Abstract
The availability of new potent systemic therapies for urothelial carcinoma may change the way we use standard chemotherapy perioperatively. In particular, identifying which patients with muscle-invasive bladder cancer (MIBC) would benefit from adjuvant chemotherapy (AC) is compelling. From a multicenter database we selected 950 patients with cT2–4N0M0 MIBC treated with radical cystectomy (RC), with or without neoadjuvant chemotherapy (NAC), and AC. We used Kaplan-Meier analyses to test 1-yr recurrence-free survival (RFS) rates according to AC use. Nomogram-derived probabilities of 1-yr recurrence after RC were plotted against actual recurrence rates according to AC use. Overall, we did not see evidence of an AC effect on the 1-yr RFS rate (p = 0.6). Conversely, the 1-yr RFS rate was higher among patients with pT3–4 or pN1 disease who received AC (75% vs 54%; p < 0.001). We were unable to demonstrate a difference between AC and no AC among patients who received prior NAC (1-yr RFS 57% vs 76%; p = 0.057). As the most important finding, AC was associated with incremental RFS benefits only for patients with a nomogram-derived 1-yr recurrence probability of >40%.
Patient summary:
Maximizing disease control with adjuvant chemotherapy was beneficial for patients with muscle-invasive bladder cancer who had a calculated recurrence risk of >40% and did not impact cancer recurrence in lower-risk disease. Therefore, patient stratification using the nomogram available for predicting recurrence is advisable pending external validation.
Keywords: Immunotherapy, Muscle-invasive bladder cancer, Adjuvant chemotherapy, Nomogram, Recurrence-free survival
Neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) represents the standard treatment for muscle-invasive bladder cancer (MIBC) [1]. Besides the established platinum-based regimens, immune checkpoint inhibitors have gained momentum after proof that they are active and safe in locally advanced and metastatic urothelial carcinoma [2], with several clinical trials now testing these agents in the perioperative setting [3]. While we await the results of ongoing adjuvant immunotherapy (IO) trials, there is still debate regarding the criteria to use to recommend adjuvant chemotherapy (AC), especially after NAC. For example, the EORTC 30994 trial, which did not achieve full accrual, failed to show a significant overall survival (OS) benefit for adjuvant versus deferred chemotherapy, but did show prolonged progression-free survival [4]. There are even fewer prospective data on an AC benefit for patients treated with NAC who show high-risk residual disease at RC, although at least one retrospective analysis suggests a benefit in the time to recurrence and the risk of relapse with AC, especially for ≥pT4 or node-positive disease [5]. Therefore, refinement of the selection criteria for AC administration through individualized risk estimation would optimize treatment for patients and decrease unnecessary toxicity due to ineffective chemotherapy exposure, and would be of value as a framework for interpreting results from the ongoing adjuvant IO trials. We recently developed a tool for estimating 1-yr recurrence-free survival (RFS) after RC based on clinical and pathological factors [6]. The data come from the San Raffaele Hospital (Milan, Italy) institutional database (n = 1067) and the Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC) database (n = 3024). We postulated that only patients with an elevated nomogram-predicted risk of 1-yr recurrence might be suitable candidates for AC. Therefore, the aim of our analyses was to identify which patients benefit the most from AC according to their postoperative risk of recurrence. We examined the AC effect among MIBC patients treated with RC, with and without perioperative chemotherapy, from the same databases. These findings obtained for patients who received standard AC may help clinicians to contextualize the results of the next adjuvant and neoadjuvant IO studies.
Overall, 950 patients with cT2–4N0M0 MIBC who received RC, with or without NAC and/or AC, were selected (Table 1). We used Kaplan-Meier analyses to estimate 1-yr RFS rates according to AC use for the overall population, subgroups of patients with pT3–4 or pN1 disease, and NAC-treated patients. A probability of 1-yr recurrence after RC was calculated according to a predefined nomogram [6] that accounted for surgical margin status, pathological tumor and nodal stage, and previous NAC administration. We tested the interaction between AC and 1-yr RFS derived from the aforementioned nomogram. Finally, we used a 2000-bootstrapped nonparametric curve-fitting method to graphically explore the relationship between the nomogram-derived risk of recurrence and the actual recurrence rate, with corresponding 95% confidence intervals (CIs). Our aim was to establish a nomogram-derived threshold to identify patients who would benefit from AC.
Table 1 –
General characteristics for patients in the overall cohort and for patients with a predicted probability of 1-yr recurrence of 20% according to our nomogram
| Paramter | Statistics | Overall population (n = 950) | Patients with predicted probability of 1-yr recurrence of >20% (n = 585) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | No NAC or AC (n = 545) | NAC (n = 242) | AC (n = 146) | NAC+AC (n = 17) | Overall | No NAC or AC (n = 346) | NAC (n = 90) | AC (n = 135) | NAC + AC (n =14) | ||
| Age at surgery (yr) | Median | 68 | 71 | 64 | 65 | 62 | 68 | 71 | 64 | 64 | 61.5 |
| IQR | 60–74 | 63–77 | 57–70 | 56–70 | 55–68 | 61–75 | 63–78 | 60–70 | 56–70 | 55–67 | |
| LNs removed (n) | Median | 15 | 15 | 16 | 16 | 20.5 | 16 | 16 | 16 | 16 | 22 |
| IQR | 9–22 | 9–22 | 10–22 | 10–240 | 14–27 | 10–24 | 10–23 | 9–22 | 10–24 | 15–25 | |
| Positive LNs (n) | Median | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 1 | 3 |
| IQR | 0–1 | 0–1 | 0–0 | 0–3 | 0–4.5 | 0–2 | 0–2 | 0–2 | 0–3 | 2–6 | |
| Race, n (%) | Caucasian | 850 (90) | 479 (88) | 229 (95) | 128 (88) | 14 (82) | 518 (89) | 307 (89) | 80 (89) | 119 (88) | 12 (86) |
| Hispanic or Latino | 69 (7) | 51 (9) | 1 (0.4) | 16 (11) | 1 (6) | 42 (7) | 26 (7) | 1 (1) | 14 (10) | 1 (7) | |
| Black | 22 (2) | 9 (2) | 9 (3.6) | 2 (1) | 2 (12) | 20 (3) | 9 (3) | 8 (9) | 2 (2) | 1 (7) | |
| Other or mixed | 9 (1) | 6 (1) | 3 (1) | 0 (0) | 0 (0) | 5 (1) | 4 (1) | 1 (1) | 0 (0) | 0 (0) | |
| Smoking status, n (%) | Never smoker | 192 (20) | 111 (20) | 55 (23) | 22 (15) | 4 (24) | 121 (21) | 76 (22) | 19 (21) | 22 (16) | 4 (29) |
| Current smoker | 215 (23) | 110 (20) | 62 (25) | 39 (27) | 4 (24) | 124 (21) | 68 (20) | 15 (17) | 38 (28) | 3 (21) | |
| Former smoker | 351 (37) | 179 (33) | 108 (45) | 57 (39) | 7 (41) | 220 (38) | 113 (33) | 50 (55) | 51 (38) | 6 (43) | |
| Unknown | 192 (20) | 145 (27) | 17 (7) | 28 (19) | 2 (11) | 120(20) | 89 (25) | 6 (7) | 24 (18) | 1 (7) | |
| CCI, n (%) | 0 | 288 (30) | 133 (25) | 100 (41) | 47 (32) | 8 (47) | 173 (29) | 85 (24) | 36 (40) | 45 (33) | 7 (50) |
| ≥1 | 567 (60) | 377 (69) | 89 (37) | 92 (63) | 9 (53) | 366 (63) | 241 (70) | 35 (39) | 83 (62) | 7 (50) | |
| Unknown | 95 (10) | 35 (6) | 53 (22) | 7 (5) | 0 (0) | 46 (8) | 20 (6) | 19 (21) | 7 (6) | 0 (0) | |
| Histology, n (%) | Pure UC | 826 (87) | 474 (87) | 206 (85) | 129 (88) | 17 (100) | 504 (86) | 292 (84) | 78 (87) | 120 (89) | 14 (100) |
| UC with DH | 124 (13) | 71 (13) | 36 (15) | 17 (12) | 0 (0) | 81 (14) | 54 (16) | 12 (13) | 15(11) | 0 (0) | |
| cT stage, n (%) | cT2 | 567 (60) | 319 (59) | 145 (60) | 91 (62) | 12 (70) | 332 (57) | 188 (54) | 48 (53) | 86 (64) | 10 (72) |
| cT3–4 | 223 (23) | 100 (18) | 91 (38) | 29 (20) | 3 (18) | 151 (26) | 80 (23) | 40 (45) | 28 (21) | 3 (2) | |
| Unknown | 160 (17) | 126 (23) | 6 (2) | 26 (18) | 2 (12) | 102 (17) | 78 (23) | 2 (2) | 21 (15) | 1 (7) | |
| pT stage, n (%) | pT0 | 96 (10) | 17 (3) | 79 (33) | 0 (0) | 0 (0) | |||||
| pT1 | 63 (7) | 43 (8) | 16 (7) | 3 (2) | 1 (6) | 3 (1) | 2 (1) | 0 (0) | 1 (1) | 0(0) | |
| pT2 | 189 (20) | 121 (22) | 42 (17) | 21 (14) | 5 (29) | 38 (6) | 17 (5) | 5 (6) | 13 (10) | 3(21) | |
| pT3 | 389 (41) | 232 (43) | 65 (27) | 84 (58) | 8 (47) | 389 (66) | 232 (67) | 65 (72) | 84 (62) | 8 (58) | |
| pT4 | 154 (16) | 94 (17) | 20 (8) | 37 (25) | 3 (18) | 154 (26) | 94 (27) | 20 (22) | 37 (27) | 3(21) | |
| pTa/pTis | 59 (6) | 38 (7) | 20 (8) | 1 (1) | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | |
| pN stage, n (%) | pN0 | 644 (68) | 379 (70) | 197 (81) | 61 (42) | 7 (41) | 298 (51) | 191 (55) | 50 (56) | 53 (39) | 4 (29) |
| pN1 | 102 (10) | 50 (9) | 20 (8) | 31 (21) | 1 (6) | 94 (16) | 47 (14) | 17 (19) | 29 (22) | 1 (7) | |
| pN2 | 160 (17) | 89 (16) | 21 (9) | 43 (30) | 7 (41) | 158 (27) | 88 (25) | 20 (22) | 43 (32) | 7 (50) | |
| pN3 | 16 (2) | 7 (1) | 1 (0.5) | 6 (4) | 2 (12) | 16 (3) | 7 (2) | 1 (1) | 6 (4) | 2 (14) | |
| pNX | 28 (3) | 20 (4) | 3 (1.5) | 5 (3) | 0 (0) | 19 (3) | 13 (4) | 2 (2) | 4 (3) | 0 (0) | |
| pTpN, n (%) | pT0–2pN0-X | 382 (40) | 207 (38) | 154 (64) | 16 (11) | 5 (29) | 25 (4) | 11 (3) | 5 (6) | 7 (5) | 2 (14) |
| pT3–4 or pN1 | 568 (60) | 338 (62) | 88 (36) | 130 (89) | 12 (71) | 560 (96) | 335 (97) | 85 (94) | 128 (95) | 12 (86) | |
| Margins status, n (%) | Negative | 866 (91) | 497 (91) | 222 (92) | 134 (92) | 13 (77) | 503 (86) | 300 (87) | 70 (78) | 123 (91) | 10 (71) |
| Positive | 84 (9) | 48 (9) | 20 (8) | 12 (8) | 4 (23) | 82 (14) | 46 (13) | 20 (22) | 12 (9) | 4 (29) | |
| NAC, n (%) | No | 691 (73) | 545 (100) | 0 (0) | 146 (100) | 0 (0) | 481 (82) | 346 (100) | 0 (0) | 135 (100) | 0 (0) |
| Yes | 259 (27) | 0 (0) | 242 (100) | 0 (0) | 17 (100) | 104(18) | 0 (0) | 90 (100) | 0 (0) | 14(100) | |
| NAC platinum regimen, n (%) | Carboplatin | 22 (2) | 0 (0) | 20 (8) | 0 (0) | 2 (12) | 15 (3) | 0 (0) | 13 (15) | 0 (0) | 2 (14) |
| Cisplatin | 214 (23) | 0 (0) | 203 (84) | 0 (0) | 11 (65) | 76 (13) | 0 (0) | 66 (73) | 0 (0) | 10 (72) | |
| No NAC | 691 (73) | 545 (100) | 0 (0) | 146 (100) | 0 (0) | 481 (82) | 346 (100) | 0 (0) | 135 (100) | 0 (0) | |
| Unknown regimen | 23 (2) | 0 (0) | 19 (8) | 0 (0) | 4 (23) | 13 (2) | 0 (0) | 11 (12) | 0 (0) | 2 (14) | |
| AC, n (%) | No | 787 (83) | 545 (100) | 242 (100) | 0 (0) | 0 (0) | 436 (75) | 346 (100) | 90 (100) | 0 (0) | 0 (0) |
| Yes | 163 (17) | 0 (0) | 0 (0) | 146 (100) | 17 (100) | 149 (25) | 0 (0) | 0 (0) | 135 (100) | 14 (100) | |
| AC platinum regimen, n (%) | Carboplatin | 20 (2) | 0 (0) | 0 (0) | 18 (12) | 2 (12) | 19 (3) | 0 (0) | 0 (0) | 17 (13) | 2 (14) |
| Cisplatin | 83 (9) | 0 (0) | 0 (0) | 73 (50) | 10 (59) | 79 (13) | 0 (0) | 0 (0) | 70 (52) | 9 (64) | |
| No AC | 787 (83) | 545 (100) | 242 (100) | 0 (0) | 0 (0) | 436 (75) | 346 (100) | 90 (100) | 0 (0) | 0 (0) | |
| Unknown regimen | 60 (6) | 0 (0) | 0 (0) | 55 (38) | 5 (29) | 51 (9) | 0 (0) | 0 (0) | 48 (35) | 3 (22) | |
AC = adjuvant chemotherapy; CCI = Charlson comorbidity index; DH = divergent histology; IQR: interquartile range; LN = lymph node; NAC = neoadjuvant chemotherapy; UC = urothelial carcinoma.
Within our sample, 259 patients (27%) received NAC, whereas 163 patients (17%) received AC. In both settings, cisplatin-based regimens were most commonly administered (83% in NAC, 51% in AC). We did not see evidence of an AC effect (75% vs 71%; p = 0.6) on 1-yr RFS rates in the overall patient population (rate difference 4%, 95% CI −5% to +13%; Supplementary Fig. 1). Considering NAC-treated patients only, we were unable to demonstrate a difference in 1-yr RFS between AC and no AC (57% vs 76%; rate difference 19%, 95% CI −50% to +12%; p = 0.057; Supplementary Fig. 2), as previously reported [5]. Then we stratified patients according to pathological stage at RC as pT0–2N0 (n = 382; 40%) versus pT3–4 or pN1 (n = 568; 60%). The 1-yr RFS rate was higher (rate difference 21%, 95% CI 10–32%; p < 0.001) among patients with pT3–4 or pN1 disease who received AC (75%) than among those who did not (54%). Conversely, we did not see evidence of an AC benefit (p = 0.07) among patients with pT0–2N0-X disease (AC vs no AC: 76% vs 89%; rate difference 13%, 95% CI −35% to +9%; Supplementary Figs. 3 and 4).
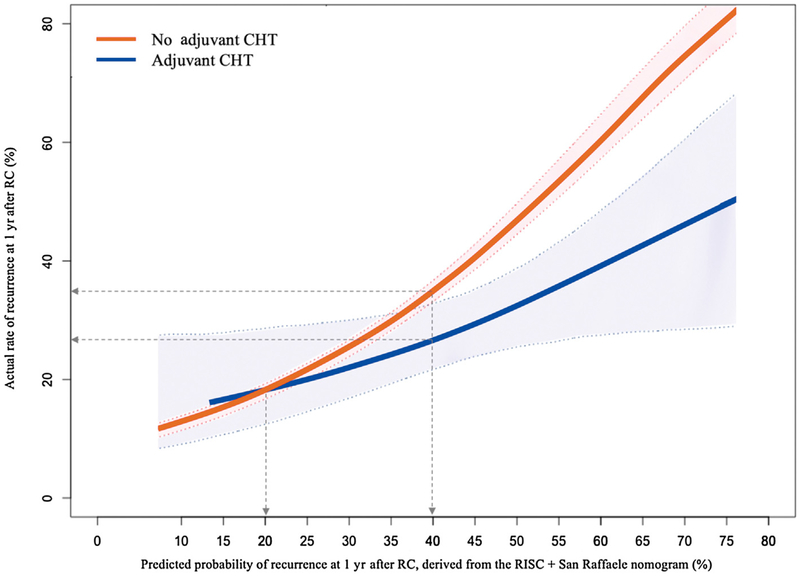
The interaction test for the hypothesis that the impact of AC on the 1-yr recurrence rate will vary according to the 1-yr recurrence risk derived from our nomogram was statistically significant (p = 0.048). Observed 1-yr recurrence rates were plotted against the predicted probability of 1-yr recurrence according to AC administration. Fig.1 shows that AC was associated with an RFS benefit only for patients with a calculated risk of 1-yr recurrence of >20%. Specifically, the recurrence risk decreased by 5% for predicted 1-yr recurrence of 35%, and by 10% for predicted risk of 43%. In fact, a statistically significant and clinically meaningful difference in the 1-yr recurrence rate was observed from the 40% cutoff onwards, for which a separation of the CI boundaries was observed: the 1-yr recurrence risk was 26% (95% CI 21–32%) with AC and 35% (95% CI 33–36%) without AC, corresponding to a net decrease in 1-yr relapse risk of 9%.
Fig. 1 –
Actual rate of cancer recurrence at 1 yr after radical cystectomy (RC) plotted against the nomogram-predicted probability of recurrence at 1 yr after RC. Confidence intervals were generated from a 2000-repetition bootstrap of the logistic models. CHT = chemotherapy.
The possibility of quantifying the individual relapse risk and the AC benefit based on nomogram using readily available clinical parameters is appealing. Here, we observed that maximizing disease control with AC started to be beneficial for patients with a calculated risk of 1-yr recurrence of >20%, but the AC effect was most clinically meaningful for patients with a risk of >40% (eg, patients with positive surgical margins, high pathological stage, and a suboptimal response to NAC). These features, and the corresponding relapse risk, may not necessarily apply to different adjuvant therapeutic strategies such as checkpoint blockade. Therefore, future attempts should identify different cutoffs linked to different survival benefits from adjuvant therapies (eg, chemotherapy vs immunotherapy).
From a biological perspective, AC might have maximal activity in treatment-naive patients, with no cytotoxic selective pressure that might eventually enrich the residual tumor in chemoresistant cancer cells [7,8]. Nevertheless, addressing this hypothesis in clinical practice is not trivial owing to the lack of widely accessible personalized genomic tools to predict the response to chemotherapy in vivo and because the majority of patients now included in adjuvant checkpoint inhibitor trials have disease that has previously progressed through NAC. Until such genomic tests are available to routinely identify optimal AC candidates, use of the 40% nomogram-derived threshold for AC represents a more accurate and reliable benchmark than single clinical and pathological factors (e.g., evidence of lymph node involvement [9]).
Limitations of this study, beside the retrospective nature of our analyses, include the lack of external validation of our model and our threshold probabilities, the heterogeneity of eligibility criteria for NAC/AC and of administered regimens, and the lack of data on cisplatin-based regimen eligibility. Considering the ongoing investigation of IO therapeutics in perioperative settings, another limitation could be that high pathologic tumor and/or nodal stage after checkpoint inhibition might not necessarily be associated with similar recurrence risk features, as indicated in the literature. Lastly, like all retrospective studies that compare patients offered or not offered an intervention (such as AC), selection bias skews the findings towards a benefit of the intervention. This typically does not bear out in randomized trials, as in the case of EORTC 30994 [4,10].
In conclusion, pending validation with data from prior randomized trials, our proposed 40% nomogram-predicted 1-yr recurrence risk could guide clinicians in stratifying patients for AC therapy. If validated, this finding could also help to contextualize results from the next adjuvant and neoadjuvant studies with targeted therapy or immunotherapy, especially if no OS benefit is obtained. In future studies we aim to compare the observed 1-yr RFS estimates with those predicted by our nomogram among patients receiving various perioperative therapeutic sequences within clinical trials, such as the sequential neoadjuvant immunotherapy and AC published in the pivotal PURE-01 trial [3]. These analyses may eventually allow us to test the survival improvement and the biological effects of different multimodal systemic therapies and to further risk-stratify patients to optimize their care.
Supplementary Material
Acknowledgments:
The authors thank Dr. Michele Marchioni, Dr. Andrea Forconi, and Dr. Zhe Tian for their insightful comments.
Footnotes
Please visit www.eu-acme.org/europeanurology to answer questions on-line. The EU-ACME credits will then be attributed automatically.
Financial disclosures: Filippo Pederzoli certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Lauren C. Harshman has received consulting fees from Exelixis Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Corvus, Merck, Novartis, Michael J. Hennessy Associates (a healthcare communications company for several brands such as OncLive and PER), Jounce, and EMD Serono; research funding from Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valient, Janssen, Medivation/Astellas, Genentech, Pfizer, and Endocyte (Novartis); and support for travel from Bayer. The remaining authors have nothing to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eururo.2019.06.032.
References
- [1].Witjes JA, Bruins M, Compérat E, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer. Arnhem, The Netherlands: European Association of Urology; 2018. [Google Scholar]
- [2].Powles T, Necchi A, Rosen G, Hariharan S, Apolo AB. Anti-programmed cell death 1/ligand 1 (PD-1/PD-L1) antibodies for the treatment of urothelial carcinoma: state of the art and future development. Clin Genitourin Cancer 2018;16:117–29. 10.1016/j.clgc.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018;36:3353–60. 10.1200/JCO.18.01148. [DOI] [PubMed] [Google Scholar]
- [4].Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86. 10.1016/S1470-2045(14)71160-X. [DOI] [PubMed] [Google Scholar]
- [5].Chanza NM, Werner L, Plimack E, et al. Incidence, patterns, and outcomes with adjuvant chemotherapy for residual disease after neoadjuvant chemotherapy in muscle-invasive urinary tract cancers. Eur Urol Oncol. In press. 10.1016/j.euo.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bandini M, Briganti A, Plimack ER, et al. Modeling 1-year relapse-free survival after neoadjuvant chemotherapy and radical cystectomy in patients with clinical T2–4N0M0 urothelial bladder carcinoma: endpoints for phase 2 trials. Eur Urol Oncol 2019;2:248–56. 10.1016/j.euo.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer 2006;6:924–35. 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- [8].Pietzak EJ, Zabor EC, Bagrodia A, et al. Genomic differences between “primary” and “secondary” muscle-invasive bladder cancer as a basis for disparate outcomes to cisplatin-based neoadjuvant chemotherapy. Eur Urol 2019;75:231–9. 10.1016/j.eururo.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bandini M, Fossati N, Briganti A. Nomograms in urologic oncology, advantages and disadvantages. Curr Opin Urol 2019;29:42–51. 10.1097/MOU.0000000000000541. [DOI] [PubMed] [Google Scholar]
- [10].Soni PD, Hartman HE, Dess RT, et al. Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol 2019;37:1209–16. 10.1200/JCO.18.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.