Abstract
Aims
To evaluate efficacy of Saccharomyces cerevisiae fermentate prebiotic (EH) in protection of intestinal barrier integrity in rats during heat stress, to analyze the impact of heat stress and preventive treatment with EH on the structure of the gut microbiota.
Methods and Results
Two groups of rats were treated orally with EH or phosphate‐buffered saline for 14 days. On day 15, half of the rats in each group were exposed to heat stress conditions, while control animals were kept at room temperature. Histological and Western blot analyses of the intestine, culture‐based microbiological analysis and high‐throughput 16S rRNA sequencing for the gut microbiota were performed for each rat. Exposure of animals to heat stress conditions resulted in inhibition of tight junction (TJ) proteins expression, decrease of Paneth and goblet cells, decrease of beneficial and increase of pathogenic bacteria. Oral treatment of rats with EH before stress significantly prevents these adverse effects by elevation of the gut beneficial bacteria, particularly butyrate‐producing bacteria.
Conclusions
Essential effect of EH in protection of intestinal barrier integrity during heat stress is connected with beneficial modulation of the gut microbiota.
Significance and Impact of the Study
Our results will contribute to the development of new approaches to prevention of heat stress‐related complications.
Keywords: gut microbiota, heat stress, rats, Saccharomyces cerevisiae fermentate, tight junction proteins
Introduction
Heat stress, as other types of stress, seriously impacts gastrointestinal physiology, which result in intestinal ulceration, development of irritable bowel syndrome and inflammatory bowel disease (Soderholm and Perdue, 2001; Yu et al., 2010). It was shown that stress significantly affects intestinal barrier function resulted in gut permeability and systemic inflammation (Lambert, 2009). One of the mechanisms connecting stress and gastrointestinal diseases is stress‐induced effects on mucosal barrier function (Konturek et al., 2011). Intestinal barrier function is the ability to control uptake across the mucosa and to protect the inner environment from potentially harmful compounds present in the intestinal lumen. This barrier is achieved by the intracellular junctional complexes: tight junctions (TJ), adherens junctions, gap junctions and desmosomes (Suzuki, 2013). The TJ are the apical‐most junctional complex, responsible for sealing the intercellular space. They act as a primary barrier to the diffusion of solutes through the intercellular space. The main types of transmembrane proteins in TJ are occludin and claudins, which link adjacent enterocytes (Ohland and MacNaughton, 2010). Zonula occludens (ZO) proteins are important intracellular TJ proteins that link the transmembrane TJ proteins: claudins, occludin and junctional adhesion molecules (JAM) to the actomyosin cytoskeleton (Grootjans et al., 2010). Disruption of the intestinal TJ barrier, induces activation of the mucosal immune system and inflammation, and can act as a trigger for the development of intestinal and systemic diseases (Suzuki, 2013). Various factors may cause destabilization of TJ proteins: enteric pathogens and their toxins, anti‐inflammatory drugs, alcohol (Groschwitz and Hogan, 2009). Heat stress was shown to disrupt intestinal barrier function (Hall et al., 2001) and to change the expression of TJ proteins (Xiao et al., 2013). Usually, the effect of heat stress on TJ proteins is assessed in vitro in epithelial cell monolayers. Recently, Pearce et al. (2013) showed changes in the TJ proteins composition in pigs, exposed to heat stress, but authors did not propose approaches to prevent/reduce this adverse effect of heat stress. It was found that Paneth and goblet cells are critical for maintenance of intestinal barrier (Vaishnava et al., 2008; Bevins and Salzman, 2011; Johansson and Hansson, 2016). Goblet cells are responsible for production of mucins, forming the basic skeleton of mucus layer, which serves as a first line of innate defence. Paneth cells produce different antimicrobial compounds essential for control intestinal barrier and limit bacterial penetration to host tissues. Keeping the integrity of the intestinal barrier is a key for intestinal homeostasis and overall for the health status of the host. It was shown that microbiota and its metabolites can regulate the gut barrier function (Kelly et al., 2015; Jakobsson et al., 2015). Exposure to various types of stress results in significant changes in the composition of the gut microbiota and associated complications (Bailey et al., 2011). Prebiotics and probiotics have been proposed as a promising approach to normalize microbiota and, as a result to improve intestinal barrier function (Russo et al., 2012; Wilms et al., 2016). Our previous study showed that fermentate of Saccharomyces cerevisiae was very effective in prevention of heat stress‐related complications in rats (traumatic changes of the gut morphology, elevation of serum lipopolysaccharides, pathology of erythrocytes) (Ducray et al., 2016). These beneficial effects of yeast fermantate are due to prebiotic activity of this product, previously confirmed in vitro (Possemiers et al., 2013) and in clinical trials (Pinheiro et al., 2017). We hypothesize that EH can protect the gut microbiota and improve intestinal barrier function during heat stress conditions, thus preventing adverse effects of heat. The main objectives of this study were to evaluate efficacy of EH in protection of intestinal barrier integrity during heat stress, to analyze the impact of heat stress and preventive treatment with EH on the structure of the gut microbiota.
Materials and methods
Ethics statement
All animal procedures were approved by the Auburn University Institutional Animal Care and Use Committee (protocol number 2016‐2853). The study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Animals
Adult male Sprague–Dawley rats weighing 250–300 g were purchased from Harlan Laboratories (Indianapolis, IN). Animals were housed under specific pathogen free conditions with a 12‐h light/dark cycle at (20 ± 1)°C, and were provided with standard food (2018 Teklad Global 18% Protein Rodent Diet; Harlan) and water ad libitum.
Saccharomyces cerevisiae fermantate
The powder form of S. cerevisiae fermentate (EH) was provided by the manufacturer (Embria Health Sciences, Ankeny, IA). EH is rich in yeast cell fragments and various metabolites, including polyphenols, polysaccharides such as beta glucan, trace minerals, amino acids and peptides (Pinheiro et al., 2017). Before oral treatment of rats yeast fermentate was diluted in phosphate‐buffered saline (PBS) at the rate 7 mg kg−1 of animal weight in 1 ml of PBS.
Antibodies
Primary rabbit polyclonal antibodies against zonula occludence (ZO‐1) (#40‐2200), occludin (#40‐4700), mouse anti‐claudin‐1‐monoclonal antibody (#37‐4900) and beta‐Actin Loading Control antibody (# MA5‐15739) were from ThermoFisher Scientific (Waltham, MA), rabbit polyclonal antibodies against JAM‐A (#ab125886) were from Abcam (Cambridge, MA). IRDye 800CW goat anti‐rabbit (#926‐32211) and IRDye 800CW goat anti‐mouse (#926‐32210) secondary antibody were from LiCor (Lincoln, NE).
Experimental design
Animal model of heat stress was successfully used in our previous study (Ducray et al., 2016). Briefly, two groups of male Sprague–Dawley rats weighing 250–300 g (16 rats in each group) were treated by oral gavage with 1 ml of yeast fermentate prebiotic (EH group) or with 1 ml of PBS (PBS group) once a day for 14 days (Fig1). On day 15, rats in each group were subdivided (eight rats in each group): PC—control (PBS/room temperature), EC—control prebiotic (EH/room temperature), PS—PBS + stress (PBS/45°C) and ES—prebiotic + stress (EH/45°C). Animals from group PS and ES were exposed for 25 min to heat stress conditions (45°C, relative humidity 55%) in a climatic chamber (Environmental Chamber 6020‐1; Caron, Marietta, OH). Control animals (groups PC and EC) were kept at room temperature. Rectal temperature was measured in each rat before and immediately after the experiment. Animals were allowed to stand 4 h at room temperature after the experiment, because it was showed that maximal effect of stress on epithelial function was 4 h after exposure to stress conditions (Soderholm et al., 2002; Zareie et al., 2006). Four hours after the stress experiments, rats were anesthetized with isoflurane (2–4%) and euthanized by rapid decapitation. Samples of small intestine from each rat were taken for morphological analysis and Western blot. Faecal matter from the colon was immediately placed in anaerobic broth for culture‐based microbiological analysis. For 16S rRNA sequencing of the gut microbiota faecal samples were placed at −80°C until the experiment.
Figure 1.
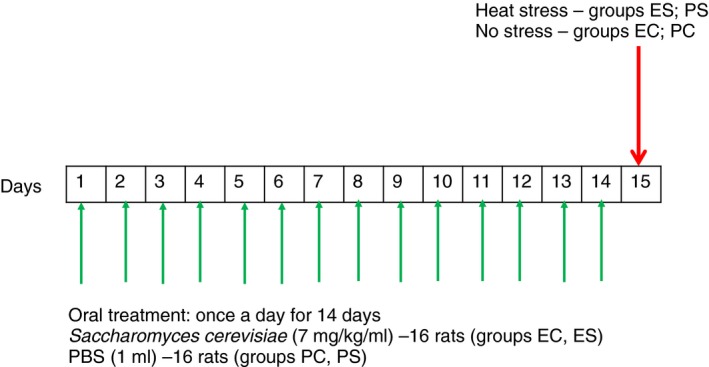
Experimental design. Two groups of rats (16 rats in each group) were treated by oral gavage with 1 ml of Saccharomyces cerevisiae fermentate prebiotic (EH) or with 1 ml of PBS once a day for 14 days (Fig. 1). On day 15, rats in each group were subdivided (eight rats in each group): PC—control (PBS/room temperature), EC—control prebiotic (EH/room temperature), PS—PBS + stress (PBS/45°C) and ES—prebiotic + stress (EH/45°C). Animals from group PS and ES were exposed for 25 min to heat stress conditions (45°C, relative humidity 55%) in a climatic chamber. Control animals (groups PC and PS) were kept at room temperature. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Histological analysis
Samples of the small intestine were prepared as it was previously described (Ducray et al., 2016). Briefly, samples were fixed in Bouin’s fixative (Electron Microscopy Sciences, Hatfield, PA) embedded in paraffin, sectioned at 6 μm, slide mounted, haematoxylin and eosin stained, and cover‐slipped. Haematoxylin‐eosin staining was performed according to the standard protocol (Stevens, 1990).
Counting of goblet cells
Four sections from each rat were stained as previously described (Trevizan et al., 2016). Briefly, sections were subjected to a series of deparaffinization, stained with Alcian Blue (Electron Microscopy Sciences) for 30 min, washed with tap and distilled water, treated with 0·5% periodic acid (Electron Microscopy Sciences), washed with distilled water for 2 min, stained with Schiff’s Reagent (Electron Microscopy Sciences) for 20 min, washed with tap water for 5 min, stained with haematoxylin (1 min), washed with tap water for 2 min, dehydrated, cleared in HemoDi (Fisher Scientific, Pittsburgh, PA) and mounted in Eukitt Mounting Medium (Electron Microscopy Sciences). Eight images from each section were taken with a digital camera (Pro series 3CCD camera) coupled to an optical microscope (Olympus BX50, Microscope Central, Feasterville, PA) with a 20x objective. The number of goblet cells presented in 0·96 mm2 in the mucosa of each animal were quantified using ImagePro Plus software (Media Cybernetics, Rockville, MD).
Paneth cells counting
Phloxine‐tartrazine technique were used to analyze Paneth cells, as previously reported (Di Sabatino et al., 2008). Briefly, sections were treated with alum haematoxylin (5 min), washed with tap water (5 min), stains in phloxine B‐ calcium carbonate (Electron Microscopy Sciences) for 20 min, rinsed in tap water, blot dried, stained saturated solution of tartrazine saturated cellosolve (Electron Microscopy Sciences) for 10 min, rinsed in 95% alcohol, dehydrated in absolute alcohol, cleared in HemoDi (Fisher Scientific) and mounted in Eukitt Mounting Medium (Electron Microscopy Sciences). The amount of Paneth cells were counted for each sample using a high resolution microscope system (Vainrub et al., 2006). Four sections from each rat were analyzed.
SDS‐PAGE and Western Blotting
Intestinal tissues were snap‐frozen in liquid nitrogen and kept at −80°C until study. Tissues were homogenized using T‐PER Reagent with Protease Inhibitor Cocktail (Thermo Scientific, Rockford, IL). Samples were centrifuged at 15 000 g for 30 min at 4°C and supernatants were collected. A protein assay (Bio‐Rad, Hercules, CA) was conducted to determine the protein concentration for each sample. An equal amount of proteins (50 µg) were separated by SDS–PAGE (10%) and transferred to nitrocellulose membranes. The membranes were blocked for 1 h in Odyssey blocking buffer (LiCor) and incubated overnight at 4°C with primary antibodies against β‐actin, claudin, occludin, ZO‐1 or JAM‐A proteins. The membranes were washed with PBS/0·1% Tween‐20 three times and incubated with goat anti‐rabbits IRDye 800CW secondary antibodies for 1 h, then washed with PBS/0·1% Tween‐20 four times. Membranes were imaged by LiCor Odyssey scanner, and blots were analyzed by Image Studio 2.0 analytical software (LiCor). The procedure was repeated at least four times for each protein. Bands were standardized to the density of actin and were represented as a ratio of each protein to actin.
Analysis of the gut microbiota
Culture‐based microbiological study
Determination of the gut microbiota was performed according to methods described previously (Sudo et al., 2004; Nishino et al., 2013). Faecal matter was removed from the colon of each rat using sterile technique, placed in sterile preweighted tubes with anaerobic broth, weighted and vortexed until homogenous. Serial 10‐fold dilutions from 10−1 to 10−7 were prepared and from the appropriate dilution, a 0·1 ml aliquot was then spread on four plates with different media: Anaerobic Basal Agar (Alfa Aesar, Tewksbury, MA) for total anaerobic bacteria; Brain Heart Infusion Agar (Hardy Diagnostic, Santa Maria, CA) for total aerobes; Blood agar (Hardy Diagnostic) for haemolytic bacteria; Violet Red Bile Agar (Hardy Diagnostic) for Enterobacteriaceae ; Bifidobacterium agar (HiMedia Laboratories, West Chester, PA) for Bifidobacterium; Difco Lactobacilli MRS agar (Becton Dickinson, Sparks, MD) for Lactobacillus; BBL Mannitol Salt agar (Becton Dickinson) for Staphylococcus; Brucella agar with hemin and vitamin K1 (HiMedia Laboratories) for Bacteroides; Reinforced Clostridial Medium (Hardy Diagnostic, Santa Maria, CA) for Clostridium; Sabouraud agar (HiMedia Laboratories) for yeasts. For isolation of anaerobic bacteria plates were placed in an anaerobic chamber in a microaerophilic environment generated by a GasPak EZ Anaerobe Container System (Becton Dickinson and Co). All plates were incubated at 37°C and colonies were counted after incubation for 24 h for aerobes and 48 h for anaerobes. The number of colony‐forming units per gram of faecal matter was calculated. Bacterial cultures and yeasts were identified by morphology of colonies, microscopical analysis of cells’ morphology, Gram staining, formation of spores, aerobic and anaerobic growth, as it was recommended elsewhere (Benno and Mitsuoka, 1992; Sudo et al., 2004).
High‐throughput 16S rRNA sequencing for the gut microbiota
Faecal samples were submitted to MR DNA (Shallowater, TX) for DNA isolation and sequencing. Genomic DNA was isolated from samples using a QIAamp DNA stool mini kit (Qiagen, Germantown, MD) following the manufacturer's instructions. The purified DNA was eluted from the spin filter using 50 μl of solution C6 and stored at −20°C until PCR amplification.
Amplicon sequencing using next generation technology (bTEFAP) was originally described by Dowd et al. (2008). The 16s rRNA V1‐V3 primers, 27F AGRGTTTGATCMTGGCTCAG and 519R GTNTTACNGCGGCKGCTG, were utilized to evaluate the microbial ecology of each sample on the MiSeq with methods via the bTEFAP DNA analysis service. Each sample underwent a single‐step 30 cycle PCR using HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA) were used under the following conditions: 94°C for 3 min, followed by 28 cycles of 94°C for 30 s; 53°C for 40 s and 72°C for 1 min; after which a final elongation step at 72°C for 5 min was performed. Following PCR, all amplicon products from different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, MA). Samples were sequenced utilizing the Illumina MiSeq chemistry following manufacturer’s protocols. The Q25 sequence data derived from the sequencing process was processed using a proprietary analysis pipeline (MR DNA, Shallowater, TX). Sequences were depleted of barcodes and primers then short sequences <200 bp were removed, sequences with ambiguous base calls removed, and sequences with homopolymer runs exceeding 6bp removed. Sequences were then denoised and chimeras removed. Operational taxonomic units (OTUs) were defined after removal of singleton sequences, clustering at 3% divergence (97% similarity). OTUs were then taxonomically classified using blastn against a curated NCBI database.
Bioinformatics analysis
Statistical analysis of sequence results was performed using a variety of computer packages including XLstat, NCSS 2007, ‘R’ and NCSS 2010. Significance reported for any analysis is defined as P < 0·05.
Statistical analysis
All results were presented as a mean and standard deviation. The difference between groups was analyzed by the one‐way anova, followed by the Bonferroni test (Baurhoo et al., 2007; Possemiers et al., 2013). The significance level was set at 0·05 to define statistical significance. Statistical calculations and graph plotting were carried out using Microcal™ Origin ver. 9.0 (Northhampton, MA) and 2010 Microsoft Excel.
Results
Body temperature
Body temperature of rats, exposed to heat stress conditions (PS and ES groups) significantly increased. The mean body temperature was 37·55 ± 0·16°C before and 40·98 ± 0·43°C immediately after stress (P < 0·05) in PS group and 37·66 ± 0·73°C before and 40·50 ± 0·60°C after (P < 0·05) in ES group. No change in body temperature of control rats, not exposed to stress (PC and EC groups) was found.
Tight junction proteins expression
Expression of TJ proteins (occludin, claudin, ZO‐1 and JAM‐A) in the intestine of all rats was analyzed by Western blot. Expression of all tested proteins was significantly depressed in animals from PS group in comparison with other groups (P < 0·05) (Fig2). Pretreatment with EH before exposure to heat stress (group ES) resulted in significantly increased level of all proteins in comparison with PS group (P < 0·05), though lower in comparison with EC group.
Figure 2.
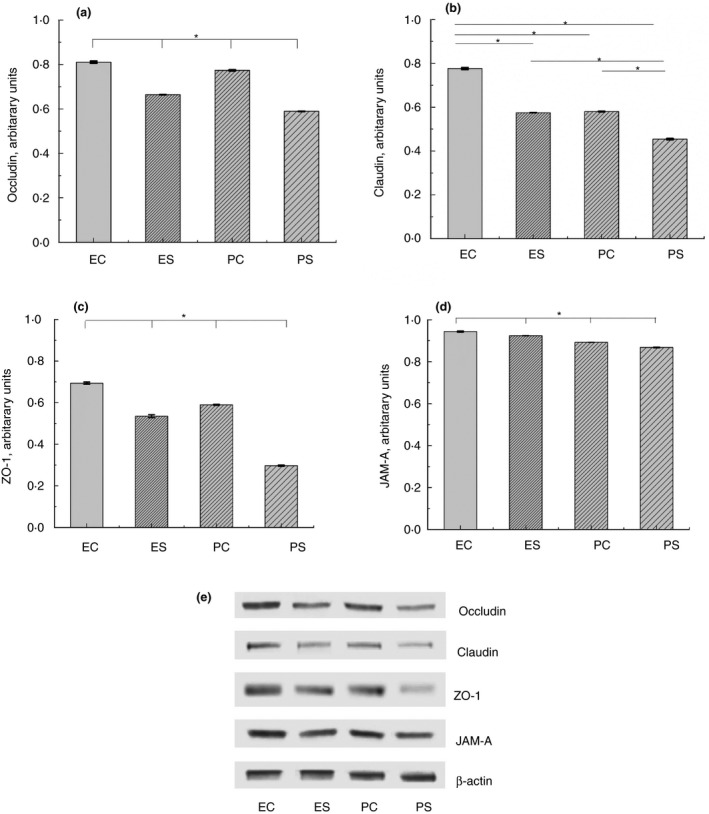
Expression of TJ proteins in the intestine of rats from different experimental groups: ES—rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC—rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; PS—rats were orally gavaged with PBS and exposed to heat stress; PC—rats were orally gavaged with PBS and kept at room temperature; *P < 0·05.
Paneth cells number
The number of Paneth cells in rats, exposed to heat stress (groups PS and ES), was significantly lower in comparison with control groups (PC and EC). Supplementation of rats with EH before heat stress (ES group) prevented the loss of Paneth cells in comparison with rats, pretreated with PBS (PS group) (1·61 ± 0·07 and 1·12 ± 0·07 accordingly, P < 0·05) (Fig. 3a,b).
Figure 3.
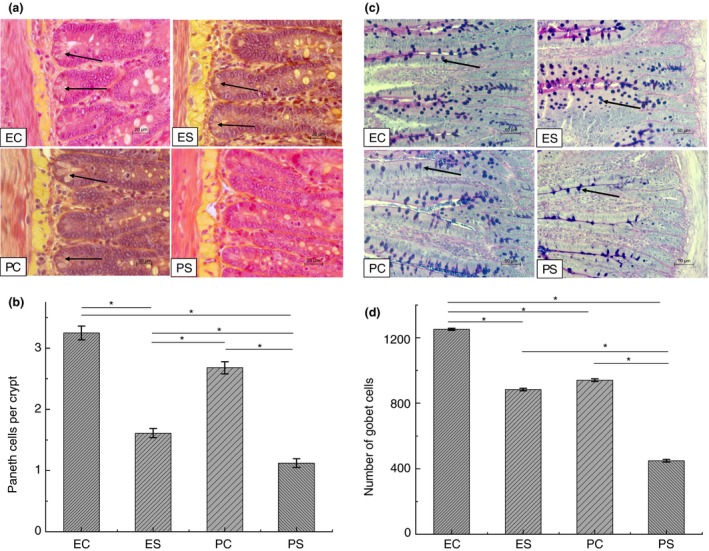
Paneth and goblet cells in the intestine of rats from different experimental groups. (a) Histological samples of the small intestine were stained with phloxine‐tartrazine to analyze Paneth cells, scale bar = 20 µm; arrows show Paneth cells; (b) number of Paneth cells, *P < 0·05; (c) Alcian blue staining of goblet cells in histological samples of the small intestine, scale bar = 50 µm; Arrows show goblet cells; (d) Goblet cells number, *P < 0·05. ES—rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC—rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; PS—rats were orally gavaged with PBS and exposed to heat stress; PC—rats were orally gavaged with PBS and kept at room temperature. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Number of goblet cells
The number of goblet cells was significantly decreased in rats from PS group in comparison with control rats (group PC) (448·8 ± 8·4 and 940·8 ± 8·4 accordingly, P < 0·05). Goblet cell count in intestine of heat stressed rats pretreated with EH (group ES) was lower than in nonstressed rats from EC group (883·8 ± 7·8 and 1251 ± 6·6 accordingly, P < 0·05), but significantly higher than in animals pretreated with PBS before exposure to heat stress (PS group) (Fig. 3c,d). Treatment of control rats with EH (EC group) resulted in significant elevation of goblet cells in comparison with control PC group.
Culture‐based analysis of the gut microbiota
Analysis of the gut microbial community in rats from different experimental groups revealed significant decrease of anaerobic to aerobic bacteria ratio in rats from PS group in comparison with all other groups. No difference in this ratio was found in rats, treated with EH (Fig. 4a). Significant elevation of Escherichia spp. (Fig. 4b), haemolytic bacteria (Fig. 4c) and Staphylococcus spp. (Fig. 4d) was found in rats from PS group. Number of Staphylococcus spp. and haemolytic bacteria was significantly higher in animals from ES group in comparison with PC group, but significantly lower than in animals from PS group. No difference in Bifidobacterium spp. number was observed in groups of animals, pretreated with PBS (PC, PS), but treatment with EH resulted in significant elevation of these bacteria (groups ES, EC) (Fig. 4e). The highest number of Lactobacullus spp. was revealed in rats pretreated with PBS before exposure to heat stress conditions (Fig4f). Treatment with EH did not affect Lactobacillus spp. number.
Figure 4.
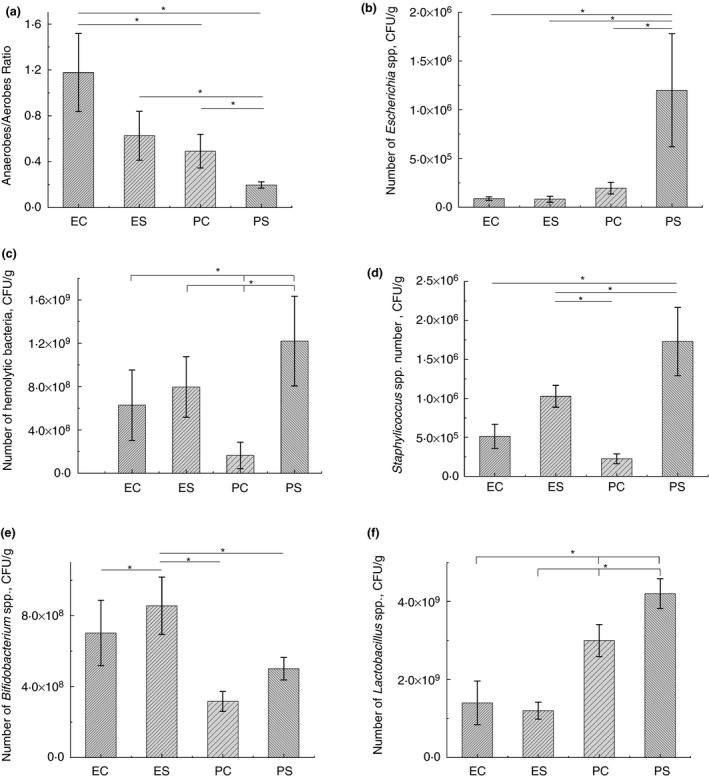
Analysis of the gut microbiota of rats by a culture‐based method. ES—rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC—rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; PS—rats were orally gavaged with PBS and exposed to heat stress; PC—rats were orally gavaged with PBS and kept at room temperature; *P < 0·05.
16S rRNA sequencing of the gut microbiota
After stringent quality sequence curation, a total of 1 565 513 sequences were parsed and 1 382 946 were then clustered. 1 382 796 sequences identified within the Bacteria and Archaea domains were utilized for final microbiota analyses. The average reads per sample was 60 121. Ten different phyla were identified. The most abundant phyla in the gut microbiota of rats from different experimental groups were Firmicutes, Bacteroidetes and Actinobacteria (Fig. 5). Firmicutes was a dominant phylum (68·3%) followed by Bacteroidetes (23·6%) and Actinobacteria (5·5%). Significantly higher number of Actinobacteria was found in PC group (11·3 ± 1·8%, P < 0·05), Bacteroidetes were prevalent in PS group (29·7 ± 4·8%, P < 0·05).
Figure 5.
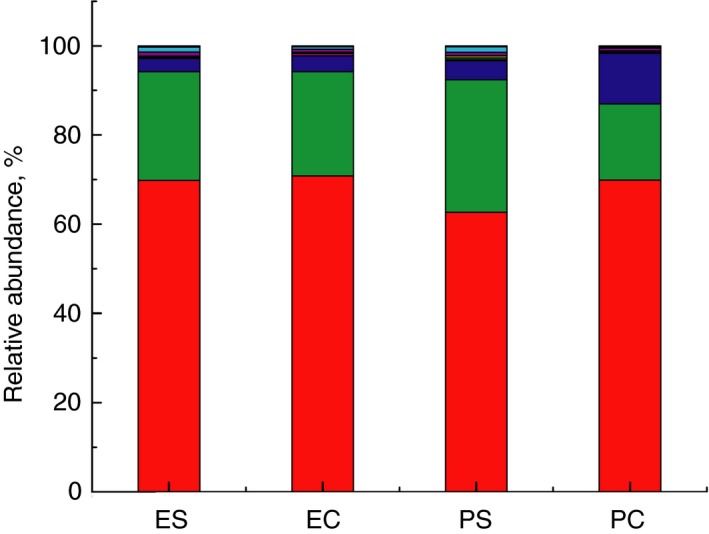
Composition of the gut microbiota of rats from different experimental groups at the phylum level. All phyla present in abundance of <0·1% are included as other. ES—rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC—rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; PS—rats were orally gavaged with PBS and exposed to heat stress; PC—rats were orally gavaged with PBS and kept at room temperature (( ) other; (
) other; ( ) Verrucomicrobia; (
) Verrucomicrobia; ( ) Tenericutes; (
) Tenericutes; ( ) TM‐7; (
) TM‐7; ( ) Deferribacteres; (
) Deferribacteres; ( ) Proteobacteria; (
) Proteobacteria; ( ) Actinobacteria; (
) Actinobacteria; ( ) Bacteriodetes; (
) Bacteriodetes; ( ) Firmicutes). [Colour figure can be viewed at http://wileyonlinelibrary.com]
) Firmicutes). [Colour figure can be viewed at http://wileyonlinelibrary.com]
At the genus level the most significant changes were found in PS group in comparison with control PC group (Fig. 6; Table 1). Totally 14 genera were affected by heat stress. Some genera considerably increased (Acetanaerobacterium, Akkermansia, Allistipes, Allobaculum, Bacteroides, Johnsonella, Oscillibacter, Staphylococcus, Tannerella), whereas others (Bifidobacterium, Enterorhabdus, Holdemanella, Pedobacter) significantly decreased. Bilophila was absent in rats from PC group, but detected in PS rats. Treatment of rats with yeast fermentate before exposure to heat stress (ES group) resulted in less changes of gut microbiota. Only nine genera were significantly changed: relative abundance of Bifidobacterium and Allobaculum were declined, while Acetanaerobacterium, Bacteroides, Eubacterium, Johnsonella, Lactococcus, Oscillospira, Roseburia and Vallitalea, substantially increased. Akkermansia and Staphylococcus were significantly higher only in rats from PS group in comparison with animals from PC group. Minor changes in the gut microbiota were found in EC group of rats in comparison with PC group—only Bifidobacterium significantly decreased.
Figure 6.
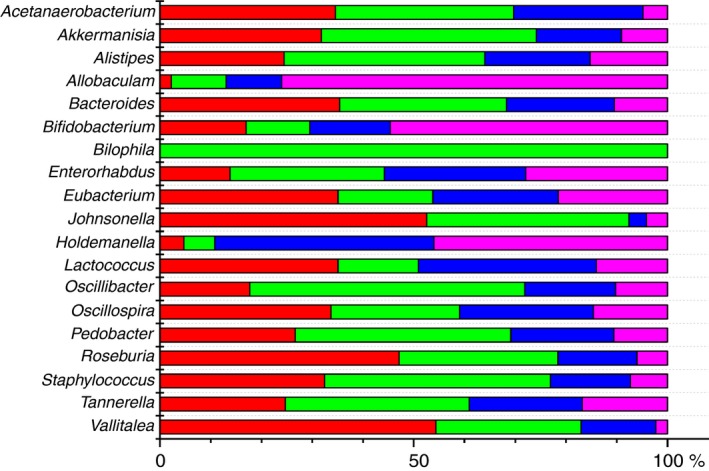
Microbial composition of the gut microbiota in different groups is presented as a per cent of abundance. ( ) ES—rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; (
) ES—rats were orally gavaged with Saccharomyces cerevisiae fermentate and exposed to heat stress; ( ) EC—rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; (
) EC—rats were orally gavaged with Saccharomyces cerevisiae fermentate and kept at room temperature; ( ) PS—rats were orally gavaged with PBS and exposed to heat stress; (
) PS—rats were orally gavaged with PBS and exposed to heat stress; ( ) PC—rats were orally gavaged with PBS and kept at room temperature. [Colour figure can be viewed at http://wileyonlinelibrary.com]
) PC—rats were orally gavaged with PBS and kept at room temperature. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Changes in the gut microbiota genera after different treatments
| Genera | PC | PS | PS vs PC changes, % | P value | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Acetanaerobacterium | 0·0035 | 0·0009 | 0·0252 | 0·0061 | 619·4603 | 0·0053 |
| Akkermansia | 0·3169 | 0·1184 | 1·4911 | 0·4477 | 370·1025 | 0·0219 |
| Alistipes | 2·8117 | 0·5532 | 7·2977 | 1·5431 | 159·7039 | 0·0183 |
| Allobaculum | 4·7639 | 1·2169 | 0·5713 | 0·4029 | −88·0252 | 0·0113 |
| Bacteroides | 0·8846 | 0·1283 | 2·7718 | 0·5332 | 214·7727 | 0·0063 |
| Bifidobacterium | 10·6204 | 0·6910 | 2·4397 | 0·8783 | −77·0245 | 0·0015 |
| Bilophila | 0 | 0 | 0·0016 | 0·0007 | PS* | |
| Enterorhabdus | 0·0537 | 0·0102 | 0·0265 | 0·0034 | −50·5617 | 0·0302 |
| Johnsonella | 0·0007 | 0·0003 | 0·0081 | 0·0036 | 995·8904 | 0·0404 |
| Holdemanella | 3·5545 | 0·8240 | 0·4755 | 0·2157 | −86·6236 | 0·0047 |
| Oscillibacter | 0·0071 | 0·0026 | 0·0352 | 0·0108 | 402·4449 | 0·0274 |
| Pedobacter | 0·0623 | 0·0021 | 0·0241 | 0·0052 | −59·7735 | 0·0094 |
| Tannerella | 0·0932 | 0·0178 | 0·2137 | 0·0243 | 137·5029 | 0·0034 |
| Staphylococcus | 0·3586 | 0·0809 | 2·2036 | 0·7185 | 514·4869 | 0·0341 |
| Genera | PC | ES | ES vs PC Changes, % | P value | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Acetanaerobacterium | 0·0035 | 0·0009 | 0·0248 | 0·0081 | 615·9931 | 0·0258 |
| Allobaculum | 4·7639 | 1·2169 | 0·2186 | 0·0691 | −95·4110 | 0·0257 |
| Bacteroides | 0·8846 | 0·1283 | 2·9801 | 0·4812 | 236·8742 | 0·0018 |
| Bifidobacterium | 10·6204 | 0·6910 | 3·2922 | 1·7735 | −69·0207 | 0·0026 |
| Eubacterium | 4·0526 | 0·5370 | 6·5942 | 0·6038 | 62·7175 | 0·0104 |
| Johnsonella | 0·0007 | 0·0003 | 0·0092 | 0·0014 | 1156·9830 | 0·0001 |
| Lactococcus | 0·0050 | 0·0016 | 0·1045 | 0·0415 | 214·1153 | 0·0437 |
| Oscillospira | 0·9808 | 0·1301 | 2·2579 | 0·3157 | 130·2043 | 0·0038 |
| Roseburia | 0·0268 | 0·0049 | 0·2091 | 0·0471 | 679·2159 | 0·0032 |
| Vallitalea | 0·0005 | 0·0003 | 0·0114 | 0·0038 | 2231·2421 | 0·0169 |
| Genera | PC | EC | EC vs PC Changes, % | P value | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Bifidobacterium | 10·6204 | 0·6910 | 3·0878 | 0·9778 | −70·9260 | 0·0001 |
PC—rats were pretreated with PBS and kept at room temperature; PS—rats were pretreated with PBS and exposed to heat stress; ES—rats were pretreated with Saccharomyces cerevisiae fermentate and exposed to heat stress; EC—rats were pretreated with Saccharomyces cerevisiae fermentate and kept at room temperature.
Genus was found only in this group.
Discussion
This study aimed to evaluate the efficacy of the S. cerevisiae fermentate in protection of the intestinal barrier function and in modulating the gut microbiota during heat stress. Exposure of rats, pretreated with PBS, to heat stress conditions resulted in significant decrease of occludin, claudin, ZO‐1 and JAM‐A expression. Decreased expression of TJ proteins during heat stress was found in Caco‐2 cells (Gupta et al., 2017) and in animal studies (Wu et al., 2018). Inhibition of these proteins expression indicates the disturbance of the TJ barrier functions and accompanied by intestinal permeability (He et al., 2016). Our results showed that oral administration of S. cerevisiae fermentate to rats before heat stress significantly enhanced TJ proteins expression. In previous studies, this fermentate demonstrated prebiotic activity by protection against inflammation (Possemiers et al., 2013) and improvement of gastrointestinal discomfort in patients (Pinheiro et al., 2017). The findings of other authors revealed a positive role of prebiotics in supporting of normal intestinal barrier function. Thus, the dietary use of inulin‐enriched pasta by healthy volunteers protected intestinal barrier functioning during physical exercise (Russo et al., 2012). Cani et al. (2009) found that oligofructose‐enriched diet contributed to the improvement of gut barrier function in obese mice by up‐regulation of TJ proteins expression.
We observed that heat stress resulted in significant decrease of Paneth and goblet cells in the intestine of rats. Paneth and goblet cells are essential components of the intestinal epithelium and contribute to the barrier function of epithelium (Furness et al., 2013). Depletion of these cells may lead to the development of an epithelial barrier defect (Estienne et al., 2010). Reduction of Paneth and goblet cells was shown to increase sensitivity of mice to TNF‐induced toxicity, accompanied by increased hypothermia, lethality and intestinal permeability (Van Hauwermeiren et al., 2015). Decrease of these cells was induced by different stress conditions, such as neonatal maternal separation (Bessette et al., 2016), chronic and heat stress (Deng et al., 2012; Gao et al., 2018). Our results revealed that pretreatment of rats with S. cerevisiae fermentate before exposure to heat stress prevented decline of Paneth and goblet cells. Beneficial effect of cell wall from S. cerevisiae as a dietary supplement for stabilization of goblet cells in chickens was demonstrated by Baurhoo et al. (2009). Prebiotic inulin in combination with rutin reduced inflammatory status and endoplasmic reticulum stress in Paneth cells (Guo et al., 2018).
Paneth and goblet cells are indispensable for maintaining homeostasis with enteric microbes (Baurhoo et al., 2007; Vaishnava et al., 2008) as they promote the removal of microbes from the mucosal surface (Chairatana and Nolan, 2017). Reduction in number or defects in activity of these cells lead to microbiota disbiosis (Baurhoo et al., 2007; Riba et al., 2017). Our data showed significant changes in the gut microbiota only in rats from PS group with substantial depletion of Paneth and goblet cells. Thus, culture‐based bacteriological analysis of the gut microbiota revealed decrease of anaerobic to aerobic bacteria ratio in these animals. It is well known that most microorganisms in the distal small intestine and colon are anaerobes (Weng and Walker, 2013), which numerously exceed aerobic bacteria in the gut (Maity et al., 2012). The predominance of aerobic bacteria in the gut microbiota has been found in the patients with colon cancer (Vargo et al., 1980), necrotizing fasciitis (Saini et al., 2004), in malnutrition (Million et al., 2016) and in severely burned patients (Chen et al., 1998) indicating an imbalance of the intestinal microbiota. We also found significant increase of haemolytic bacteria, Escherichia spp. and Staphylococcus spp. in rats of PS group. Elevated number of bacteria with haemolytic activity indicates the microbiota disorder (Popova et al., 2017) as these bacteria can be a potentiator of intestinal inflammation and epithelial dysfunction in the gut (Wiegand et al., 2017). Imbalance in quantitative composition of Escherichia spp. and Staphylococcus spp. also specifies dysbiotic changes of the gut microbiota (Popova et al., 2017; Itani et al., 2018). The number of Lactobacillus spp. was significantly higher in rats of PS group in comparison with other groups of animals. The effect of stress on lactobacilli in the gut is estimated differently by researchers. Some of them observed an increase of Lactobacillus spp. during chronic stress (Wong et al., 2016), while others reported about depleting of these bacteria in stressed animals (Marin et al., 2017). Treatment with EH did not change the relative abundance of Lactobacillus spp. The same result was obtained with EH in clinical trial (Pinheiro et al., 2017). We did not find the difference in Bifidobacterium spp. number in groups of rats pretreated with PBS (PS and PC groups). But administration of EH significantly increased the number of bifidobacteria. Positive effect of EH on Bifidobacterium was previously observed in vitro study (Possemiers et al., 2013). Stimulation of bifidobacteria in the gut of elderly people by prebiotic supplementation was found in clinical trials (Guigoz et al., 2002).
High‐throughput 16S rRNA gene sequencing revealed that in all groups of rats Firmicutes was a dominant phylum that is in accordance with the data of other authors (Golubeva et al., 2015; Byerley et al., 2017). Significant changes of the gut microbiota in different groups were found at the genus taxonomic level. Exposure of rats to heat stress conditions (PS group) resulted in substantial decrease of beneficial bacteria (Allobaculum, Bifidobacterium) in comparison with control (PC) group. Beneficial effects of these bacteria were shown in many studies. Thus, Allobaculum was associated with prevention of obesity and insulin resistance (Everard et al., 2014), Bifidobacterium are known as a normal component of the gut microbiota and as probiotics for human and animal consumption (Russell et al., 2011). Enterorhabdus and Pedobacter were also decreased in PS group of rats. Enterorhabdus was shown to be associated with autism spectrum disorder in a murine model (de Theije et al., 2014) and with a genetic variant of the human leukocyte antigen complex that has been related to inflammatory diseases (Opstelten et al., 2016). Pedobacter, heparinase‐produsing bacteria, are a normal component of the gut microbiota of healthy fish (Wang et al., 2018a) and the medicinal leech (Ott et al., 2015). Significant increase of pathogenic bacteria (Alistipes, Bacteroides, Bilophila, Johnsonella, Oscillibacter, Tannerella and Staphylococcus) was found in PS group. This result corresponds to our data from the culture‐based analysis of the microbiota, testifying that elevation of pathogenic bacteria was observed only in rats from PS group. Alistipes, Bacteroides and Bilophila were overrepresented in the carcinoma patients (Feng et al., 2015). Bilophila is one of the most common anaerobic bacteria recovered from patients with perforated and gangrenous appendicitis (Baron, 1997). It was shown, that increased number of Bilophila induces systemic inflammation and contribute to the commencement of the chronic diseases (Feng et al., 2017). Johnsonella was highly associated with tumour site (Pushalkar et al., 2012) and with chronic obstructive pulmonary disease (Wu et al., 2017), Tannerella was found to be a predisposing factor in atherosclerosis progression (Lee et al., 2014). Our data show that stress results in significant increase of Oscillibacter, which is known as a potential opportunistic pathogen, positively correlated with gut permeability (Lam et al., 2012). We hypothesize that Oscillibacter bacteria could be related to the disturbance in the TJ proteins expression, observed in PS group. Two genera (Acetanaerobacterium and Akkermansia) were elevated after heat stress. There are some evidence of beneficial effects of Acetanaerobacterium, associated with the high production of enterolactone (Hullar et al., 2015), which may protect against hormone‐dependent cancers and cardiovascular diseases (Kilkkinen et al., 2001). Akkermansia muciniphila is a mucin‐degrading bacterium, considered by some authors as an important member of the gut microbiota for control of physiological and homeostatic functions during obesity and type 2 diabetes (Everard et al., 2013). Conversely, other studies showed that increased abundance of A. muciniphila is related to hypertension (Tain et al., 2018) and can impair intestinal barrier function after using mucin by these bacteria as a nutrient (Desai et al., 2016). Depletion of the mucus layer by enriched A. muciniphila was associated with higher susceptibility to a gastrointestinal pathogen. Analysis of the microbiota in PS group indicates that disturbance in the microbial community is mostly by increase of pathogenic bacteria. Our results revealed that Akkermansia number was considerably higher only in PS group, where intestinal barrier function was disrupted. Previously we showed that exposure of rats to heat stress conditions significantly decreases the total thickness of intestinal mucosa (Ducray et al., 2016). Treatment with EH before stress (group ES) prevented increase of Akkermansia and destruction of intestinal barrier. These results are consistent with data from Desai et al. (2016), who found that abundance of A. muciniphila increased rapidly in the absence of prebiotic. We found significant decrease of two genera (Allobaculum, Bifidobacterium) and increase of Acetanaerobacterium, Bacteroides, Johnsonella in microbiota of rats from ES group vs PC group. Same trend presents in PS group that indicates specific effect of stress on these groups of bacteria. Essential impact of the EH on microbiota during heat stress is manifested in elevated number of beneficial bacteria (Eubacterium, Lactococcus, Oscillospira, Roseburia, Vallitalea). Roseburia, Eubacterium and Oscillospira are butyrate‐producing bacterial genera, positively correlate with antioxidant activities and negatively correlate with inflammation (Gophna et al., 2017; Wang et al., 2018b). Our results are consistent with previously in vitro study of Possemiers et al. (2013), who showed that yeast fermentate induces butyrate production and possess anti‐inflammatory activity. Butyrate is recognized as an essential host energy source (Donohoe et al., 2011), which can protect the mucus layer from injury (van der Beek et al., 2017). Positive contribution of Lactococcus and Vallitalea to the change of microbiota was noticed by other authors in humans and animals (Borrelli et al., 2017; Savage et al., 2018; Mao et al., 2018). We did not find significant change of the microbiota in EC group, except decreased abundance of Bifidobacterium. Data about lower number of Bifidobacterium in PS, ES and EC groups are in contrast with culture‐based results. Other authors also reported that species, isolated from culture did not generally correspond with the most abundant genera in microbiome analysis (Koeller et al., 2018). For example, increased Bifidobacterium abundance was detectable only with an in vitro culture method, and not pyrosequencing (Finegold et al., 2014). It was shown that the abundance of Bifidobacterium in humans and animals is underestimated with 16S rRNA gene‐based approach (Hooda et al., 2012).
Our results revealed substantial effect of S. cerevisiae fermentate prebiotic in prevention of heat stress‐related complications. Oral treatment of rats with prebiotic before exposure to heat stress conditions protected disruption of Paneth and goblet cells homeostasis, maintained expression of TJ proteins. We suggest that these effects are associated with beneficial modulation of the gut microbiota by prebiotic.
Conflict of Interest
No conflict of interest declared.
Acknowledgement
This work was supported in part by Embria Health Sciences LLC and by Lake Erie College of Osteopathic Medicine.
References
- Bailey, M.T. , Dowd, S.E. , Galley, J.D. , Hufnagle, A.R. , Allen, R.G. and Lyte, M. (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav Immun 25, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, E.J. (1997) Bilophila wadsworthia: a unique gram‐negative anaerobic rod. Anaerobe 3, 83–86. [DOI] [PubMed] [Google Scholar]
- Baurhoo, B. , Phillip, L. and Ruiz‐Feria, C.A. (2007) Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the caeca and litter of broiler chickens. Poult Sci 86, 1070–1078. [DOI] [PubMed] [Google Scholar]
- Baurhoo, B. , Goldflus, F. and Zhao, X. (2009) Purified cell wall of Saccharomyces cerevisiae increases protection against intestinal pathogens in broiler chickens. Int J Poult Sci 8, 133–137. [Google Scholar]
- Benno, Y. and Mitsuoka, T. (1992) Impact of Bifidobacterium longum on human fecal microflora. Microbiol Immunol 36, 683–694. [DOI] [PubMed] [Google Scholar]
- Bessette, C. , Henry, G. , Sekkal, S. , Benoit, B. , Bruno, J. , Meugnier, E. , Ferrier, L. , Theodorou, V. et al (2016) Oral administration of a casein matrix containing beta‐casofensin protects the intestinal barrier in two preclinical models of gut diseases. J Funct Food 27, 223–235. [Google Scholar]
- Bevins, C.L. and Salzman, N.H. (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9, 356–368. [DOI] [PubMed] [Google Scholar]
- Borrelli, L. , Coretti, L. , Dipineto, L. , Bovera, F. , Menna, F. , Chiariotti, L. , Nizza, A. , Lembo, F. et al. (2017) Insect‐based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci Rep 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerley, L.O. , Samuelson, D. , Blanchard, E. , Luo, M. , Lorenzen, B.N. , Banks, S. , Ponder, M.A. , Welsh, D.A. et al (2017) Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem 48, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P.D. , Possemiers, S. , Van De Wiele, T. , Guiot, Y. , Everard, A. , Rottier, O. , Geurts, L. , Naslain, D. et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP‐2‐driven improvement of gut permeability. Gut 58, 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chairatana, P. and Nolan, E.M. (2017) Defensins, lectins, mucins, and secretory immunoglobulin A: microbe‐binding biomolecules that contribute to mucosal immunity in the human gut. Crit Rev Biochem Mol Biol 52, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Zhang, Y.P. and Xiao, G.X. (1998) A preliminary clinical study of bifidobacteria preparation on the treatment of diarrhea in severely burned patients. Chin Med J 111, 381–382. [PubMed] [Google Scholar]
- De Theije, C.G.M. , Wopereis, H. , Ramadan, M. , Van Eijndthoven, T. , Lambert, J. , Knol, J. , Garssen, J. , Kraneveld, A.D. et al (2014) Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37, 197–206. [DOI] [PubMed] [Google Scholar]
- Deng, W. , Dong, X.F. , Tong, J.M. and Zhang, Q. (2012) The probiotic Bacillus licheniformis ameliorates heat stress‐induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci 91, 575–582. [DOI] [PubMed] [Google Scholar]
- Desai, M.S. , Seekatz, A.M. , Koropatkin, N.M. , Kamada, N. , Hickey, C.A. , Wolter, M. , Pudlo, N.A. , Kitamoto, S. et al (2016) A dietary fiber‐deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino, A. , Miceli, E. , Dhaliwal, W. , Biancheri, P. , Salerno, R. , Cantoro, L. , Vanoli, A. , De Vincenzi, M. et al (2008) Distribution, proliferation, and function of Paneth cells in uncomplicated and complicated adult celiac disease. Am J Clin Pathol 130, 34–42. [DOI] [PubMed] [Google Scholar]
- Donohoe, D.R. , Garge, N. , Zhang, X.X. , Sun, W. , O'connell, T.M. , Bunger, M.K. and Bultman, S.J. (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, S.E. , Callaway, T.R. , Wolcott, R.D. , Sun, Y. , Mckeehan, T. , Hagevoort, R.G. and Edrington, T.S. (2008) Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag‐encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol 8, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducray, H.A.G. , Globa, L. , Pustovyy, O. , Reeves, S. , Robinson, L. , Vodyanoy, V. and Sorokulova, I. (2016) Mitigation of heat stress‐related complications by a yeast fermentate product. J Therm Biol 60, 26–32. [DOI] [PubMed] [Google Scholar]
- Estienne, M. , Claustre, J. , Clain‐Gardechaux, G. , Paquet, A. , Tache, Y. , Fioramonti, J. and Plaisancie, P. (2010) Maternal deprivation alters epithelial secretory cell lineages in rat duodenum: role of CRF‐related peptides. Gut 59, 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard, A. , Belzer, C. , Geurts, L. , Ouwerkerk, J.P. , Druart, C. , Bindels, L.B. , Guiot, Y. , Derrien, M. et al (2013) Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci USA 110, 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard, A. , Lazarevic, V. , Gaia, N. , Johansson, M. , Stahlman, M. , Backhed, F. , Delzenne, N.M. , Schrenzel, J. et al. (2014) Microbiome of prebiotic‐treated mice reveals novel targets involved in host response during obesity. ISME J 8, 2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Q. , Liang, S.S. , Jia, H.J. , Stadlmayr, A. , Tang, L.Q. , Lan, Z. , Zhang, D.Y. , Xia, H.H. et al (2015) Gut microbiome development along the colorectal adenoma‐carcinoma sequence. Nat Commun 6, 13. [DOI] [PubMed] [Google Scholar]
- Feng, Z. , Long, W.M. , Hao, B.H. , Ding, D. , Ma, X.Q. , Zhao, L.P. and Pang, X.Y. (2017) A human stool‐derived Bilophila wadsworthia strain caused systemic inflammation in specific‐pathogen‐free mice. Gut Pathog 9. 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold, S.M. , Li, Z.P. , Summanen, P.H. , Downes, J. , Thames, G. , Corbett, K. , Dowd, S. , Krak, M. et al (2014) Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct 5, 436–445. [DOI] [PubMed] [Google Scholar]
- Furness, J.B. , Rivera, L.R. , Cho, H.J. , Bravo, D.M. and Callaghan, B. (2013) The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10, 729–740. [DOI] [PubMed] [Google Scholar]
- Gao, X.H. , Cao, Q.H. , Cheng, Y. , Zhao, D.D. , Wang, Z. , Yang, H.B. , Wu, Q.J. , You, L.J. et al (2018) Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA 115, E2960–E2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubeva, A.V. , Crampton, S. , Desbonnet, L. , Edge, D. O'Sullivan, O. , Lomasney, K.W. , Zhdanov, A.V. , Crispie, F.M. et al. (2015) Prenatal stress‐induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 60, 58–74. [DOI] [PubMed] [Google Scholar]
- Gophna, U. , Konikoff, T. and Nielsen, H.B. (2017) Oscillospira and related bacteria ‐ from metagenomic species to metabolic features. Environ Microbiol 19, 835–841. [DOI] [PubMed] [Google Scholar]
- Grootjans, J. , Thuijls, G. , Verdam, F. , Derikx, J.P.M. , Lenaerts, K. and Buurman, W.A. (2010) Non‐invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg 2, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz, K.R. and Hogan, S.P. (2009) Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124, 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigoz, Y. , Rochat, F. , Perruisseau‐Carrier, G. , Rochat, I. and Schiffrin, E.J. (2002) Effects of oligosaccharide on the faecal flora and non‐specific immune system in elderly people. Nutr Res 22, 13–25. [Google Scholar]
- Guo, X.L. , Tang, R.Y. , Yang, S.Y. , Lu, Y.R. , Luo, J. and Liu, Z.H. (2018) Rutin and its combination with inulin attenuate gut dysbiosis, the inflammatory status and endoplasmic reticulum stress in paneth cells of obese mice induced by high‐fat diet. Front Microbiol 9, 2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Chauhan, N.R. , Chowdhury, D. , Singh, A. , Meena, R.C. , Chakrabarti, A. and Singh, S.B. (2017) Heat stress modulated gastrointestinal barrier dysfunction: role of tight junctions and heat shock proteins. Scand J Gastroenterol 52, 1315–1319. [DOI] [PubMed] [Google Scholar]
- Hall, D.M. , Buettner, G.R. , Oberley, L.W. , Xu, L.J. , Matthes, R.D. and Gisolfi, C.V. (2001) Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol‐Heart Circul Physiol 280, H509–H521. [DOI] [PubMed] [Google Scholar]
- He, S.S. , Liu, F.H. , Xu, L. , Yin, P. , Li, D.Y. , Mei, C. , Jiang, L.S. , Ma, Y.F. et al. (2016) Protective effects of ferulic acid against heat stress‐induced intestinal epithelial barrier dysfunction in vitro and in vivo . PLoS ONE 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda, S. , Boler, B.M.V. , Serao, M.C.R. , Brulc, J.M. , Staeger, M.A. , Boileau, T.W. , Dowd, S.E. , Fahey, G.C. et al. (2012) 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr 142, 1259–1265. [DOI] [PubMed] [Google Scholar]
- Hullar, M.A.J. , Lancaster, S.M. , Li, F. , Tseng, E. , Beer, K. , Atkinson, C. , Wahala, K. , Copeland, W.K. et al (2015) Enterolignan‐producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol Biomarkers Prev 24, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani, T. , Moubareck, C.A. , Melki, I. , Rousseau, C. , Mangin, I. , Butel, M.J. and Karam‐Sarkis, D. (2018) Preterm infants with necrotising enterocolitis demonstrate an unbalanced gut microbiota. Acta Paediatr 107, 40–47. [DOI] [PubMed] [Google Scholar]
- Jakobsson, H.E. , Rodriguez‐Pineiro, A.M. , Schutte, A. , Ermund, A. , Boysen, P. , Bemark, M. , Sommer, F. , Backhed, F. et al. (2015) The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16, 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, M.E.V. and Hansson, G.C. (2016) Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 16, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, J.R. , Kennedy, P.J. , Cryan, J.F. , Dinan, T.G. , Clarke, G. and Hyland, N. (2015) Breaking down the barriers: the gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Front Cell Neurosci 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkkinen, A. , Stumpf, K. , Pietinen, P. , Valsta, L.M. , Tapanainen, H. and Adlercreutz, H. (2001) Determinants of serum enterolactone concentration. Am J Clin Nutr 73, 1094–1100. [DOI] [PubMed] [Google Scholar]
- Koeller, K. , Herlemann, D.P.R. , Schuldt, T. , Ovari, A. , Guder, E. , Podbielski, A. , Kreikemeyer, B. and Olzowy, B. (2018) Microbiome and culture based analysis of chronic rhinosinusitis compared to healthy sinus mucosa. Front Microbiol 9, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek, P.C. , Brzozowski, T. and Konturek, S.J. (2011) Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 62, 591–599. [PubMed] [Google Scholar]
- Lam, Y.Y. , Ha, C.W.Y. , Campbell, C.R. , Mitchell, A.J. , Dinudom, A. , Oscarsson, J. , Cook, D.I. , Hunt, N.H. et al (2012) Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet‐induced obese mice. PLoS ONE 7, e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, G.P. (2009) Stress‐induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 87, E101–E108. [DOI] [PubMed] [Google Scholar]
- Lee, H.R. , Jun, H.K. and Choi, B.K. (2014) Tannerella forsythia BspA increases the risk factors for atherosclerosis in ApoE(‐/‐) mice. Oral Dis 20, 803–808. [DOI] [PubMed] [Google Scholar]
- Maity, C. , Adak, A. , Pathak, T.K. , Pati, B.R. and Mondal, K.C. (2012) Study of the cultivable microflora of the large intestine of the rat under varied environmental hyperbaric pressures. J Microbiol Immunol Infect 45, 281–286. [DOI] [PubMed] [Google Scholar]
- Mao, N. , Cubillos‐Ruiz, A. , Cameron, D.E. and Collins, J.J. (2018) Probiotic strains detect and suppress cholera in mice. Sci Transl Med 10, eaao2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, I.A. , Goertz, J.E. , Ren, T.T. , Rich, S.S. , Onengut‐Gumuscu, S. , Farber, E. , Wu, M. , Overall, C.C. et al (2017) Microbiota alteration is associated with the development of stress‐induced despair behavior. Sci Rep 7. 43859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million, M. , Alou, M.T. , Khelaifia, S. , Bachar, D. , Lagier, J.C. , Dione, N. , Brah, S. , Hugon, P. et al (2016) Increased gut redox and depletion of anaerobic and methanogenic prokaryotes in severe acute malnutrition. Sci Rep 6. 26051 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nishino, R. , Mikami, K. , Takahashi, H. , Tomonaga, S. , Furuse, M. , Hiramoto, T. , Aiba, Y. , Koga, Y. et al (2013) Commensal microbiota modulate murine behaviors in a strictly contamination‐free environment confirmed by culture‐based methods. Neurogastroenterol Motil 25, 521–e371. [DOI] [PubMed] [Google Scholar]
- Ohland, C.L. and Macnaughton, W.K. (2010) Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol‐Gastroint Liver Physiol 298, G807–G819. [DOI] [PubMed] [Google Scholar]
- Opstelten, J.L. , Plassais, J. , Van Mil, S.W.C. , Achouri, E. , Pichaud, M. , Siersema, P.D. , Oldenburg, B. and Cervino, A.C.L. (2016) Gut microbial diversity is reduced in smokers with crohn's disease. Inflamm Bowel Dis 22, 2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, B.M. , Rickards, A. , Gehrke, L. and Rio, R.V.M. (2015) Characterization of shed medicinal leech mucus reveals a diverse microbiota. Front Microbiol 5, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, S.C. , Mani, V. , Boddicker, R.L. , Johnson, J.S. , Weber, T.E. , Ross, J.W. , Rhoads, R.P. , Baumgard, L.H. et al. (2013) Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 8, e70215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro, I. , Robinson, L. , Verhelst, A. , Marzorati, M. , Winkens, B. , Van Den Abbeele, P. and Possemiers, S. (2017) A yeast fermentate improves gastrointestinal discomfort and constipation by modulation of the gut microbiome: results from a randomized double‐blind placebo‐controlled pilot trial. BMC Complement Altern Med 17, 10.1186/s12906-017-1948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova, A.Y. , Kaftyreva, L.A. , Suzhaeva, L.V. , Voitenkova, E.V. , Zabrovskaia, A.V. , Egorova, S.A. , Makarova, M.A. , Matveeva, Z.N. et al (2017) Comparative characteristics of intestine microbiome of Republic of Guinea and Russian Federation residents. Infektsiya Immun 7, 375–382. [Google Scholar]
- Possemiers, S. , Pinheiro, I. , Verhelst, A. , Van Den Abbeele, P. , Maignien, L. , Laukens, D. , Reeves, S.G. , Robinson, L.E. et al (2013) A dried yeast fermentate selectively modulates both the luminal and mucosal gut microbiota and protects against inflammation, as studied in an integrated in vitro approach. J Agric Food Chem 61, 9380–9392. [DOI] [PubMed] [Google Scholar]
- Pushalkar, S. , Ji, X.J. , Li, Y.H. , Estilo, C. , Yegnanarayana, R. , Singh, B. , Li, X. and Saxena, D. (2012) Comparison of oral microbiota in tumor and non‐tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol 12, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba, A. , Olier, M. , Lacroix‐Lamande, S. , Lencina, C. , Bacquie, V. , Harkat, C. , Gillet, M. , Baron, M. et al (2017) Paneth cell defects induce microbiota dysbiosis in mice and promote visceral hypersensitivity. Gastroenterology 153, 1594–1606.e2. [DOI] [PubMed] [Google Scholar]
- Russell, D.A. , Ross, R.P. , Fitzgerald, G.F. and Stanton, C. (2011) Metabolic activities and probiotic potential of bifidobacteria. Int J Food Microbiol 149, 88–105. [DOI] [PubMed] [Google Scholar]
- Russo, F. , Linsalata, M. , Clemente, C. , Chiloiro, M. , Orlando, A. , Marconi, E. , Chimienti, G. and Riezzo, G. (2012) Inulin‐enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon‐like peptide 2 in healthy young volunteers. Nutr Res 32, 940–946. [DOI] [PubMed] [Google Scholar]
- Saini, S. , Gupta, N. , Aparna, Lokveer and Griwan, M.S. (2004) Surgical infections: a microbiological study. Braz J Infect Dis 8, 118–125. [DOI] [PubMed] [Google Scholar]
- Savage, J.H. , Lee‐Sarwar, K.A. , Sordillo, J. , Bunyavanich, S. , Zhou, Y.J. , O'Connor, G. , Sandel, M. , Bacharier, L.B. et al. (2018) A prospective microbiome‐wide association study of food sensitization and food allergy in early childhood. Allergy 73, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm, J.D. and Perdue, M.H. (2001) Stress and the gastrointestinal tract II. Stress and intestinal barrier function. Am J Physiol‐Gastroint Liver Physiol 280, G7–G13. [DOI] [PubMed] [Google Scholar]
- Soderholm, J.D. , Yang, P.C. , Ceponis, P. , Vohra, A. , Riddell, R. , Sherman, P.M. and Perdue, M.H. (2002) Chronic stress induces mast cell‐dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 123, 1099–1108. [DOI] [PubMed] [Google Scholar]
- Stevens, A. (1990) The haematoxylins In Theory and Practice of Histological Techniques ed. Bancrorft J.D., Stevens A. and Turner D.R. pp. 99–112 Edinburg, London, Melbourne and New York: Churchill Livingstone. [Google Scholar]
- Sudo, N. , Chida, Y. , Aiba, Y. , Sonoda, J. , Oyama, N. , Yu, X.N. , Kubo, C. and Koga, Y. (2004) Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol‐London 558, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T. (2013) Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 70, 631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain, Y.L. , Lee, W.C. , Wu, K.L.H. , Leu, S. and Chan, J.Y.H. (2018) Resveratrol prevents the development of hypertension programmed by maternal plus post‐weaning high‐fructose consumption through modulation of oxidative stress, nutrient‐sensing signals, and gut microbiota. Mol Nutr Food Res 62, e1800066. [DOI] [PubMed] [Google Scholar]
- Trevizan, A.R. , Vicentino‐Vieira, S.L. , Watanabe, P.D. , Gois, M.B. , De Melo, G.D.N. , Garcia, J.L. , Araujo, E.J.D. and Sant'ana, D.D.G. (2016) Kinetics of acute infection with Toxoplasma gondii and histopathological changes in the duodenum of rats. Exp Parasitol 165, 22–29. [DOI] [PubMed] [Google Scholar]
- Vainrub, A. , Pustovyy, O. and Vodyanoy, V. (2006) Resolution of 90 nm (lambda/5) in an optical transmission microscope with an annular condenser. Opt Lett 31, 2855–2857. [DOI] [PubMed] [Google Scholar]
- Vaishnava, S. , Behrendt, C.L. , Ismail, A.S. , Eckmann, L. and Hooper, L.V. (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host‐microbial interface. Proc Natl Acad Sci USA 105, 20858–20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Beek, C.M. , Dejong, C.H.C. , Troost, F.J. , Masclee, A.M. and Lenaerts, K. (2017) Role of short‐chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev 75, 286–305. [DOI] [PubMed] [Google Scholar]
- Van Hauwermeiren, F. , Vandenbroucke, R.E. , Grine, L. , Lodens, S. , Van Wonterghem, E. , De Rycke, R. , De Geest, N. , Hassan, B. et al. (2015) TNFR1‐induced lethal inflammation is mediated by goblet and Paneth cell dysfunction. Mucosal Immunol 8, 828–840. [DOI] [PubMed] [Google Scholar]
- Vargo, D. , Moskovitz, M. and Floch, M.H. (1980) Faecal bacterial flora in cancer of the colon. Gut 21, 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Sun, G.X. , Li, S.S. , Li, X. and Liu, Y. (2018a) Intestinal microbiota of healthy and unhealthy Atlantic salmon Salmo salar L. in a recirculating aquaculture system. J Oceanol Limnol 36, 414–426. [Google Scholar]
- Wang, Y.B. , Xie, Q.H. , Sun, S. , Huang, B.J. , Zhang, Y. , Xu, Y. , Zhang, S.M. and Xiang, H.Y. (2018b) Probiotics‐fermented Massa Medicata Fermentata ameliorates weaning stress in piglets related to improving intestinal homeostasis. Appl Microbiol Biotechnol 102, 10713–10727. [DOI] [PubMed] [Google Scholar]
- Weng, M. and Walker, W.A. (2013) The role of gut microbiota in programming the immune phenotype. J Dev Orig Health Dis 4, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand, S. , Zakrzewski, S.S. , Eichner, M. , Schulz, E. , Gunzel, D. , Pieper, R. , Rosenthal, R. , Barmeyer, C. et al (2017) Zinc treatment is efficient against Escherichia coli alpha‐haemolysin‐induced intestinal leakage in mice. Sci Rep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms, E. , Gerritsen, J. , Smidt, H. , Besseling‐Van Der Vaart, I. , Rijkers, G.T. , Fuentes, A.R.G. , Masclee, A.A.M. and Troost, F.J. (2016) Effects of supplementation of the synbiotic ecologic (R) 825/FOS P6 on intestinal barrier function in healthy humans: a randomized controlled trial. PLoS ONE, 11, e0167775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, M.L. , Inserra, A. , Lewis, M.D. , Mastronardi, C.A. , Leong, L. , Choo, J. , Kentish, S. , Xie, P. et al (2016) Inflammasome signaling affects anxiety‐ and depressive‐like behavior and gut microbiome composition. Mol Psychiatry 21, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X.W. , Chen, J.Z. , Xu, M. , Zhu, D.T. , Wang, X.Y. , Chen, Y.L. , Wu, J. , Cui, C.G. et al (2017) 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J Oral Microbiol 9. 1324725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q.J. , Liu, N. , Wu, X.H. , Wang, G.Y. and Lin, L. (2018) Glutamine alleviates heat stress‐induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult Sci 97, 2675–2683. [DOI] [PubMed] [Google Scholar]
- Xiao, G.Z. , Tang, L.Q. , Yuan, F.F. , Zhu, W. , Zhang, S.H. , Liu, Z.F. , Geng, Y. , Qiu, X.W. et al (2013) Eicosapentaenoic acid enhances heat stress‐impaired intestinal epithelial barrier function in Caco‐2 cells. PLoS ONE 8, e73571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Yin, P. , Liu, F.H. , Cheng, G.L. , Guo, K.J. , Lu, A. , Zhu, X.Y. , Luan, W.L. et al (2010) Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp Biochem Physiol A Mol Integr Physiol 156, 119–128. [DOI] [PubMed] [Google Scholar]
- Zareie, M. , Johnson‐Henry, K. , Jury, J. , Yang, P.C. , Ngan, B.Y. , Mckay, D.M. , Soderholm, J.D. , Perdue, M.H. et al (2006) Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55, 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]


