Abstract
X-linked Charcot–Marie-Tooth (CMTX) disease is a common inherited degenerative disorder of the peripheral nerve. Previously, our laboratory identified a large New Zealand/United Kingdom (NZ/UK) family mapping to the CMTX3 locus (Xq26.3–27.1). We have now identified a second large, Australian X-linked CMT family that links to the CMTX3 locus. This new family has the same phenotype as our previously described CMTX3family, with slightly milder disease in males than CMTX1and asymptomatic carrier females. This study also includes he re-analysis of one of the original US pedigrees reporting the CMTX3 locus. The large Australian family shared the complete disease haplotype with our original NZ/UK family, while the American family shared only the distal portion of the disease haplotype. Comparison of the frequency of the CMTX3 haplotype to the normal population showed strong statistical evidence (p<0.0001) indicating that the smaller shared haplotype is identical by descent. This suggests that the new CMTX3 family, our previously reported family, and the original American CMTX3 family have a common ancestor, and the disease in these families is caused by a founder mutation. The ancestral recombination observed in the American family refines the CMTX3 interval to a 2.5 Mb region betweenDXS984 and DXS8106. In this region, 11 out of the 15annotated genes have been excluded for pathogenic mutations.
Keywords: X-linked Charcot–Marie-Tooth disease, Founder effect, Linkage analysis
Introduction
Charcot–Marie-Tooth (CMT) disease is a degenerative disorder of the peripheral nerve. Clinical characteristics include progressive distal limb weakness, muscle atrophy, loss of deep tendon reflexes, and sensory abnormalities. CMT disease is the most common inherited peripheral neuropathy with one in 2,500 people being affected [1]. X-linked Charcot–Marie-Tooth (CMTX) accounts for up to 15% of all CMT cases, making it the second most common form of CMT after CMT1A [2, 3]. There are five loci so far reported for CMTX: CMTX1 (OMIM 304040), CMTX2 (OMIM 302801), CMTX3 (OMIM 302802), CMTX5 (OMIM 311070), and Cowchock syndrome (OMIM 310490). These syndromes have associated mental retardation and/or spasticity except for CMTX1. The dominant form of X-linked CMT (CMTX1) is the most commonly reported X-linked form of CMT. CMTX1 is caused by mutations in the gap junction beta 1/connexin32 (GJB1/Cx32) gene and is believed to account for more than 50% of all X-linked CMT cases [4]. Recently, mutations in the phosphoribosyl pyrophosphate synthetase 1 (PRPS1) gene were found to cause CMTX5 [5].
CMTX3 was first reported by Ionasescu et al. in 1991 [6]. The locus was mapped to Xq26-q28 in two American families with a phenotype including mental retardation and spasticity. Our laboratory confirmed and refined the CMTX3 locus in a large New Zealand and UK family (CMT623) in which clinical features were suggestive of CMTX but without mental retardation or spasticity [7]. The CMTX3 locus was refined to a 5.7 Mb region flanked by the markers DXS1041 and DXS8106.
In this study, we report another large multigenerational family with X-linked CMT mapping to the CMTX3 locus. To determine if our locus is the previously described CMTX3 locus, we re-analyzed one of the original American CMTX3 families reported by Ionasescu et al. [6]. Interestingly, a common disease haplotype was identified in all three CMTX3 families, suggesting that the disease in these families is caused by a founder mutation.
Materials and methods
Participants in the study gave informed consent in accordance with protocols approved by the Sydney South West Area Health Service Ethics Review Committee (Sydney, Australia). Genomic DNA was extracted from peripheral blood using the Puregene kit (Gentra Systems) according to the manufacturer’s instructions. DNA from an affected male from family US Ped2 was sent to our laboratory for genotype analysis. Access to the genotype data of the remaining family members was made possible through our collaborators (A.A. and K.F.).
Linkage and haplotype analysis
Individuals from a large multigenerational X-linked CMT family (CMT193-Ext) and the original CMTX3 family (US Ped2) were genotyped for the CMTX3 locus using the microsatellite markers DXS1041, DXS1062, DXS1192, DXS1232, DXS984, DXS1205, DXS1227, and DXS8106. Forward primers contained a 5′-FAM fluorescence label. Microsatellite markers were polymerase chain reaction (PCR) amplified in 20 μl reactions containing 25 ng DNA, 0.5 U perpetual Taq polymerase (Trend-Bio), 1.5 mM MgCl2, 200 μM each dNTP, 10 pmol primers and 1× PCR Enhancer (Invitrogen). PCR was performed on either a 9700 thermocycler (Applied Biosystems) with a Touchdown cycle or a MasterCycler (Eppendorf) with an initial denaturation of 95°C for 15 min followed by 35 cycles of 95°C for 30 s, appropriate Ta for 30 s and 68°C for 40 s with a final hold of 68°C for 5 min. Microsatellite markers were sent to the Australian Cancer Research Foundation Facility, Garvan Institute of Medical Research (NSW, Australia), for size fractionation using the GeneScan LIZ 600 size standard. Genotypes were analyzed using GeneMarker v1.5 (SoftGenetics LLC) software.
Two-point linkage analysis was performed using the MLINK program of the linkage package (V5.1) [8] in the Fastlink implementation (version 4.1p) [9]. Fully penetrant X-linked recessive inheritance was assumed with a disease allele frequency of 0.0001. Male and female recombination rates were considered equal. Marker alleles were set at 1/n, where n is the number of alleles observed. Extended haplotypes of family individuals were constructed according to the order of the Rutgers combined linkage-physical map of the human genome [10] and based on the minimal number of intermarker recombinations.
Alleles spanning the shared CMTX3 haplotype were determined in 152 unaffected control chromosomes and compared to the haplotype identified in families CMT193-ext, CMT623, and Ped2 US.Statistical analysis was performed using a two-tailed Fisher’s exact test for a 2×2 contingency table.
SNP genotyping using high resolution melt analysis
The single nucleotide polymorphism (SNP) markers rs1012777, rs176029, rs764198, and rs1997686 were genotyped using high resolution melt analysis methods established in our laboratory [11]. Primers were designed to amplify 50 to 100 bp on either side of the SNP. Samples were amplified in 10 μl reactions containing 25 ng DNA, 0.5 U perpetual Taq polymerase (TrendBio), 1× LC Green Plus (Idaho Technology), 3 mM MgCl2, 200 μM dNTP, 8 pmol primers and 1× PCR Enhancer (Invitrogen). To facilitate heteroduplex formation, all male samples were mixed 1:1 (v/v) with a known non-affected male control. PCR was performed on a MasterCycler (Eppendorf) with an initial denaturation of 95°C for 15 min followed by 35 cycles of 95°C for 30 s, appropriate Ta for 30 s and 68°C for 40 s with a final hold of 68°C for 5 min. To aid heteroduplex formation, a two-hold step of 95°C for 5 s and 50°C for 5 min was performed immediately after PCR amplification.
Results
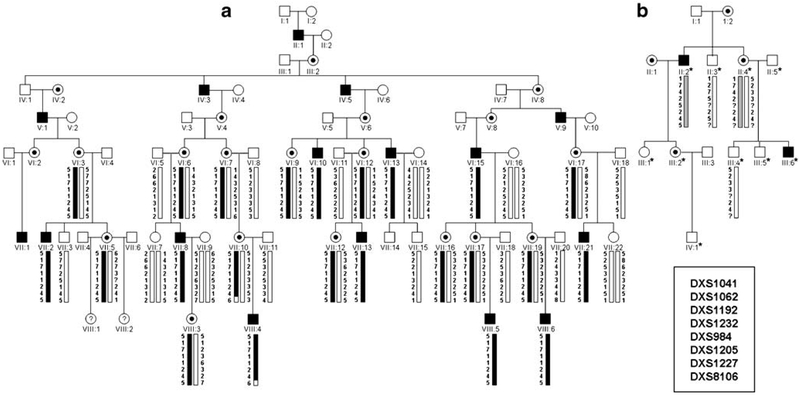
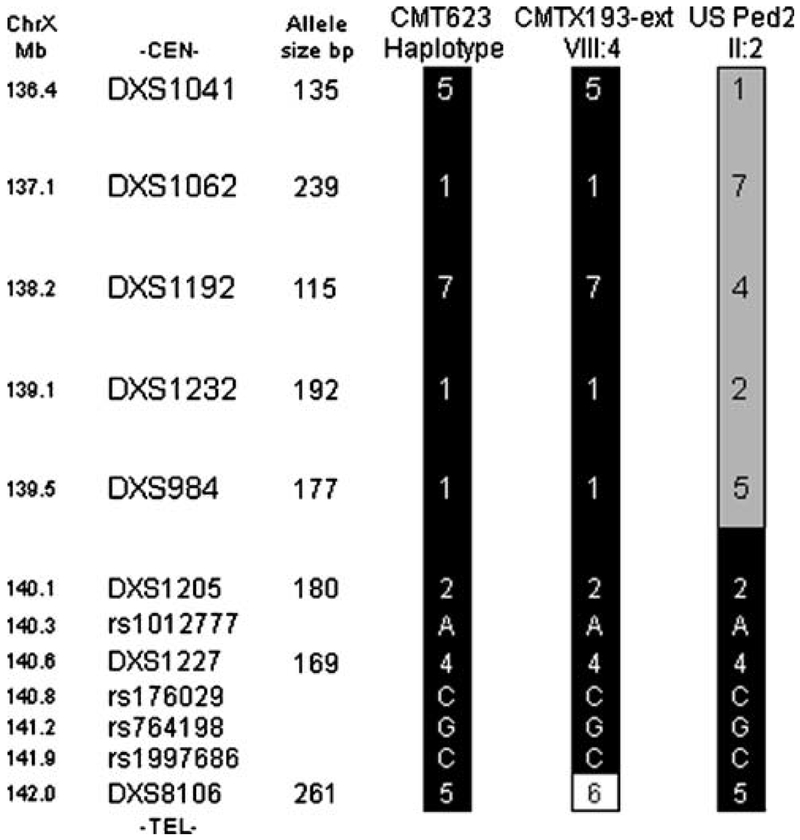
We have identified a second large, X-linked CMT family (CMT193-ext) mapping to the CMTX3 locus. Significant logarithm of the odds (LOD) scores (≥+2) obtained for seven of the markers, with a maximum LOD score of z=4.75 at θ=0.0 at the marker DXS984, establish linkage between the disease and this locus in this family (Table 1). The haplotype segregating with the disease (5-1-7-1-1-2-4-5) is identical to the haplotype we previously reported for CMTX3 family CMT623 (Figs. 1 and 2). While the family does not further refine the proximal flanking marker, the distal boundary for the CMTX3 locus was confirmed by the recombination observed in an affected individual (VIII:4; Fig. 1a). Haplotype analysis of four family members (one affected, one carrier, and two non-affected individuals) from one of the original American CMTX3 families, US Ped2, also identified a haplotype segregating with the affected and carrier individuals (Fig. 1b). Interestingly, for the distal portion of the haplotype (Cen-DXS1205-DXS1227-DXS8106-Tel), the American family had the same allele pattern as the two large CMTX3 families (Fig. 2). This suggests that the gene mutation in the three families may be derived from a common ancestor.
Table 1.
Two point LOD scores for the CMTX3 locus and CMT193-Ext
| Recombination fraction | |||||||
|---|---|---|---|---|---|---|---|
| Marker | 0 | 0.01 | 0.05 | 0.1 | 0.2 | 0.3 | 0.4 |
| DXS1041 | 2.86 | 2.80 | 2.56 | 2.26 | 1.64 | 1.03 | 0.46 |
| DXS1062 | 4.26 | 4.18 | 3.86 | 3.45 | 2.59 | 1.69 | 0.81 |
| DXS1192 | 4.17 | 4.10 | 3.78 | 3.37 | 2.53 | 1.66 | 0.80 |
| DXS1232 | 4.30 | 4.22 | 3.88 | 3.45 | 2.56 | 1.66 | 0.78 |
| DXS984 | 4.75 | 4.67 | 4.31 | 3.85 | 2.89 | 1.89 | 0.91 |
| DXS1205 | 3.28 | 3.21 | 2.94 | 2.60 | 1.91 | 1.22 | 0.57 |
| DXS1227 | 3.84 | 3.77 | 3.47 | 3.08 | 2.30 | 1.50 | 0.72 |
| DXS8106 | −inf | −1.45 | 0.38 | 0.94 | 1.13 | 0.89 | 0.49 |
Figure 1.
Haplotype analysis for families CMT193-ext (a) and US Ped2 (b) for markers spanning the CMTX3 locus. Males are represented by squares, females as circles. Blackened squares and black dots represent affected males and female carriers, respectively. Haplotypes were constructed using the genotype information of microsatellite markers ordered centromere (top) to telomere (bottom). The shared haplotype segregating with the disease in CMT193-ext and US Ped2 are marked with black and gray bars, respectively. The asterisk denotes individuals from US Ped2 that were genotyped in the original study by Ionasescu et al. [6]. For this study, DNA samples from four individuals were available for genotyping.
Figure 2.
Comparison of haplotypes constructed for the CMTX3 locus in affected males from CMT623, CMT193-ext, and US Ped2. Allele sizes (based on the GeneScan LIZ600 size standard) are listed to the left of the CMT623 haplotype. Black shading indicate alleles shared by all families in the distal portion of the haplotype
To further explore this possibility, additional markers between DXS1205 and DXS8106 were analyzed. All affected individuals showed an identical disease haplotype (2-A-4-C-G-C-5) for the seven markers (DXS1205, rs1012777, DXS1227, rs176029, rs764198, rs1997686, and DXS8106; Fig. 2). The frequency of the haplotype spanning these markers was determined in 152 control chromosomes. Three of the controls had the CMTX3 haplotype. Comparison of the disease-carrying haplotype to that of the controls using the Fisher’s exact test for a 2×2 contingency table showed statistically significant evidence ( p<0.0001) that the occurrence of the same distal haplotype in the three CMTX3 families is highly unlikely to have arisen by chance and is shared identity by descent.
Discussion
The locus for CMTX3 was first reported in 1991 by Ionasescu et al. [6]; however, it was over a decade before the locus was confirmed and refined in a large NZ/UK family (CMT623) by our laboratory [7]. The original CMTX3 locus was mapped to chromosome Xq26-q28 in two American families using restriction fragment length polymorphism (RFLP) markers. The combined LOD scores in these families established significant linkage and defined a 31.2 Mb interval flanked by the markers DXS37 and DXS15 [6]. As the smaller locus mapped in CMT623 lay within the original reported CMTX3 locus, it was assumed that the families in both studies would be caused by mutations in the same gene [7].
We have now identified a second large, multi-generational family (CMT193-Ext) mapping to the CMTX3 locus. Although the linkage results do not further refine the CMTX3 locus, a recombination in an affected individual (VIII:4) confirms DXS8106 as the distal flanking marker (Fig. 1a). Re-analysis of members from one of the original American families (US Ped2) identified a haplotype segregating with the disease based on the new microsatellite markers (Fig. 1b) and further suggests that the disease in this family is at the same locus.
The haplotype segregating with the disease in family CMT193-ext was found to be identical to the disease haplotype reported in the NZ/UK family (CMT623). Furthermore, the American family was shown to share the distal portion of the disease haplotype (Fig. 2). Comparison of the small haplotype to the controls provided statistically significant data indicating the sharing of alleles between the affected individuals of CMT623, CMT193-ext, and US Ped2 was a non-random association. We conclude that these families have a common ancestor and that CMTX3 disease is caused by a founder mutation. In our initial study, we reported an unaffected male carrying the distal portion of the CMTX3 haplotype [7]. This information was used to prioritize gene mutation scanning between the markers DXS1041 and DXS1205. The exclusion of genes in this region and new evidence of a founder mutation suggests that this individual is non-penetrant for the CMTX3 disease.
Cases of founder mutations have been reported for CMT including founder mutations in the CMTX1 gene, connexin32 [12]. A major focus in positional cloning projects is the refinement of the disease locus for candidate gene analysis. The combination of genetic linkage studies and haplotype association analysis has proven useful for fine mapping a disease locus. Evidence of a founder effect seen in several unrelated families of European descent refined the recessive CMT4C locus [13] originally reported in two consanguineous Algerian families [14]. Similarly in this study, the use of genetic linkage analysis on the two large families established linkage to a 5.7 Mb region on chromosome Xq26.3-q27.1 and evidence of a founder effect in the three families has refined the CMTX3 locus to a 2.5 Mb region between the markers DXS984 and DXS8106 through an ancestral recombination observed in the US pedigree.
The refined 2.5 Mb interval contains 15 annotated genes and 18 expressed sequence tag (EST) clusters that represent either novel transcripts or alternate splice variants for the known genes (UCSC Genome Browser, Human Mar. 06 Assembly). The coding region and splice sites for LDOC1 were previously excluded [7], and this study excludes another ten of the annotated genes (RP1–177G6.2, CDR1, SPANXB2, SPANXC, SPANXA2, SPANXA1, BC042039, SPANXE, MAGEC2, and SPANXN4). Mutation analysis of the remaining genes is continuing. In the event that a pathogenic mutation is not identified in the remaining annotated genes, our research will focus on the validation and characterization of the EST clusters and screening of these novel transcripts. The gene mutation causing CMTX3 could potentially be a novel undescribed gene.
In summary, we have identified a second family linked to the CMTX3 locus and re-analyzed one of the original families reporting the CMTX3 locus. The identification of a founder haplotype for CMTX3 disease and the presence of an ancestral recombination have refined the CMTX3 locus to a 2.5 Mb region. This has prioritized our search for the pathogenic gene, which when found, will contribute to our understanding of axonal degeneration in peripheral nerve diseases.
Acknowledgments
The authors thank the families CMT193-ext and US Ped2 for participating in this study. The authors also thank Mrs. Annette Berryman for tracing the genealogy of the families and the laboratory of Dr. Eric Green for fluorescence genotyping support. This research was supported by the National Health and Medical Research Council (grant ID 512443), the USA Muscular Dystrophy Association (grant ID MDA4314), and the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health (USA). M.B. is a recipient of an Australian Postgraduate Award (Australia). All experiments comply with the current laws of Australia.
References
- 1.Skre H (1974) Genetic and clinical aspects of Charcot–Marie-Tooth’s disease. Clin Genet 6:98–118 [DOI] [PubMed] [Google Scholar]
- 2.Nelis E, Van BC, De JP, Lofgren A, Vandenberghe A, Latour P, Le GE, Brice A, Mostacciuolo ML, Schiavon F, Palau F, Bort S, Upadhyaya M, Rocchi M, Archidiacono N, Mandich P, Bellone E, Silander K, Savontaus ML, Navon R, Goldberg-Stern H, Estivill X, Volpini V, Friedl W, Gal A (1996) Estimation of the mutation frequencies in Charcot–Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur J Hum Genet 4:25–33 [DOI] [PubMed] [Google Scholar]
- 3.Dubourg O, Tardieu S, Birouk N, Gouider R, Leger JM, Maisonobe T, Brice A, Bouche P, Leguern E (2001) The frequency of 17p11.2 duplication and Connexin 32 mutations in 282 Charcot–Marie-Tooth families in relation to the mode of inheritance and motor nerve conduction velocity. Neuromuscul Disord 11:458–463 [DOI] [PubMed] [Google Scholar]
- 4.Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH (1993) Connexin mutations in X-linked Charcot–Marie-Tooth disease. Science 262:2039–2042 [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Sohn KM, Shy ME, Krajewski KM, Hwang M, Park JH, Jang SY, Won HH, Choi BO, Hong SH, Kim BJ, Suh YL, Ki CS, Lee SY, Kim SH, Kim JW (2007) Mutations in PRPS1, which encodes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5). Am J Hum Genet 81:552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ionasescu VV, Trofatter J, Haines JL, Summers AM, Ionasescu R, Searby C (1991) Heterogeneity in X-linked recessive Charcot– Marie-Tooth neuropathy. Am J Hum Genet 48:1075–1083 [PMC free article] [PubMed] [Google Scholar]
- 7.Huttner IG, Kennerson ML, Reddel SW, Radovanovic D, Nicholson GA (2006) Proof of genetic heterogeneity in X-linked Charcot–Marie-Tooth disease. Neurology 67:2016–2021 [DOI] [PubMed] [Google Scholar]
- 8.Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottingham RW, Idury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- 10.Kong X, Murphy K, Raj T, He C, White PS, Matise TC (2004) A combined linkage-physical map of the human genome. Am J Hum Genet 75:1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennerson ML, Warburton T, Nelis E, Brewer M, Polly P, De JP, Timmerman V, Nicholson GA (2007) Mutation scanning the GJB1 gene with high-resolution melting analysis: implications for mutation scanning of genes for Charcot–Marie-Tooth disease. Clin Chem 53:349–352 [DOI] [PubMed] [Google Scholar]
- 12.Claramunt R, Sevilla T, Lupo V, Cuesta A, Millan JM, Vilchez JJ, Palau F, Espinos C (2007) The p.R1109X mutation in SH3TC2 gene is predominant in Spanish Gypsies with Charcot–Marie- Tooth disease type 4. Clin Genet 71:343–349 [DOI] [PubMed] [Google Scholar]
- 13.Gabreels-Festen A, van BS, Eshuis L, Leguern E, Gabreels F, van EB, Mariman E (1999) Study on the gene and phenotypic characterisation of autosomal recessive demyelinating motor and sensory neuropathy (Charcot–Marie-Tooth disease) with a gene locus on chromosome 5q23-q33. J Neurol Neurosurg Psychiatry 66:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessali M, Zemmouri R, Guilbot A, Maisonobe T, Brice A, Leguern E, Grid D (1997) A clinical, electrophysiologic, neuropathologic, and genetic study of two large Algerian families with an autosomal recessive demyelinating form of Charcot–Marie-Tooth disease. Neurology 48:867–873 [DOI] [PubMed] [Google Scholar]