Abstract
Aims
We evaluated the relationship between body mass index (BMI), including low BMI, and nocturia in Japanese women.
Methods
We collected data on 18 952 women who participated in a multiphasic health screening in Fukui, Japan, in 2006. The participants were asked to report any current or previous disease. Self‐reported current body weight and height were used to calculate the BMI. We analyzed the relationship between nocturia, as assessed by a questionnaire, and other variables including age, BMI, and comorbidities.
Results
The participants’ mean age was 60.6 years. Overall, the prevalence of nocturia (two or more voids/night) was 4.3% and increased in an age‐dependent manner. BMI did not affect nocturia in the young participants. The prevalence of nocturia was higher in the high‐BMI women (>25.0 kg/m 2) in their fifth and sixth decades, but the prevalence was higher in the low‐BMI (<18.5 kg/m 2) in the women more than 80‐years old. A multivariate analysis revealed a significant association between nocturia and the following: age, BMI, sleep disturbance, arteriosclerosis, cerebrovascular disease, chronic pulmonary disease, diabetes mellitus, and hypertension. Not only high BMI (which is already reported as a risk of nocturia) but also low BMI was a factor related to nocturia.
Conclusion
Our findings indicate that in addition to obesity, low BMI is a factor of nocturia in women.
Keywords: low BMI, nocturia, women
1. INTRODUCTION
An increased frequency of nocturnal voiding (ie, nocturia) is one of the most bothersome urinary tract symptoms affecting an individual's quality of life, particularly when two or more episodes occur per night.1, 2 Studies of the etiology of nocturia and its consequences have indicated that comorbidities such as cerebrovascular disease, chronic pulmonary disease, diabetes, and hypertension are risk factors for nocturia.3 Epidemiologic studies have shown that obesity is a risk factor for nocturia,4, 5, 6 but an investigation conducted in Korea indicated that body mass index (BMI) was not associated with an increased likelihood of nocturia in women.7 Differences in BMI distribution among study cohorts may explain this discrepancy, since low percentages of participants with a BMI less than 18.5 kg/m2, that is, the underweight (BMI <18.5 kg/m2) and non‐overweight (BMI, 18.5‐24.9 kg/m2) were combined in some of the previous studies. There is little information concerning the relationship between low BMI and nocturia. We conducted the present investigation to evaluate the relationship between BMI—including low BMI—and nocturia in Japanese women.
2. STUDY DESIGN, PARTICIPANTS, AND METHODS
We collected data on 18 952 women who participated in a multiphasic health screening in Fukui, Japan, in 2006. The participants were asked to report any current or previous disease. Self‐reported current body weight and height were used to calculate each participant's BMI (body weight in kg divided by the square of height in meters). We analyzed the relationship between nocturia (two or more voids/night) as assessed by a questionnaire, and other variables including age, BMI, hypertension, sleep disturbance, cardiovascular disease, arteriosclerosis, cerebrovascular disease, chronic pulmonary disease, chronic hepatic disease, chronic renal failure, and diabetes mellitus. All data from 18 952 women were analyzed. A logistic regression model was used for the statistical analysis. P < .05 was considered significant.
3. RESULTS
3.1. Population characteristics
The participants’ mean age was 60.6 years (18‐97 years, standard deviation [SD] 13.5). The mean BMI was 22.4 kg/m2 (13.5‐53.1 kg/m2, SD 3.1), and the percent distributions of BMI were 8.5% for less than 18.5 kg/m2 (underweight), 72.6% for 18.5 to 24.9 kg/m2 (non‐overweight), 11.0% for 25.0 to 27.0 kg/m2 (overweight), and 7.9% for more than 27.0 kg/m2 (obesity). The distributions of age and BMI in this study population are shown in Figure 1.
Figure 1.
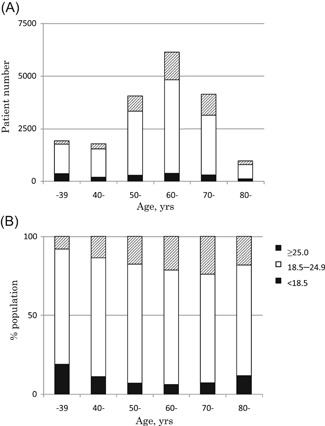
Distribution of age and body mass index (BMI) values in the study population of 18 952 Japanese women
The prevalence of the diseases that are thought to affect nocturia were 20.4% for hypertension, 13.0% for sleep disturbance, 5.4% for cardiovascular disease, 1.9% for cerebrovascular disease, 3.3% for chronic pulmonary disease, 3.3% for chronic hepatic disease, 15.3% for hyperlipidemia, 0.4% for chronic renal failure, and 3.9% for diabetes. Overall, the prevalence of nocturia was 4.3%.
3.2. Age and nocturia
The age‐specific overall prevalence of nocturia was 0.6% for the participants less than 39‐years old, 0.8% for those aged 40 to 49 years, 2.1% for those 50‐ to 59‐years old, 5.5% for the 60‐ to 69‐year old group, 6.7% for the participants aged 70 to 79 years, and 9.9% for those more than 80‐years old; the proportion of nocturia thus increased with advancing age (Figure 2). The prevalence of nocturia according to age and BMI is illustrated in Figure 3. BMI did not affect nocturia in the young population. Interestingly, while the prevalence of nocturia is higher in the high‐BMI women in their fifth and sixth decades of life, the prevalence in the participants with a low BMI (<18.5 kg/m2) catches up with the prevalence in the high‐BMI group in the seventh decade, and finally the prevalence is higher in the low‐BMI in the women more than 80‐years old.
Figure 2.
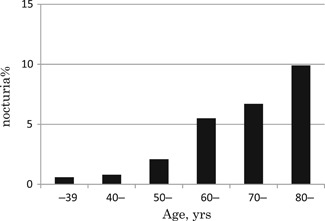
Prevalence of nocturia according to the participants’ age
Figure 3.
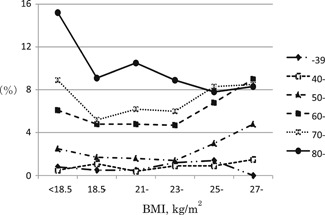
Odds ratio (OR) of nocturia according to BMI. BMI, body mass index
3.3. Factors of nocturia
As shown in Table 1, the multivariate analysis of factors for nocturia indicated a significant association between nocturia and the following: age (50‐59 years, odds ratio [OR] 2.5; 60‐69 years, OR, 6.0; 70‐79 years, OR, 7.9; and >80 years, OR, 13.2; reference group, women aged <39 years); BMI <18.5 (OR, 1.5; reference group, women with BMI 18.5‐24.9), BMI 25.0 to 26.9 (OR, 1.4), BMI >27.0 (OR, 1.8), sleep disturbance (OR, 6.0), arteriosclerosis (OR, 2.1), cerebrovascular disease (OR, 1.7), chronic pulmonary disease (OR, 1.7), diabetes (OR, 1.4), and hypertension (OR, 1.3).
Table 1.
Multivariate analysis of risk factors for nocturia (two or more voids/night) in Japanese women
| OR | 95% CI | P‐value | |
|---|---|---|---|
| Age, y | |||
| ≤39 | Ref. | ||
| 40‐44 | – | – | .839 |
| 45‐49 | – | – | .310 |
| 50‐54 | 2.9 | – | .059 |
| 55‐59 | 5.0 | 1.5‐5.3 | .001 |
| 60‐64 | 7.0 | 2.7‐9.2 | <.001 |
| 65‐69 | 8.4 | 3.8‐12.6 | <.001 |
| 70‐74 | 7.7 | 4.6‐15.3 | <.001 |
| 75‐79 | 13.3 | 4.2‐14.3 | <.001 |
| ≥80 | 7.2‐24.8 | <.001 | |
| BMI, kg/m2 | |||
| 18.5‐24.9 (normal) | Ref. | ||
| <18.5 (underweight) | 1.5 | 1.1‐1.9 | .005 |
| 25.0‐26.9 (overweight) | 1.4 | 1.1‐1.8 | .007 |
| ≥27.0 (obesity) | 1.8 | 1.4‐2.4 | <.001 |
| Sleep disturbance | 6.0 | 3.3‐10.8 | <.001 |
| Arteriosclerosis | 2.1 | 1.1‐4.1 | .029 |
| Chronic pulmonary disease | 1.7 | 1.3‐2.3 | .001 |
| Cerebrovascular disease | 1.7 | 1.2‐2.4 | .004 |
| Hypertension | 1.3 | 1.1‐1.6 | .002 |
| Diabetes mellitus | 1.4 | 1.0‐1.8 | .083 |
| Cardiovascular disease | – | – | .920 |
| Hepatic disease | – | – | .826 |
| Renal disease | – | – | .362 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Cardiovascular disease, liver disease, hyperlipidemia, renal dysfunction, and gynecological disease did not affect nocturia. There was also an excess risk of nocturia among the low‐BMI women in addition to those of a high BMI, showing a J‐shaped association between BMI and nocturia in Japanese women (Figure 4).
Figure 4.
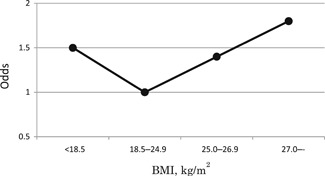
Odd ratios of nocturia according to BMI. BMI, body mass index; OR, odds ratio
4. DISCUSSION
Nocturia may cause sleep disorders, mood disturbances, a reduced quality of life, and distractibility. The reported prevalence of nocturia varies due in part to the definitions of nocturia used. The International Continence Society defined nocturia as the complaint of needing to wake at night one or more times to void.8 It has also been indicated that it is troublesome if an individual regularly experiences more than two voids during sleep hours.9 Schatzl et al10 noted that among women, only 12.3% with nocturia of 1×/night reported that it was “quite a problem” or “a serious problem” and this percentage increased to 39.4% (2×/night), 42.9% (3×/night), and 80.0% (≥4×/night).10 We defined nocturia as the complaint of ≥2 voids/night, to identify the nocturia patients whose quality of life was impaired. The shortage of this research is that the population selected might be not representative of all women. The overall prevalence of nocturia in our cohort is very low compared to a study from Asia (31.7% in Chinese inhabitants aged ≥40).11
We examined the prevalence and factors related to female nocturia in Japan. Our analyses revealed that age and sleep disturbance had the strongest impact on the prevalence of nocturia in the women, in addition to factors previously reported to be associated with nocturia, that is, cerebrovascular disease, chronic pulmonary disease, diabetes, hypertension, and high BMI, which is consistent with a previous report.3 Several studies indicated high BMI as a risk factor for nocturia.4, 5, 6 However, an analysis of the data of 1005 women in Korea showed that BMI was not associated with an increased likelihood of nocturia in the women.7
One explanation of this discrepancy in results concerning BMI is the differences in BMI distribution among countries and ethnicities. We did not make diagnosis of frailty in this study, but assumed that many of frailty may be low BMI patients. According to the 1998 National Nutrition Survey of Japan, the prevalence of persons with a BMI ≥30 kg/m2 among women aged ≥15 years was 3.6%.12 This prevalence is markedly lower than that in the United States (18.1% for women in 1998).13 Because of the relatively low percentage of individuals with a BMI less than 18.5 kg/m2, some studies combined their underweight (BMI <18.5 kg/m2) and non‐overweight (BMI 18.5‐24.9 kg/m2) groups. Therefore, the relationship between low BMI and nocturia could not be elucidated.
We were able to distinguish a low‐BMI group from normal and obese groups among Japanese women. Interestingly, our analyses revealed low BMI as a new independent related factor for female nocturia in addition to high BMI, resulting in a J‐shaped association between nocturia and BMI. Prevalence estimates vary due to the age range considered. Our findings indicate that BMI did not affect nocturia among the participants less than 50‐years old.
The mechanism underlying the relationship between low BMI and nocturia remains unknown, but it may be related to increased incidences of cognitive decline, dementia, obstructive sleep apnea, nocturnal polyuria, and/or polypharmacy, and to lifestyle changes or possible changes to the bladder itself that have yet to be identified. Low BMI is also associated with frailty in older people.14 Associations between frailty and lower urinary tract symptoms, including urinary incontinence,15 and over active bladder (OAB)16 were recently reported. Suskind et al16 showed that frailty, when adjusted for age, race, sex, and number of medications, was a significant predictor of OAB.
A particular subset of older individuals has added vulnerabilities such as pre‐existing illness, poor functional status, polypharmacy, cognitive deficits, and limited social support, placing them at a particular risk for frailty and associated adverse events. Frailty is a state of vulnerability that has been conceptualized as a biologic syndrome of decreased reserve or resistance to stressors, resulting in adverse outcomes such as disability, institutionalization, and mortality. Frailty is considered to be potentially reversible and therefore a target for care strategies to improve outcomes. Depending on the measurement used, frailty exists in anywhere from 36% to 88% of older individuals.17 It is reported that the prevalence of frailty in Japanese women was 2.1%, 3.8%, 10.1%, 22.3%, and 37.2% for those aged 65 to 69, 70 to 74, 75 to 79, 80 to 84, and ≥85 years, respectively.18 A single operational definition of frailty has yet to be defined, however. A theoretical cycle of frailty based on five criteria has been proposed: (a) slow walking speed, (b) weakness (defined as impaired grip strength), (c) low physical activity, (d) self‐reported exhaustion, and (e) unintended weight loss. Some (but not all) individuals with a low BMI may be patients with frailty.
To best of our knowledge, this is the first study to demonstrate that low BMI is an independent factor related to female nocturia. A large study conducted in Japan showed that low BMI in elderly people was associated with an increased risk of all‐cause mortality, and the results were essentially unchanged even when the analyses were conducted in the subjects who did not have cancer, cardiovascular disease, or stroke.19 Nocturia may be a marker of frailty. Patients with nocturia, especially the very elderly, should be monitored and treated to avoid or limit the effects of frailty such as malnutrition, falls, and the consequent accumulation of disabilities.
5. CONCLUSION
Our findings suggest that in addition to obesity, low BMI is an independent factor associated with nocturia in women. Because nocturia is associated with various factors, multiple approaches are required for the treatment of patients with nocturia. Nocturia may be a marker of frailty especially among the very elderly.
Ito H, Aoki Y, Oe H, Taga M, Tsuchiyama K, Yokoyama O. Low and high body mass index values are associated with female nocturia. Neurourology and Urodynamics. 2019;38:2250‐2254. 10.1002/nau.24126
References
REFERENCES
- 1. van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardisation of terminology in nocturia: report from the standardisation sub‐committee of the International Continence Society. Neurourol Urodyn. 2002;21:179‐183. [DOI] [PubMed] [Google Scholar]
- 2. Häkkinen JT, Hakama M, Shiri R, et al. Incidence of nocturia in 50 to 80‐year‐old Finnish men. J Urol. 2006;176:2541‐2545. [DOI] [PubMed] [Google Scholar]
- 3. Weiss JP, Blaivas JG. Nocturia. J Urol. 2000;163:5‐12. [PubMed] [Google Scholar]
- 4. Hsieh CH, Chen HY, Hsu CS, et al. Risk factors for nocturia in Taiwanese women aged 20–59 years. Taiwan J Obstet Gynecol. 2007;46:166‐170. [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald MP, Litman HJ, Link CL, McKinlay JB. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. 2007;177:1385‐1389. [DOI] [PubMed] [Google Scholar]
- 6. Vaughan CP, Auvinen A, Cartwright R, et al. Impact of obesity on urinary storage symptoms: Results from the FINNO study. J Urol. 2013;189:1377‐1382. [DOI] [PubMed] [Google Scholar]
- 7. Choo MS, Ku JH, Park CH, et al. Prevalence of nocturia in a Korean population aged 40 to 89 years. Neurourol Urodyn. 2008;27:60‐64. [DOI] [PubMed] [Google Scholar]
- 8. Abrams P, Cardozo L, Fall M, Griffiths D, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub‐committee of the International Continence Society. Neurourol Urodyn. 2002;21:167‐178. [DOI] [PubMed] [Google Scholar]
- 9. Weiss JP. Nocturia: “Do the Math. J Urol. 2006;175:S16‐S18. [DOI] [PubMed] [Google Scholar]
- 10. Schatzl G, Temml C, Schmidbauer J, et al. Cross‐sectional study of nocturia in both sexes: analysis of a voluntary health screening project. Urology. 2000;56:71‐75. [DOI] [PubMed] [Google Scholar]
- 11. Wen L, Wen YB, Wang ZM, et al. Risk factors of nocturia (two or more voids per night) in Chinese people older than 40 years: risk factors of nocturia in China. Neurourol Urodyn. 2015;34:566‐570. [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Health and Welfare. (Kokumin Eiyo no Genjou) Results of National Nutrition Survey, 1998. Daiichi Shuppan: Tokyo; 2000. [in Japanese].
- 13. Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991‐1998. JAMA. 1999;282:1519‐1522. [DOI] [PubMed] [Google Scholar]
- 14. Pessanha FP1, Lustosa LP, Carneiro JA, et al. Body mass index and its relationship with disability, chronic diseases and frailty in older people: a comparison of the Lipschitz and WHO classifications. J Frailty Aging. 2017;6(1):24‐28. 10.14283/jfa.2016.113 [DOI] [PubMed] [Google Scholar]
- 15. Wagg A, Gibson W, Ostaszkiewicz J, et al. Urinary incontinence in frail elderly persons: report from the 5th International Consultation on Incontinence: urinary incontinence in frail elderly persons. Neurourol Urodyn. 2015;34:398‐406. [DOI] [PubMed] [Google Scholar]
- 16. Suskind AM, Quanstrom K, Zhao S, et al. Overactive bladder is strongly associated with frailty in older individuals. Urology. 2017;106:26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Iersel MB, Rikkert MG. Frailty criteria give heterogenous results when applied in clinical practice. J Am Geriatr Soc. 2006;54:728‐729. [DOI] [PubMed] [Google Scholar]
- 18. Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: a systematic review and meta‐analysis. J Epidemiol. 2017;27:347‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamakoshi A, Yatsuya H, Lin Y, et al. JACC Study Group . BMI and all‐cause mortality among Japanese older adults: findings from the Japan collaborative cohort study. Obesity. 2010;18:362‐369. [DOI] [PubMed] [Google Scholar]


