs were more frequent in the t‐NPWT group (41 AEs from 29 patients) than in the s‐NPWT group (16 AEs from 12 patients). The s‐NPWT system met noninferiority and achieved statistical superiority vs. t‐NPWT in terms of wound progression toward healing over the treatment period. When NPWT is being considered for the management of challenging VLUs and DFUs, s‐NPWT should be considered a first choice over other types of NPWT.
List of Abbreviations
- BWAT‐m
modified Bates‐Jensen Wound Assessment Tool
- EQ‐5D‐5 L
EuroQoL 5 Dimensions, 5 Levels
- MVTR
Moisture Vapor Transmission Rate
- SSC
Surgical Site Complications
- SSI
Surgical Site Infection
- s‐NPWT
Single‐use Negative Pressure Wound Therapy
- t‐NPWT
Traditional Negative Pressure Wound Therapy
INTRODUCTION
The most common types of chronic wounds worldwide are venous leg ulcers (VLUs), diabetic foot ulcers (DFUs), and pressure ulcers. More than 40 million new chronic wounds are reported annually.1
Most leg ulcers (at least 70%) are the result of chronic venous insufficiency2 and others are due to mixed venous and arterial disease.3 Estimated to occur in 1–2% of the population worldwide, the prevalence of leg ulcers may rise to 5% in the elderly.4 Assuming good arterial blood supply, graduated compression therapy is the gold standard treatment for VLU.
DFU affect 15–25% of all diabetic patients during their lifetime2, 5, 6 and precede 84% of all lower leg amputations.7 Everett et al8 found a 5% mortality rate in the first 12 months following the development of a DFU, and 5‐year mortality rates have been estimated at 42%. In addition, patients with DFUs also have a 2.5‐fold increased risk of death compared with their diabetic counterparts without foot wounds.8
The management of DFUs is based on the three principles: off‐loading, appropriate local wound management (including surgical debridement), and infection control.8 For DFUs that fail to improve (>50% wound area reduction) after 4 weeks of standard wound therapy, the Society for Vascular Surgery and others recommend to consider the use of adjunctive wound therapy options, which include negative pressure wound therapy (NPWT).9
Over 20 years ago, Argenta and Morykwas, published the results of their experimental work with negative pressure,10 as well as the results of a clinical trial11 on the treatment of wounds with NPWT—including 175 chronic wounds. They concluded that NPWT, with both continuous and intermittent application, enhanced granulation tissue formation and helped bacterial clearance. Armstrong and Lavery confirmed these findings in their 2005 paper,12 comparing NPWT vs. standard moist wound care after partial foot amputation in patients with diabetes. More patients in the NPWT group healed and showed a faster rate of granulation tissue formation.
The pump in traditional negative pressure wound therapy (t‐NPWT)1 systems is designed for several years of use with numerous individual patients.13 t‐NPWT has been described as an effective treatment for acute and chronic wounds of different etiologies.14, 15
While many t‐NPWT systems are available, comparative studies have demonstrated equivalent clinical outcomes.16, 17 t‐NPWT provides consistent management of wounds across diverse clinical settings and as a result, it has been widely adopted as a treatment of choice in many different clinical circumstances. Some t‐NPWT systems have been adapted and received approval for use in the home setting. Still, the dressing may be complicated to apply, and the size of the pump and canister may be intrusive and limit patient mobility.17
Evidence supports NPWT as adjunctive care in patients with DFUs,18, 19, 20, 21, 22, 23, 24 however, high quality clinical evidence on the management of chronic wounds is still scarce for VLUs.25, 26, 27
Kieser et al28 demonstrated that the addition of t‐NPWT in the management of resistant chronic venous ulcers being treated with compression therapy produced rapidly improving ulcers with a clean, granulating base. In another group of patients with nonhealing venous ulcers previously treated with elastic compression Kucharzewski et al29 achieved complete healing in all ulcers by 20 weeks.
In 2012, Armstrong et al30 showed noninferiority of a single‐use NPWT mechanically powered system when compared to an electrically powered t‐NPWT system for the management of lower extremity ulcers. The size and how the vacuum was produced being different, both types of devices used similar types of dressing including a filler.
More recently, a battery‐operated single‐use NPWT (s‐NPWT)2 system have become available. Based on their reliability to deliver negative pressure consistently,17 single‐use NPWT systems are expected to simplify the application and management of NPWT and make the therapy accessible to more patients, including very active as well as homebound individuals.13
The investigation product (PICO, Smith & Nephew, Fort Worth, TX) is a single‐use NPWT system; many recent publications support its clinical efficacy in the postoperative management of closed surgical incisions to reduce the incidence of surgical site complications (SSC)3, and surgical site infections (SSI)4.31, 32, 33, 34, 35 However, limited data existed for this device in the treatment of chronic wounds.35, 36, 37, 38, 39
A prospective pilot study was undertaken in which chronic lower extremity wounds (two DFUs and nine VLUs) were treated with s‐NPWT for four weeks.36 In this study, DFUs decreased in size on average 62%, VLUs by 32%. All VLUs were treated with concurrent compression bandaging.
Hampton37 showed in a small group of nonhealing VLU that the use of s‐NPWT led to an average weekly reduction in size of 21%. During the six weeks of the study, the wound size achieved with s‐NPWT was reached on average 10 weeks earlier than predicted.
In a multicountry study using s‐NPWT on hard to heal wounds which included 12 VLUs,38 there was a statistically significant improvement in the healing trajectory resulting in a 33% reduction in cost.
In a small pilot study comparing the use of NPWT with historic controls in the management of VLUs not responding to compression,39 there was a significant reduction in time to heal (p = 0.024).
A clinical study was undertaken to determine the clinical efficacy of a NPWT system in postsurgical open diabetic foot amputation wounds.40 To allow for comparison, the study specifically mimicked the inclusion and exclusion criteria and endpoints of the 2005 Armstrong and Lavery paper12 cited above. They found that the tested NPWT system was well tolerated and effective; the results confirmed noninferiority when compared to results from the paper by Armstrong and Lavery.12
The purpose of this study was to clinically compare for efficacy and safety, one portable battery‐powered, disposable, canisterless s‐NPWT system that delivers −80 mmHg of negative pressure with traditional NPWT systems, which include a canister, require mains electricity supply and deliver negative pressure at −125 mmHg in the management of lower extremity ulcers, including both VLUs and DFUs.
MATERIALS AND METHODS
This was a randomized, controlled, multicenter study designed to compare the clinical efficacy of two types of NPWT systems (single‐use NPWT and traditional NPWT) to promote wound healing in patients with chronic lower extremity wounds, either VLUs or DFUs.
Patients were initially screened, and then received two weeks of standard care (either compression or offloading), in order to verify two weeks later if the implementation of standard of care had a significant effect on wound healing, and to avoid bias from potentially including “quick healers”. If the target ulcer reduced in area > 30% during the run‐in period, the subject was not included in the study. Those remaining were randomized to either s‐NPWT or t‐NPWT and were followed weekly for 12 weeks or until ulcer healed, whichever occurred first. Patients whose wounds had closed prior to the 12th week of treatment were instructed to return for one further visit to confirm the wound healing for purposes of reporting.
The study was performed in compliance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice (GCP), ISO 14155:2011, and under governing IRB/IEC review and approval of the study protocol. Recruited patients signed an informed consent form before starting their participation in the study.
The 18 centers participating in the trial (16 in the USA and 2 in Canada) were a good representation of clinical institutions where wound care is provided.
The Study was registered at http://clinicaltrial.gov under the number NCT02470806.
Objectives
The primary objective was to assess a single‐use NPWT system vs. traditional NPWT (different brands) for the percentage change in target wound area over a 12‐week period from baseline. Secondary endpoints were the percentage change in the target ulcer depth and volume, time (in days) to achieve complete target ulcer closure, and the proportion of patients that achieved confirmed complete target ulcer closure.
Safety was assessed on all randomized patients. The safety analysis was based on an evaluation of the extent of exposure to study therapies and the occurrence of adverse events (AE). The duration of exposure was calculated as the number of treatment days during the course of the study. Adverse events associated with the target ulcer and all serious adverse events (SAE) were recorded.
Study device
The study device is a single‐use NPWT system, composed of a battery‐powered pump set to deliver continuous negative pressure at an average of ‑80 mmHg. The device is small, lightweight and does not require a canister. Instead uses a dressing which incorporates (1) a silicone interface, (2) an incompressible layer that transmits pressure evenly across the whole wound bed, (3) a superabsorbent core to lock exudate away from the wound and a high MVTR5 top film layer, which allows the evaporation of up to 80% of absorbed exudate. The dressing can be used with or without wound fillers (foams, gauze) depending on individual clinical characteristics and needs of the wound. The system is intended for use in wound sizes up to 400 cm3 in volume, which are low to moderately exuding. The system is expected to work for up to 7 days. Dressings can be changed as required (e.g. every 2–3 days).
Traditional NPWT devices used for comparison
All t‐NPWT systems used as comparators during this trial had the following characteristics: (1) capable of generating a range of negative pressures; (2) a canister; (3) connective tubing; (4) foam or gauze fillers. All t‐NPWT devices had the corresponding approvals for home use.
ActiV.A.C. ® NPWT System, Kinetic Concepts Inc., (KCI) an Acelity Company, San Antonio TX. Negative pressure range from −25 to −200 mmHg.
Invia® Liberty NPWT System, Medela Inc., McHenry IL. Negative pressure range from −40 to −200 mmHg.
Avance® NPWT System, Mölnlycke Health Care, Goteborg, Sweden. Negative pressure range from −60 to −180 mmHg.
Renasys◊ GO NPWT System, Smith & Nephew Inc., Fort Worth TX. Negative pressure range from −40 to −200 mmHg.
The t‐NPWT systems were used according to their specific instructions for use and following local protocols. Investigators were allowed to decide about the type and level of negative pressure to be applied over the target wounds.
Inclusion criteria
Criteria for screening and recruitment included adult patients of both genders with either a VLU present for more than 4 weeks and measuring 2 to 36 cm2 in surface area or a DFU present for more than 4 weeks and measuring 0.5 to 10 cm2 in surface area. Patients must have provided signed consent, be capable and willing to comply with protocol instructions, and be in acceptable health state. Inclusion criteria also required a confirmed adequate arterial supply defined as either an ABI ≥0.7 ≤ 1.2, great toe pressure ≥ 40 mmHg, or a transcutaneous perfusion of oxygen ≥30 mmHg on the foot.
Exclusion criteria
Exclusion criteria included suspected or known allergies to the components of the different NPWT systems; pregnancy; participation in other research within 30 days of screening; ulcers deemed by the investigator to be highly exuding; anatomic location not amendable to the creation of an airtight seal; malignancy in the target ulcer; concurrent diagnosis of vasculitis or claudication; current administration of systemic chemotherapy or corticosteroids. In addition, patients who had previous treatment with NPWT or hyperbaric oxygen within 7 days of screening, leukopenia, thrombocytopenia, anemia, two‐fold or higher increase in bilirubin levels, three times or higher increase in hepatic enzymes were also excluded.
For patients with nondiabetic ulceration, exclusion criteria also included: ulcers whose etiology was nonvenous (e.g. sickle‐cell anemia, pyoderma gangrenosum, vasculitis), the presence of deep vein thrombosis, the refusal or inability to tolerate compression therapy, exposure of muscle, tendon or bone in the target ulcer, the size of the target ulcer was >15 cm in one linear direction. For patients with DFU, exclusion criteria also included: diagnosis of active Charcot foot syndrome and the location of the target wound on the toes.
Furthermore, at the end of the run‐in period and prior to randomization, the following exclusion criteria applied to all patients: a reduction of the target‐ulcer area ≥ 30% during the run‐in period, the use of excluded medications, therapies or procedures during the run‐in period, a clinical infection of the target ulcer requiring treatment, and the investigator's judgment that the subject was not appropriate for the trial.
Wound management
Ulcers were treated following good clinical practice and standardized approved protocols for the management of wound care. In addition, patients with VLUs were also required to wear multilayer compression bandages, and patients with DFUs to use offloading.
Patients were seen weekly at the wound centers for assessment and procedures including wound debridement if required and the study intervention, which consisted in the application/change of the corresponding s‐NPWT or t‐NPWT system/dressing. Patients returned home with clear instructions about when to contact the center in case of problems between the visits.
Assessment methods
Wound dimensions were measured using the ARANZ–Silhouette® wound imaging and measurement device at each study visit.
Target ulcers were assessed using a modified Bates‐Jensen wound assessment tool (BWAT‐m)6. This included information related to the presence of undermining, necrotic tissue type, necrotic tissue amount, exudate type, exudate amount, skin color surrounding the skin, granulation tissue and epithelialization. Each element was used as required by the BWAT tool to obtain a score ranging from 1 to 5. The validity of this approach was guaranteed by the individual validity of the elements when used as described by the BWAT tool. The separate measurements were combined to produce a wound status score on a scale of 8–40. A higher status score reflected a worse wound status. Baseline scores obtained at the randomization visit were compared to scores obtained at the exit visit.
The condition of the periwound skin was assessed using a custom designed 7‐category descriptive scale: Normal, Erythematous, Edematous, Eczematous, Excoriated, Macerated, and Indurated.
Wound closure was defined as complete reepithelialization, without drainage or the need for a dressing, and assessed at two different visits. When full closure was seen at one visit, patients were required to attend one additional visit, one week later to confirm 100% wound closure and epithelium stability.
DFU were additionally categorized using the Wagner classification.
Quality of life was assessed using the cardiff wound impact schedule (CWIS) and the EQ‐5D‐5L7 instrument. Study patients completed both instruments at the randomization visit (baseline) and at the end of the treatment period.
A survey designed to assess the impact of the NPWT devices on aspects of day living was completed by patients at the exit visit.
Randomization
Allocation of patients to treatment group was conducted using an online randomization system (Sealed Envelope™) and was stratified by ulcer type and ulcer size (cm2) at baseline. Each stratification factor contained two levels: VLU or DFU and small or large ulcer. The surface size to consider an ulcer as large was >12 cm2 for VLU, and more than 2 cm2 for DFU.
Statistical analysis
The study was intended to test for noninferiority in the percentage change of target‐ulcer area with s‐NPWT vs. t‐NPWT over a 12‐week treatment period. During a review of five published studies with VLU and DFU, treated with s‐NPWT or with t‐NPWT, the mean percentage change in ulcer area was found to be approximately 47–62%, in cases where it was considered an appropriate measure; the standard deviation ranged between 21% and 24.5%. Using a noninferiority margin of 12.5%, a sample size of 128 patients will provide 80% statistical power at the (cumulative) 0.025 one‐sided significance level assuming a weighted average mean healing of 60% with a worst‐case standard deviation of 24.5%. To allow for a 20% drop out rate throughout the 12‐week treatment period, it was determined that 160 patients (80 per treatment group) would need to be randomized. Unless otherwise stated, all significance tests and hypothesis testing are two‐sided and performed at the 5% significance level. Resulting p‐values are quoted and 95% two‐sided confidence intervals are provided where appropriate. All statistical analyses were completed using SAS for Windows version 9.4.
For noninferiority analysis, the primary analysis was performed with the per protocol population so that drop outs do not drive noninferiority and then the analysis was repeated using the full analysis set (intention to treat population). For the efficacy results, the difference between treatment groups was analyzed using a stepwise regression method considering treatment and all baseline characteristics for the model, which at a minimum contained treatment, pooled site, baseline area, and wound type. Variables were required to have an F‐value significant at the 10% level to be entered into the model.
RESULTS
A total of 217 patients were screened for inclusion into the study. Of these, 164 patients were enrolled (60 with DFU and 104 with VLU), randomized to treatment following successful completion of the screened period, and received at least one of the trial treatments; they constituted the safety population (SAF). Details of the different study populations are presented in Figure 1. The intention‐to‐treat (ITT) population included 161 patients who were randomized, received trial treatment, and attended at least one follow‐up post baseline visit.
Figure 1.
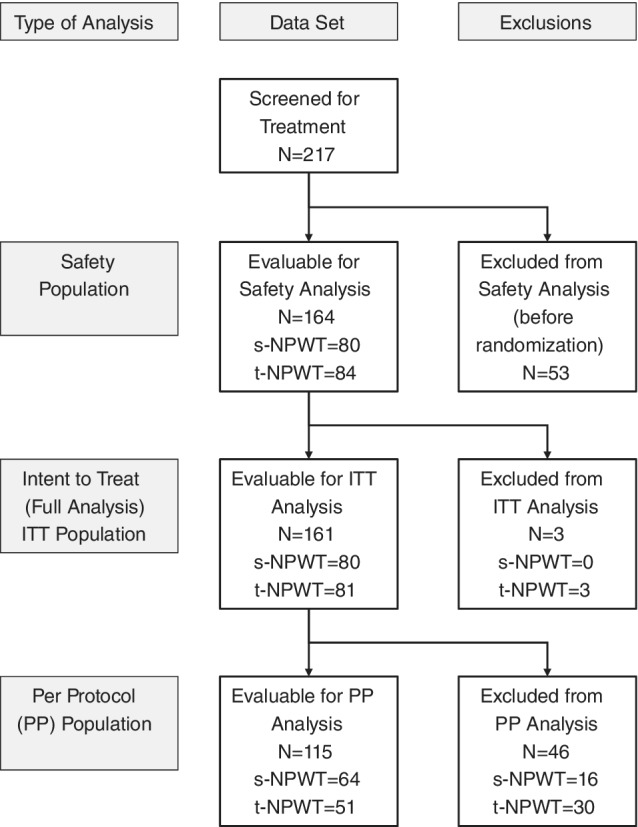
The distribution of the randomized subjects by statistical analysis population: safety and efficacy (ITT and PP).
The per protocol population (PP) included 115 patients randomized to trial treatment, who continued treatment and had no significant protocol deviations. Patients who achieved closure were included regardless of time on therapy unless they were deemed to have significant protocol deviations.
Demographic characteristics
Table 1 summarizes the demographic characteristics of the ITT population. The average age for all patients was 61.5 years, and 36.6% of patients were women. The demography of treatment groups was statistically similar.
Table 1.
Demographic characteristics (ITT population)
| Characteristics | Total | Treatment group | p‐value | ||
|---|---|---|---|---|---|
| s‐NPWT N = 80 | t‐NPWT N = 81 | ||||
| Age (years) | Mean | 61.5 | 62.5 | 60.4 | 0.317 |
| SD | 13.4 | 14.7 | 11.9 | ||
| BMI | Mean | 33.8 | 33.7 | 33.9 | 0.867 |
| SD | 8.9 | 8.8 | 9.0 | ||
| Gender | Female | 59 | 32 | 27 | 0.380 |
| Male | 102 | 48 | 54 | ||
Medical history and subject mobility
Relevant medical history details are presented in Table 2. There were no statistically significant differences between the two groups.
Table 2.
Distribution of preexisting medical conditions (ITT population)
| Medical condition | Treatment group | Overall N = 161 | |
|---|---|---|---|
| s‐NPWT N = 80 | t‐NPWT N = 81 | ||
| Anemia | 13 (16.3%) | 12 (14.8%) | 25 (15.5) |
| Stroke (CVA) | 5 (6.3%) | 6 (7.4%) | 11 (6.8%) |
| Peripheral vascular disease | 14 (17.5%) | 10 (12.3%) | 24 (14.9%) |
| Congestive heart failure | 7 (8.8%) | 13 (16%) | 20 (12.4%) |
| Rheumatoid arthritis | 2 (2.5%) | 4 (4.9%) | 6 (3.7%) |
| Osteoarthritis | 15 (18.8%) | 17 (21%) | 32 (19.9%) |
| Deep vein thrombosis | 9 (11.3%) | 13 (16%) | 22 (13.7%) |
| Varicose veins | 22 (27.5%) | 29 (35.8%) | 51 (31.7%) |
| Immunodeficiency | 1 (1.3%) | 2 (2.5%) | 3 (1.9%) |
| Steroid use | 1 (1.3%) | 1 (1.2%) | 2 ((1.2%) |
| Hypertension | 59 (73.8%) | 54 (66.7%) | 113 (70.2%) |
| Neuropathy | 38 (47.5%) | 42 (51.9%) | 80 (49.7%) |
Ninety‐five patients of the ITT population had diabetes, all of them Type‐II; similarly distributed between treatment groups, s‐NPWT = 46, t‐NPWT = 49.
A majority of patients in the ITT population had good mobility and were able to walk unaided. The remainder of the patients were either able to walk with aid or were chair‐bound. No patients were bed bound. The distribution of mobility status was similar between treatment groups.
Wounds types
Since the randomization included stratification, similar numbers of each wound type were distributed between treatment groups. No significant differences were found regarding wound type, wound location, and Ankle‐Brachial Index at baseline. Average wound duration prior to inclusion was 23 weeks in the s‐NPWT group and 29 weeks in the t‐NPWT group; this difference was not statistically significant. More ulcers were located in the left limb (57%), and the lesions were primary in 70% of the patients, these finding were similar in both treatment groups.
There was no difference between treatment groups in the percentage of patients having received previous treatment for their reference wound. The most frequently used devices were foam dressings, antimicrobial dressings, gauze, and compression therapy bandages.
Considering DFU patients only, 29 had Wagner grade 1 ulcers and 31 had Wagner grade 2 ulcers. Forty‐one ulcers were located in the plantar area, 11 in the dorsum of the foot, and 8 on the heel.
Prior to randomization, healing trajectories for reference wounds between screening and randomization were analyzed by group after the randomization took place. There was no statistically significant difference in the percentage change in wound area (p = 0.252), suggesting that healing trajectories were similar while undergoing treatment with standard care prior to study treatment.
Fillers were used in all patients in the t‐NPWT as per manufacturers’ instructions. In the s‐NPWT group, fillers were used in less than 20% of the dressing changes, mainly in DFUs.
The t‐NPWT systems were used in continuous mode 98.1% of the time and in intermittent mode 1.9% of the time; negative pressure was set at an average − 118.3 mmHg (median: −125 mmHg, SD: 23.4 mmHg).
The mean wound area for all the patients in the ITT population was 6.6 cm2 (6.7 and 6.5 for s‐NPWT and t‐NPWT, respectively). In addition, the mean wound depth at baseline was 3.2 mm, and the mean volume was 0.3 cm3. There were no significant differences between the treatment groups in baseline wound dimensions.
Primary efficacy, wound area improvement
In the per‐protocol (PP) analysis set, the average baseline wound area was similar between the two treatment groups though slightly larger in the t‐NPWT group, 6.2 cm2 for s‐NPWT and 7.8 cm2 for t‐NPWT. By the end of the 12‐week treatment period, the average change in wound area favored s‐NPWT (0.7 cm2) when compared to t‐NPWT (3.8 cm2). The mean percent reduction in area was therefore 88.7% for s‐NPWT and 58.6% for t‐NPWT, which was statistically significant (p = 0.003). More details are presented in Table 3.
Table 3.
Change in wound area over 12‐week treatment period by treatment group. All wounds (PP population)
| Characteristic | Parameter | Total | Treatment group | |
|---|---|---|---|---|
| s‐NPWT N = 64 | t‐NPWT N = 51 | |||
| Baseline wound area (cm2) | Mean | 6.9 | 6.2 | 7.8 |
| SD | 6.1 | 5.9 | 6.3 | |
| Final wound area (cm2) | Mean | 2.1 | 0.7 | 3.8 |
| SD | 4.9 | 1.8 | 6.7 | |
| Change in wound area (cm2) | Mean | (−) 4.9 | (−) 5.5 | (−) 4.0 |
| SD | 5.8 | 5.4 | 6.2 | |
| Percentage change in wound area (%) | Mean | (−) 75.3 | (−) 88.7 | (−) 58.6 |
| SD | 48 | 24 | 64 | |
After adjustment for baseline wound area, pooled site, wound type (DFU/VLU), and wound duration at baseline in the PP population, the least squares (LS) mean percentage change in wound area over the 12‐week treatment period was 96.9% for s‐NPWT and 69.9% for t‐NPWT. The 27% area reduction difference in favor of s‐NPWT meets the preestablished definition of noninferiority using a 12.5% margin. In addition, this difference was statistically significant (p = 0.003).
The above analyses were also repeated using the ITT population. The change in wound area at the end of the 12‐week treatment period, and the percentage change in wound area over the same period are presented in Table 4. The results remained in favor of s‐NPWT and the difference between the two treatments (s‐NPWT: t‐NPWT) increased compared to the results from the PP population analysis. When using the ITT population, the LS‐mean percent reduction was 90.24% for s‐NPWT and 51% for t‐NPWT. The difference of 39.1% in favor of s‐NPWT was statistically significant (p < 0.001).
Table 4.
Change in wound area over 12‐week treatment period by wound type and treatment group. (ITT population)
| Characteristic | Parameter | Total | Treatment group | ||
|---|---|---|---|---|---|
| s‐NPWT | t‐NPWT | ||||
|
ALL wounds N = 161 |
Baseline wound area (cm2) | Mean | 6.6 | 6.7 | 6.5 |
| SD | 6.3 | 6.6 | 6.1 | ||
| Final wound area (cm2) | Mean | 3.3 | 2.2 | 4.5 | |
| SD | 7.4 | 6.6 | 8.1 | ||
| Change in wound area (cm2) | Mean | (−) 3.3 | (−) 4.5 | (−) 2.0 | |
| SD | 6.5 | 6.3 | 6.5 | ||
| Percentage change in wound area (%) | Mean | (−) 52.1 | (−) 73.1 | (−) 31.3 | |
| SD | 68 | 45 | 81 | ||
|
VLU N = 101 |
Change in wound area (cm2) | Mean | (−) 4.2 | (−) 5.7 | (−) 2.6 |
| SD | 7.8 | 7.3 | 8.0 | ||
| Percentage change in wound area (%) | Mean | (−) 55.3 | (−) 74.5 | (−) 35.8 | |
| SD | 68 | 45 | 81 | ||
|
DFU N = 60 |
Change in wound area (cm2) | Mean | (−) 1.7 | (−) 2.4 | (−) 1.0 |
| SD | 2.7 | 2.6 | 2.7 | ||
| Percentage change in wound area (%) | Mean | (−) 46.6 | (−) 70.8 | (−) 24 | |
| SD | 69 | 45 | 80 | ||
Further, in both ITT and PP analyses, wound duration at baseline was found to be a significant factor (p = 0.001 and p = 0.003, respectively) affecting inversely the percentage change in wound area.
Subset analyses were performed using data from VLU and DFU patients separately. Results are presented in Table 4. While the percentage change over the 12‐week treatment period was generally larger for VLUs, similar differences between treatment groups (treatment effect) were observed when compared to results across all wound types combined. The difference in LS‐mean percent change in wound area over the 12‐week treatment period was a bigger reduction of 36.15% for VLUs, and a bigger reduction of 38.8% for DFUs in patients of the s‐NPWT group. The differences between treatment groups for both subsets, VLU (p = 0.007) and DFU (p = 0.031), were statistically significant.
Key secondary efficacy and wound depth variation
In the PP analysis set, the average baseline wound depth was 4.0 mm for s‐NPWT and 2.4 mm for t‐NPWT. By the end of the 12‐week treatment period, the average change in wound depth favored s‐NPWT (−3.4 mm) when compared to t‐NPWT (−0.9 mm). The mean percent change in depth was a 68.8% reduction for s‐NPWT and a 38.8% reduction for t‐NPWT. Detailed data are presented in Table 5. When using the PP population, the LS‐mean percent change in wound depth over the 12‐week period was a 72.4% reduction for s‐NPWT, and a 41.6% reduction for t‐NPWT after adjustment for baseline wound depth, pooled site, wound type, and wound duration at baseline. The difference of 30.8% in favor of s‐NPWT was statistically significant (p = 0.018).
Table 5.
Change in wound depth over 12‐week treatment period by treatment group. All wounds (PP population)
| Characteristic | Parameter | Total | Treatment group | |
|---|---|---|---|---|
| s‐NPWT N = 64 | t‐NPWT N = 51 | |||
| Baseline wound depth (mm) | Mean | 3.3 | 4.0 | 2.4 |
| SD | 5.3 | 6.8 | 1.3 | |
| Final wound depth (mm) | Mean | 1.0 | 0.6 | 1.5 |
| SD | 1.6 | 1.1 | 2.1 | |
| Change in wound depth (mm) | Mean | (−) 2.3 | (−) 3.4 | (−) 0.9 |
| SD | 5.6 | 7.1 | 1.9 | |
| Percentage change in wound depth (%) | Mean | (−) 56 | (−) 68.8 | (−) 38.8 |
| SD | 66 | 59 | 71 | |
These analyses were also performed using the ITT population. The average baseline wound depth was similar for both groups: 3.7 mm for s‐NPWT and 2.7 mm for t‐NPWT. The mean percentage change in depth was a 48.1% reduction in the s‐NPWT group and a 12.7% reduction in the t‐NPWT group. The adjusted LS‐mean percent change was a 45.6% reduction for s‐NPWT and a 13.2% reduction for t‐NPWT. The difference of 32.5% in favor of s‐NPWT remained statistically significant (p = 0.014).
Secondary efficacy and wound volume variation
In the PP analysis set, the average baseline wound volume was closely similar between treatment groups: 0.3 cm3 for both s‐NPWT and t‐NPWT. By the end of the 12‐week treatment period, the average percentage change in wound volume favored s‐NPWT (98% reduction) when compared to t‐NPWT (10% reduction). Results from the ITT population showed a reduction of volume in the s‐NPWT group (61% smaller) and an increase in volume in the t‐NPWT group (30% bigger).
The LS‐mean percentage change in the PP population was a 77.9% reduction for the s‐NPWT and a 1.6% increase for t‐NPWT after adjustment for baseline wound volume, pooled site, wound type an wound duration at baseline. The difference of 79.5% in favor of s‐NPWT was statistically significant (p = 0.01). The same analyses with the ITT population showed a percentage reduction of 48.6% for the s‐NPWT group and 42.5% increase for the t‐NPWT group. The difference of 91.1% in favor of s‐NPWT remained statistically significant (p = 0.013).
Secondary efficacy and time to wound closure
In the ITT population, across all wound types, 54 (33.5%) patients achieved confirmed wound closure. This was comprised of 36 (45%) patients in the s‐NPWT group, and 18 (22%) patients in the t‐NPWT group. Table 6 presents the distribution of confirmed wound closure over the 12‐week period by treatment group.
Table 6.
Confirmed wound closure over the 12‐week follow‐up period by treatment group. (ITT population)
| Wound type | Confirmed closure | Total | Treatment group | |
|---|---|---|---|---|
| s‐NPWT | t‐NPWT | |||
| VLU and DFU | Yes | 54 (33.5%) | 36 (45%) | 18 (22.2%) |
| No | 107 (66.5%) | 44 (55%) | 63 (77.8%) | |
| VLU | Yes | 37 (36.6%) | 23 | 14 |
| No | 64 (63.4%) | 28 | 36 | |
| DFU | Yes | 17 (28.3%) | 13 | 4 |
| No | 43 (71.7%) | 16 | 27 | |
The odds ratio (t‐NPWT: s‐NPWT) for the incidence of confirmed wound closure, after adjusting for wound type, pooled site, and wound duration; across all wound types was 0.294 (0.135, 0.638) (p = 0.002) in favor of s‐NPWT. The odds ratios for wound types, individually, and combined are presented in Table 7.
Table 7.
Logistic regression model for confirmed wound closure
| Incidence of confirmed wound closure | Odds ratio (t‐NPWT: s‐NPWT) | 95% confidence interval | p‐value | |
|---|---|---|---|---|
| Lower | Upper | |||
| ITT–All wounds | 0.294 | 0.135 | 0.638 | 0.002 |
| ITT–VLU | 0.398 | 0.152 | 1.044 | 0.061 |
| ITT–DFU | 0.161 | 0.035 | 0.754 | 0.020 |
The PP population analysis resulted in a similar conclusion to the ITT analysis across all wound types; with significant difference in wound closure during the 12‐week treatment period. However, when analyzed as separate wound types, while the treatment difference for VLUs and DFUs remained in favor of s‐NPWT, due to lower numbers of subjects available for analysis in the PP, subset analyses did not find a statistical significance.
Using a cox proportional hazards model, the hazard ratio (t‐NPWT: s‐NPWT) for the time to achieve confirmed wound closure across all wound types was 0.493 (0.273, 0.891) in the ITT group. This ratio was statistically significant (p = 0.019) in favor of s‐NPWT. Wound duration at baseline was found to be a significant covariate in the model. The survival plots for achieving confirmed wound closure comparing treatment groups is presented in Figure 2. The median estimate of the time to achieve confirmed wound closure was 77 days for s‐NPWT (49, undefined limit). No estimate could be provided for t‐NPWT due to the low number of patients achieving confirmed wound closure during the 12‐week follow‐up period.
Figure 2.
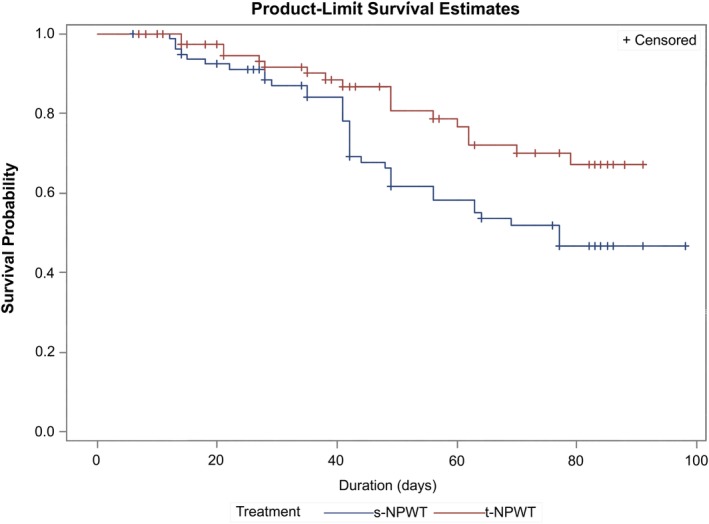
The survival plots for probability of achieving confirmed wound closure (ITT).
Wound assessment
The wound status BWAT‐m scores were similar across treatment groups at the baseline assessment; 18.5 for s‐NPWT, compared with 17.9 for t‐NPWT. This difference was not statistically significant. However, the mean change in wound status score between the baseline and exit visit was −3.2 for s‐NPWT, compared with −1.8 for t‐NPWT. A negative change in score suggests an improving wound. The difference between treatment groups, in terms of the change in wound status score was statistically significant (p = 0.021), suggesting that target wounds in the s‐NPWT group had improved by a greater amount compared to t‐NPWT, between the baseline and exit visit.
Dressing wear time
Across all wound types, patients in the s‐NPWT group required on average 8.5 dressing changes, while those in the t‐NPWT required 15.3 changes. The mean s‐NPWT dressing wear time was 6.5 days compared with the mean t‐NPWT dressing wear time of 3.1 days. The difference in mean dressing wear time was statistically significant in favor of s‐NPWT, compared to t‐NPWT. Similar wear times were recorded for each wound type.
Health‐related quality of life
CWIS: There was no statistical evidence of difference at the baseline assessment for any of the individual measures or domains assessed. There was no statistical evidence of a difference between treatment groups during the treatment period.
EQ‐5D: Similar scores were recorded for the EQ‐VAS (visual assessment scale) between treatment groups at the baseline visit. There were no significant differences between treatment groups between the baseline and exit visits.
Satisfaction with the device
The questionnaire assessed the overall satisfaction with the device, the willingness to use the device again on another wound in the future, the comfort of use, the interference with mobility and the impact of the device on sleep. The difference in trend of agreement between treatment groups was found to be statistically significant for all the parameters explored in favor of s‐NPWT.
Wound infection during 12‐week follow‐up period
Across all patients, 12 (7.5%) became infected during the treatment period. Between treatment groups, this corresponded to 7 (8.8%) patients in the s‐NPWT treatment group, compared to 5 (6.3%) patients in the t‐NPWT treatment group. This was not statistically significant (p = 0.766). Across treatment groups, 10 of the 12 wounds that became infected during the treatment period were DFU.
Safety assessment
Performed with data from the safety population (N = 164 patients). Within the group randomized to receive s‐NPWT, the mean duration of exposure to the device was 59.0 days, the median was 56 days, and the longest duration of exposure was 98 days. Within the group exposed to t‐NPWT, the mean duration of exposure to the device was 52.8 days, the median was 55 days, and the longest duration of exposure was 91 days.
In total, 86 (52.4% of 164 patients) reported 196 AE. Of this total, 139 were considered nontreatment related.
From the remaining 57 AEs, 16 related to s‐NPWT were reported for 12 patients and 41 related to t‐NPWT were reported for 29 patients.
Eleven subject reported twelve AEs on the target ulcers, which were considered as related to the study treatments (3 with s‐NPWT and 9 with t‐NPWT) and resulted in subject discontinuation from the study. Conditions included an increase in the target ulcer size, inability to tolerate NPWT, and periwound skin maceration.
The majority of the AEs severity was mild or moderate. There were two deaths during the course of the study. Neither death was considered study devices related. There were no deaths related to the study treatments.
Ten device‐related events were reported in the s‐NPWT group and 14 in the t‐NPWT group. These included: loud noise, light indicator malfunction, device stop working, alarm malfunction, leakage, signal of blockage/full when canister was empty, persistent beeping, and battery malfunction.
DISCUSSION
In this study, the s‐NPWT system met noninferiority and further achieved statistical superiority in terms of wound progression toward healing measured by reductions in wound dimensions (area, depth, and volume) over the treatment period of 12 weeks.
The randomization resulted in two very similar groups of patients in terms of basic demographic characteristics, distribution of concomitant health conditions, and physical characteristics of the target wounds.
As the original intention was to assess for noninferiority, the initial analyses were performed using the PP population to assure no differences existed in the group of patients that actually used the devices as intended. The reduction in wound area, depth, and volume was significantly more important in the s‐NPWT group.
Given these results, the analyses were also repeated using the ITT population and adjusted for factors such as wound type, wound site, and wound duration at baseline. The results of the extended analyses were consistent with the conclusions drawn when using the PP population.
While the estimates of the individual treatment effect are similar to the results for the combined analysis (VLUs and DFUs), it should be noted that due to smaller numbers of patients present in the subset analyses, confidence intervals, and p‐values would naturally be larger than in the primary analysis even when effect sizes are found to be consistent. The rate of confirmed healed wounds at 12 weeks in the s‐NPWT group was similar to healing rates reported in the literature for studies with a follow‐up of 16 or more weeks.18, 30
The study was designed to compare two types of NPWT, not to compare NPWT against standard of care or standard dressings nor to assess the clinical value of the negative pressure produced by the s‐NPWT system by using the dressing with and without applying negative pressure. As there exists evidence on the clinical benefits of the study device for the proposed indications,35, 36, 37, 38, 39 it was considered more appropriate to compare the study device with t‐NPWT.
Results on wound progression were analyzed and presented individually (surface area, depth, and volume). When considered together, the fact that average wound volume increased in the t‐NPWT group, when analyzing data from the ITT population, deserves further consideration. The main factor responsible for this apparent increase seems to be the integration in the analyses of data from wounds that got worse during the follow‐up period. A baseline average volume of 0.3 cm3 may seem small, but ARANZ Silhouette allowed for a precise and objective measurement of wounds, thus even small variations could be identified and become significant.
At the same time, this needs to be correlated with data from the depth assessment. Average depth reduction was 3.4 mm in the s‐NPWT group and 0.9 mm in the t‐NPWT group. The smaller wound improvement in the t‐NPWT group could be linked to dressing characteristics (because a filler is required) or to the higher level of negative pressure (−125 mmHg). This study was not designed to assess the potential mechanical impact of fillers on the wound bed of chronic wounds; however, it is worth considering that the possibility of using a dressing with or without filler may be an advantage for the management of VLUs and DFUs. As the wound evolves and the granulation tissue fills the wound, the filler under negative pressure may become a physical constraint.
Armstrong et al30 were able to demonstrate noninferiority in the combined set of ulcers, but for both types of tested devices, the dressings required the use of a filler. Our evidence suggest that there may be some factor(s) intrinsic to the s‐NPWT dressing that could also help to explain the superiority results.
As the purpose of the present study was primarily around clinical outcomes, further research is necessary to explore in detail and understand the mechanism of action of different NPWT systems on chronic wounds.
Published evidence seems to recommend the use of NPWT in the management of patients with challenging DFUs showing delayed healing, at the same time, evidence is growing for stalled VLUs. For the requirements of this RCT, the dressings were used for 12 weeks or up to complete healing; however, further research is needed to confirm the most appropriate length of treatment with s‐NPWT for DFUs and VLUs in real world conditions, and to define what patients would benefit the most from it.
Patients included in this trial were screened and then randomized applying strict criteria to avoid bias. The average duration for all ulcers prior to entering in this study was 187 days, and the baseline average surface area was 7 cm2. Treating patients who have suffered ulcers for long periods must lead the treating clinician to consider ethically, independently from the ulcer's size, what to do in cases where appropriately implemented standard care has not promoted healing. We agree that NPWT is not indicated for every DFU or VLU patient; however, its use should be considered in cases where correctly administered standard care has not been successful.
Differences in dressing wear times can be partly explained by what it is recommended in the corresponding instructions for use (IFU), as well as the natural evolution of chronic wounds where the level of exudate decreases as the wound progresses to healing.
The instruments used to assess quality of life may not have been the most appropriate; CWIS and EQ‐D5 measure the impact of the disease on the patient's QoL but are less specific to assess the impact of a treatment. This is more evident when QoL results are compared to the results of the acceptance evaluation, where patients clearly prefer the advantages of a quieter and less intrusive system. Maybe a general tool like SF 36 would have been more appropriate.
Adverse events described as wound area increasing and considered treatment‐related were responsible for the discontinuation of eight patients in the t‐NPWT group vs. only one in the s‐NPWT group. Safety results are in‐line with the existing safety profiles for negative pressure system/devices, and the majority of AEs present in this population are linked to patients’ health condition and comorbidities. Infection rates on the target wounds were similar between treatment groups and consistent with the larger population.
In conclusion, results from this study support the use of s‐NPWT system for the management of chronic leg ulcers (VLUs and DFUs). When appropriate standard of care has not been successful, and NPWT is being considered for the management of challenging or stalled DFUs or VLUs, s‐NPWT (PICO) should be considered a first choice over other types of NPWT.
DISCLOSURES
This clinical trial was performed thanks to an unrestricted grant from Smith & Nephew. Dr. Jaimes is an employee of Smith & Nephew.
ACKNOWLEDGMENTS
Dr Alexander Reyzelman, DPM, Center for Clinical Research, San Francisco CA; Dr Robert Kirsner, MD, University of Miami Department of Dermatology, Miami, FL; Dr Cyaandi Dove, DPM, Advanced Foot and Ankle Center, Las Vegas, NV; Dr Joseph Cavorsi, Center for Advanced Wound Care, Wyomissing, PA; Dr Joseph Caporusso, DPM, Futuro Clinical Trials, McAllen, TX; Dr Robert Galiano, MD, Northwestern University, Feinberg School of Medicine, Chicago, IL; Dr Dean Vayser, DPM, ILD Consulting, Carlsbad, CA; Dr Travis Motley, DPM, Acclaim Bone & Joint Institute, Fort Worth, TX; Dr Jane Yang, MD, Olive View‐UCLA Medical Center, Sylmar, CA; Dr Stephanie Wu, DPM, Center for Lower Extremity Ambulatory Research, Chicago, IL; Dr Bradley Lamm, DPM, and Dr Norman Siddiqu, DPM, Rubins Institute for Advanced Orthopedics – Sinai Hospital, Baltimore, MD; Dr John Clements, DPM, Carillion Clinic, Roanoke, VA; Dr Noah Oliver, DPM, Ochsner Clinic Foundation, New Orleans, LA; Dr Emily Oji, MD, Valley Foot & Ankle Speciality Providers, Fresno, CA; Dr Xingbo Sun, DPM, The Sun Healthcare & Surgery Group, Martinez, CA; Dr Christina Morin, DPM, Centre Podiatrique et Soins de Plaies, Boucherville, QC, Canada; Dr Perry Mayer, MD, The Mayer Institute, Hamilton, ON, Canada.
ENDNOTES
t‐NPWT: traditional Negative Pressure Wound Therapy.
s‐NPWT: single‐use Negative Pressure Wound Therapy.
SSC: Surgical Site Complications.
SSI: Surgical Site Infection.
MVTR: Moisture Vapor Transmission Rate.
BWAT‐m: modified Bates‐Jensen Wound Assessment Tool.
EQ‐5D‐5 L: EuroQoL 5 dimensions, 5 levels.
Contributor Information
Robert Kirsner, Email: rkisner@med.miami.edu.
Henry Jaimes, Email: henry.jaimes@smith-nephew.com.
REFERENCES
- 1. Driscoll P. Wound prevalence and wound management, 2012–2020. Mediligence; 2013. Available at http://blog.mediligence.com (accessed May 20, 2018).
- 2. Singer A, Tassiopoulos A, Kirsner R. Evaluation and management of lower‐extremity ulcers. N Engl J Med 2017; 377: 1559–67. [DOI] [PubMed] [Google Scholar]
- 3. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987; 294: 1389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chi YW, Raffetto JD. Venous leg ulceration pathophysiology and evidence based treatment. Vasc Med 2015; 20: 168–81. [DOI] [PubMed] [Google Scholar]
- 5. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int 2015; 39: 29–39. [DOI] [PubMed] [Google Scholar]
- 6. Markakis K, Bowling FL, Boulton AJ. The diabetic foot in 2015: an overview. Diabetes Metab Res Rev 2016; 32 Suppl 1: 169–78. [DOI] [PubMed] [Google Scholar]
- 7. Brem H, Tomic‐Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117: 1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann New York Acad. Sci 2018; 1411: 153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the society for vascular surgery in collaboration with the American podiatric medical association and the society for vascular medicine. J Vasc Surg 2016; 63Suppl: 3S–21S. [DOI] [PubMed] [Google Scholar]
- 10. Argenta L, Morykwas M. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997; 38: 553–62. [DOI] [PubMed] [Google Scholar]
- 11. Argenta L, Morykwas M. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38: 563–77. [PubMed] [Google Scholar]
- 12. Armstrong D, Lavery L. Negative pressure wound therapy after partial diabetic foot amputation: a multicenter, randomized controlled trial. Lancet 2005; 366: 1704–10. [DOI] [PubMed] [Google Scholar]
- 13. Delhougne G, Hogan C, Tarka K, Nair S. A retrospective, cost‐minimization analysis of disposable and traditional negative pressure wound therapy medicare paid claims. Ostomy Wound Manage 2018; 64: 26–33. 10.25270/owm.2017.6. [DOI] [PubMed] [Google Scholar]
- 14. Vig S, Dowsett C, Berg L, Caravaggi C, Rome P, Birke‐Sorensen H, et al. International expert panel on negative pressure wound therapy. Evidence‐based recommendations for the use of negative pressure wound therapy in chronic wounds: steps towards an international consensus. J Tissue Viability 2011; 20: S1–S18. [DOI] [PubMed] [Google Scholar]
- 15. Birke‐Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, et al. International expert panel on negative pressure wound therapy. Evidence‐based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)—steps towards an international consensus. J Plast Reconstr Aesthet Surg 2011; 64 Suppl: S1–S16. [DOI] [PubMed] [Google Scholar]
- 16. Rahmanian‐Schwarz A, Wilkomm L, Gonser P, Hirt B, Schaller H. A novel option in negative pressure wound therapy (NPWT) for chronic and acute wound care. Burns 2012; 38: 573–7. [DOI] [PubMed] [Google Scholar]
- 17. Hurd T, Trueman P, Rossington A. Use of a portable, single use negative pressure wound therapy device in home care patients with low to moderately exuding wounds: a case series. Ostomy Wound Manage 2014; 60: 30–6. [PubMed] [Google Scholar]
- 18. Blume P, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008; 31: 631–6. [DOI] [PubMed] [Google Scholar]
- 19. Sajid M, Mustafa Q, Shaheen N, Hussain S, Shurk I, Ahmed M. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers. J Coll Physicians Surg Pak 2015; 25: 789–93. [PubMed] [Google Scholar]
- 20. Guffanti A. Negative pressure wound therapy in the treatment of diabetic foot ulcers: a systematic review of the literature. J Wound Ostomy Continence Nurs 2014; 41: 233–7. [DOI] [PubMed] [Google Scholar]
- 21. Wang R, Feng Y, Di B. Comparisons of negative pressure wound therapy and ultrasonic debridement for diabetic foot ulcers: a network meta‐analysis. Int J Clin Exp Med 2015; 8: 12548–56. [PMC free article] [PubMed] [Google Scholar]
- 22. Dale AP, Saeed K. Novel negative pressure wound therapy with instillation and the management of diabetic foot infections. Curr Opin Infect Dis 2015; 28: 151–7. [DOI] [PubMed] [Google Scholar]
- 23. Meloni M, Izzo V, Vainieri E, Giurato L, Ruotolo V, Uccioli L. Management of negative pressure wound therapy in the treatment of diabetic foot ulcers. World J Orthop 2015; 6: 387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vassallo I, Formosa C. Comparing calcium alginate dressings to vacuum‐assisted closure: a clinical trial. Wounds 2015; 27: 180–90. [PubMed] [Google Scholar]
- 25. Mouës C, Heule F, Hovius S. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg 2011; 201: 544–56. [DOI] [PubMed] [Google Scholar]
- 26. Dumville J, Land L, Evans D, et al. Negative pressure wound therapy for treating leg ulcers. Cochrane Database Syst Rev 2015; 7: Cd011354 10.1002/14651858.CD011354.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vuerstaek J, Vainas T, Wuite J, Nelemans P, Neumann M, Veraart J. State‐of‐rhe‐art treatment of chronic leg ulcers: a randomised controlled trial comaring vacuum‐assisted closure (VAC) with modern wound dressings. J Vasc Surg 2006; 44: 1029–37. [DOI] [PubMed] [Google Scholar]
- 28. Kieser DC, Roake JA, Hammond C, Lewis DR. Negative pressure wound therapy as an adjunct to compression for healing chronic venous ulcers. J Wound Care 2011; 20: 35–7. [DOI] [PubMed] [Google Scholar]
- 29. Kucharzewski M, Mieszcza P, Wilemska‐Kucharzewska K, Taradaj J, Kuropatnicki A, Sliwinski Z. The application of negative pressure wound therapy in the treatment of chronic venous leg ulceration: authors experience. Biomed Res Int. 2014: Article ID 297230 10.1155/2014/297230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong D, Marston W, Reyzelman A, Kirsner R. Comparative effectiveness of mechanically and electrically powerwed negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen 2012; 20: 332–41. [DOI] [PubMed] [Google Scholar]
- 31. Chaboyer W, Anderson V, Webster J, Sneddon A, Thalib L, Gillespie BM. Negative pressure wound therapy on surgical site infections in women undergoing elective caesarean sections: a pilot RCT. Healthcare (Basel, Switzerland) 2014; 2: 417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karlakki S, Hamad AK, Whittall C, Graham NM, Banerjee RD, Kuiper JH. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: a randomised controlled trial. Bone Joint Res 2016; 5: 328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galiano R, Hudson D, Shin J, Van Der Hults R, Tanaydin V, Djohan R, et al. Incisional negative pressure wound therapy for prevention of wound healing complications following reduction mammaplasty. PRS Global Open 2018; 6: e1560 10.1097/GOX.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. NICE (National Institute for Health and Care Excellence) . PICO negative pressure wound therapy for closed surgical incision wounds, Medtech innovation briefing.Available at: http://nice.org.uk/guidance/mib149 (Accessed June 15, 2018).
- 35. Hudson D, Adams K, Van Huyssteen A, Martin R, Huddleston E. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no‐canister system. Int Wound J 2015; 12: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz J, Goss S, Facchin F, Gendics C, Lantis JC. Single‐use negative pressure wound therapy for the treatment of chronic lower leg wounds. J Wound Care 2015; 24: S4–9. [DOI] [PubMed] [Google Scholar]
- 37. Hampton J. Providing cost‐effective treatment of hard‐to‐heal wounds in the community through use of NPWT. Brit J Comm Nurs 2015; 20: S14–20. [DOI] [PubMed] [Google Scholar]
- 38. Dowsett C, Hampton J, Myers D, Styche T. Use of PICO™ to improve clinical and economic outcomes in hard‐to‐heal wounds. Wounds Int 2017; 8: 52–8. [Google Scholar]
- 39. Wang E, Tang R, Walsh N, Stoper L, Bharat C, Ponosh S, et al. Topical negative pressure therapy and compression in the management of venous leg ulcers: a pilot study. Wound Prac Res 2017; 25: 36–40. [Google Scholar]
- 40. Schwartz JA, Fuller A, Avdagic E, Gendics C, Lantis JC. Use of NPWT with and without soft port technology in infected foot wounds undergoing partial diabetic foot amputation. J Wound Care. 2015; 24: S4–S12. [DOI] [PubMed] [Google Scholar]


