Abstract
Background
Thrombin generation testing has been used to provide information on the coagulation phenotype of patients. The most used technique is the calibrated automated thrombogram (CAT) but it suffers from a lack of standardization, preventing its implementation in routine. The ST Genesia is a new analyzer designed to assess thrombin generation based on the same principle as the CAT. Unlike the CAT system, the ST Genesia is a benchtop, fully automated analyzer, able to perform the analyses individually and not by batch, with strict control of variables such as temperature and volumes, ensuring, theoretically, maximal reproducibility.
Objectives
This study aimed at assessing the performance of the STG‐DrugScreen application on the ST Genesia analyzer. We also aimed at exploring stability of plasma samples after freezing and defining a reference normal range.
Results
Results demonstrated the excellent interexperiment precision of the ST Genesia and confirmed that the use of a reference plasma helps reducing the inter‐experiments variability. Stability revealed that plasma samples are stable for at least 11 months at −70°C or lower, except for those containing low molecular weight heparins which have to be tested within 6 months. Freezing had no effect on the majority of thrombin generation parameters except on time to peak.
Conclusions
Our results suggest an easy implementation of thrombin generation with the use of ST Genesia in the routine laboratory. This will facilitate the design of multicentric studies and enable the establishment of reliable and evidence‐based thresholds, which may improve the management of patients treated with anticoagulants.
Keywords: anticoagulants, blood coagulation tests, clinical laboratory techniques, normal range, reproducibility
Essentials.
Thrombin generation testing is widely used to provide information on the coagulation phenotype of patients.
Some limitations have been highlighted such as the lack of established standardization.
This is the first study reporting precision results of the ST Genesia.
Normal range of the STG‐DrugScreen application has been assessed in healthy subjects.
The ST Genesia may facilitate the implementation of thrombin generation in the routine laboratory.
1. INTRODUCTION
Thrombin generation has been used since the early 1950s.1 At the start, whole blood or plasma was supplemented with triggers such as tissue factor or activator of the intrinsic pathway of the coagulation combined with calcium chloride to initiate coagulation. Thrombin generation was then evaluated by sampling the clotting mixture at regular intervals into a test tube containing fibrinogen. The clotting times were then recorded and the quantity of thrombin generated was derived from a calibration curve constructed with a known amount of thrombin.2 Changes have been made and the test was improved by replacing fibrinogen by a chromogenic substrate specific for thrombin. This required the use of defibrinated plasma and a computer was used to calculate the parameters stemming from the thrombogram.3 Finally, the chromogenic substrate was replaced by a fluorogenic substrate, which permits the measurement of thrombin in samples that were not defibrinated because fluorescence is not hampered by the turbidity generated during the clotting process. These improvements have laid the groundwork for what is now known as calibrated automated thrombogram (CAT).4 Currently, different solutions are available on the market to measure thrombin generation.5 These are the Innovance ETP (Siemens Healthcare), the only one still based on the cleavage of a chromogenic substrate as readout, the Technothrombin TGA (Technoclone) and the Thrombinoscope CAT (Diagnostica Stago). These last two both use a fluorogenic substrate5; however, some limitations have been highlighted, such as the lack of established standardization of the methods and reagents, and missing quality controls. In addition, batch‐to‐batch variations have been reported which further hampers study‐to‐study comparisons.6, 7, 8
As a consequence, efforts have been made since the past decade to reduce the interlaboratory variation, such as the use of a reference plasma to normalize the results.9 Without the use of this reference plasma, coefficient of variation (CVs) for the most global parameter of CAT (i.e., the endogenous thrombin potential [ETP]), were often higher than 15%. This also raised the question of different local practices which may explain, in part, this high CV.10 Even if this strategy appeared to efficiently reduce the interlaboratory variation and showed the potential benefit of results normalization, improvements are still needed to ensure a proper standardization of the method and its implementation in the routine daily care of patient. Namely, it has been reported that standardization of the tested conditions (e.g., control of the temperature throughout the process, preheating of the sample and the reagents, collection tube or centrifugation) may significantly affect the CAT parameters.8, 9, 10, 11 Thus, for its implementation in routine daily care, the CAT system had to move to a next generation of analyzers able to perform the analyses individually and not by batch, with strict control of all variables to ensure maximal reproducibility.
The newly developed ST Genesia is a fully automated system intended to measure thrombin generation. It provides capacity to continuously load patients’ samples for unitary testing. This new system has been designed to offer enhanced reproducibility compared with CAT and also to provide traceability and standardization, two criteria necessary to fulfill the requirements of an in vitro diagnostics format and to enter the clinical laboratory.
Numerous studies showed that thrombin generation measurement is sensitive enough to detect the presence of anticoagulants in patients’ plasma and to evaluate the intensity of the treatment12, 13, 14, 15, 16, 17, 18, 19 therefore, Diagnostica Stago aimed at developing a kit for the assessment of thrombin generation in anticoagulated patients, the STG‐DrugScreen. The first aim of this study was to determine the precision of the device with this kit and the utility of the normalization of the results. Second, this study aimed at assessing the stability of the plasma samples over time after freezing as well as defining the normal range of the STG‐DrugScreen application in a normal healthy population.
2. MATERIALS AND METHODS
The study intends to evaluate the precision performance of the device under final user setting and investigate the stability of thrombin generation results on samples stored at −70°C or lower. The study was in accordance with the Declaration of Helsinki and has been approved by the Ethical Committee of the CHU UCL Namur, Yvoir, Namur, Belgium. The study took place from June 2016 to September 2017 at the CHU UCL Namur, Yvoir, Belgium.
2.1. Plasma collection of patients’ and healthy donors’ samples
Blood was taken by venipuncture in the antecubital vein and collected into 0.109 M sodium citrate (9:1 v/v) tubes (Vacuette, Greiner, Austria) without corn trypsin inhibitor using a 21‐gauge needle (Terumo). The first tube was always discarded and the first centrifugation was performed within 30 minutes. The platelet‐poor plasma (PPP) was obtained from the supernatant fraction of blood tubes after a double centrifugation for 15 minutes at 1500 × g at room temperature. The centrifuge brake was set to the minimum position at the end of the process. The residual platelet count was assessed every week to ensure the centrifugation procedure provides plasma with a platelet count <10,000 platelets/μL. Immediately after centrifugation, PPP was aliquoted by 600 μL (n = 12 for the stability study, n = 4 for the normal range definition study) and frozen at ≤−70°C without any delay (except for one aliquot tested fresh in the stability study). Frozen PPP samples were thawed, heated to 37°C for 2‐3 minutes and mixed gently just before the experiment. All tests were performed within 4 hours after thawing.
2.2. Normal and targeted population and samples
Six healthy individuals and 23 samples from patients treated with an anticoagulant have been included in the stability study (apixaban n = 4; dabigatran n = 3; low‐molecular‐weight heparins [LMWH] n = 5; rivaroxaban n = 5; vitamin K antagonists [VKA] n = 6). For patients on apixaban, dabigatran, and rivaroxaban, plasma had to be taken at peak (i.e., between 30 minutes and 4 hours after drug intake) to obtain the highest effect on thrombin generation. For patients under LMWH, the blood had to be taken between 1 and 6 hours after the administration while for patients under VKA, the targeted international normalized ratio (INR) was between 2 and 3.
For the definition of the normal range, 42 healthy individuals (mean age = 20 ± 3 years; min‐max range = 18‐32 years), not taking any antithrombotic therapy and not having any hemostasis disorders were included in the study.
2.3. Assessment of thrombin generation
Thrombin generation has been assessed on an ST Genesia analyzer using the STG‐DrugScreen application. More information about the analyzer, its methodology and the reagents are provided in the supplementary material.
2.4. Assessment of anticoagulant activity with specific tests
For patients on apixaban, rivaroxaban, and LMWH, the estimated concentrations or the anti‐Xa activity have been measured using the STA‐Liquid Anti‐Xa (Diagnostica Stago) and the corresponding calibrators and controls according to the recommendations of the manufacturer on an STA‐R Max analyzer (Diagnostica Stago). For dabigatran samples, the estimated dabigatran plasma concentrations have been measured using the STA‐ECA II (Diagnostica Stago) with the corresponding calibrators and controls. For VKA patients, the INRs have been measured with the STA–Neoplastine R (Diagnostica Stago) according to the recommendations of the manufacturer. For healthy individuals, the prothrombin time (PT) and the activated partial thromboplastin time (aPTT) have been assessed using the STA–Neoplastine R (Diagnostica Stago) and the STA‐C.K. Prest (Diagnostica Stago) according to the recommendations of the manufacturer.
2.5. Determination of the precision of the ST Genesia in its STG‐DrugScreen application
Each day of testing (n = 97), a new calibration is set and the quality controls (two levels) and the reference plasma are assessed. During the study period, two batches of reagent kits were used (batch 201797, from 21 June 2016 to 3 November 2016, and batch 202028, from 4 November 2016 to 29 September 2017). The precision has been assessed by collecting the daily values of the quality controls (two levels [i.e., the STG‐QualiTest Norm DS and the STG‐QualiTest Low DS]) and the reference plasma (i.e., the STG‐RefPlasma DS) over this period. Both the absolute and normalized results of the lag time, time‐to‐peak, ETP, and peak height were extracted, and the mean, standard deviation and CV computed from those results.
2.6. Determination of the stability after freezing of the plasma samples for thrombin generation measurement
Fresh plasma from the six healthy individuals, six patients on VKA, five on LMWH, four on apixaban, three on dabigatran, and five on rivaroxaban were tested at different time points to assess the stability of the different thrombin generation parameters over time. Testing was performed on follows.
On a fresh sample (the day of plasma collection after centrifugation)
At day 1, month 1, month 3, month 6, month 9, month 10, and month 11 (for healthy individuals only) after freezing at −70°C or lower. The maximal deviation tolerated was 7 days except for day 1 for which no deviation was tolerated.
The stability of thrombin generation results once the plasma has been frozen at −70°C or lower was assessed on the lag time, time‐to‐peak, ETP, and peak height using the method of the rejection limit (RL). This method aims to establish the length of time during which the measurement of the thrombin generation is acceptable, when carefully following laboratory's established sample handling and storage conditions. Briefly, the percent bias (or the %RL) was plotted vs time for each time point. The regression line of this plot represents the average percent bias (proportional change) over time. For each parameter, the 100% RL is defined based on the maximal allowable error (MAE). To take into account method precision, reagents aging, and sample aging, the MAE was set at 20% for the ETP and at 18% for the lag time, time‐to‐peak, and the peak height. The RL is also adjusted according to the value of the intercept of the linear regression with the y axis. The maximal sample stability is defined as the intercept of the 95% confidence interval (CI) of the linear regression with the adjusted rejection limit. If there is no interception, the maximal duration limit is defined as the last point of measurement (see the Results section and Figures 1 and 2 for graphical representation).
Figure 1.
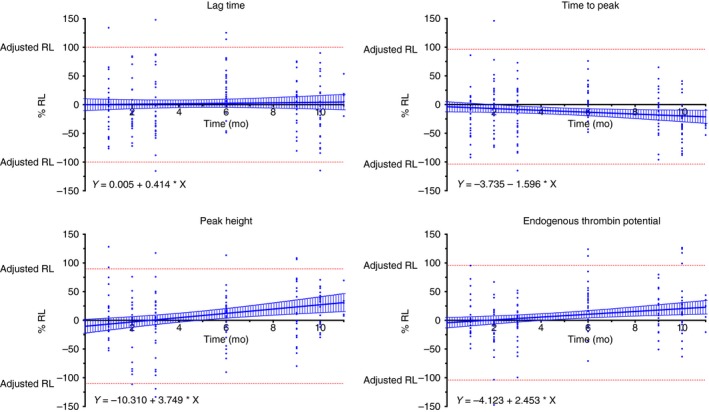
Stability of the different thrombin generation parameters. The 100% rejection limit (RL) has been defined as the maximal allowable error (MAE). For the endogenous thrombin potential (ETP), the MAE was 20% and for the lag time, time to peak, and the peak height; the MAE was 18% based on the data from the precision study. The Y‐intercept was used to determine the adjusted RL (upper adjusted RL = 100 + Y‐intercept – lower adjusted RL = −100 + Y‐intercept). The 95% confidence interval of the linear regression (blue hashed zone) do not cross the adjusted RL, which means that the samples are stable over the analyzed period (i.e., 11 months for healthy subject and 10 months for anticoagulated patients)
Figure 2.
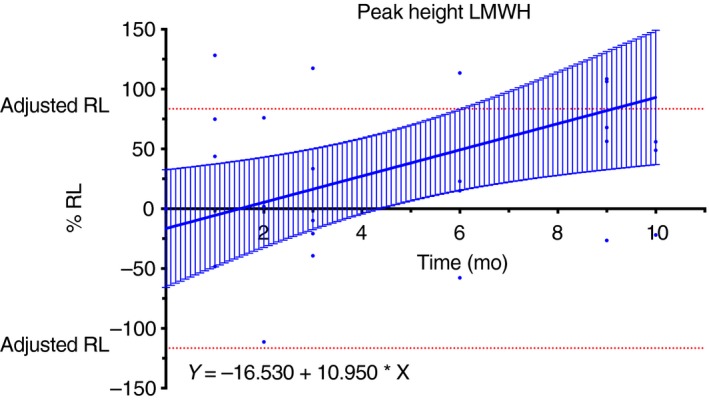
Stability results for samples from patients treated with low molecular weight heparin (LMWH). The upper 95% confidence interval limit of the linear regression crosses the adjusted rejection limit (RL) after 6 months determining the maximal stability of the samples
The impact of freezing was assessed comparing the results of each sample at day 0 (i.e., fresh sample) to the results obtained at day 1. To provide evidence of the normal distribution of the results, a Shapiro‐Wilk test using an α‐risk of 5% (two‐sided CI) has been performed. If the population had not a normal distribution, then the paired results were compared using a Wilcoxon matched rank‐signed test. If the distribution was normal, then the means were compared with a t test for paired samples. In both cases, a P value below 5% leads to the conclusion of significant impact of freezing on results.
3. RESULTS
3.1. Thrombin generation values of the reference plasma and controls and precision of the ST‐Genesia
The within‐batch variability was always below the between‐batches relative differences, except for the normalized ETP of the STG‐QualiTest Low DS (Table 1). The within‐batch variability was always below 6%. All results are summarized in Table 1.
Table 1.
Coefficient of variation of the different quality controls over 2 batches of STG®‐DrugScreen kit
| STG‐QualiTest Norm DS | STG‐QualiTest Low DS | STG‐RefPlasma DS | Mean coefficient of variation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean value | SD | CV | Mean value | SD | CV | Mean value | SD | CV | |||||
| Lag time (min – absolute/ratio – normalized) | |||||||||||||
| Absolute | |||||||||||||
| All (n = 97) | 0.87 min | 0.06 min | 6.53% | 1.05 min | 0.12 min | 11.03% | 0.95 min | 0.05 min | 5.11% | 7.56% | |||
| Batch 201797 (n = 37) | 0.93 min | 0.04 min | 4.03% | 1.19 min | 0.05 min | 3.97% | 0.98 min | 0.05 min | 5.25% | 4.42% | |||
| Batch 202028 (n = 60) | 0.84 min | 0.04 min | 4.36% | 0.96 min | 0.03 min | 3.14% | 0.93 min | 0.03 min | 3.38% | 3.63% | |||
| Difference, % | 11 | 24 | 5 | ||||||||||
| Normalized | |||||||||||||
| All (n = 97) | 0.92 | 0.04 | 4.71% | 1.10 | 0.09 | 8.47% | 6.59% | ||||||
| Batch 201797 (n = 37) | 0.95 | 0.04 | 3.83% | 1.21 | 0.05 | 4.22% | 4.03% | ||||||
| Batch 202028 (n = 60) | 0.91 | 0.04 | 4.42% | 1.04 | 0.04 | 3.69% | 4.06% | ||||||
| Difference, % | 4 | 16 | |||||||||||
| Time‐to‐peak (min – absolute/ratio– normalized) | |||||||||||||
| Absolute | |||||||||||||
| All (n = 97) | 1.91 min | 0.08 min | 4.07% | 2.14 min | 0.17 min | 7.85% | 2.13 min | 0.07 min | 3.26% | 5.06% | |||
| Batch 201797 (n = 37) | 1.97 min | 0.07 min | 3.60% | 2.33 min | 0.10 min | 4.25% | 2.17 min | 0.08 min | 3.61% | 3.82% | |||
| Batch 202028 (n = 60) | 1.86 min | 0.05 min | 2.60% | 2.02 min | 0.05 min | 2.28% | 2.11 min | 0.05 min | 2.50% | 2.46% | |||
| Difference, % | 6 | 15 | 3 | ||||||||||
| Normalized | |||||||||||||
| All (n = 97) | 0.90 | 0.02 | 2.70% | 1.00 | 0.06 | 6.19% | 4.45% | ||||||
| Batch 201797 (n = 37) | 0.91 | 0.02 | 2.09% | 1.08 | 0.03 | 2.55% | 2.69% | ||||||
| Batch 202028 (n = 60) | 0.89 | 0.02 | 2.58% | 0.96 | 0.03 | 2.56% | 2.57% | ||||||
| Difference, % | 2 | 13 | |||||||||||
| Peak height (nmol/L – absolute/% – normalized) | |||||||||||||
| Absolute | |||||||||||||
| All (n = 97) | 480.1 nmol/L | 22.6 nmol/L | 4.71% | 215.9 nmol/L | 13.3 nmol/L | 6.17% | 472.5 nmol/L | 18.6 nmol/L | 3.95% | 4.94% | |||
| Batch 201797 (n = 37) | 501.8 nmol/L | 16.5 nmol/L | 3.30% | 206.2 nmol/L | 10.1 nmol/L | 4.89% | 485.8 nmol/L | 17.0 nmol/L | 3.50% | 3.90% | |||
| Batch 202028 (n = 60) | 466.7 nmol/L | 13.7 nmol/L | 2.93% | 221.9 nmol/L | 11.5 nmol/L | 5.16% | 464.3 nmol/L | 14.5 nmol/L | 3.12% | 3.74% | |||
| Difference, % | 8 | 7 | 5 | ||||||||||
| Normalized | |||||||||||||
| All (n = 97) | 101.6% | 2.9% | 2.81% | 45.7% | 3.2% | 6.94% | 4.88% | ||||||
| Batch 201797 (n = 37) | 103.2% | 2.2% | 2.08% | 42.4% | 1.4% | 3.30% | 2.69% | ||||||
| Batch 202028 (n = 60) | 100.5% | 2.8% | 2.75% | 47.8% | 2.0% | 4.15% | 3.45% | ||||||
| Difference, % | 3 | 11 | |||||||||||
| Endogenous thrombin potential (ETP) (nmol/L/min – absolute/% – normalized) | |||||||||||||
| Absolute | |||||||||||||
| All (n = 97) | 1531 nmol/L/min | 96 nmol/L/min | 6.26% | 523 nmol/L/min | 22 nmol/L/min | 4.27% | 1722 nmol/L/min | 72 nmol/L/min | 4.19% | 4.91% | |||
| Batch 201797 (n = 37) | 1607 nmol/L/min | 65 nmol/L/min | 4.07% | 524 nmol/L/min | 19 nmol/L/min | 3.53% | 1736 nmol/L/min | 64 nmol/L/min | 3.67% | 3.76% | |||
| Batch 202028 (n = 60) | 1483 nmol/L/min | 80 nmol/L/min | 5.40% | 522 nmol/L/min | 25 nmol/L/min | 4.69% | 1714 nmol/L/min | 76 nmol/L/min | 4.45% | 4.85% | |||
| Difference, % | 8 | 0 | 1 | ||||||||||
| Normalized | |||||||||||||
| All (n = 97) | 88.9% | 4.9% | 5.56% | 30.4% | 1.3% | 4.18% | 4.87% | ||||||
| Batch 201797 (n = 37) | 92.6% | 3.7% | 3.95% | 30.2% | 1.1% | 3.47% | 3.71% | ||||||
| Batch 202028 (n = 60) | 86.6% | 4.2% | 4.85% | 30.5% | 1.4% | 4.55% | 4.70% | ||||||
| Difference, % | 7 | 1 | |||||||||||
Results are presented as CV expressed in percentage. For the 2 levels of controls, results are presented as absolute or normalized (against STG ‐RefPLasma DS). The use of a reference plasma titrated by a manufacturer reduces the interbatch variation. Difference in % represents the relative difference between the two batches of reagents and controls for each TGT parameter.
CV, coefficient of variation; SD, standard deviation; TGT, thrombin generation test.
3.2. Impact on freezing on thrombin generation parameters
On the total cohort (n = 29 samples), Shapiro‐Wilk normality test showed normal distribution for the lag time and the ETP parameters, whereas the normality failed for the peak height and the time to peak. This means that, according to the statistical analysis plan, a t test for paired samples was used for the lag time and the ETP, whereas a Wilcoxon matched rank‐signed test was used for the peak height and the time‐to‐peak. Because of a small sample size, the Wilcoxon matched rank‐signed test was used when the results were stratified by treatment (Table 2).
Table 2.
Impact of freezing on thrombin generation parameters
| Fresh (D0) vs frozen (D1) | Passed normality testing? | Mean difference (D1‐D0) | Wilcoxon matched‐pairs signed rank test or paired t test (P value) | Significant? |
|---|---|---|---|---|
| All results (n = 29) | ||||
| Lag timea | Yesa | −0.08 min | 0.7179a | No |
| Peak height | No | 3.29 nmol/L | 0.096 | No |
| Time‐to‐peak | No | −0.15 min | 0.020 | Yes |
| ETPa | Yesa | 1 nmol/L/min | 0.9702a | No |
| Apixaban (n = 4) | ||||
| Lag time | N too small | −0.09 min | 0.375 | No |
| Peak height | 19.38 nmol/L | 0.250 | No | |
| Time‐to‐peak | −0.24 min | 0.250 | No | |
| ETP | −4 nmol/L/min | 0.875 | No | |
| Dabigatran (n = 3) | ||||
| Lag time | N too small | −0.25 min | 0.250 | No |
| Peak height | 7.53 nmol/L | 0.500 | No | |
| Time‐to‐peak | −0.23 min | 0.500 | No | |
| ETP | 27 nmol/L/min | 0.250 | No | |
| Healthy (n = 6) | ||||
| Lag time | N too small | −0.02 min | >0.999 | No |
| Peak height | −20.17 nmol/L | 0.688 | No | |
| Time‐to‐peak | −0.03 min | >0.999 | No | |
| ETP | −116 nmol/L/min | 0.438 | No | |
| LMWH (n = 5) | ||||
| Lag time | N too small | −0.00 min | >0.999 | No |
| Peak height | 2.78 nmol/L | 0.625 | No | |
| Time‐to‐peak | −0.09 min | 0.474 | No | |
| ETP | 28 nmol/L/min | 0.313 | No | |
| Rivaroxaban (n = 5) | ||||
| Lag time | N too small | −0.21 min | 0.063 | No |
| Peak height | 14.77 nmol/L | 0.125 | No | |
| Time‐to‐peak | −0.53 min | 0.063 | No | |
| ETP | 79 nmol/L/min | 0.188 | No | |
| Vitamin K antagonists (n = 6) | ||||
| Lag time | N too small | −0.02 min | 0.750 | No |
| Peak height | 4.79 nmol/L | 0.313 | No | |
| Time‐to‐peak | −0.04 min | 0.313 | No | |
| ETP | 22 nmol/L/min | 0.219 | No | |
Wilcoxon matched pairs signed‐rank test have been performed to compare the impact on freezing (i.e., at D0 on fresh and at D + 1 once the samples have been frozen). When considering all results (n = 29), freezing has an effect on time to peak. Results are also stratified by treatment but the number of samples is too small to drive any conclusion.
Abbreviations: CI, confidence interval; ETP, endogenous thrombin potential; LMWH, low‐molecular‐weight heparin; RL, rejection limit; SE, standard error; VKA, vitamin K antagonist.
For these analyses, a paired t test was used as the data passed the normality testing using the Shapiro‐Wilk test.
Wilcoxon matched rank‐signed test revealed no significant difference between the two conditions on lag time, peak height, and ETP, whereas the time‐to‐peak was impaired (mean of difference = −0.15 minutes). When results are analyzed by treatment, there is no significant difference between fresh and frozen plasma (Table 2).
3.3. Stability of the plasma samples once conserved at −70°C or below
For the lag time, the adjusted RLs are −100% to 100%, the slope of the linear regression was equal to 0.414 (95% CI, −1.358 to 2.185). The 95% CI of the linear regression did not cross the adjusted RLs (Figure 1) and the maximal storage duration was defined as the latest point assessed in this study (i.e., 11 months for healthy volunteers and 10 months for anticoagulated patients). Stratification by treatment did not provide different results (Table 3). For the time to peak, the adjusted RLs were −104% to 96%, the slope of linear regression was equal to −1.596 (95% CI, −3.113 to −0.078; significant nonzero). The 95% CI of the linear regression did not cross the adjusted RLs (Figure 1) and the maximal storage duration for this parameter was also defined at the latest point assessed in this study (i.e., 11 months). Again, stratification by treatment did not provide different results (Table 3). For the peak height, the adjusted RLs were −110% to 90%, the slope of linear regression was equal to 3.749 (95% CI, −1.675 to 5.822). The 95% CI of the linear regression did not cross the adjusted RLs (Figure 1) and the maximal storage duration for this parameter was also defined at the latest point assessed in this study (i.e., 11 months). Stratification by treatment revealed that the peak height is only stable for 6 months in samples from patients with LMWH (Table 3 and Figure 2). For the ETP, the adjusted RLs were −104% to 96%, the slope of linear regression was equal to 2.453 (95% CI, 0.886‐4.019). The 95%CI of the linear regression did not cross the adjusted RLs (Figure 1) and the maximal storage duration for this parameter was defined at the latest point assessed in this study (i.e., 11 months). Stratification by treatment did not provide different results (Table 3).
Table 3.
Stability of plasma samples once frozen at −80°C
| Parameter | Linear regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | SE | 95% CI | Slope significantly nonzero? | Intercept | SE | 95% CI | Adjusted RL (%) | Time at which RL is reacheda | |
| Lag time | |||||||||
| All | 0.414 | 0.898 | −1.358 to 2.185 | No | 0.005 | 5.219 | 10.290 to 10.300 | −99.995 to 100.005 | 11 mo |
| Apixaban | −3.210 | 3.243 | −9.903 to 3.483 | No | 20.180 | 19.250 | −19.540 to 59.910 | −79.820 to 120.18 | 10 mo |
| Dabigatran | −1.412 | 1.853 | −5.340 to 2.516 | No | 6.077 | 11.150 | −17.560 to 29.720 | −93.923 to 106.08 | 10 mo |
| Healthy | 2.165 | 1.316 | −0.504 to 4.834 | No | −17.280 | 8.207 | −33.920 to −0.632 | −117.280 to 82.72 | 11 mo |
| LMWH | −0.383 | 2.028 | −4.520 to 3.753 | No | −9.564 | 11.810 | −33.830 to 14.320 | −109.564 to 90.436 | 10 mo |
| Rivaroxaban | 1.453 | 2.485 | −3.603 to 6.509 | No | −0.721 | 13.190 | −27.560 to 26.120 | −100.721 to 99.279 | 10 mo |
| VKA | 2.216 | 2.069 | −1.994 to 6.426 | No | 9.868 | 11.590 | −13.710 to 33.450 | −90.132 to 109.87 | 10 mo |
| Time to peak | |||||||||
| All | −1.596 | 0.769 | −3.113 to −0.078 | Yes | −3.735 | 4.471 | −12.560 to 5.086 | −103.735 to 96.265 | 11 mo |
| Apixaban | −0.031 | 2.089 | −4.334 to 4.271 | No | 2.476 | 12.190 | −22.640 to 27.590 | −97.524 to 102.746 | 10 mo |
| Dabigatran | −3.204 | 3.012 | −9.589 to 3.181 | No | −21.720 | 18.13 | −60.140 to 16.710 | −121.720 to 78.280 | 10 mo |
| Healthy | −1.465 | 1.197 | −3.892 to 0.9618 | No | −17.370 | 7.463 | −32.510 to 2.238 | −117.37 to 82.630 | 11 mo |
| LMWH | −3.527 | 1.870 | −7.342 to 0.2870 | No | −3.726 | 10.890 | −25.930 to 18.480 | −103.726 to 96.274 | 10 mo |
| Rivaroxaban | −0.9155 | 1.585 | −4.140 to 2.309 | No | 0.691 | 8.412 | −16.420 to 17.810 | −99.309 to 100.691 | 10 mo |
| VKA | −0.055 | 1.737 | −3.592 to 3.483 | No | 7.920 | 9.870 | −12.180 to 28.020 | −92.080 to 107.920 | 10 mo |
| ETP | |||||||||
| All | 2.453 | 0.794 | 0.886 to 4.019 | Yes | −4.123 | 4.615 | −13.230 to 4.980 | −104.123 to 95.877 | 11 mo |
| Apixaban | 2.873 | 1.989 | −1.191 to 6.937 | No | −4.348 | 11.690 | −28.470 to 19.770 | −104.348 to 95.652 | 10 mo |
| Dabigatran | −1.850 | 2.721 | −7.618 to 3.918 | No | 17.650 | 16.380 | −17.060 to 52.360 | −82.350 to 117.65 | 10 mo |
| Healthy | 3.015 | 1.097 | 0.7903 to 5.240 | Yes | −6.215 | 6.842 | −20.090 to 7.661 | −106.215 to 93.785 | 11 mo |
| LMWH | 5.053 | 2.586 | −0.221 to 10.330 | No | −12.630 | 15.050 | −43.330 to 18.060 | −112.630 to 87.37 | 10 mo |
| Rivaroxaban | 3.280 | 2.321 | −1.441 to 8.002 | No | −9.106 | 12.320 | −34.170 to 15.950 | −109.106 to 90.894 | 10 mo |
| VKA | 1.697 | 7.429 | −2.515 to 2.882 | No | 1.697 | 7.429 | −13.420 to 16.810 | −98.303 to 101.7 | 10 mo |
| Peak height | |||||||||
| All | 3.749 | 1.051 | −1.675 to 5.822 | Yes | −10.310 | 6.108 | −22.360 to 1.745 | −110.310 to 89.69 | 11 mo |
| Apixaban | −0.064 | 2.204 | −4.612 to 4.484 | No | −20.860 | 13.080 | −47.860 to 6.136 | −120.860 to 79.14 | 10 mo |
| Dabigatran | 0.952 | 2.244 | −3.805 to 5.708 | No | 11.470 | 13.500 | −17.150 to 40.100 | −88.530 to 111.47 | 10 mo |
| Healthy | 3.252 | 0.682 | 1.870 to 4.634 | Yes | 7.126 | 4.250 | −1.494 to 15.750 | −92.874 to 107.13 | 11 mo |
| LMWH | 10.950 | 4.141 | 2.506 to 19.400 | Yes | −16.530 | 24.100 | −65.690 to 32.630 | −116.530 to 83.47 | 6 mo |
| Rivaroxaban | 1.657 | 2.245 | −2.911 to 6.224 | No | −22.670 | 11.920 | −46.910 to 1.573 | −122.670 to 77.33 | 10 mo |
| VKA | 2.904 | 1.591 | −0.334 to 6.141 | No | −10.460 | 8.912 | −28.590 to 7.673 | −110.460 to 89.54 | 10 mo |
Abbreviations: ETP, endogenous thrombin potential; LMWH, low‐molecular‐weight heparin; VKA, vitamin K antagonists.
When the regression slope is not statistically significant (P > 0.05) from zero, this indicates parameter is not changing as a function of time and the maximum storage time is equal to T MAX (last point of the linear regression.
When the regression slope is statistically significant (P ≤ 0.05) from zero, and the one‐sided 95% confidence limit of the regression line intersects the adjusted rejection limit before reaching T MAX, the maximum storage time is determined as this intersection.
3.4. Thrombin generation in the normal and targeted population
The reference values obtained in the healthy population revealed thrombin generation values close to the value of the reference plasma (Table 4). Screening coagulation testing, namely the PT and aPTT, were normal in all healthy individuals (PT: mean = 13.7 ± 0.7 seconds; min‐max range = 11.9‐15.10 seconds; and aPTT: mean = 31.5 ± 2.5 seconds; min‐max range = 27.1‐36.7 seconds). For apixaban, measured drug plasma levels through anti‐Xa method were between 158 and 370 ng/mL, the normalized thrombin generation parameters were all affected by the treatment (Table 5). No clear correlation could be drawn from this small population (n = 4) between thrombin generation parameters and the plasma level. For dabigatran, peak plasma levels were between 51 and 187 ng/mL, the normalized lag time and time to peak were affected in all subjects, whereas the ETP and the peak height were not different from the healthy subjects at the lowest concentration observed (i.e., 51 ng/mL) (Table 5). For LMWH, the peak anti‐Xa effect were between 0.35 IU/mL and 1.37 IU/mL. All thrombin generation parameters were affected. In the patient with the highest exposure (i.e., 1.37 IU/mL), the peak height and the ETP were highly affected (8% and 14% the value of the reference plasma, respectively) (Table 5). For rivaroxaban, the peak plasma concentrations were between 197 and 300 ng/mL. The most affected thrombin generation parameters were the peak height and the time to peak (Table 5). In patient 1, the rivaroxaban concentration was 229 ng/mL, the peak height dropped to 37% with significant prolongation of the time to peak but the ETP was not affected (i.e., 101% the value of the reference plasma). In patients treated with VKA, the INR varied between 1.86 and 3.31. The peak and the ETP were the most influenced thrombin generation parameters while lag time and time to peak were less influenced (Table 5).
Table 4.
Normal range definition of the STG‐DrugScreen application on the ST Genesia system using plasma sample from 42 healthy individuals
| Healthy (n = 42) | Lag time absolute | Peak height absolute | Time‐to‐peak absolute | ETP absolute | Lag time normalized (ratio) | Peak height normalized | Time‐to‐peak normalized (ratio) | ETP normalized |
|---|---|---|---|---|---|---|---|---|
| Mean | 0.93 min | 456.20 nmol/L | 2.03 min | 1418 nmol/L | 1.16 | 97% | 0.98 | 83% |
| SD | 0.09 min | 56.35 nmol/L | 0.16 min | 270 nmol/L | 0.10 | 12% | 0.08 | 15% |
| CV (%) | 9.8 | 12.4 | 7.9 | 19.1 | 9.6 | 12.1 | 7.9 | 18 |
| Minimum | 0.73 min | 355.90 nmol/L | 1.80 min | 1027 nmol/L | 0.81 | 75% | 0.87 | 56% |
| Maximum | 1.22 min | 571.40 nmol/L | 2.69 min | 1993 nmol/L | 1.33 | 120% | 1.29 | 118% |
| 10% | 0.82 min | 380.20 nmol/L | 1.87 min | 1122 nmol/L | 0.90 | 81% | 0.90 | 68% |
| 90% | 1.00 min | 538.90 nmol/L | 2.17 min | 1851 nmol/L | 1.11 | 115% | 1.29 | 109% |
All samples were tested on the same day, explaining the few differences in term of variability between absolute and normalized results.
Abbreviations: CV, coefficient of variation; ETP, endogenous thrombin potential; SD, standard deviation.
Table 5.
Thrombin generation results at T0 of all patients and healthy donors included in the stability study
| Patient no. | Lag time absolute | Peak height absolute | Time‐to‐peak absolute | ETP absolute | Lag time normalized (ratio) | Peak height normalized | Time‐to‐peak normalized (ratio) | ETP normalized | Anticoagulant ng/mL or IU/mL or INR |
|---|---|---|---|---|---|---|---|---|---|
| Healthy 1 | 0.96 min | 561.90 nmol/L | 2.12 min | 1903 nmol/L/min | 0.98 | 113% | 0.96 | 105% | |
| Healthy 2 | 1.11 min | 382.90 nmol/L | 2.37 min | 1263 nmol/L/min | 1.12 | 77% | 1.08 | 69% | |
| Healthy 3 | 1.00 min | 395.00 nmol/L | 2.36 min | 1509 nmol/L/min | 1.01 | 80% | 1.07 | 83% | |
| Healthy 4 | 0.95 min | 488.20 nmol/L | 2.01 min | 1844 nmol/L/min | 0.96 | 98% | 0.91 | 101% | |
| Healthy 5 | 1.14 min | 410.00 nmol/L | 2.64 min | 1589 nmol/L/min | 1.15 | 83% | 1.20 | 87% | |
| Healthy 6 | 1.06 min | 427.10 nmol/L | 2.38 min | 1716 nmol/L/min | 1.07 | 86% | 1.08 | 94% | |
| Apixaban 1 | 2.48 min | 153.90 nmol/L | 4.69 min | 1295 nmol/L/min | 2.62 | 31% | 2.23 | 77% | 307 ng/mL |
| Apixaban 2 | 1.90 min | 112.40 nmol/L | 6.79 min | 1214 nmol/L/min | 2.01 | 23% | 3.23 | 72% | 269 ng/mL |
| Apixaban 3 | 2.77 min | 247.90 nmol/L | 4.82 min | 1437 nmol/L/min | 2.83 | 46% | 2.19 | 74% | 158 ng/mL |
| Apixaban 4 | 2.14 min | 169.20 nmol/L | 4.29 min | 995 nmol/L/min | 2.27 | 33% | 2.08 | 56% | 195 ng/mL |
| Dabigatran 1 | 2.71 min | 325.30 nmol/L | 3.84 min | 1150 nmol/L/min | 2.64 | 69% | 1.74 | 68% | 89 ng/mL |
| Dabigatran 2 | 2.22 min | 401.50 nmol/L | 3.35 min | 1361 nmol/L/min | 2.22 | 87% | 1.50 | 83% | 51 ng/mL |
| Dabigatran 3 | 2.64 min | 312.80 nmol/L | 3.66 min | 1015 nmol/L/min | 2.86 | 68% | 1.78 | 60% | 187 ng/mL |
| LMWH 1 | 1.87 min | 43.83 nmol/L | 3.81 min | 260 nmol/L/min | 1.93 | 8% | 1.81 | 14% | 1.37 IU/mL |
| LMWH 2 | 1.41 min | 331.70 nmol/L | 2.81 min | 1179 nmol/L/min | 1.45 | 64% | 1.34 | 65% | 0.35 IU/mL |
| LMWH 3 | 1.33 min | 334.00 nmol/L | 2.54 min | 1092 nmol/L/min | 1.39 | 69% | 1.25 | 64% | 0.35 IU/mL |
| LMWH 4 | 1.38 min | 259.70 nmol/L | 2.79 min | 879 nmol/L/min | 1.41 | 57% | 1.27 | 49% | 0.52 IU/mL |
| LMWH 5 | 1.35 min | 122.70 nmol/L | 3.49 min | 661 nmol/L/min | 1.51 | 27% | 1.64 | 40% | 1.26 IU/mL |
| Rivaroxaban 1 | 2.47 min | 177.90 nmol/L | 7.01 min | 1695 nmol/L/min | 2.49 | 37% | 3.23 | 101% | 229 ng/mL |
| Rivaroxaban 2 | 3.33 min | 93.67 nmol/L | 9.67 min | 965 nmol/L/min | 3.65 | 20% | 4.61 | 58% | 197 ng/mL |
| Rivaroxaban 3 | 2.88 min | 191.30 nmol/L | 6.29 min | 1454 nmol/L/min | 3.12 | 38% | 3.01 | 82% | 218 ng/mL |
| Rivaroxaban 4 | 3.28 min | 146.10 nmol/L | 7.35 min | 1187 nmol/L/min | 3.43 | 30% | 3.62 | 70% | 296 ng/mL |
| Rivaroxaban 5 | 1.95 min | 106.40 nmol/L | 8.03 min | 1064 nmol/L/min | 2.12 | 23% | 3.90 | 63% | 300 ng/mL |
| VKA 1 | 2.06 min | 104.80 nmol/L | 3.43 min | 451 nmol/L/min | 2.01 | 22% | 1.55 | 27% | 2.57 |
| VKA 2 | 2.14 min | 109.80 nmol/L | 3.35 min | 391 nmol/L/min | 2.24 | 23% | 1.65 | 23% | 3.31 |
| VKA 3 | 2.87 min | 137.90 nmol/L | 4.56 min | 525 nmol/L/min | 3.01 | 29% | 2.16 | 29% | 1.86 |
| VKA 4 | 2.39 min | 118.70 nmol/L | 3.74 min | 451 nmol/L/min | 2.44 | 26% | 1.71 | 25% | 2.91 |
| VKA 5 | 2.10 min | 91.17 nmol/L | 3.26 min | 260 nmol/L/min | 2.14 | 20% | 1.49 | 15% | 2.68 |
| VKA 6 | 1.82 min | 130.90 nmol/L | 3.36 min | 459 nmol/L/min | 2.04 | 29% | 1.60 | 27% | 1.79 |
Results of the intensity of anticoagulation (expressed as ng/mL for DOACs, IU/mL for LMWH, and INR for VKA) are provided.
Abbreviations: DOACs, direct oral anticoagulants (i.e., apixaban, dabigatran, rivaroxaban); INR, international normalized ratio; LMWH, low‐molecular‐weight heparin; VKA, vitamin K antagonist.
4. DISCUSSION
This is the first study evaluating the performances of the ST Genesia, a benchtop analyzer for thrombin generation in the routine setting. This instrument aims at introducing thrombin generation testing in the routine laboratory. Indeed, to date, the CAT system is probably one of the most used thrombin generation technique in research laboratories but the use of a microtiter plate, the manual placing of the sample and the reagents into the wells, added to the lack of standardized procedure and reagents have prevented its introduction into the routine practice.2 By providing a complete kit of reagent and controls, the STG‐DrugScreen application of the ST Genesia simplifies the evaluation of the thrombin generation in patients treated with anticoagulants in the routine laboratory. This is made possible by the standardized and validated methodology of the ST Genesia. The ST Genesia is derived from the CAT principle, but is not strictly equivalent. Yet, as already shown by others,20 there is a direct correlation between the two systems under comparable triggering conditions. This study is not intended to provide the clinical performance of the STG‐DrugScreen application to predict any clinical events, but to provide the analytical performance of the system in the routine setting and to evaluate the stability of the samples once conserved at −70°C or below.
The precision of the ST Genesia has been evaluated using the data from the daily calibration and the value of the two levels of control and reference plasma (i.e., the STG‐QualiTest Low DS and STG‐QualiTest Norm DS and STG‐RefPlasma DS, respectively) over a 15‐month period. Two batches of reagents were provided by the manufacturer during the course of this study. Main results revealed that the coefficient of variation of the different parameters were always <10% and for the majority of these results the CV was even <5% (Table 1). As previously reported,10 this study also demonstrates that the use of a reference plasma reduced the interexperiment variability. Regarding the stability of the samples, our results demonstrate that freezing has no impact on most thrombin generation parameters except the time to peak, especially in samples containing apixaban, dabigatran, or rivaroxaban. Our data suggest that the plasma, once frozen, is stable during at least 11 months, except for LMWH samples, for which the maximal duration storage is 6 months.
As mentioned, this study has limitations. First, this is a monocentric study and, thus, the interlaboratory performance of the ST Genesia has not been assessed. Second, results of external quality assurance program have not been included in this validation because the current thrombin generation EQA program is not designed for thrombin generation assessment of anticoagulant treatment21 and thus the high tissue factor activity of the STG‐DrugScreen trigger may not be comparable to that of the other reagents and methodologies. Finally, the study was not powered to drive any conclusions regarding the effect of anticoagulants on thrombin generation because only peak plasma samples were collected for this part of the study. Only trends can be drawn on the overall effect of the different anticoagulant agents; further work is necessary to define the minimal concentration of anticoagulants that would yield results outside the reference range.
4.1. Reference plasma
Many previous publications recommended the use of a reference plasma to reduce the interlaboratory variability.6, 7, 9, 10 The choice of providing a reference plasma within the STG‐DrugScreen kit agrees with the literature because it has been reported that the use of local plasma (i.e., a reference plasma performed at the laboratory facilities) were generally unable to improve the interlaboratory variability or the CV is even worsen after normalization.6, 8, 10 The choice of a commercially available and certificated plasma is therefore more appropriate than using local plasma. On the other hand, it has been reported a certain heterogeneity between the different reference plasma which may impede the benefit of the use of such reference plasma.6 Therefore, the best approach would be to use a reference plasma specifically dedicated for the application. This strategy improves the between‐batch variation. In this study, we report relative differences between batches from 0% to 24% when results are not normalized (Table 1; see results of the controls). Once results are normalized most of the parameters show better inter‐batch CVs (except for the peak height with the STG‐QualiTest Low DS), demonstrating the efficiency of this approach. The choice of a freeze‐dried plasma can be challenged because it has been reported that these plasmas have higher thrombin generation capacity than frozen plasma from multiple preanalytical reasons.6, 22 In any case, the conditions in which these reference plasmas are produced are probably far from the best recommendations of blood sample collection for thrombin generation testing because these reference plasmas have to be produced on a large scale. Thus, one solution may be to assess the response of the commercial reference plasma to a smaller pool of plasma from healthy donors collected in ideal conditions and then to apply a correction factor to the commercial plasma to compensate for these differences. In the package insert of the STG‐DrugScreen kit, an assay value of the reference plasma is provided by the manufacturer to correct the reference plasma activity. The results of this study demonstrate that this approach is efficient since our healthy subjects, collected in ideal conditions for this application, were close to 100% or a ratio of 1 depending on the parameter (Table 5). In addition, the difference between the two batches of the STG‐RefPlasma DS was ≤5% for all thrombin generation parameters demonstrating that the use of a correction factor specific to each batch of reagent and reference plasma is useful to reduce the batch‐to‐batch variation (Table 1).
4.2. Quality controls
It has been reported that internal quality control is essential to produce consistent results for thrombin generation23 however, in the era of thrombin generation, the confusion between internal quality control and a reference is often made. Namely, most studies have proposed to normalize the results according to a reference plasma,6, 7, 9, 10, 23 which is not the same as true control plasma. To comply with local legislation, regulations, guidelines, or standards issued by relevant bodies, at least two levels of controls are recommended. Thus, this is of upmost importance for thrombin generation to propose such different level of controls to allow the implementation of thrombin generation into routine testing. This will ensure the technique is under control for both normal and abnormal sample analysis.
Over our series of 97 measurements of the reference plasma and the quality controls, we demonstrate that the CVs of the normalized results were always below 10% and for the majority of the parameters were even below 5%. These interassay precision results are in line with the intra‐ and interassay reproducibility data provided by the manufacturer in the insert‐sheet of the STG‐DrugScreen kit (Table S2). All of these data demonstrate that the STG‐DrugScreen performed on the ST Genesia is a precise thrombin generation application, which will allow thrombin generation to be performed routinely to assess patients treated with anticoagulants. All the requirements are now met to implement this technique in the routine laboratory.
4.3. Impact of freezing and stability of the samples
A previous study evaluating the effect of preanalytical parameters on the measurement of circulating microparticles with thrombin generation revealed that plasmas are stable for 1 year at −80°C.24 Apart from this information, few studies been done to assess the stability of plasma samples for thrombin generation testing. In this previous study of Lacroix et al., the authors assessed the stability of thrombin generation using the CAT system for the measurement of the procoagulant activity of circulating microparticles with the MP‐reagent from Thrombinoscope bv, a reagent that does not contain tissue factor to trigger the coagulation. There is a different setting than the one assessed in this study because the STG‐DrugScreen reagent contains tissue factor at concentration higher than 5 pM. This higher tissue factor concentration provides robustness to the analytical procedure. Indeed, in another study of Loeffen et al., thrombin generation triggered with tissue factor at a concentration of 5 pM has not demonstrated to be affected by the two centrifugation protocols tested nor by the temperature at which the plasma is stored until analysis (4°C, room temperature or 37°C).8
Freezing has no effect on the majority of the thrombin generation parameters using the STG‐DrugScreen application, except for time to peak (Table 2). Once frozen, plasma samples are stable during at least 11 months, except for LMWH samples for which an impact is seen after 6 months (Table 3, Figure 2). A previous report also demonstrates that LMWH samples are not stable beyond 6 months.25 This may be explained by the release of PF‐4 from residual platelets. Namely, according to the CLSI document H21‐A5, heparin neutralization may occur in sample not collected on CTAD tubes from the release of PF4 from platelets.26 It is possible that residual platelets released PF4 during storage and thus neutralize heparin. This is consistent with the increase of the peak observed. For the other subgroups, all parameters were stable which is in correlation with the findings of Woodhams et al.,27 who demonstrated that all plasma coagulation proteins were stable for at least 18 months once stored at −74°C. If all the coagulation proteins are stable at −74°C, it can be expected that a global test such as thrombin generation, which relies on these coagulation proteins, can also be stable for a such long period. Our results confirm this assumption for a period of 11 months for healthy subjects and 10 months for anticoagulated patients.
4.4. Normal range definition
In this study, 42 samples from healthy donors were tested to provide grounds for the definition of a normal for the STG‐DrugScreen application on the ST‐Genesia. All these samples were normal according to PT and aPTT screening. The small correction between absolute and normal value is certainly because all 42 samples were tested on the same day with the first batch of reagents (i.e., batch 201797). For all the parameters, the interindividual variation was always below 20% and was below 10% for the lag time and the time‐to‐peak. When comparing with the results obtained in patients treated with an anticoagulant, the 10th‐90th percentile range (Table 4) could serve as a basis for the classification of normal and abnormal plasma. It could be proposed that samples below the 10th percentile (for peak height and ETP) and above the 90th percentile (for lag time and time to peak) may represent samples in which anticoagulant therapy is suspected. However, further studies in larger cohorts of patients taking anticoagulants are needed before adopting this assumption.
4.5. Usefulness of thrombin generation to assess anticoagulated patients
Tailoring drug dosages to fit the needs of individual patients could be helpful to make old and new drugs more effective and safer.2 Preliminary observations showed that thrombin generation testing is affected by all anticoagulant drugs and therefore it could be the candidate assay.18, 19, 28, 29, 30, 31 The test has been found to be very sensitive to all kind of anticoagulants 13, 14, 15, 16, 18, 32, 33 and may best represent the interindividual response than just exploring plasma drug concentrations.28 In addition to considering the interindividual response to an antithrombotic drug, thrombin generation testing is also able to explore in more detail the effect of anticoagulants on the coagulation process. Namely, depending the type of drug, our results confirmed that the fingerprint of the thrombin generation differs, revealing their different pharmacodynamics (Table 5).17, 18, 32, 34 This is of particular importance because bleeding or thrombosis have been reported within the “on‐therapy” range, demonstrating that the drug level alone may not be sufficient to identify those who are more at risk.35 However, further investigation on patients who bleed or who have recurrent thrombosis while on a fixed dose of anticoagulants is needed to show the benefit of thrombin generation and provide cutoffs for bleeding and thrombotic complications. Thrombin generation has also been reported as an informative tool to document on antidote administration in polytrauma models with direct implication for patient care.36 This is particularly important because it may help in adjusting the dose of prothrombin complex concentrate to administer.37 The ST Genesia will provide a step forward to this aim in allowing thrombin generation to be done routinely with reduced interexperiments and interlaboratory variations. In this way, it will be easier to recruit patients, design multicenter studies, and provide data for the establishment of reliable and evidence‐based thresholds.
5. CONCLUSIONS
This is the first study presenting performance data of the ST Genesia, a new benchtop analyzer for thrombin generation testing. The study aimed at evaluating the performances of the STG‐DrugScreen application, a kit of reagents designed for the quantitative determination of thrombin generation in plasma from patients treated with an anticoagulant. Results revealed an excellent interexperiment precision thanks to the standardized methodology, the excellent reproducibility of the analyzer and the use of the reference plasma included in the kit. Stability study revealed that plasma samples are stable for at least 11 months after freezing and storage at −70°C or lower, except for those containing LMWH, which have to be tested within 6 months. Freezing has no impact of the majority of thrombin generation parameters except on time‐to‐peak. Thus, our results support the hypothesis that the ST Genesia may facilitate the implementation of thrombin generation in the routine laboratory; this way, it may offer a serious input in the individual screening of patients requiring anticoagulants. However, multicentric studies are required to assess the interlaboratory reproducibility of the ST Genesia and confirm its usefulness for standardizing thrombin generation testing. In addition, the STG‐DrugScreen application of the ST Genesia should be part of the propositions of external quality assurance programs.
CONFLICTS OF INTERESTS
The study was financed by Diagnostica Stago group. Among the authors, J. Douxfils is chief executive officer and founder of QUALIblood s.a. and reports personal fees from Diagnostica Stago, Roche, Roche Diagnostics, and Daiichi‐Sankyo, outside the submitted work. F. Mullier reports institutional fees from Diagnostica Stago, Werfen, Nodia, Sysmex, and Bayer. He also reports speaker fees from Boehringer Ingelheim, Bayer Healthcare, and Bristol‐Myers Squibb‐Pfizer, all outside the submitted work. The other authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Among the authors, J. Douxfils and F. Mullier designed the study. C. Devroye, M. Guldenpfennig, and J. Baudar performed the analyses. J‐M. Dogné, C. Devroye, M. Guldenpfennig, and J. Baudar collected data. J. Dogné, L. Morimont, and C. Bouvy analyzed and interpreted data. J. Douxfils, L. Morimont, and C. Bouvy performed statistical analysis. J. Dogné, L. Morimont, C. Bouvy, M. de Saint‐Hubert, B. Devalet, A‐S. Dincq, J‐M. Dogné, A‐S. Larock, S. Lessire, and F. Mullier wrote the manuscript.
Supporting information
Douxfils J, Morimont L, Bouvy C, et al. Assessment of the analytical performances and sample stability on ST Genesia system using the STG‐DrugScreen application. J Thromb Haemost. 2019;17:1273–1287. 10.1111/jth.14470
Manuscript handled by: Robert Gosselin
Final decision: Robert Gosselin, 29 April 2019
REFERENCES
- 1. Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62:699–707. [DOI] [PubMed] [Google Scholar]
- 3. Hemker HC, Willems GM, Beguin S. A computer assisted method to obtain the prothrombin activation velocity in whole plasma independent of thrombin decay processes. Thromb Haemost. 1986;56:9–17. [PubMed] [Google Scholar]
- 4. Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. [DOI] [PubMed] [Google Scholar]
- 5. Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost. 2018;2:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, et al. Standardisation of thrombin generation test–which reference plasma for TGT? An international multicentre study. Thromb Res. 2010;125:353–6. [DOI] [PubMed] [Google Scholar]
- 7. Dargaud Y, Luddington R, Gray E, Negrier C, Lecompte T, Petros S, et al. Effect of standardization and normalization on imprecision of calibrated automated thrombography: an international multicentre study. Br J Haematol. 2007;139:303–9. [DOI] [PubMed] [Google Scholar]
- 8. Loeffen R, Kleinegris MC, Loubele ST, Pluijmen PH, Fens D, van Oerle R, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10:2544–54. [DOI] [PubMed] [Google Scholar]
- 9. Dargaud Y, Wolberg AS, Luddington R, Regnault V, Spronk H, Baglin T, et al. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: an international multicentre study. Thromb Res. 2012;130:929–34. [DOI] [PubMed] [Google Scholar]
- 10. Perrin J, Depasse F, Lecompte T; French‐speaking CAT group and under the aegis of GEHT; French‐speaking CATgroup (all in France unless otherwise stated); French‐speaking CAT group all in France unless otherwise stated . Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136:125–30. [DOI] [PubMed] [Google Scholar]
- 11. Spronk HM, Dielis AW, De Smedt E, van Oerle R, Fens D, Prins MH, et al. Assessment of thrombin generation II: validation of the Calibrated Automated Thrombogram in platelet‐poor plasma in a clinical laboratory. Thromb Haemost. 2008;100:362–4. [PubMed] [Google Scholar]
- 12. Gerotziafas GT, Elalamy I, Depasse F, Perzborn E, Samama MM. In vitro inhibition of thrombin generation, after tissue factor pathway activation, by the oral, direct factor Xa inhibitor rivaroxaban. J Thromb Haemost. 2007;5:886–8. [DOI] [PubMed] [Google Scholar]
- 13. Douxfils J, Chatelain B, Chatelain C, Dogne JM, Mullier F. Edoxaban: impact on routine and specific coagulation assays. A practical laboratory guide. Thromb Haemost. 2016;115:368–81. [DOI] [PubMed] [Google Scholar]
- 14. Douxfils J, Chatelain C, Chatelain B, Dogne JM, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110:283–94. [DOI] [PubMed] [Google Scholar]
- 15. Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogne JM. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956–66. [DOI] [PubMed] [Google Scholar]
- 16. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–97. [DOI] [PubMed] [Google Scholar]
- 17. Bloemen S, Zwaveling S, Douxfils J, Roest M, Kremers R, Mullier F. The anticoagulant effect of dabigatran is reflected in the lag time and time‐to‐peak, but not in the endogenous thrombin potential or peak, of thrombin generation. Thromb Res. 2018;171:160–6. [DOI] [PubMed] [Google Scholar]
- 18. Dale B, Eikelboom JW, Weitz JI, Young E, Paikin JS, Coppens M, et al. Dabigatran attenuates thrombin generation to a lesser extent than warfarin: could this explain their differential effects on intracranial hemorrhage and myocardial infarction? J Thromb Thrombolysis. 2013;35:295–301. [DOI] [PubMed] [Google Scholar]
- 19. Hacquard M, Perrin J, Lelievre N, Vigneron C, Lecompte T. Inter‐individual variability of effect of 7 low molecular weight antithrombin‐dependent anticoagulants studied in vitro with calibrated automated thrombography. Thromb Res. 2011;127:29–34. [DOI] [PubMed] [Google Scholar]
- 20. Siguret V, Foulon G, Abdoul J, Carlo A, Lecompte T, Gouin‐Thibault I. PB584/Thrombin generation analysis with a new automated system (ST‐Genesia): inter‐series performances during DRIVING study and comparison with CAT system. Res Pract Thromb Haemost. 2018;2:285. [Google Scholar]
- 21. Kluft C, Meijer P. External quality assessment for thrombin generation tests: an exploration. Semin Thromb Hemost. 2010;36:791–6. [DOI] [PubMed] [Google Scholar]
- 22. Rodgers SE, Wong A, Gopal RD, Dale BJ, Duncan EM, McRae SJ. Evaluation of pre‐analytical variables in a commercial thrombin generation assay. Thromb Res. 2014;134:160–4. [DOI] [PubMed] [Google Scholar]
- 23. Bagot CN, Leishman E. Establishing a reference range for thrombin generation using a standard plasma significantly improves assay precision. Thromb Res. 2015;136:139–43. [DOI] [PubMed] [Google Scholar]
- 24. Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, et al. Impact of pre‐analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10:437–46. [DOI] [PubMed] [Google Scholar]
- 25. Rojnuckarin P, Akkawat B, Juntiang J. Stability of plasma anti‐Xa activity in low‐molecular‐weight heparin monitoring. Clin Appl Thromb Hemost. 2010;16:313–7. [DOI] [PubMed] [Google Scholar]
- 26. Adcock Funk DM, Hoefner D, Kottke‐Marchant K, Marlar RA, Szamosi DI, Warunek DJ. H21‐A5: Collection, Transport, and Processing of Blood Specimens for Testing Plasma‐Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline — Fifth Edition. Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 27. Woodhams B, Girardot O, Blanco MJ, Colesse G, Gourmelin Y. Stability of coagulation proteins in frozen plasma. Blood Coagul Fibrinolysis. 2001;12:229–36. [DOI] [PubMed] [Google Scholar]
- 28. Rigano J, Ng C, Nandurkar H, Ho P. Thrombin generation estimates the anticoagulation effect of direct oral anticoagulants with significant interindividual variability observed. Blood Coagul Fibrinolysis. 2018;29:148–54. [DOI] [PubMed] [Google Scholar]
- 29. Chowdary P, Adamidou D, Riddell A, Aghighi S, Griffioen A, Priest P, et al. Thrombin generation assay identifies individual variability in responses to low molecular weight heparin in pregnancy: implications for anticoagulant monitoring. Br J Haematol. 2015;168:719–27. [DOI] [PubMed] [Google Scholar]
- 30. Radulovic V, Hyllner M, Ternstrom L, Karlsson M, Bylock A, Hansson KM, et al. Sustained heparin effect contributes to reduced plasma thrombin generation capacity early after cardiac surgery. Thromb Res. 2012;130:769–74. [DOI] [PubMed] [Google Scholar]
- 31. Dieri R al, Alban S, Beguin S, Hemker HC. Thrombin generation for the control of heparin treatment, comparison with the activated partial thromboplastin time. J Thromb Haemost. 2004;2:1395–401. [DOI] [PubMed] [Google Scholar]
- 32. Robert S, Ghiotto J, Pirotte B, David JL, Masereel B, Pochet L, et al. Is thrombin generation the new rapid, reliable and relevant pharmacological tool for the development of anticoagulant drugs? Pharmacol Res. 2009;59:160–6. [DOI] [PubMed] [Google Scholar]
- 33. Siriez R, Evrard J, Dogne JM, Pochet L, Gheldof D, Chatelain B, et al. Betrixaban: impact on routine and specific coagulation assays‐a practical laboratory guide. Thromb Haemost. 2018;118:1203–14. [DOI] [PubMed] [Google Scholar]
- 34. Bloemen S, Hemker HC, Al Dieri R. Large inter‐individual variation of the pharmacodynamic effect of anticoagulant drugs on thrombin generation. Haematologica. 2013;98:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sennesael AL, Larock AS, Douxfils J, Elens L, Stillemans G, Wiesen M, et al. Rivaroxaban plasma levels in patients admitted for bleeding events: insights from a prospective study. Thromb J. 2018;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Honickel M, Braunschweig T, Rossaint R, Stoppe C, Ten Cate H, Grottke O. Reversing dabigatran anticoagulation with prothrombin complex concentrate versus idarucizumab as part of multimodal hemostatic intervention in an animal model of polytrauma. Anesthesiology. 2017;127:852–61. [DOI] [PubMed] [Google Scholar]
- 37. Neal MD, Levy JH. Precision correction of coagulopathy or prothrombin complex concentrates?: reversal options for dabigatran following trauma. Anesthesiology. 2017;127:744–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


