Summary
Male reproductive development involves a complex series of biological events and precise transcriptional regulation is essential for this biological process in flowering plants. Several transcriptional factors have been reported to regulate tapetum and pollen development, however the transcriptional mechanism underlying Ubisch bodies and pollen wall formation remains less understood. Here, we characterized and isolated a male sterility mutant of TDR INTERACTING PROTEIN 3 (TIP3) in rice. The tip3 mutant displayed smaller and pale yellow anthers without mature pollen grains, abnormal Ubisch body morphology, no pollen wall formation, as well as delayed tapetum degeneration. Map‐based cloning demonstrated that TIP3 encodes a conserved PHD‐finger protein and further study confirmed that TIP3 functioned as a transcription factor with transcriptional activation activity. TIP3 is preferentially expressed in the tapetum and microspores during anther development. Moreover, TIP3 can physically interact with TDR, which is a key component of the transcriptional cascade in regulating tapetum development and pollen wall formation. Furthermore, disruption of TIP3 changed the expression of several genes involved in tapetum development and degradation, biosynthesis and transport of lipid monomers of sporopollenin in tip3 mutant. Taken together, our results revealed an unprecedented role for TIP3 in regulating Ubisch bodies and pollen exine formation, and presents a potential tool to manipulate male fertility for hybrid rice breeding.
Keywords: rice, male sterility, TIP3, PHD‐finger, Ubisch bodies, pollen exine
Significance Statement
TIP3, a PHD‐finger protein, functions as a transcriptional activator and physically interacts with TDR participating in tapetum programmed cell death, Ubisch body morphogenesis and pollen wall formation. Our results provide an insight into the molecular mechanism underlying tapetal programmed cell death (PCD) and pollen wall formation in rice.
Introduction
Male sterility refers to the phenomenon in which stamens are degenerated or malformed, and viable male gametes are not produced, while female gametes are fully fertile in flowering plants. Although it is an unfavorable trait for an individual plant, male sterility has been widely used for hybrid seed production in agriculture (Fan and Zhang, 2017). The distinguishing character between male fertility and sterility is whether mature pollen grains are produced. The formation of the pollen wall is a key step required for pollen viability. Primexine, callose wall formation and sporopollenin precursors are three essential factors for pollen wall formation.
Primexine is indispensable for exine patterning and is thought to function as a scaffold, matrix, or template for initial sporopollenin accumulation (Paxson‐Sowders et al., 2001; Ariizumi et al., 2004, 2005). The undulating structure of the plasma membrane might be associated with normal primexine formation (Ariizumi and Toriyama, 2011; Sun et al., 2013). Several genes such as DEX1 (Paxson‐Sowders et al., 2001), OsDEX1 (Yu et al., 2016), NEF1 (Ariizumi et al., 2004), HKM (Ariizumi et al., 2005), NPU (Chang et al., 2012), RPG1 (Sun et al., 2013) and Exine Formation Defect (EFD) (Hu et al., 2014) are reportedly involved in primexine and the undulating structure of plasma membrane formation during anther development. Disrupting this gene expression can cause aberrant undulation of the plasma membrane and impaired primexine patterning, leading to disorganized deposition of sporopollenin on the plasma membrane and eventually male sterility. During meiosis, microsporocytes are surrounded by a thick and specialized cell wall called the callose, which acts as the mold for primexine (Ariizumi and Toriyama, 2011; Shi et al., 2015). In rice, GSL5 is required for callose wall formation during microspore formation, while DCM1 plays an essential role in preserving the callose from premature dissolution (Zhang et al., 2018).
Sporopollenin precursors, such as lipids and their derivatives, phenolic compounds and polysaccharides, are biosynthesized and processed in tapetal cells (Piffanelli et al., 1998; Bubert et al., 2002). Therefore, the tapetum requires the coordinated expression of a large number of genes to regulate this complex biological process and to function normally. Several transcriptional factors (TFs) have been reported to play vital roles in this process. GAMYB was reported to directly interact with the promoter regions of CYP703A3 and β‐ ketoacyl reductase in vitro (Kaneko et al., 2004). CYP703A3 belongs to the cytochrome P450 hydroxylase family and preferably catalyzes lauric acid to generate 7‐hydroxylated lauric acid. Disruption of GAMYB and CYP703A3 showed similar defects in exine and Ubisch body formation (Aya et al., 2009; Liu et al., 2010; Yang et al., 2014). TDR, a basic helix−loop−helix (bHLH) TF, not only modulates tapetum development and degeneration, but also functions in aliphatic metabolism and pollen formation by directly activating the expression of its target genes, OsCP1, OsC6 and CYP703A3 (Li et al., 2006; Zhang et al., 2008, 2010; Yang et al., 2014). Another two bHLH TFs, TIP2/bHLH142 and EAT1/DTD, interact with TDR and together form a regulatory cascade in determining the differentiation and development of anther wall and pollen exine (Niu et al., 2013b; Fu et al., 2014; Ko et al., 2014; Yi et al., 2016). PTC1 and its Arabidopsis ortholog MS1 encode PHD‐finger TFs and regulate programmed tapetal development and functional pollen formation by affecting the expression of tapetum‐ and microspore‐expressed genes (Wilson et al., 2001; Ito et al., 2007; Yang et al., 2007; Li et al., 2011a). Simultaneously, various enzymes are indispensable for de novo synthesis of fatty acids in tapetal plastids. ACOS5 and its rice ortholog OsACOS12, encoding acyl‐CoA synthetase, catalyze fatty acyl‐containing substrates to form the sporopollenin acyl‐CoA ester of polymer precursors in tapetal cells (Souza et al., 2009; Li et al., 2016; Yang et al., 2017). Cytochrome P450 fatty acid hydroxylase, such as Arabidopsis CYP704B1, CYP703A2 and rice CYP704B2, CYP703A3, catalyzes hydroxylation of fatty acids both in Arabidopsis and rice (Morant et al., 2007; Dobritsa et al., 2009; Li et al., 2010; Chen et al., 2011; Yang et al., 2014, 2018). MS2 and DPW encode fatty acid reductase to convert palmitoyl‐acyl carrier protein to the corresponding C16:0 alcohol (Chen et al., 2011; Shi et al., 2011). DPW2, a BAHD acyltransferase, specifically transfers hydroxycinnamic acid moieties from hydroxycinnamoyl‐CoAs to ω‐hydroxy fatty acids (Xu et al., 2017). All the sporopollenin precursors synthesized by enzymatic reaction are allocated for pollen wall development from tapetal cells to the anther locule. Lipid transfer protein OsC6 and ABC subfamily G (ABCG) transporters OsABCG15 and OsABCG26 may be responsible for the transport of sporopollenin precursors (Zhang et al., 2010; Niu et al., 2013a; Wu et al., 2014; Zhao et al., 2015; Chang et al., 2016).
The plant homeodomain (PHD) fingers, comprised of ~60 amino acids with a Cys4−His−Cys3 motif, are widely distributed and conserved throughout eukaryotes (Bienz, 2006). Increasing evidence shows that PHD proteins are involved in the control of gene transcription and chromatin remodeling. Arabidopsis HAT3.1 has been isolated as a PHD‐finger protein and is capable of interacting with specific DNA fragments in vitro (Schindler et al., 1993). MS1 encodes a PHD transcriptional factor and its ortholog in rice PTC1, barley HvMS1 and maize ZmMs7, all of which play key roles in regulating transcription during programmed tapetum development and pollen wall formation (Wilson et al., 2001; Ito et al., 2007; Yang et al., 2007; Li et al., 2011a; Gomez and Wilson, 2014; Zhang et al., 2017a). MMD1/DUET, another PHD protein, is required for the organization and progression of chromosomes during male meiosis (Reddy et al., 2003; Yang, 2003; Andreuzza et al., 2015; Wang et al., 2016). In this work, we identified a PHD‐finger protein (TIP3) in rice by a map‐based cloning strategy. TIP3 null confers delayed tapetum degradation and defects in Ubisch body morphogenesis and pollen wall formation. In addition, TIP3 functions as a transcriptional activator and physically interacted with TDR, affecting the expression of genes related to tapetum programmed cell death (PCD) and pollen wall formation. Our results provide insight into the molecular mechanism underlying tapetal PCD and pollen wall formation in rice.
Results
Rice tip3 is a no‐pollen male sterility mutant
To further understand the molecular mechanism of male sterility, a no‐pollen male‐sterile mutant was isolated from our rice mutant library on the background indica rice cv. Zh8015 (Yang et al., 2018). This mutant was later designated as tip3 because the gene product interacted with TDR (TDR INTERACTING PROTEIN 3) (see below). Compared with wild‐type plants, the tip3 mutant displayed normal vegetative growth and similar morphology of spikelets as those of wild‐type plants (Figure 1a,b). But the anthers of tip3 mutant were shorter, pale yellow (Figure 1c) and without viable pollen grains (Figure 1d). When tip3 mutant plants were pollinated with wild‐type pollen grains, all F1 progenies were fertile, and the F2 plants presented an approximate 3:1 ratio for phenotype segregation (fertility: sterility = 209: 77, χ2 = 0.56 < χ2 0.05 = 3.84). This demonstrates that tip3 developed a normal female fertility and the sterile phenotype was controlled by a single recessive locus.
Figure 1.

Phenotypic comparison between wild‐type and the tip3 mutant. (a) A wild‐type plant (left) and tip3 mutant plant (right) after heading. (b) A wild‐type spikelet (left) and tip3 spikelet (right) before anthesis. (c) A wild‐type anther (left) and tip3 anther (right) at stage 13 of anther development.(d) I2‐KI staining of wild‐type and tip3 pollen grains at the mature stage.gl, glume; le, lemma; pa, palea; pi, pistil; st, stamen. Bars: 15 cm in (a), 1.5 mm in (b), 200 μm in (c) and 5 μm in (d).
Ubisch body morphogenesis and pollen wall formation defect in tip3
To characterize the cytological defects in tip3, the semi‐thin section technique was used for the analysis of anther development in the mutant and wild‐type according to anther development stages (Zhang and Wilson, 2009; Zhang et al., 2011). Microsporocytes underwent meiosis producing dyads and tetrads at stage 8 (Figure S1). Tapetal cells became vacuolated and the cytoplasm was darkly stained. There were no morphological differences between the wild‐type and mutant at this stage (Figure 2a,b,d,e). Up to stage nine, wild‐type tetrads released spherical haploid microspores. As vacuoles were reabsorbed, the cytoplasm in tapetal cells became condensed and deeply stained (Figure 2c). Although microsporocytes released haploid microspores, the haploid microspores presented a messy cytoplasm with many small vacuoles in tip3 mutants. Another distinct difference was that vacuolated tapetal cells still remained in the mutant (Figure 2f). At stage 10, wild‐type microspores vacuolated with a round‐shaped morphology and exhibited thicker exine deposition on the outer surface of the microspores (Figure 2g). Then vacuolated microspores underwent asymmetric mitotic division and displayed falcate shapes at the beginning of stage 11 (Figure 2h). In contrast, microspores in tip3 mutants appeared to struggle to complete vacuolization and asymmetric mitosis at stages 10–11, but the most striking phenotypic abnormality was the lack of the typical pollen exine deposition on the outer surface of so‐called uninucleate microspores and binucleate pollen grains (Figure 2j,k). At stage 12, wild‐type anthers produced mature microspores filled with starch (Figure 2i), while tip3 microspores gradually degraded leaving only remnants in their locules (Figure 2l).
Figure 2.
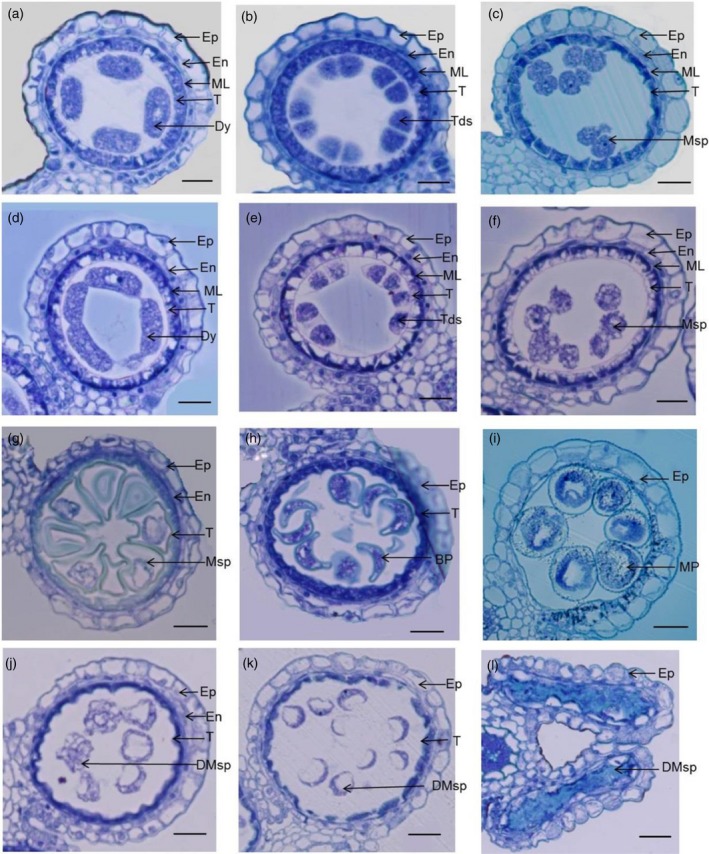
Transverse section analysis of the anther development in wild‐type and the tip3 mutant. (a–c, g–i) Images of anthers in wild‐type at stages 8a, 8b and 9–12, respectively. (d–f, j–l) Images of anthers in tip3 mutant at stages 8a, 8b and 9–12, respectively. Ep, epidermis; En, endothecium; ML, middle layer; T, tapetum; Dy, dyad; Tds, tetrads; Msp, microspore; DMsp, degenerated microspores; BP, bicellular pollen; MP, mature pollen. Bars represent 20 μm.
To reveal the tip3 developmental defects in detail, transmission electron microscopy (TEM) was conducted to observe anther development. At stage 8b, defined organelles such as the nucleus and large vacuole were apparent in wild‐type and mutant cytoplasm (Figure 3a–d). Microspores were enclosed as tetrads by the callose wall, primexine began to deposit and regular plasma membrane undulation was observed (Figure 3q,r). There was no distinct difference between wild‐type and tip3 mutants at this stage. At late stage nine, the wild‐type tapetal cytoplasm became condensed and large vacuoles were diminished. Tapetal cells produced and secreted abundant Ubisch bodies on the inner surface of the tapetum (Figure 3e,f). Meanwhile, a darkly stained layer of exine appeared on the microspore surface (Figure 3s). However, the tip3 tapetal cells still maintained the vacuolated state, and there were no Ubisch bodies emerging on the inner surface of the tapetum (Figure 3g,h). Therefore, no sporopollenin precursors were available for the formation of exine; what remained was a light abnormal exine layer on tip3 microspores (Figure 3t). At stage 10, wild‐type tapetal cells continued to degrade and generated more Ubisch bodies along the inner surface of tapetal cells. Ubisch bodies exhibited an electron‐transparent central kernel surrounded by a few electron‐dense particles (Figure 3i,j). Whereas the degradation of the tapetum and middle layer was delayed in tip3 mutant and its tapetal cells remained visible nucleus in the cytoplasm. Ubisch bodies appeared as completely electron‐opaque spheres with varying size in tip3 mutant (Figure 3k,l). At late stage 10, many more Ubisch bodies of irregular shapes and sizes deposited on the wild‐type pollen exine, which formed with well organized electron‐dense layers including sexine, tectum and nexine (Figure 3u). In contrast, no exine was formed with electron‐dense remnants and abnormal Ubisch bodies in tip3 anther locules (Figure 3v). At late stage 12, the tapetum was thoroughly degraded and spherical microspores were clearly observable in wild‐type anther locules because of the accumulation of starch and lipidic materials in pollen grains (Figure 3m,n). However, there were no pollen grains generated in tip3 anther locules, abnormal Ubisch bodies appeared collapsed and squeezed into an irregular line (Figure 3o,p). A hair‐like cuticle layer deposited on the wild‐type anther epidermis with relatively wide spacing (Figure 3w), while the tip3 anther epidermis showed a dense, hair‐like cuticle layer (Figure 3x). These observations indicated abnormal Ubisch body morphogenesis and pollen wall formation in the tip3 mutant.
Figure 3.
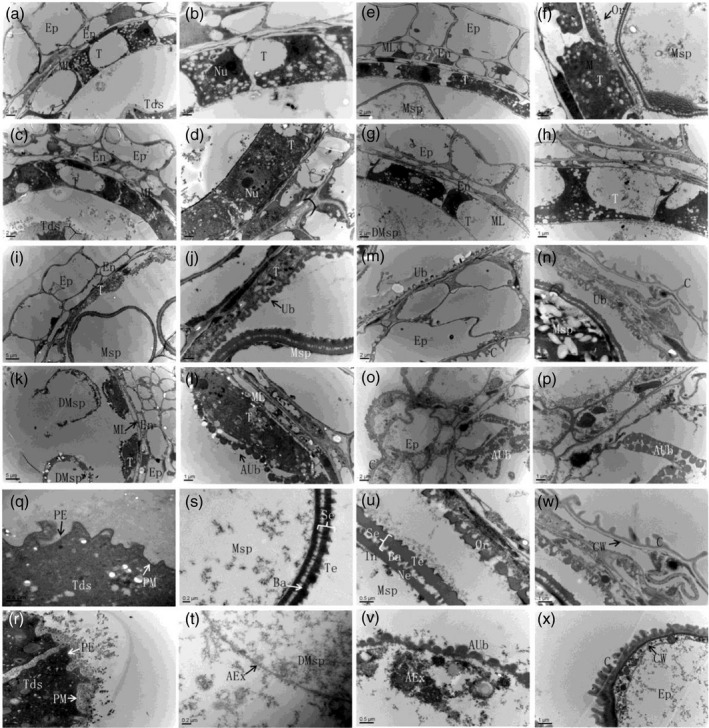
Transmission electron microscopy analysis of wild‐type and the tip3 mutant anther development. (a–d) TEM analysis of the anther development in wild‐type (a, b) and tip3 (c, d) at stage 8b. (e–h) TEM analysis of the anther development in wild‐type (e, f) and tip3 (g, h) at stage 9. (i–l) TEM analysis of the anther development in wild‐type (i, j) and tip3 (k, l) at stage 10. (m–p) TEM analysis of the anther development in wild‐type (m, n) and tip3 (o, p) at late stage 13. (q–v) TEM analysis of the pollen exine in wild‐type (q, s, u) and tip3 (r, t, v) at stages 8b, late 9 and late 12, respectively. (w–x) Comparison of anther epidermis cuticle layer between wild‐type (w) and tip3(x) at late stage 12. Ep, epidermis; En, endothecium; ML, middle layer; T, tapetum; Tds, tetrads; Msp, microspore; DMsp, degenerated microspores; MP, mature pollen; PE, primexine; Ex, exine; Ba, bacula; Te, tectum; Nu, nucleus; Ne, nexine; Se, sexine; In, intine; Ub, Ubisch body; AUb, abnormal Ubisch body; AE, abnormal exine; C, cuticle; CW, cell wall. Bars represent 5 μm in (i, k); 2 μm in (a, c, e, g, m, o); 1 μm in (b, d, f, h, j, j, n, p, w, x); 0.5 μm in (q, r, u, v); 0.2 μm in (s, t).
Scanning electron microscopy (SEM) was also used to analyze morphological differences in anther and pollen grains between the wild‐type and tip3 mutant. Consistent with the phenotypic analysis (Figure 1c), lengths of tip3 anthers were almost half as short as wild‐type anthers at stage 13 (Figure 4a,b). Three‐dimensional spaghetti‐like cutin layers with relatively looser ridges deposited on the wild‐type epidermis (Figure 4c,e), while the tip3 epidermis displayed a relatively smooth appearance because of a dense arrangement of cutin layers (Figure 4d,f). This defective phenotype is supported by TEM results (Figure 3w,x). The inner surface of the wild‐type anther was densely distributed with Ubisch bodies (Figure 4g), while that of tip3 was like a tangled net of randomly distributed unknown remnants (Figure 4h). At this stage, the wild‐type microspore displayed a round shape and germinal aperture, and its surface was covered with evenly distributed apophyses (Figure 4i,j). In contrast, microspores in tip3 were gradually degraded with extremely small electron‐opaque granules which were randomly distributed in the anther locules (Figure 4k–n).
Figure 4.
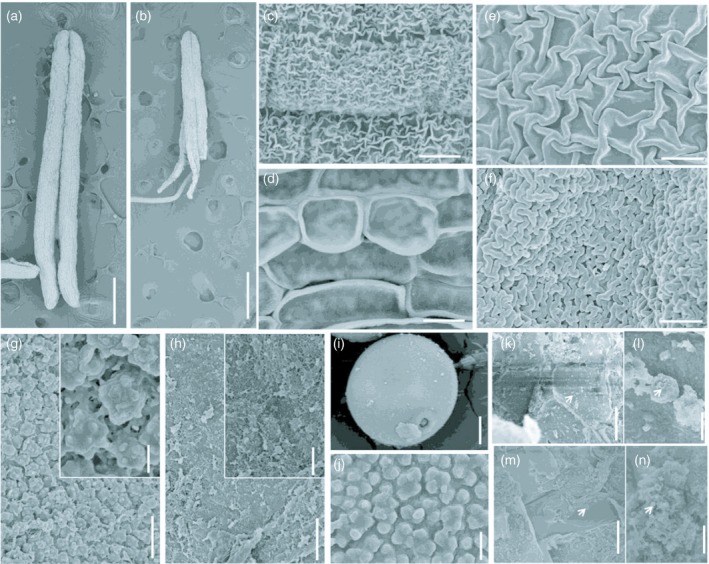
Scanning electron microscopy analysis of anthers and pollen grains in wild‐type and the tip3 mutant. (a, b) Anthers from the wild‐type (a) and tip3 (b) at stage 13. (c–f) Anther epidermis observation of the wild‐type (c, e) and tip3 (d, f) at stage 13. (g, h) Inner surface of epidermis in the wild‐type (g) and tip3 (h) at stage 13. (i, j) Wild‐type pollen grain (i) and the enlarged surface on the pollen exine (j) at stage 13. (k–n) Observation of pollen grains degradation of in tip3 at stages 10 (k, l) and 13 (m, n). An enlarged observation of degraded pollen grains (l, n). The white arrow refers to degraded particles. Bars represent 500 μm in (a, b); 10 μm in (c, d, i); 2.5 μm in (e−h, k, m); 0.5 μm in (j, l, n).
The degradation of tapetal cells is delayed in tip3
Cytological observation showed that tip3 tapetal cells still remained vacuolated (Figure 2f) at stage nine and visible nucleus in cytoplasm (Figure 3l) at stage 10. Therefore, we assumed that the normal degradation of tapetal cells and the middle layer was delayed in tip3. TUNEL assay is generally used to detect programmed cell death (Li et al., 2006). In wild‐type tapetal cells, PCD is initiated with nuclear DNA fragmentation at stage 8a. The expected positive TUNEL signals were detected in the wild‐type tapetal cells (Figure 5a). As the degradation of tapetal cells continued, positive TUNEL signals became stronger at stages 8b and 9 (Figure 5b,c). Positive TUNEL signals were also detected in the middle layer in close proximity to the tapetum (Figure 5b). At stage 10, TUNEL signals in the degenerating wild‐type tapetal cells became weaker, but other anther somatic layers and vascular bundles continued to degrade (Figure 5d). However, no TUNEL signals were detected in tip3 tapetal cells at stage 8a (Figure 5e). From stage 8b onwards, there were few TUNEL signals scattered across the tapetum, but relatively more positive signals were detected in other anther somatic layers and vascular bundles (Figure 5f–h). These TUNEL assay results suggested that tapetum degradation in tip3 is delayed.
Figure 5.
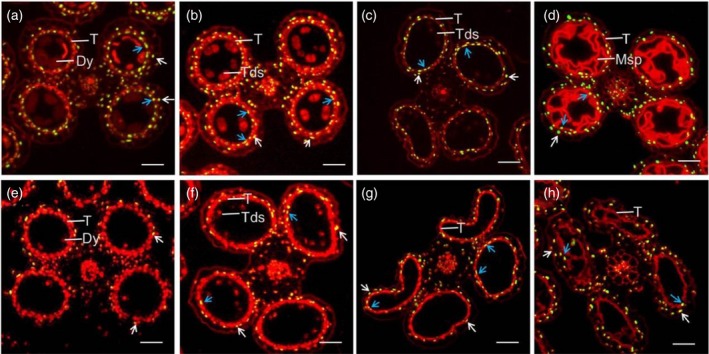
Detection of DNA fragmentation in wild‐type and tip3 anthers using the TUNEL assay. (a–d) DNA fragmentation in wild‐type anthers at stages 8a, 8b, 9 and 10, respectively. (e–h) DNA fragmentation in tip3 anthers at stages 8a, 8b, 9 and 10, respectively. The red fluorescence indicates background signal stained by propidium iodide (PI), yellow fluorescence indicates a positive TUNEL signal merged by red fluorescence and green fluorescence from TUNEL‐positive nuclei staining. The blue arrows show the TUNEL‐positive signal in tapetum and the white arrows show the TUNEL‐positive signal in the middle layer. T, tapetum; Dy, dyad cell; Tds, tetrads; Msp, microspore. Bars represent 40 μm.
Map‐based cloning of TIP3
To identify the mutated gene that controlled the male sterility phenotype, we employed a map‐based cloning strategy. Using 93 male‐sterile individuals from the F2 population obtained by crossing the tip3 mutant and 02428, the tip3 locus was initially mapped between two markers RM15721 and RM15894 on chromosome 3 according to molecular marker linkage analysis (Figure 6a). Then, nine new InDel makers and 1377 recessive plants were used for fine mapping (Figure 6b). Finally, the tip3 locus was delimited to a 21.4‐kb region between the markers S20–29 and S20–35, which contained three open reading frames (ORFs) (Figure 6c). Subsequent sequence analysis revealed a 2‐bp deletion (at position 1418–1419 bp from the ATG start codon) in the third exon of ORF3 (LOC_Os03g50780) in tip3 (Figure 6d). LOC_Os03g50780 was characterized to have three exons and two introns and encodes a total 698‐amino acid protein with a putative PHD‐finger domain at the amino acid residue position 641–685 (http://rice.plantbiology.msu.edu/). This mutation caused a frame shift of the protein and led to a premature translation termination at position 473 of the amino acid sequence, resulting in a deletion of the PHD‐finger domain.
Figure 6.
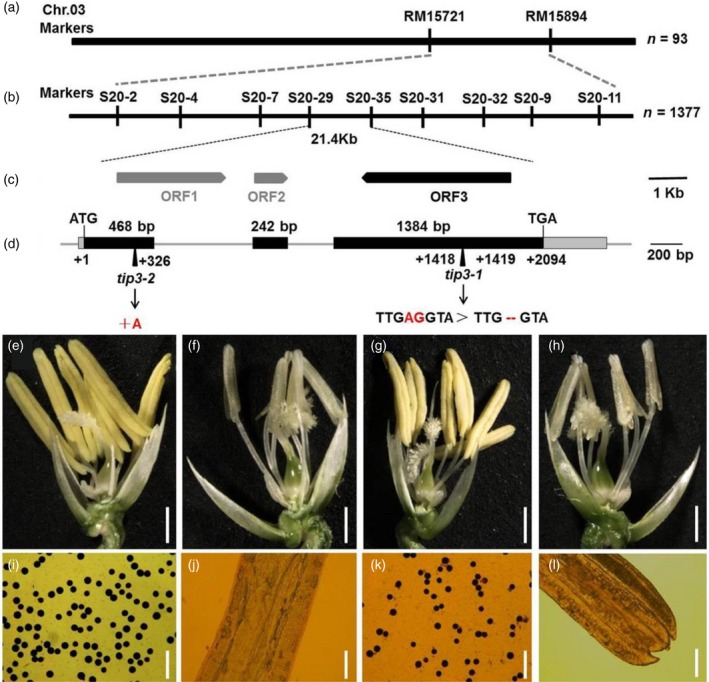
Map‐based cloning of TIP3. (a) Genetic linkage map for the primary location of the TIP3 locus on chromosome 3. (b) The TIP3 locus was fine‐mapped to a 21.4 kb region. (c) Prediction of three putative open reading frames (ORFs) in the fine mapping genomic region. (d) Gene schematic of the TIP3 (ORF3). Black boxes represent exons; the gray lines between exons represent introns. (e–h) The spikelet phenotype of wild‐type, tip3,TIP3‐complemented line and tip3‐2 (homozygous line knocked out by CRISPR/Cas9), respectively. Bars represent 500 μm. (i–l) The pollen grains were stained with 1% I2‐KI solution at stage 13 from the wild‐type, tip3,TIP3‐complemented line and tip3‐2, respectively. Bars represent 5 μm.
To confirm that the mutation in LOC_Os03g50780 is responsible for the tip3 male sterility phenotype, a plasmid containing the wild‐type genomic fragment of LOC_Os03g50780 was transformed into tip3 calli (Figure S2a). As expected, the transgenic positive plants had restored male fertility with normal anthers and visible pollen grains (Figure 6g,k and Figure S2b). Furthermore, LOC_Os03g50780 was further confirmed as the TIP3 gene by targeted knockout using a CRISPR/Cas9 system. The gRNA/Cas9 vector targeting the specific site of endogenous LOC_Os03g50780 was introduced into indica rice cv. Zh8015. Sequencing analysis exhibited one single nucleotide (A) insertion at the 326 bp from the ATG start codon in a homozygous knocked out line, named tip3‐2 (Figures 6d and S3a,b). This mutation also caused a frame shift mutation and premature termination of protein translation at 109 of the amino acid sequence, leading to the absence of the PHD‐finger domain. The tip3‐2 mutant showed complete male sterility with pale yellow anthers, no visible pollen grains and a lack of seeds, resembling the tip3 phenotype (Figure 6h,l and Figure S3c). Taken together, these results confirmed that LOC_Os03g50780/TIP3 is the target gene whose mutation can cause the male sterility phenotype.
TIP3 belongs to the PHD‐finger family
To further investigate the biological function of the TIP3 protein, we analyzed the conserved domain using the NCBI CD‐search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). TIP3 contains a Cys4‐His‐Cys3 domain, which is characterized by a canonical PHD‐finger (Figure 7a). Several PHD proteins have been reported in plants, such as MMD1/DUET (Reddy et al., 2003; Yang, 2003), MS1 (Wilson et al., 2001; Ito et al., 2007), PTC1 (Li et al., 2011a) and ZmMs7 (Zhang et al., 2017a). Therefore, we conducted multiple sequence alignment between these four known PHD proteins and TIP3. The results showed that Cys4‐His‐Cys3 amino acid residues at the carboxyl terminal were conserved in these five PHD proteins (Figure 7b). To determine whether TIP3 and its related homologs are also evolutionarily conserved in flowering plants, phylogenetic analysis was performed using the full‐length amino acid sequences of TIP3 and its 12 closest‐related orthologs obtained by BLASTP in the NCBI database. MS1, PTC1 and ZmMs7 were located in the same clade, which have been reported to be required for tapetum and microspore development (Wilson et al., 2001; Ito et al., 2007; Yang et al., 2007; Li et al., 2011a; Zhang et al., 2017a) (Figure 7c). Arabidopsis MMD1, which is required for male meiosis (Yang, 2003), and homologs from other dicots, such as Glycine max, Theobroma cacao and Gossypium raimondii, were grouped into the same clade (Figure 7c). TIP3 was grouped into a clade of monocots including Oryza brachyantha, Brachypodium distachyon, Sorghum bicolor, Setaria italica and Panicum hallii (Figure 7c). Our results demonstrated that, although TIP3, PTC1, MMD1, MS1 and ZmMs7 showed a common PHD‐finger domain, TIP3 and other monocots formed a separate clade distinct from other known PHD proteins. This implies that TIP3 might function in its own unique manner.
Figure 7.
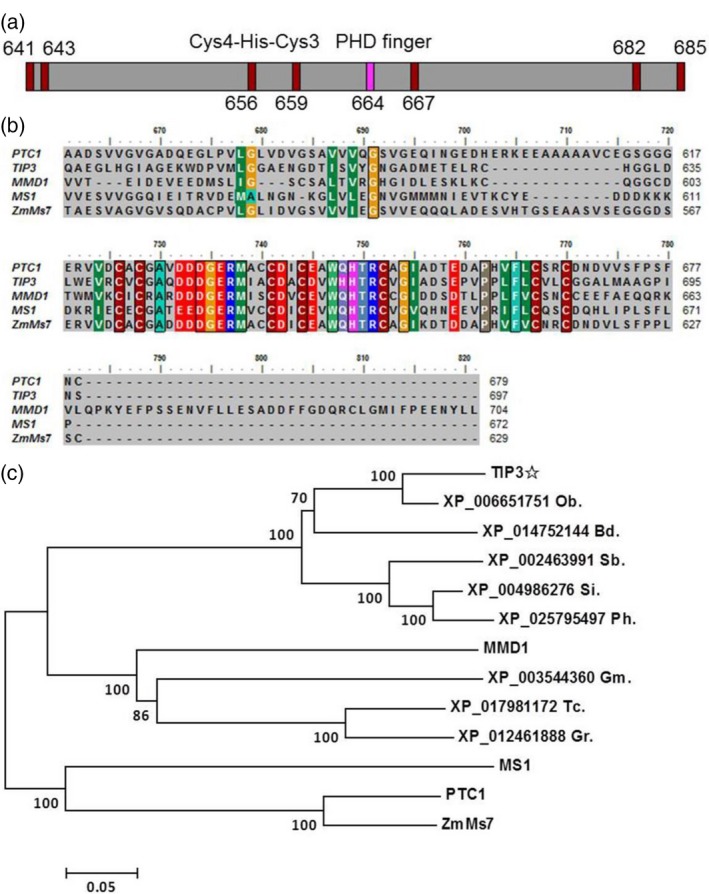
Bioinformatics analysis of TIP3. (a) Diagram of PHD‐finger domain in TIP3 protein. The numbers indicate the position of the amino acid residue at the TIP3 amino acid sequence. Red rectangle represents Cys and pink rectangle represents His. (b) Multiple sequence alignment between TIP3 amino acid sequence and other known PHD proteins at the carboxyl terminal. (c) Phylogenetic analysis of TIP3 and 12 homologs in flowering plants. The tree was constructed using MEGA7 software based on the neighbor‐joining method. Ob, Oryza brachyantha; Bd, Brachypodium distachyon; Sb, Sorghum bicolor; Si, Setaria italica; Ph, Panicum hallii; Gm, Glycine max; Tc, Theobroma cacao; Gr, Gossypium raimondii.
TIP3 is mainly expressed in tapetum and microspores
To further elucidate the function of rice TIP3 during male reproductive development, we analyzed the expression pattern of TIP3 in anthers and vegetative tissues in wild‐type plants using quantitative RT‐PCR (qRT‐PCR). The results indicated that TIP3 was constitutively expressed in all tested issues, including root, stem, leaf, sheath and relatively higher levels in anther (Figure 8a), and are consistent with the expression profile data from the Rice Expression Profile Database (RiceXPro) (http://ricexpro.dna.affrc.go.jp/) (Figure S4). In anther tissues, the peak of expression appeared to correspond to a spikelet length of <2.9 mm, and then the expression declined gradually. In later stages, expression of TIP3 increased again and remained at the length of 5.0–8.9 mm (Figure 8a and Figure S5), which corresponded to the dyad, tetrad and microspore development stages. In transgenic plants carrying the pTIP3::GUS construct, GUS signals were only detectable in the anthers at the spikelet length of <8.9 mm (Figure 8b). Further transverse section analysis of GUS‐stained anthers indicated that TIP3 was highly expressed in whole anther tissues at the early stage (Figure 8c), and then preferentially expressed in the tapetum and microspores (Figure 8d) until no GUS expression was visible at the length of more than 10 mm (Figure 8e). To further precisely determine the spatial and temporal patterns of TIP3 expression in the anthers, we performed RNA in situ hybridization on wild‐type anther sections. TIP3 expression was initially detected mainly in anther somatic layers at stage six (Figure 8f), and then the strong expression signal was detected predominantly in the tapetum and microspores from stage 8a to 10 (Figure 8g–j). From stage 11 onward, the expression of TIP3 transcripts decreased (Figure 8k). By contrast, no expression signals were detected in anthers sections with the sense probe at stage 6 and 8a (Figure 8l–m).This expression pattern well supported the idea that TIP3 plays a specific function in tapetum development and pollen wall formation.
Figure 8.
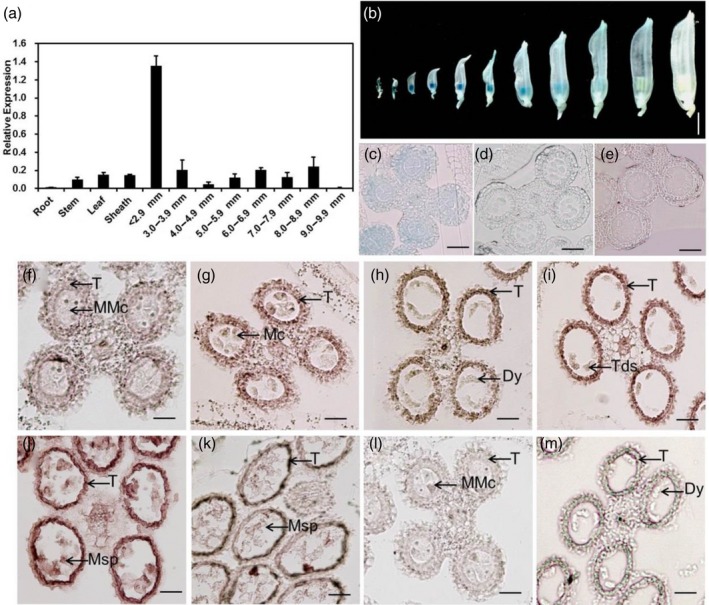
Expression pattern of the TIP3 gene. (a) qRT‐PCR analysis of TIP3. OsACTIN was used as the reference. Data are presented as mean ± SE. According to the length of spikelet, anthers were divided into different groups. (b) GUS staining of transgenic anthers containing pTIP3::GUS. Bar represents 3 mM. (c, d) Transverse section analysis of GUS‐stained anthers at stages 6, 9 and 11, respectively. Bar represents 50 μm. (f–m) In situ hybridization analysis of TIP3 in wild‐type anthers. Hybridized with TIP3 antisense probe at stage 6–11; (f–k), sense probe at stage 6 (l) and stage 8a (m). MMC, microspore mother cell; MC, meiotic cell; T, tapetum; Dy, dyad cell; Tds, tetrads; Msp, microspore. Bar represents 40 μm.
TIP3 acts as a transcriptional activator in yeast
As mentioned above, TIP3 encodes a putative protein containing a PHD‐finger domain, which usually is found in nuclear proteins. Therefore, to verify whether TIP3 is a nuclear protein, we performed a subcellular localization experiment in the rice protoplast. The p35S:TIP3−GFP and p35S:OsMADS3−mCherry fusion constructs were transiently co‐expressed in rice protoplast. In cells co‐expressing GFP and RFP, the green and red fluorescence signals were visible and only observed in the protoplast nucleus (Figure 9a–d). This result demonstrated that TIP3 is a nuclear‐localized protein. To verify whether TIP3 functions as a transcription factor, a transcriptional activity assay was conducted in yeast. According to the domain analysis, the full‐length and truncated fragments were fused to the DNA‐binding domain (BD) of yeast GAL4 (Figure 9e). All the constructs and the empty vector were transformed into yeast and cultured on control medium (SD/−Trp) and selective medium (SD/−Trp/−His/−Ade). Interestingly, the full‐length sequence of the TIP3 protein, the N‐terminal region (BD‐ΔC:1–640 residues) and the C‐terminal region (BD‐ΔN: 641–698 residues) were found to be sufficient for activation of the reporter, while its PHD‐finger domain (BD‐PHD: 641–685 residues) and empty vector were unable to activate the reporter for survival on the selective medium (Figure 9e). To further validate the results, we quantitatively measured expression activity of the β‐galactosidase (β‐gal) reporter gene in yeast. Consistent with the above results, relative higher β‐gal activity was detected from the full‐length TIP3 protein, BD‐ΔC and BD‐ΔN, while no activity was detected from the BD‐PHD construct (Figure 9e). Taken together, these results demonstrated that TIP3 is a nucleus‐localized transcription factor with transcriptional activation activity and the PHD domain is not required for transcription activation. Hence, we speculated that the PHD domain may be functionally associated with binding to DNA or specific nuclear protein partners.
Figure 9.
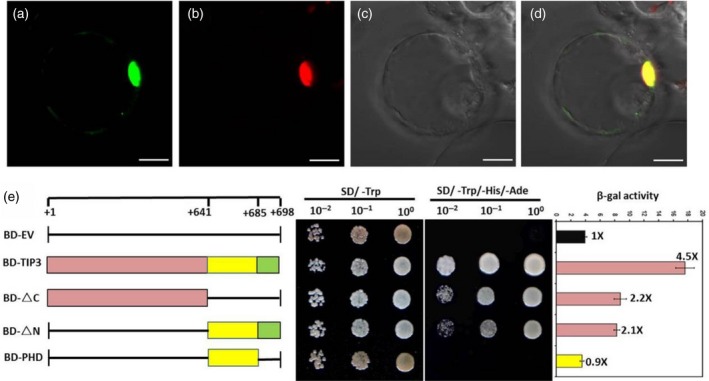
Subcellular localization and transcriptional activity analysis of TIP3. (a–d) Subcellular localization of TIP3 in rice protoplast. TIP3−GFP fusion protein (a), nuclear marker OsMADS3‐mCherry (b), bright field (c) and the merged image (d). Bar represents 10 μm. (e) Transcriptional activation assay of TIP3 in yeast. Full‐length TIP3 amino acid sequence and truncated proteins were fused with the DNA‐binding domain in pGBKT7 (showed on the left). Yeast cells expressed various constructs and empty pGBKT7 grew on control medium (SD/−Trp) and selective medium (SD/−Trp/−His/−Ade), respectively. BD, GAL4‐DNA‐binding domain; EV, empty vector. Amino acid positions are labeled on the top of diagrams. The empty pGBKT7 was used as a negative control. The β‐galactosidase (β‐gal) activity was measured using the substrate chlorophenol red‐β‐d‐galactopyranoside (CPRG). Error bars indicate SD.
TIP3 physically interacts with TDR
The above results demonstrated that the full‐length TIP3 protein is a transcription factor and can autonomously activate expression of the reporter gene. Therefore, using ChIP‐seq technology to identify target genes is the most straightforward approach to explore the role of TIP3 in tapetum development and pollen wall formation. However, we have not yet synthesized a suitable TIP3 antibody for this purpose. Bienz (2006) previously reported that a PHD‐finger, a nuclear protein‐interaction domain, can bind to specific nuclear protein partners. Therefore, a yeast two‐hybrid assay was performed to determine whether TIP3 also physically interacts with nuclear proteins, such as TDR (Li et al., 2006; Zhang et al., 2008), EAT1 (Niu et al., 2013b) or TIP2 (Fu et al., 2014; Ko et al., 2014), which has also been reported to regulate tapetum development and pollen wall formation. Because only the PHD domain in the TIP3 protein did not possess the ability of autonomous activation (Figure 9e), we used this domain as the bait. As expected, the yeast strains co‐transformed TIP3 (BD‐PHD) and prey proteins (TDR, EAT1 and TIP2) that were, respectively, grown on control medium (SD/−LT) or selective medium (SD/−LTHA). This result showed that the PHD domain of the TIP3 protein interacts with the three bHLH TFs, TDR, EAT1 and TIP2, in yeast (Figure 10a). To further verify this interaction, we performed the bimolecular fluorescence complementation (BiFC) assay in Nicotiana benthamiana mesophyll cells. TIP3 was fused with the N‐terminus of yellow fluorescent protein (YFP) (YN), and other proteins were fused with the C‐terminus of YFP (YC). After infiltrating plants with Agrobacterium cells carrying appropriate constructs, the reconstituted YFP fluorescence was observed in the nucleus of tobacco leaf epidermal cells. Interestingly, the reconstituted YFP fluorescence was only detected in plants transformed with the constructs of a combination of YN‐TIP3/YC‐TDR, indicating that TIP3 interacts with TDR in the nucleus in vivo (Figure 10b). YN‐HAL3 associated with YC‐HAL3 served as a positive control with the whole cell expressing the YFP fluorescence (Su et al., 2016). In contrast, no YFP fluorescence was detected in the co‐expression of YN‐TIP3/YC or YN/YC‐TDR, which served as the negative controls. Together, these results indicated that TIP3 interacts with TDR in the nucleus in vivo, implying that TIP3 may function in regulating tapetum development and pollen wall formation through its interaction with TDR in the nucleus.
Figure 10.
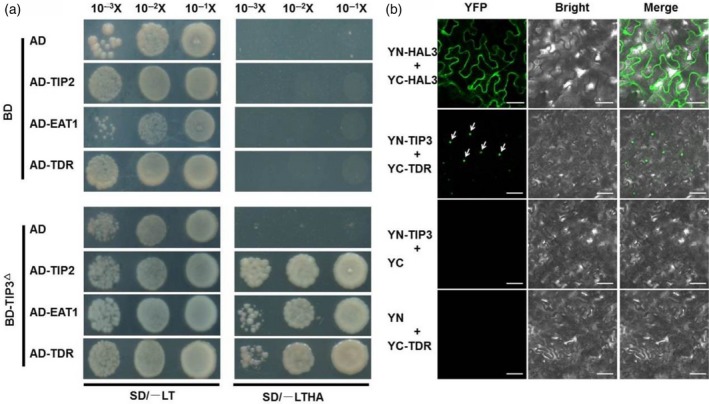
Interaction between TIP3 and TDR (a) Yeast two‐hybrid assay for the interaction between TIP3 and TIP2, EAT1,TDR. Empty vector pGBKT7 and pGADT7 were used as the negative controls. SD/−LT: medium lacking Leu, Trp; SD/−LTHA: medium lacking Leu, Trp, His and Ade. (b) Bimolecular fluorescence complementation verified the interaction between TIP3 and TDR in tobacco leaf epidermal cells. Top panel: the combination of YN‐HAL3 and YC‐HAL3 used as the positive control, bars represent 50 μm. Second panel: the positive interaction between YN‐TIP3 and YC‐TDR, the fluorescent nucleus was indicated by white arrows, bars represent 100 μm. The last two panels: the combination of YN‐TIP3 and YC (third panel) and YN with YC‐TDR (bottom panel) was used as the negative control, bars represent 100 μm.
Expression levels of known tapetum and pollen exine development genes are altered in tip3
Based on observations of delayed tapetum degradation in tip3 and the interaction between TIP3 and TDR, we speculated that TIP3 might affect the expression of genes involved in tapetum development and programmed cell death. Therefore, we examined the expression profiles of tapetum‐related genes between wild‐type and tip3 mutant anthers at the spikelet length of 5.0–8.9 mm. Compared with wild‐type, the expression levels of MSP1 and GAMYB were significantly reduced in tip3 (Figure 11a,b). MSP1 plays a critical role in early sporogenic development as well as normal anther wall and tapetal layer development (Nonomura et al., 2003). GAMYB, encoding a MYB transcriptional factor, was reported to directly activate the expression of CYP703A3 to regulate tapetum PCD and exine formation (Aya et al., 2009). API5 encodes a putative homolog of antiapoptosis protein Api5 in animals and participates in the tapetal PCD process in rice (Li et al., 2011b), its expression level, however, was not significantly altered in tip3 (Figure 11c). The expression of three bHLH TF (UDT1, TDR and EAT1) showed alteration at different anther stages (Figure 11d–f). UDT1 is required for the differentiation of secondary parietal cells to mature tapetal cells (Jung et al., 2005) and both TDR (Li et al., 2006) and EAT1 (Niu et al., 2013b) mainly participate in normal tapetum PCD at early stages. CP1 (Cysteine protease), AP25 and AP37 (Aspartate protease) are required for inducing programmed cell death in both yeast and plants (Li et al., 2006; Niu et al., 2013b). Our results showed that the expression levels of these three genes were significantly altered in tip3 (Figure 11g–i). We also examined the expression of genes involved in sporopollenin precursor biosynthesis and Ubisch body transportation. Compared with wild‐type, three sporopollenin precursor biosynthesis genes (DPW, CYP703A3 and CYP704B2) and three sporopollenin precursor transport genes (OsC6, OsABCG15 and OsABCG26) were significantly altered in tip3, while the expression of WDA was not significantly changed (Figure 11j–p). Taken together, TIP3 may regulate anther development and viable pollen formation by affecting the expression of genes involved in tapetum PCD, sporopollenin precursor biosynthesis and transportation during male reproductive development.
Figure 11.

Expression analysis of the genes required for anther development using qPCR at different stages. Anthers were divided into different groups according to the length of spikelet. Error bars indicate the SD of three biological replicates. Student's t test: *(P < 0.05), **(P < 0.01).
Discussion
TIP3 regulates Ubisch bodies and pollen wall formation
Mature pollen grains are typically covered by a chemically and physically resistant outer cell wall called the exine. Increasing numbers of studies have contributed to our understanding of mechanisms underlying pollen exine formation and revealed that callose wall formation, primexine formation and sporopollenin precursor biosynthesis are three vital developmental processes during pollen exine formation. In this study, we characterized and isolated a no‐pollen male sterility mutant named tip3 (Figures 1 and 2). According to cytological observations, tip3 was able to produce normal callose walls and primexine as well as undulated plasma membranes during meiosis (Figure 3q,r). However, Ubisch bodies composed of sporopollenin exhibited abnormal morphology in tip3 anther locules (Figure 3j,l,u,v). Ubisch bodies normally exhibited an electron‐transparent central kernel surrounded by a few electron‐dense particles (Figure 3j,u), while in tip3 they exhibited completely electron‐opaque spheres of varying size (Figure 3l,v). The abnormal structure of Ubisch bodies may affect the assembly and deposition of sporopollenin on the primexine leading to a lack of pollen exine. This mutant phenotype is reminiscent of the phenotypes of np1 in rice (Liu et al., 2017). Ubisch body kernels of the np1‐4 mutant were surrounded by abundant electron‐dense materials, leaving only a small hole on one side. This mutation was caused by NP1, a putative glucose‐methanol‐choline (GMC) oxidoreductase, and was likely to be involved in the production of lipidic molecules required for polymerization or assembly (or both) of sporopollenin precursors (Liu et al., 2017). Furthermore, smaller Ubisch bodies have been observed in ptc1 with the reduction of both the electron‐opaque central region and the surrounding electron‐dense particles (Li et al., 2011a). We speculated that the abnormal Ubisch body morphology might be due to the dysfunction of genes involved in the synthesis of sporopollenin precursors. A previous study has reported that sporopollenin precursors are composed of complex biopolymers derived mainly from saturated precursors such as long‐chain fatty acids or long aliphatic chains (Ariizumi and Toriyama, 2011). A large number of enzymes such as ACOS5, MS2, DPW2, DPW, OsGPAT3 and cytochrome P450 family members directly catalyze this biological process (Souza et al., 2009; Li et al., 2010; Shi et al., 2011; Yang et al., 2014; Wallace et al., 2015; Men et al., 2017; Xu et al., 2017). In our study, we detected that the expression levels of CYP703A3 and CYP704B2 were significantly altered in the tip3 mutant (Figure 11). This result, to some extent, revealed a potential cause of the abnormal sporopollenin precursor synthesis. In addition, the tip3 mutant demonstrated delayed tapetum degradation (Figure 5), which might affect lipids and their biosynthesis of derivatives in tapetal cells. The expression levels of tapetum‐related genes such as GAMYB, EAT1, TDR, CP1, AP25 and AP37 correspond to the delayed degradation of tapetum in tip3 (Li et al., 2006; Zhang et al., 2008; Aya et al., 2009; Liu et al., 2010; Niu et al., 2013b; Ko et al., 2014). We surmise that disruption of TIP3 might affect the expression of its downstream genes, and directly catalyzes the synthesis of sporopollenin precursors, conferring defects in Ubisch bodies and pollen wall formation as well as delayed degradation of the tapetum.
TIP3 plays diversified roles in male reproductive development
The protein conserved domain analysis determined that TIP3 contains a canonical PHD‐finger with typical Cys4‐His‐Cys3 amino acid residues at the carboxyl terminal (Figure 7a). The PHD‐finger is comprised of about 60 amino acids and extremely conserved in TIP3, MMD1, MS1, PTC1 and ZmMs7 (Figure 7b) (Wilson et al., 2001; Yang, 2003; Li et al., 2011a; Zhang et al., 2017a). However, evolutionary analysis showed that TIP3 was grouped into a separate clade with other monocots from other known PHD proteins (Figure 7c). We deduced that TIP3 may play a diversified role in male reproductive development. As reported, MS1 and its orthologues PTC1, HvMS1 and ZmMs7 show conservative functions in tapetal cell death and pollen development (Wilson et al., 2001; Yang, 2003; Ito et al., 2007; Yang et al., 2007; Li et al., 2011a; Gomez and Wilson, 2014; Zhang et al., 2017a). PTC1, especially, was able to partially rescue the mutant phenotype of MS1 in the dicot Arabidopsis. However both tip3 and ptc1 mutants showed complete male sterility in rice, implying that the two genes are not functionally redundant. Therefore, TIP3 and PTC1 may have different functions in male reproductive development. This hypothesis was further supported by the different spatial and temporal expression profiles of the two genes. TIP3 expressed across many stages of anther development, while PTC1 specifically expressed during stages 8 and 9 of anther development (Figure 8). TIP3 is likely to have other undiscovered functions in male reproductive development. Another PHD‐finger protein, MMD1/DUET, is reportedly involved in chromosome organization and progression during male meiosis in Arabidopsis (Reddy et al., 2003; Yang, 2003). Recent studies revealed that MMD1/DUET directly activates its target genes CAP‐D3 and TDM1 to regulate, respectively, chromosome condensation and microtubule organization, cell cycle transitions during male meiosis (Andreuzza et al., 2015; Wang et al., 2016). However, our results showed that tip3 could normally undergo meiosis and form dyads and tetrads, as observed by DAPI staining (Figure S1). Therefore, we speculated that TIP3 plays a diversified function that differs from other known PHD‐finger protein functions in regulating Ubisch bodies and pollen wall formation during male reproductive development.
The proposed mechanism of TIP3 in tapetum degradation and pollen wall formation
Several transcription factors have been reported to precisely regulate programmed tapetum development and pollen wall formation. GAMYB and UDT1 have been reported to work in parallel to regulate anther development at early stages and may positively activate the expression of TDR (Liu et al., 2010). Both gamyb‐4 and udt1 develop abnormal enlarged tapetum and do not form microspores (Jung et al., 2005; Liu et al., 2010). TDR regulates rice tapetum development and degeneration by directly activating the expression of its targets genes, OsCP1 and OsC6 (Li et al., 2006). TDR also physically interacts with EAT1 and EAT1 directly regulates the expression of OsAP25 and OsAP37, which induce programmed cell death in both yeast and plants (Niu et al., 2013b). TIP2/bHLH142, another bHLH TF interacting with TDR, acts downstream of UDT1, but upstream of TDR and EAT1, and TIP2/bHLH142 can form a dimer with TDR to further activate EAT1 transcription by binding to its promoter (Fu et al., 2014; Ko et al., 2014). This is a commonly accepted transcriptional cascade in regulating tapetum development and pollen grain formation (Figure s6). Our results demonstrated that TIP3 encoded a conserved PHD‐finger transcription factor with transcriptional activation activity (Figure 9e). Based on the defects of Ubisch bodies and pollen wall formation and the altered expression of tapetum‐ and microspore‐expressed genes in tip3, we speculated that TIP3 may activate specific target genes involved in de novo fatty acid synthesis and modification in tapetal cells or processing and deposition of sporopollenin to regulate sculptured pollen wall formation. Conversely, Y2H and BiFC assays demonstrated that TIP3 physically interacted with TDR in vivo. This interaction may be required for normal tapetum degradation and pollen wall formation. When the normal interaction was disturbed, the expression levels of TFs or its targets genes may be altered accordingly, further causing delayed tapetum degradation and defective pollen walls in the tip3 mutant. We propose that TIP3, or TIP3 interaction with TDR, may directly or indirectly activate specific downstream genes to regulate tapetum degradation and pollen wall formation in rice.
Experimental procedures
Plant materials and growth conditions
All rice plants used in this study were grown in paddy fields. The tip3 mutant was identified from a 60Coγ‐ray radiation‐induced population derived from indica rice cv. Zhonghui8015 (Zh8015). The F2 mapping population was derived from a cross between tip3 and 02428 (japonica). The BC1F2 population was generated from a cross between tip3 and Zh8015 for genetic analysis.
Phenotypic characterization of tip3 mutants
Plants and spikelets were photographed with an E995 digital camera (Nikon, Tokyo, JAPAN) and OLYMPUS MVX10 stereomicroscope, respectively. Pollen grains were observed and photographed with a Leica DM2500 microscope. Spikelets at different developmental stages were selected to perform semi‐thin sections and TEM assay as described previously (Li et al., 2006; Yang et al., 2018, 2019). Development stages of rice anther were determined as previously described (Zhang and Wilson, 2009; Zhang et al., 2011). Scanning electron microscopy assay was performed as described by Yang et al. (2019).
Map‐based cloning of TIP3 and complementation analysis
To fine map the TIP3 locus, bulked segregation analysis (Liu, 2005) was used. In total, 1377 individuals with the mutant phenotype were selected from the F2 population and insertion−deletion (InDel) markers (listed in Table S1) were designed based on the reference sequence japonica Nipponbare and indica 9311 (http://www.gramene.org) for fine mapping. TIP3 was finally mapped to a 21.4 kb region between S20–S29 and S20–S35.
A 6.42‐kb genomic fragment of TIP3 was obtained by PCR amplification and subcloned into the binary vector pCAMBIA13001. Then the pCAMBIA13001‐TIP3 construct was transformed into tip3 mutants through Agrobacterium‐mediated transformation. The CRISPR/Cas9 system was used to knock out the TIP3 gene as previously described (Miao et al., 2013; Huang et al., 2017). The pCAS9‐AarI binary vector with target sequence was transformed into Zh8015 callus tissue through Agrobacterium‐mediated transformation. The mutants were screened from T0 transgenic plants and confirmed by sequencing for further phenotypic analysis. Primers used for vector construction and sequencing are listed in Table S2.
Phylogenetic analysis
The conservative domain of TIP3 protein was analyzed with the NCBI CD‐search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The full‐length protein sequences of TIP3 and its homologs in other plant species were obtained from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/). Multiple alignments were performed using BioEdit 7.0.9.0. The phylogenetic tree was constructed using mega 6.0 software based on the neighbor‐joining method (bootstrap = 1000).
RNA extraction and qPCR analysis
Anthers of wild‐type and tip3 mutants at all developmental stages were collected in liquid nitrogen (Zhang et al., 2017b). Total RNA was isolated and cDNA was synthesized as reported previously (Yang et al., 2019). qPCR was performed on a Roche LightCycler 480 device using SYBR Premix Ex Taq II (TaKaRa, Kyoto, JAPAN). The rice actin gene was used as the internal reference gene. Each experiment was performed in three replicates. Specific primers for each target gene are listed in Table S3.
GUS staining
A 2225‐bp upstream DNA fragment before the initiation codon of the TIP3 gene was amplified as the promoter of TIP3. The TIP3 promoter fragment was ligated into pCAMBIA1305::GUS, then the construct was introduced into Zh8015 plants through Agrobacterium‐mediated transformation. The spikelets at different development stages from transgenic positive plants were collected for GUS staining analysis as described in a previous report (Jefferson et al., 1987; Bi et al., 2017). After staining and washing with 70% (v/v) ethanol, the spikelets were photographed using an Epson Scan V330 instrument. Primers used for pTIP3::GUS construction are listed in Table S2.
RNA in situ hybridization
Wild‐type spikelets at different developmental stages were fixed in 3.7% FAA (Formalin acetic alcohol) solution (5% acetic acid, 50% ethanol, 3.7% formaldehyde) for 16 h at 4°C. After being dehydrated through the ethanol series, anthers were embedded in paraplast plus (Sigma, St. Louis, USA) and sectioned to 8 μm thickness using an RM2245 rotary microtome (Leica, Wetzlar, Germany). A 231‐bp specific fragment of TIP3 coding sequence (CDS) was amplified by primers (Table S3) and used as the template for probe labeling. The antisense and sense probes were transcribed in vitro under the T7 promoter with RNA polymerase using the DIG RNA labeling kit (Roche, Rotkreuz, Switzerland). RNA hybridization and immunological detection of the hybridized probes were performed as described previously (Li et al., 2006; Huang et al., 2017). Images were obtained using a Leica DM2000 light microscope.
TUNEL analysis
A TUNEL (terminal‐deoxynucleoitidyl transferase‐mediated nick‐end labeling) assay was performed as described previously to investigate the DNA fragmentation in wild‐type and tip3 mutant anthers (Yang et al., 2019). All sections were dewaxed in xylene and digested in proteinase K. In situ nick‐end labeling of nuclear DNA fragmentation was performed using the DeadEnd Fluorometric TUNEL system kit (Promega, Wisconsin, USA) according to the manufacturer's instructions. Images were obtained using an Imager D2 microscope (Carl Zeiss, Oberkochen, Germany).
Subcellular localization
The full‐length coding sequence of TIP3 without the stop codon was amplified and inserted into the transient expression vector pAN580 at the BamHI site. The p35S:TIP3−GFP and p35S:OsMADS3−mCherry fusion constructs were transiently co‐transformed into the rice protoplast as described previously (Wu et al., 2017). The fluorescence signal was detected with a laser scanning confocal microscope (Zeiss LSM700, Oberkochen, Germany). The primers used here are listed in Table S2.
Transcriptional activity assay
The transcriptional activity assay was performed according to the Matchmaker GAL4 Yeast Two‐Hybrid System 3 (Clontech, California, USA). The coding sequence of TIP3 and three truncated sequences of TIP3 were amplified by PCR and inserted into the pGBKT7 plasmid linearized by EcoRI. All constructs were separately transformed into the yeast strain AH109. The pGBKT7 empty vector served as a negative control. Activity of β‐galactosidase was measured as reported previously (Wu et al., 2017). The primers used for vector construction are listed in Table S2.
Yeast two‐hybrid and bimolecular fluorescence complementation assays
Depending on the transcriptional activity assay, the BD‐PHD construct was used as bait. The full‐length coding sequence of TIP2, TDR and EAT1 was cloned into the prey vector pGADT7. A yeast two‐hybrid assay was performed following the manufacturer's instructions (Clontech). For BiFC assays, the CDS of TIP3 was amplified and inserted into pCambia1300S‐YN to form the YN‐TIP3 construct. The full‐length CDS of TIP2, TDR and EAT1 was isolated and cloned into pCambia2300S‐YC to form the protein‐YC constructs. Agrobacterium strain GV3101 was used to transform various combinations of constructs and to infect tobacco leaves. The OsHAL3 was cloned into pCambia1300S‐YN and pCambia2300S‐YC as positive controls (Su et al., 2016). The BiFC fluorescence signals were observed using a laser scanning confocal microscope (Zeiss LSM700). The primers used here are listed in Table S2.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. DAPI staining analysis of meiosis in wild‐type and the tip3 mutant.
Figure S2. Complementation analysis of the tip3 by wild‐type TIP3 genomic DNA.
Figure S3. Targeted mutagenesis in TIP3 gene using the CRISPR/Cas9 system.
Figure S4. The expression profile data of TIP3 from the Rice Expression Profile Database (RiceXPro) (http://ricexpro.dna.affrc.go.jp/).
Figure S5. The information of TIP3 from the transcriptomic database Rice eFP Browser.
Figure S6. The proposed model of TIP3 in tapetal PCD and pollen exine formation.
Table S1. Primers used in fine mapping.
Table S2. Primers used in this study for vectors construction.
Table S3. Primers used in this study for in situ hybridization and qPCR assay.
Acknowledgments
We would like to thank Prof. Dabing Zhang, Prof. Wanqi Liang and Jie Wang (Shanghai Jiao Tong University, Shanghai, China) for their help in in situ assay. This work was supported by grants from the National Key Transform Program (#2016ZX08001‐002), the National Natural Science Foundation of China (#31521064 and #31801440), the Zhejiang Provincial Natural Science Foundation of China (#LQ17C130003), Independent Research subject of the State Key Laboratory of Rice Biology (#2017ZZKT10202) and the Super Rice Breeding Innovation Team and Rice Heterosis Mechanism Research Innovation Team of the Chinese Academy of Agricultural Sciences Innovation Project (CAAS‐ASTIP‐2013‐CNRRI).
Contributor Information
Liyong Cao, Email: chengshihua@caas.cn.
Shihua Cheng, Email: caoliyong@caas.cn.
References
- Andreuzza, S. , Nishal, B. , Singh, A. and Siddiqi, I. (2015) The chromatin protein DUET/MMD1 controls expression of the meiotic gene TDM1 during male meiosis in Arabidopsis. PLoS Genet. 11, e1005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi, T. and Toriyama, K. (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 62, 437–460. [DOI] [PubMed] [Google Scholar]
- Ariizumi, T. , Hatakeyama, K. , Hinata, K. , Inatsugi, R. , Nishida, I. and Sato, S. (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J. 39, 170–181. [DOI] [PubMed] [Google Scholar]
- Ariizumi, T. , Hatakeyama, K. , Hinata, K. , Sato, S. , Kato, T. , Tabata, S. and Toriyama, K. (2005) The HKM gene, which is identical to the MS1 gene of Arabidopsis thaliana, is essential for primexine formation and exine pattern formation. Sex. Plant Reprod. 18, 1–7. [Google Scholar]
- Aya, K. , Ueguchi‐Tanaka, M. , Kondo, M. , Hamada, K. , Yano, K. , Nishimura, M. and Matsuoka, M. (2009) Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell, 21, 1453–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, Z. , Zhang, Y. , Wu, W. et al (2017) ES7, encoding a ferredoxin‐dependent glutamate synthase, functions in nitrogen metabolism and impacts leaf senescence in rice. Plant Sci. 259, 24–34. [DOI] [PubMed] [Google Scholar]
- Bienz, M. (2006) The PHD finger, a nuclear protein‐interaction domain. Trends Biochem. Sci. 31, 35–40. [DOI] [PubMed] [Google Scholar]
- Bubert, H. , Lambert, J. , Steuernagel, S. , Ahlers, F. and Wiermann, R. (2002) Continuous decomposition of sporopollenin from pollen of Typha angustifolia L. by acidic methanolysis. Z. Naturforsch. C, 57, 1035–1041. [DOI] [PubMed] [Google Scholar]
- Chang, H.S. , Zhang, C. , Chang, Y.H. et al (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol. 158, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Z. , Chen, Z. , Yan, W. et al (2016) An ABC transporter, OsABCG26, is required for anther cuticle and pollen exine formation and pollen‐pistil interactions in rice. Plant Sci. 253, 21–30. [DOI] [PubMed] [Google Scholar]
- Chen, W. , Yu, X.H. , Zhang, K. , Shi, J. , de Oliveira, S. , Schreiber, L. , Shanklin, J. and Zhang, D. (2011) Male Sterile2 encodes a plastid‐localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol. 157, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobritsa, A.A. , Shrestha, J. , Morant, M. , Pinot, F. , Matsuno, M. , Swanson, R. , Moller, B.L. and Preuss, D. (2009) CYP704B1 Is a long‐chain fatty acid ‐hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 151, 574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y. and Zhang, Q. (2017) Genetic and molecular characterization of photoperiod and thermo‐sensitive male sterility in rice. Plant Reprod. 31, 3–14. [DOI] [PubMed] [Google Scholar]
- Fu, Z. , Yu, J. , Cheng, X. , Zong, X. , Xu, J. , Chen, M. , Li, Z. , Zhang, D. and Liang, W. (2014) The rice basic helix‐loop‐helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development. Plant Cell, 26, 1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, J.F. and Wilson, Z.A. (2014) A barley PHD finger transcription factor that confers male sterility by affecting tapetal development. Plant Biotechnol. J. 12, 765–777. [DOI] [PubMed] [Google Scholar]
- Hu, J. , Wang, Z. , Zhang, L. and Sun, M.X. (2014) The Arabidopsis exine formation defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol. 203, 140–154. [DOI] [PubMed] [Google Scholar]
- Huang, X. , Peng, X. and Sun, M.X. (2017) OsGCD1 is essential for rice fertility and required for embryo dorsal‐ventral pattern formation and endosperm development. New Phytol. 215, 1039–1058. [DOI] [PubMed] [Google Scholar]
- Ito, T. , Nagata, N. , Yoshiba, Y. , Ohme‐Takagi, M. , Ma, H. and Shinozaki, K. (2007) Arabidopsis MALE STERILITY1 encodes a PHD‐type transcription factor and regulates pollen and tapetum development. Plant Cell 19, 3549–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R. , Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: β‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Plant Physiol. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K.H. , Han, M.J. , Lee, Y.S. , Kim, Y.W. , Hwang, I. , Kim, M.J. , Kim, Y.K. , Nahm, B.H. and An, G. (2005) Rice undeveloped tapetum1 is a major regulator of early tapetum development. Plant Cell, 17, 2705–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, M. , Inukai, Y. , Ueguchi‐Tanaka, M. et al (2004) Loss‐of‐function mutations of the rice GAMYB gene impair alpha‐amylase expression in aleurone and flower development. Plant Cell, 16, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, S.S. , Li, M.J. and Sun‐Ben Ku, M. (2014) The bHLH142 transcription factor coordinates with TDR1 to modulate the expression of EAT1 and regulate pollen development in rice. Plant Cell, 26, 2486–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Zhang, D.S. , Liu, H.S. et al (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell, 18, 2999–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Pinot, F. , Sauveplane, V. et al (2010) Cytochrome P450 family member CYP704B2 catalyzes the {omega}‐hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell, 22, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Yuan, Z. , Vizcay‐Barrena, G. , Yang, C. , Liang, W. , Zong, J. , Wilson, Z.A. and Zhang, D. (2011a) PERSISTENT TAPETAL CELL1 encodes a PHD‐finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiol. 156, 615–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Gao, X. , Wei, Y. , Deng, L. , Ouyang, Y. , Chen, G. , Li, X. , Zhang, Q. and Wu, C. (2011b) Rice APOPTOSIS INHIBITOR5 coupled with two DEAD‐box adenosine 5′‐triphosphate‐dependent RNA helicases regulates tapetum degeneration. Plant Cell, 23, 1416–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, D. , Guo, Z. , Shi, Q. , Xiong, S. , Zhang, C. , Zhu, J. and Yang, Z. (2016) OsACOS12, an orthologue of Arabidopsis acyl‐CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol. 16, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. (2005) Genetic analysis and mapping of rice (Oryza sativa L.) male‐sterile (OsMS‐L) mutant. Chin. Sci. Bull. 50, 122. [Google Scholar]
- Liu, Z. , Bao, W. , Liang, W. , Yin, J. and Zhang, D. (2010) Identification of gamyb‐4 and analysis of the regulatory role of GAMYB in rice anther development. J. Integr. Plant Biol. 52, 670–678. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Lin, S. , Shi, J. , Yu, J. , Zhu, L. , Yang, X. , Zhang, D. and Liang, W. (2017) Rice No Pollen 1 (NP1) is required for anther cuticle formation and pollen exine patterning. Plant J. 91, 263–277. [DOI] [PubMed] [Google Scholar]
- Men, X. , Shi, J. , Liang, W. , Zhang, Q. , Lian, G. , Quan, S. , Zhu, L. , Luo, Z. , Chen, M. and Zhang, D. (2017) Glycerol‐3‐Phosphate Acyltransferase 3 (OsGPAT3) is required for anther development and male fertility in rice. J. Exp. Bot. 68, 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, J. , Guo, D. , Zhang, J. , Huang, Q. , Qin, G. , Zhang, X. , Wan, J. , Gu, H. and Qu, L.J. (2013) Targeted mutagenesis in rice using CRISPR‐Cas system. Cell Res. 23, 1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morant, M. , Jorgensen, K. , Schaller, H. , Pinot, F. , Moller, B.L. , Werck‐Reichhart, D. and Bak, S. (2007) CYP703 is an ancient cytochrome P450 in land plants catalyzing in‐chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell, 19, 1473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, B.X. , He, F.R. , He, M. , Ren, D. , Chen, L.T. and Liu, Y.G. (2013a) The ATP‐binding cassette transporter OsABCG15 is required for anther development and pollen fertility in rice. J. Integr. Plant Biol. 55, 710–720. [DOI] [PubMed] [Google Scholar]
- Niu, N. , Liang, W. , Yang, X. , Jin, W. , Wilson, Z.A. , Hu, J. and Zhang, D. (2013b) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat. Commun. 4, 1445. [DOI] [PubMed] [Google Scholar]
- Nonomura, K.I. , Miyoshi, K. , Eiguchi, M. , Suzuki, T. , Miyao, A. , Hirochika, H. and Kurata, N. (2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell, 1728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxson‐Sowders, D.M. , Dodrill, C.H. , Owen, H.A. and Makaroff, C.A. (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol. 127, 1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Piffanelli, P. , Ross, J.H.E. and Murphy, D.J. (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex. Plant Reprod. 11, 65–80. [Google Scholar]
- Reddy, T.V. , Kaur, J. , Agashe, B. , Sundaresan, V. and Siddiqi, I. (2003) The DUET gene is necessary for chromosome organization and progression during male meiosis in Arabidopsis and encodes a PHD finger protein. Development, 130, 5975–5987. [DOI] [PubMed] [Google Scholar]
- Schindler, U. , Beckmann, H. and Cashmore, A.R. (1993) HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine‐rich region. Plant J. 4, 137–150. [DOI] [PubMed] [Google Scholar]
- Shi, J. , Tan, H. , Yu, X.H. et al (2011) Defective pollen wall is required for anther and microspore development in rice and encodes a fatty acyl carrier protein reductase. Plant Cell, 23, 2225–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Cui, M. , Yang, L. , Kim, Y.J. and Zhang, D. (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 20, 741–753. [DOI] [PubMed] [Google Scholar]
- Souza, C.A. , Sookim, S. , Koch, S. , Kienow, L. and Schneider, K. (2009) A novel fatty Acyl‐CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell, 21, 507–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, L. , Shan, J.X. , Gao, J.P. and Lin, H.X. (2016) OsHAL3, a blue light‐responsive protein, interacts with the floral regulator Hd1 to activate flowering in rice. Mol Plant, 9, 233–244. [DOI] [PubMed] [Google Scholar]
- Sun, M.X. , Huang, X.Y. , Yang, J. , Guan, Y.F. and Yang, Z.N. (2013) Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod. 26, 83–91. [DOI] [PubMed] [Google Scholar]
- Wallace, S. , Chater, C.C. , Kamisugi, Y. , Cuming, A.C. , Wellman, C.H. , Beerling, D.J. and Fleming, A.J. (2015) Conservation of male sterility 2 function during spore and pollen wall development supports an evolutionarily early recruitment of a core component in the sporopollenin biosynthetic pathway. New Phytol. 205, 390–401. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Niu, B. , Huang, J. , Wang, H. , Yang, X. , Dong, A. , Makaroff, C. , Ma, H. and Wang, Y. (2016) The PHD Finger Protein MMD1/DUET ensures the progression of male meiotic chromosome condensation and directly regulates the expression of the condensin gene CAP‐D3. Plant Cell, 28, 1894–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON, Z. A. , Morroll, S.M. , DAWSON, J. et al (2001). The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD‐finger family of transcription factors. Plant J 28, 27–39. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Guan, Y. , Wu, Z. et al (2014) OsABCG15 encodes a membrane protein that plays an important role in anther cuticle and pollen exine formation in rice. Plant Cell Rep. 33, 1881–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W. , Zheng, X.M. , Chen, D. et al (2017) OsCOL16, encoding a CONSTANS‐like protein, represses flowering by up‐regulating Ghd7 expression in rice. Plant Sci. 260, 60–69. [DOI] [PubMed] [Google Scholar]
- Xu, D. , Shi, J. , Rautengarten, C. et al (2017) Defective pollen wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol. 173, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. (2003) The Arabidopsis MALE MEIOCYTE DEATH1 gene encodes a PHD‐Finger protein that is required for male meiosis. The Plant Cell Online, 15, 1281–1295. [PMC free article] [PubMed] [Google Scholar]
- Yang, C. , Vizcay‐Barrena, G. , Conner, K. and Wilson, Z.A. (2007) MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell, 19, 3530–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Wu, D. , Shi, J. et al (2014) Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J. Integr. Plant Biol. 56, 979–994. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Liang, W. , Chen, M. , Zhang, D. , Zhao, X. and Shi, J. (2017) Rice fatty acyl‐CoA synthetase OsACOS12 is required for tapetum programmed cell death and male fertility. Planta, 246, 105–122. [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Zhang, Y. , Sun, L. et al (2018) Identification of cyp703a3‐3 and analysis of regulatory role of CYP703A3 in rice anther cuticle and pollen exine development. Gene, 649, 63–73. [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Liu, L. , Sun, L. et al (2019) OsMS1 functions as a transcriptional activator to regulate programmed tapetum development and pollen exine formation in rice. Plant Mol. Biol. 99, 175–191. [DOI] [PubMed] [Google Scholar]
- Yi, J. , Moon, S. , Lee, Y.S. , Zhu, L. , Liang, W. , Zhang, D. , Jung, K.H. and An, G. (2016) Defective tapetum cell death 1 (DTC1) regulates ROS levels by binding to metallothionein during tapetum degeneration. Plant Physiol. 170, 1611–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Meng, Z. , Liang, W. et al (2016) A Rice Ca2+ binding protein is required for tapetum function and pollen formation. Plant Physiol. 172, 1772–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. and Wilson, Z.A. (2009) Stamen specification and anther development in rice. Chin. Sci. Bull. 54, 2342–2353. [Google Scholar]
- Zhang, D.S. , Liang, W.Q. , Yuan, Z. , Li, N. , Shi, J. , Wang, J. , Liu, Y.M. , Yu, W.J. and Zhang, D.B. (2008) Tapetum degeneration retardation is critical for aliphatic metabolism and gene regulation during rice pollen development. Mol. Plant, 1, 599–610. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Liang, W. , Yin, C. , Zong, J. , Gu, F. and Zhang, D. (2010) OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol. 154, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Luo, X. and Zhu, L. (2011) Cytological analysis and genetic control of rice anther development. J. Genet. Genome. 38, 379–390. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Wu, S. , An, X. et al (2017a) Construction of a multi‐control sterility system for a maize male‐sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD‐finger transcription factor. Plant Biotechnol. J. 16, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Zhang, Y. , Sun, L. et al (2017b) The rice AAA‐ATPase OsFIGNL1 is essential for male meiosis. Front Plant Sci. 8, 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Shen, Y. , Tang, D. , Shi, W. , Zhang, D. , Du, G. , Zhou, Y. , Liang, G. , Li, Y. and Cheng, Z. (2018) The zinc finger protein DCM1 is required for male meiotic cytokinesis by preserving callose in rice. PLoS Genet. 14, e1007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, G. , Shi, J. , Liang, W. , Xue, F. , Luo, Q. , Zhu, L. , Qu, G. , Chen, M. , Schreiber, L. and Zhang, D. (2015) Two ATP binding cassette G transporters, rice ATP binding cassette G26 and ATP binding cassette G15, collaboratively regulate rice male reproduction. Plant Physiol. 169, 2064–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. DAPI staining analysis of meiosis in wild‐type and the tip3 mutant.
Figure S2. Complementation analysis of the tip3 by wild‐type TIP3 genomic DNA.
Figure S3. Targeted mutagenesis in TIP3 gene using the CRISPR/Cas9 system.
Figure S4. The expression profile data of TIP3 from the Rice Expression Profile Database (RiceXPro) (http://ricexpro.dna.affrc.go.jp/).
Figure S5. The information of TIP3 from the transcriptomic database Rice eFP Browser.
Figure S6. The proposed model of TIP3 in tapetal PCD and pollen exine formation.
Table S1. Primers used in fine mapping.
Table S2. Primers used in this study for vectors construction.
Table S3. Primers used in this study for in situ hybridization and qPCR assay.


