to the editor: Infective endocarditis with Staphylococcus aureus is associated with a high mortality despite optimal antibiotic therapy.1 The synergy between bacteriophages and antibiotics has been shown in vitro and in animal studies,2 and bacteriophages have demonstrated their value in severe bacterial infections.3 AB‐SA01 (AmpliPhi Biosciences) is a bacterial DNA‐free and protein‐free highly purified preparation of three obligately lytic Myoviridae, each at 109 plaque‐forming units per dose.4 This preparation has been recently used successfully for staphylococcal sinusitis by local irrigation.5 A protocol was established for bacteriophage therapy as an adjunct to standard care of severe staphylococcal infections under the auspices of the Therapeutic Goods Administration Special Access Scheme. Here, we report the first intravenous use of AB‐SA01 in a case of severe staphylococcal sepsis with prosthetic valve endocarditis.
A 65‐year‐old man with a 30‐year‐old mechanical aortic valve presented with a week of malaise, severe exertional dyspnoea, and central pleuritic chest pain. He had been successfully treated for Haemophilus aphrophilus aortic valve endocarditis 8 years earlier with antibiotics alone. Examination revealed fever, tachypnoea, tachycardia and borderline hypotension (90–100 mmHg systolic), with a praecordial systolic murmur and click. There was no cardiac, renal or hepatic failure or any evident peripheral embolic sequelae of endocarditis (haematuria, splinter haemorrhages) at this stage. Blood cultures repeatedly grew an identical methicillin‐sensitive S. aureus determined by whole genome sequencing, and the patient received high dose intravenous flucloxacillin, ciprofloxacin and rifampicin (Box).
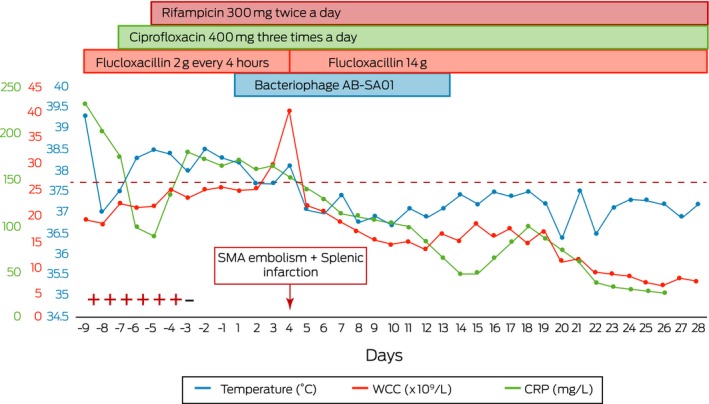
Box 1. Graphical representation of antimicrobial treatment, bacteriophage therapy and inflammatory markers.
− = negative blood cultures; + = positive blood cultures; CRP = C‐reactive protein; SMA = superior mesenteric artery; WCC = white cell count. ◆
Transoesophageal echocardiography confirmed vegetations on prosthetic aortic and native mitral valves, and the aortic root was thickened with possible paravalvular root abscess. Scheduled cardiopulmonary bypass for operative source control was postponed after a haemorrhagic infarction in the distribution of the left anterior cerebral artery on day −7 (ie, a week before starting bacteriophage therapy), despite concerns regarding development of an aortic root abscess, ongoing fevers and hypotension.
Intravenous AB‐SA01 was administered twice a day for 14 days in conjunction with the patient's prescribed antibiotics, commencing (Day 1) 9 days after his first positive blood culture. Blood cultures were negative at onset of bacteriophage therapy, and the C‐reactive protein, temperature, and white cell count results showed downward trends within 24 hours (Box). This trajectory was only interrupted by splenic infarction and occlusion of the superior mesenteric artery 48 hours after commencement, which was proven on computed tomography scan (not shown). No fevers, tachycardia, hypotension or rashes were detected after bacteriophage infusions and no adverse sequelae were attributable to the therapy.
The patient recovered after 40 days of antibiotic therapy and returned to his home state for follow‐up. A positron emission tomography scan on Day 80 showed no fluorodeoxyglucose‐avid lesions, including intracardiac lesions. Repeat echocardiogram on Day 98 for progressive heart failure showed severely dilated left ventricle with moderate mitral and trivial aortic regurgitation. A possible mechanical aortic valve vegetation and paravalvular phlegmon were again demonstrated. Blood cultures were negative. He declined surgical intervention and died on Day 103.
To our knowledge, this was the first case of staphylococcal prosthetic valve endocarditis treated with intravenous bacteriophage (AB‐SA01), which complies with good manufacturing practice standards.4 Bacteriophage infusions were well tolerated. Future controlled trials are needed to evaluate adjunctive bacteriophage therapy, especially when surgical intervention is not feasible.
Competing interests
No relevant disclosures.
Acknowledgements
This manuscript was prepared in collaboration with the Westmead Bacteriophage Therapy Team (WBTT) and AmpliPhi BioSciences. We thank Patricia Fa for pharmacy support, Ruby Lin (WBTT project manager and scientific lead) for project support, and Belinda Dillon‐Roychoudhry for technical assistance.
The copyright line for this article was changed on 7 August 2019 after original online publication.
References
- 1. Asgeirsson H, Thalme A, Weiland O. Staphylococcus aureus bacteraemia and endocarditis — epidemiology and outcome: a review. Infect Dis (Lond) 2018; 50: 175–192. [DOI] [PubMed] [Google Scholar]
- 2. Oechslin F, Piccardi P, Mancini S, et al. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis 2017; 215: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rohde C, Wittmann J, Kutter E. Bacteriophages: a therapy concept against multi‐drug‐resistant bacteria. Surg Infect (Larchmt) 2018; 19: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lehman SM, Mearns G, Rankin D, et al. Design and preclinical development of a phage product for the treatment of antibiotic‐resistant Staphylococcus aureus infections. Viruses 2019; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drilling AJ, Ooi ML, Milikovic D, et al. Long‐term safety of topical bacteriophage application to the frontal sinus region. Front Cell Infect Microbiol 2017; 7: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]


