Abstract
The multifaceted glycogen synthase kinase (GSK3) has an essential role in sperm and male fertility. Since cyclic AMP (cAMP) plays an important role in sperm function, we investigated whether GSK3 and cAMP pathways may be interrelated. We used GSK3 and soluble adenylyl cyclase (sAC) knockout mice and pharmacological modulators to examine this relationship. Intracellular cAMP levels were found to be significantly lower in sperm lacking GSK3α or GSK3β. A similar outcome was observed when sperm cells were treated with SB216763, a GSK3 inhibitor. This reduction of cAMP levels was not due to an effect on sperm adenylyl cyclase but was caused by elevated phosphodiesterase (PDE) activity. The PDE4 inhibitor RS25344 or the general PDE inhibitor IBMX could restore cAMP levels in sperm lacking GSK3α or β-isoform. PDE activity assay also showed that hyperactivated PDE4 contributes in lowering of cAMP levels in GSK3α null sperm suggesting that in wild-type sperm PDE4 activity is kept in check by GSK3. Conversely, PKA being triggered by cAMP, affected GSK3 activity through increasing its phosphorylation. Increased GSK3 phosphorylation also occurred by inhibition of sperm specific protein phosphatase type 1, PP1γ2. The relationship between cAMP, GSK3, and PP1γ2 activities was also confirmed in sperm from sAC null mice. Pull-down assay using recombinant PP1γ2 indicated that PKA, GSK3, and PP1γ2 could exist as a complex. Pharmacological inhibition of GSK3 in mature spermatozoa resulted in significantly reduced fertilization of eggs in vitro. Our results show that cAMP, PKA, and GSK3 are interrelated in regulation of sperm function.
Keywords: cAMP, fertilization, GSK3, PP1γ2, sperm
1 |. INTRODUCTION
Glycogen synthase kinase 3 (GSK3) is involved in key regulatory pathways in tissues and cells of a wide range of organisms: regulation of glucose metabolism, cell proliferation, apoptosis, embryonic development, Wnt/β-catenin signaling, growth factor action among others (Beurel, Grieco, & Jope, 2015). GSK3 typically phosphorylates a primed substrate having the consensus sequence S/T-X-X-X-S/T(p), where the C-terminal Ser/Thr is primed with a phosphate and GSK3 phosphorylates the upstream Ser/Thr residue (X being any amino acid). Phosphorylation of Ser21 and Ser9 residues of alpha and beta isoforms of GSK3, respectively, results in inhibition of GSK3 catalytic activity (Medina & Wandosell, 2011). Hence, phosphorylation by kinases and dephosphorylation by phosphatases regulate GSK3 activity (Delcommenne et al., 1998; Fang, Yu, Lu, Woodgett, & Mills, 2000; Hernandez, Langa, Cuadros, Avila, & Villanueva, 2010).
We showed that GSK3 is associated with sperm maturation in the epididymis (Somanath, Jack, & Vijayaraghavan, 2004). Catalytic activity of GSK3 is high in immature caput epididymal spermatozoa compared to mature caudal epididymal spermatozoa. However, pharmacological inhibition of GSK3 alone does not initiate motility in caput immotile sperm cells (Dudiki, Kadunganattil, Ferrara, Kline, & Vijayaraghavan, (2015). Catalytic activity of protein phosphatase PP1γ2 is substantially reduced in sperm during their transit through the epididymis (Huang & Vijayaraghavan, 2004; Vijayaraghavan et al., 1996). Inhibition of protein phosphatase by Calyculin A increase GSK3 phosphorylation and activate sperm motility (Vijayaraghavan, Mohan, Gray, Khatra, & Carr, 2000). Phosphorylation of GSK3 is substantially higher in motile caudal sperm compared to immotile caput epididymal sperm (Somanath et al., 2004). In a recent study we have shown that mice lacking GSK3α are infertile and have impaired sperm function (Bhattacharjee et al., 2015).
It is known that cAMP and protein kinase A are involved in initiation of motility and activation of metabolism of sperm during their passage through the epididymis (Vijayaraghavan & Hoskins, 1986; Vadnais, Aghajanian, Lin, & Gerton, 2013). We decided to investigate how cAMP action and GSK3 may be related in sperm. Spermatozoa, being terminally differentiated cells, are largely regulated by changes in protein phosphorylation (Dacheux & Dacheux, 2014; Freitas, Vijayaraghavan, & Fardilha, 2017). We have previously shown that GSK3 is phosphorylated during epididymal sperm maturation and that its phosphorylation could be altered in vitro under conditions that initiate or stimulate motility (Vijayaraghavan et al., 2000) confirming GSK3 in sperm appears to be under active phosphorylation and dephosphorylation. Sperm cAMP is generated by a unique soluble adenylyl cyclase (sAC). Sperm sAC is regulated by bicarbonate and calcium ions (Buffone, Wertheimer, Visconti, & Krapf, 2014; Hess et al., 2005; Vadnais et al., 2013). Similar to other cells cAMP in sperm is broken down by a phosphodiesterase. At least five subtypes of cAMP-specific PDE are present in sperm (Bender & Beavo, 2006; Omori & Kotera, 2007). Surprisingly little is known about how sperm PDEs are regulated. In somatic cells it is known that PDEs could be regulated by phosphorylation by Ser/Thr kinases (Bender & Beavo, 2006; Keravis & Lugnier, 2012). The overall aim of the present study was to elucidate how cAMP/PKA, protein phosphatase PP1γ2 and GSK3 may be interrelated in sperm.
2 |. MATERIAL AND METHODS
2.1 |. Animals and genotyping
All wild-type and transgenic mice were housed and used at Kent State University with the approval of the Kent State University Institutional Animal Care and Use Committee following the appropriate laws, guidelines and policies and performed in accordance with the NIH and National Research Council’s publication “Guide for Care and Use of Laboratory Animals”. C57BL/6 mice with floxed Gsk3α and β alleles were obtained from Dr James Woodgett (Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto). A CRE/Lox strategy was used to disrupt Gsk3β or Gsk3α expression in developing testicular germ cells. The conditional Gsk3β knockout in testis was accomplished using the Stra8-Cre, which results in deletion of floxed genes in germ cells starting from spermatocyte onwards. The primer pair used for detection of the Stra8-Cre transgene was forward primer 5′-GTGCAAGCTGAACAACAGGA-3′ and reverse primer 5′-AGGGAC ACAGCATTGGAGTC-3′ (Sinha, Puri, Nairn, & Vijayaraghavan, 2013). For the floxed Gsk3β detection, following primers were used: forward primer 5′-GGGGCAACCTTAATTTCAATT-3′ and reverse primer 5′-TC TGGGCTATAGCTATCTAGTAACG-3′. For the wild-type Gsk3α the following primers were used: forward primer 5′-GGGAGTTCTCCA GTCGTGAG-3′ and reverse primer 5′-CTTGGCGTTAAGCTCCTGTC-3′; for the global Gsk3α knockout, forward primer was 5′-GCCCAATTCCGATCATATTC-3′ and reverse primer was same as wild-type one (Bhattacharjee et al., 2015; Hoeflich et al., 2000). CD1 mice with sAC gene knocked out by homologous recombination were obtained from Dr Lonny R. Levin and Dr Jochen Buck (Department of Pharmacology, Weill Cornell Medical College, New York). Corresponding PCR reactions were performed by using the following primer pairs specific for the wild-type allele: forward 5′-GTCTGGCCACACACTAA GG-3′ and reverse 5′-CTCCAGCTCCGATGAAGG-3′; for the targeted allele: forward 5′-CTGGGCTGTCCTCAAGCTC-3′ and reverse 5′-GC AGCGCATCGCCTTCTATC-3′ (Esposito et al., 2004). The amplified genes were detected on 1.5% agarose gel.
2.2 |. Purification of recombinant proteins
Inhibitor 2 (I2 or PPP1R2) was purified from E. coli BL21 (DE52) containing PPP1R2 within a pET expression vector (Qiagen, GmbH, Hilden, Germany). In brief, IPTG-induced BL21 were pelleted and lysed in Homogenization Buffer (HB) containing 20 mM Tris, pH 7.2, 1 mM EDTA, and 1 mM EGTA (supplemented with protease inhibitor cocktail from Roche). I2 was purified from this lysate by a three-step process sequentially using 30–55% saturated ammonium sulfate precipitation, DE-52 anion-exchange chromatography and finally eluting with 200 mM NaCl through HiTrap Q anion exchange column (GE Healthcare, Uppsala, Sweden). The protein fraction was dialyzed before use in activity assays.
PPP1γ2 (His-tagged) was expressed using pTAC expression vector within BL21 (DE52) E. coli. IPTG induced bacteria were pelleted and resuspended in a binding buffer containing 20 mM Tris, 500 mM NaCl, 20 mM imidazole, pH 7.4. Sample was allowed to bind to HisTrap column (GE Healthcare) eluted with 420 mM imidazole then dialyzed into 20 mM Tris, pH 8. In the next step, the sample was purified using HiTrap Q anion exchange column.
2.3 |. Isolation of sperm cells from WT and knockout mice
The cauda epididymis and vas deferens from adult mice, aged 9–12 weeks, were isolated in PBS. The tissue was punctured with a 45-mm gauge needle and the spermatozoa were allowed to swim out and disperse into PBS for 5–10 min at 37 °C. The cell suspension was transferred to micro-centrifuge tubes using a large-bore pipette tip. It was then diluted in water sperm number was counted using a Neubauer hemocytometer using a 20× objective in a Zeiss bright field microscope.
2.4 |. Estimation of intra-sperm cAMP content
Mouse spermatozoa from cauda epididymides were washed (1 × 106 in each set) and incubated with/without stipulated doses of different pharmacological modulators: non-selective PDE inhibitor, IBMX; PDE1 specific antagonist Vinpocetine (Essayan, 2001); PDE4 selective inhibitor RS25344 (Maurice et al., 2014); PDE10 specific inhibitor Papaverine HCl (Torremans et al., 2010); PDE11 selective inhibitor BC 11–38 (Noel, Dhooghe, & Leal, 2012). After treatments of the spermatozoa, these cells were lysed with 0.1 M HCl for 40 min in ice following instructions of the Direct cAMP ELISA Kit, Enzo Life Sciences (ADI-900–066). After centrifugation, the supernatant containing cAMP was estimated using this competitive immunoassay kit for the quantitative determination of cAMP. Extracted fractions were acetylated to increase the sensitivity of the assay. The kit uses a polyclonal antibody (generated against cAMP) to bind, in a competitive manner, the cAMP in the sample or an alkaline phosphatase molecule which is covalently attached to cAMP. Samples or standards, alkaline phosphatase conjugate, and antibody were simultaneously incubated at room temperature in a secondary antibody coated multi-well plate for 2 hr with shaking. The excess reagents were then washed away and substrate was added. After around 30 min the enzyme reaction was stopped and the yellow color generated was read on a multi-well plate reader at 405 nm. The measured optical density was used to calculate the concentration of cAMP of the samples from the standard curve.
2.5 |. Estimation of intra-sperm calcium levels
Spermatozoa were isolated from WT and GSK3-double hetero (GSK3α+/−β+/−) mice in TBS, pH 7.4 supplemented with 5 mM Glucose and 1 mM CaCl2. Cells were loaded with Fura 2-AM (final conc. 4 μM) and kept at RT for 60 min. Sperm cells were then washed three times with calcium-free TBS (centrifuged at 700g for 10 min) to remove excess Fura 2-AM. At first, ratio metric fluorescence for the basal intracellular Ca2+ was recorded. CaCl2 (final conc. 1 mM) was added to the cell suspension and immediately digitonin (0.02%) was added, mixed and kept for 10 min; Fmax was measured. EGTA solution (final conc. 20 mM) was added, mixed and kept for 5 min; Fmin was measured. In each time fluorescence was measured using 340 and 380 nm excitation and 510 nm emission on a Horiba Scientific PTI QM-400Fluorescence Spectroscopy System. Continuous readings were taken from 0 to 60 s. Data were analyzed with FelixGX, version 2.0 software (Photon Technology International, Birmingham, NJ). [Ca2+]i was calculated using the standard equation (Grynkiewicz, Poenie, & Tsien, 1985):
Where, Sf is the ratio FCa2+free state excited at 340nm/ FCa2+saturated state excited at 380nm;
Kd is 220 nM for Fura 2-AM at 37 °C;
R is the F340/F380 ratio of sample;
Rmin is the F340/F380 ratio of the negative control (20 mM EGTA);
Rmax is the F340/F380 ratio of the positive control (0.02% digitonin/1 mM CaCl2).
2.6 |. Phosphodiesterase activity assay
Caudal epididymal sperm cells (3 × 107/set suspended in 200 μl of HB buffer supplemented by 10 mM Benzamidine HCl, 0.1% 2-mercaptoethanol, 1 mM PMSF) from WT and GSK3-double hetero mice were isolated. Cells were placed in microfuge tubes in ice and sonicated (QSonica) for a total of 50 s with 10 s pulse at 40 amplitude. The sonicated cell suspensions were centrifuged at 150 00g for 15 min and supernatants were obtained. These extracts were analyzed for phosphodiesterase assay using Cyclic Nucleotide Phosphodiesterase Assay Kit, Enzo Life Sciences (BML-AK800–0001). Free phosphate ions were removed from the extract using a desalting column (BML-KI100) provided by the kit. Extracts containing PDE, substrate and 5′-nucleotidase were dissolved in assay buffer (BML-KI181) in presence or absence of IBMX and RS25344. It was incubated at 30 °C for 30 min. A parallel set for standards were prepared using 5′AMP. After incubation, reactions were terminated by addition of BIOMOL® GREEN Reagent. Color was allowed to develop for 20–30 min and absorbance at 620 nm was measured on FilterMax F5 microtiter-plate reader (Molecular Devices).
2.7 |. Preparation of different types of sperm cell extracts
For preparation of whole sperm lysate, after isolation cells were centrifuged at 700g for 10 min at 4°C. The sperm pellet was resuspended in 1% SDS at a final concentration of 2 × 108 sperm/ml. The sperm suspension in 1% SDS was then boiled in a water-bath for 5 min and centrifuged at 120 00g for 15 min at room temperature. The supernatant was collected and used for Western blot analysis. To obtain soluble protein fraction, each set of pellets was resuspended in RIPA lysis buffer (containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA) supplemented with 10 mM benzamidine HCl, 0.1% 2-mercaptoethanol, 1 mM PMSF, 1 μM calyculin A, and 1 mM activated sodium orthovanadate. It was kept on ice for 30 min with occasional mixing and then was centrifuged at 160 00g for 20 min at 4 °C and supernatant was used for downstream applications.
2.8 |. Western blot analysis
The protein fractions were separated on SDS-PAGE and transferred to PVDF membrane by Bio-Rad Laboratories, Hercules, CA wet-transfer apparatus. Nonspecific binding sites were blocked with 5% skimmed milk. The PVDF paper was then incubated with primary antibody: rabbit polyclonal Anti-PDE4 antibody (Abcam, Cambridge, MA #ab14628), rabbit polyclonal phospho-GSK-3α/β (Ser21/9) antibody (Cell Signaling, Danvers, MA #9331), anti-GSK3 α/β mouse monoclonal antibody (44610; Invitrogen (Thermo Fisher Scientific), Waltham, MA), rabbit polyclonal PKA C-α antibody (Cell Signaling #4782), rabbit polyclonal Phospho-PP1α (Thr320) antibody (Cell Signaling #2581), anti-PP1gamma2 (PPP1CC2) antibody (commercially prepared using a synthetic peptide corresponding to the 22 amino acids at the carboxy terminus of PPP1CC2 as the antigen); all the primary antibodies were used at 1:1000 dilution. This was followed by horseradish peroxidase-conjugated secondary IgG (1:2500 dilution). Immunoreactive bands were visualized using a chemiluminescent substrate (Thermo Scientific Super Signal West Pico ECL). The PVDF membrane was then reprobed with either rabbit polyclonal β-tubulin (for whole cell extracts) or mouse monoclonal β-actin (for soluble protein extracts) antibody to verify the uniformity of the sample loading.
2.9 |. Kinase assays
Sperm cell extracts using RIPA was prepared as per the protocol mentioned above and used for the GSK3 assay. GSK3 activity was measured by the amount of 32PO4 transferred from [32 P] γ-adenosine triphosphate to phospho-glycogen synthase peptide-2 (GSK3 substrate, Millipore Corporation, Billercia, MA). The initial assay buffer contained 200 mM HEPES, 50 mM MgCl2, 8 μM DTT, 5 mM sodium ß-glycerophosphate, 0.4 mM ATP, and 4 μCi of gamma-P32 ATP. 5 μl of this assay buffer was added with 5 μl each of previously prepared cell extract and GS2 peptide (Millipore): YRRAAVPPSPSLSRHSSPHQ-pS-EDEEE(1 mg/ml). GSK3 activity was also measured in the presence of 1 mM LiCl. Lithium-sensitive kinase activity was considered to be due to GSK3 (Ryves, Fryer, Dale, & Harwood, 1998). The reaction mixture was incubated at 30 °C for 15 min and the reaction was stopped by cooling on ice for 10 min. A 12 μl aliquot of the reaction mixture was applied to a phosphocellulose cation exchanger (P81; Whatman Inc, Clifton, NJ) paper cut into 1.5 cm × 1.5 cm squares and washed with 0.5% (vol/vol) phosphoric acid. After three washes (5 min each) in phosphoric acid, the squares were placed into scintillation vials with 2 ml of distilled water and counted in a scintillation counter. Each of the reaction sets were done in triplicate. The GSK3 activity was measured as follows:
All assays were conducted in triplicate and the means of three or more separate experiments are shown.
For the PKA activity assay a similar approach was adopted (Vijayaraghavan, Trautman, Goueli, & Carr, 1997). Caudal sperm from WT and GSK3α knockout mice were subjected to preparation of cellular extract using RIPA lysis buffer as mentioned above. Reaction mixtures contained 50 mM Tris-Cl, pH 7.0, 250 μM [γ−32P]ATP, 250 μM Kemptide as substrate (Leu-Arg-Arg-Ala-Ser-Leu-Gly), 25 mM Na3VO4, 10 mM MgCl2, 0.25 mg/mL bovine serum albumin and where indicated, 10 μM cAMP. For each of the control sets of WT and knock out samples, 50 μM H89 was used as PKA-specific inhibitor to nullify non-specific activity. Assays were initiated by the addition of labeled ATP, incubated for 2 min at 30 °C, and stopped by addition of 1 N HCl. The reaction mixture was then spotted on phosphocellulose paper followed by three washes in 1% phosphoric acid. The papers were then analyzed by Cerenkov counting. All determinations were in triplicate.
2.10 |. Phosphatase assays
Caudal sperm sonicates of WT and sAC knockout mice were prepared as per the protocol mentioned above (section 2.7). The pellet (insoluble fraction) was resuspended in an equal volume of HB+. The soluble and insoluble fractions of the sonicates were utilized for phosphatase enzyme activity measurement on the same day. Radiolabeled phosphorylase a was used as a substrate to measure the activity of PP1 by procedures previously reported (Vijayaraghavan et al., 1996). Aliquots of the fractions of sperm extracts were pre-incubated at 30 °C for 15 min in either the presence or absence of protein phosphatase inhibitors. Purified protein phosphatase inhibitor-2 (I–2) was used at a final concentration of 25 nM to inhibit PP1 or 2 nM okadaic acid final concentration to selectively inhibit PP2A. Phosphorylase a was then added and the samples were further incubated at 30 °C for 10 min. The reaction was terminated by addition of 10% trichloroacetic acid and centrifuged for 10 min at 120 00g. Supernatants were quantitated for 32P released from phosphorylasea.
2.11 |. Pull down assay
Caudal spermatozoa from WT mice were isolated and extract was prepared in HB+ buffer (supplemented with 0.5 mM MnCl2, 2 nM calyculin A) as described above; extract was allowed to bind with recombinant PP1γ2 at a volumetric ratio of 2:1 overnight in a rotating shaker at 4 °C. Next day, washed Ni-agarose beads were added at a ratio of 2:1 to the extract and left for 2.5 hr binding at RT using the rotating shaker. After centrifugation and subsequent repeated washing with PBS/10 mM imidazole, finally pulled-down protein fraction was eluted with 200 mM imidazole in PBS; the eluate was collected after centrifugation at 500g for 5 min and checked in Western blot analysis.
2.12 |. Immuno cytochemical analysis
Caudal epididymal spermatozoa in PBS were spun down at 700g for 10 min at 4 °C. The cells were fixed in 4% paraformaldehyde, EM grade (Electron Microscopy Sciences) at 4 °C for 20 min, followed by permeabilization with 0.2% Triton–X (5 min). Fixed spermatozoa were attached to poly-L-lysine coated coverslips. The coverslips were washed three times with TTBS (Tris-buffered saline with 1% Triton–X soulution) to remove excess paraformaldehyde and incubated for 4 hr in a blocking solution containing 5% non-immune serum in TTBS at room temperature. The coverslips were then incubated overnight at 4 °C with either of the following primary antibody: anti-GSK3α antibody (1:150 dilution; SAB4300292; Sigma–Aldrich, St. Louis, MO), GSK3β (1:150 dilution; Novus Biologicals; mouse monoclonal), PPP1CC2 (1:150 dilution, rabbit polyclonal antibody), PDE4 (1:100 dilution, rabbit polyclonal antibody, ab14628), PKA Cα (1:100 dilution, rabbit polyclonal antibody, Cell Signaling 4782). Coverslips were subsequently washed three times 5 min each with TTBS, followed by incubation with the appropriate secondary antibody conjugated to Cy3 for 1 hr at room temperature. The coverslips were then washed three times, 10 min each with TTBS; cells were subsequently stained with Hoechst. Mounting medium was applied, and the sperm cells were examined by confocal laser scanning microscope (Olympus IX81 attached with FLUOVIEW FV1000).
2.13 |. In vitro fertilization
Nine to twelve-weeks old C57 BL/6 females were injected intra-peritoneally with 10 IU of pregnant mare’s serum gonadotropin (PMSG) hormone. After 52 hr, the females were injected intra-peritoneally with 10 IU of Human chronic gonadotropin (hCG) hormone. WT or GSK3α hetero (containing only one copy alpha gene) male mice (3–6 months old) were sacrificed and caudal spermatozoa was isolated in HTF medium. The cell suspension was divided into two parts to be incubated with 1% DMSO (control) and SB216763 (10 μM) in 37 °C incubator with 5% CO2 for 1 hr. The super-ovulating mature female mice were sacrificed approximately 14 hr after administering hCG. The mice were dissected and the uteruses, oviducts and ovaries, were removed and placed on PBS media. Under a stereomicroscope, the oviducts and ovaries were cleaned from any fat tissue. After that, the cleaned oviducts and ovaries were immersed in sterile mineral oil contained within a fertilization dish. A dissecting needle was used to tear and open the ampulla to release the Cumulous Oocyte Complexes (COCs). COCs were dragged from the mineral oil into a 250 μl drop of HTF medium. Fifteen microliter of treated cells were transferred from sperm collection dish into the fertilization dish that has COCs. The fertilization cultures were incubated in 5% CO2 and 37 °C for 4 hr. After that, the eggs were moved from the fertilization drop into three wash drops to remove some of the excess sperm and incubated in 5% CO2 and 37 °C overnight. After overnight incubation, the number of unfertilized eggs, fragmented eggs, and the two cells stage embryos were counted (Nagy, Gertsenstein, Vintersten, & Behringer, 2003).
2.14 |. Statistical analysis
Results are presented as means ± standard errors for at least three observations under identical condition. The data were statistically analyzed by one-way ANOVA and two-tailed unpaired t-test using Prism, version 7, Graph Pad software (La Jolla, CA). Please see figure legends for further details.
3 |. RESULTS
3.1 |. Cyclic AMP levels are reduced in sperm from mice lacking one or both alleles of Gsk3α or GsK3β
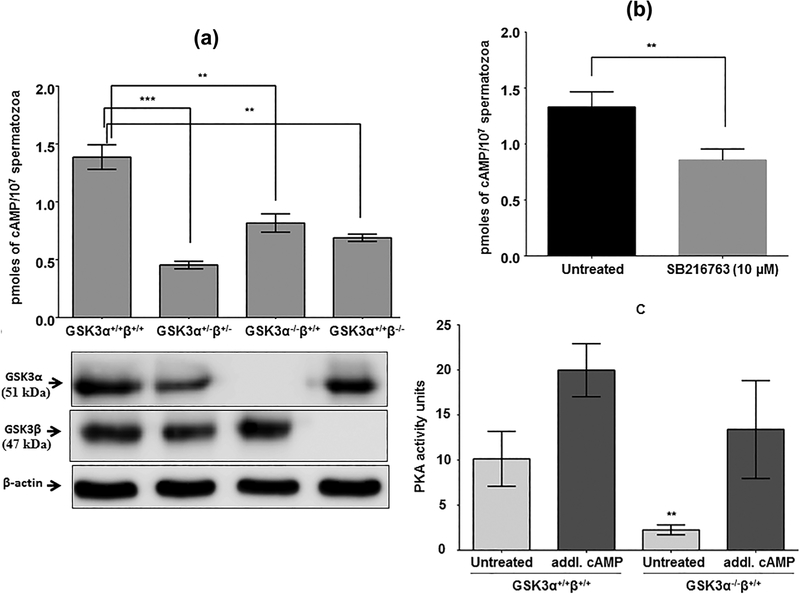
Expression of alpha and beta isoforms of Gsk3 gene were successfully disrupted by CRE/lox strategy (Figure 1a, lower panel; Supplementary Figure S1). Deletion of one copy each of alpha and beta isoform of GSK3 (Gsk3α+/−β+/−) resulted in a significant decrease of cAMP levels in sperm (Figure 1a). A similar outcome (~50% reduction) was also obtained in sperm lacking either GSK3α or GSK3β (Gsk3α−/−β+/+ or Gsk3α+/+β−/−). We have previously shown that GSK3 activity in sperm containing one allele each of GSK3α and β or sperm lacking both alleles of either one of the two isoforms is reduced about 50% of the GSK3 activity in normal wild-type sperm. These observations suggested that GSK3 may be involved in regulating the synthesis or degradation of cAMP. Following this, we examined if pharmacological inhibition of GSK3 in wild-type normal sperm also affected sperm cyclic AMP levels. Figure 1b shows that inhibition of GSK3 with the selective small molecule inhibitor (SB216763) results in significant reduction of intra-sperm cAMP levels. As expected reduced cAMP levels are reflected in lower catalytic activity of sperm PKA (Figure 1c). In extracts of both wild-type and Gsk3 knockout sperm PKA activity is restored by the addition of cAMP. Thus reduced GSK3 activity in sperm from Gsk3α knockout mice or by inhibition of GSK3 catalytic activity in wild type sperm in vitro reduced cAMP levels.
FIGURE 1.
Effect of suppression of GSK3 on intracellular cAMP titer in mouse sperm cells. (a) Effect on [cAMP]i after global deletion of copies of α and β alleles of GSK3 gene from mice. Data represent mean ± SEM of n = 7 for GSK3α+/+β+/+and GSK3α−/−β+/+each, n = 3 for GSK3α+/−β+/− and n = 3 for GSK3α+/+β−/−. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.01 versus GSK3α+/+β+/+ values. The lower panel shows the corresponding Western blot data about the levels of GSK3α and β proteins in those mice sperm. (b) Status of [cAMP]i post-treatment with cell permeable GSK3 specific inhibitor, SB216763. Incubation was done at 37 °C for 30 min. (c) Comparison of PKA activity as a function of [cAMP]i between wild-type and GSK3α knock out sperm cells in vitro. Incubation was done at 30 °C for 15 min. Data represent mean ± SEM of n = 3 for all the values. **p ≤ 0.01 versus untreated observations
3.2 |. Increased in PDE4 activity is responsible for the reduction in cAMP levels in sperm with reduced GSK3 catalytic activity
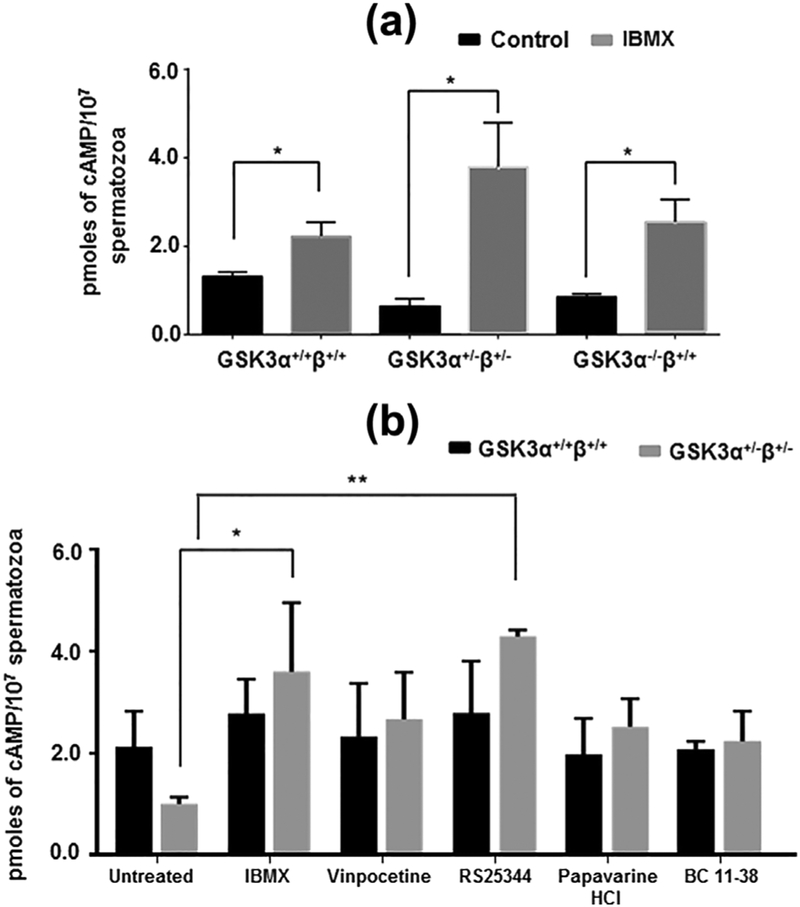
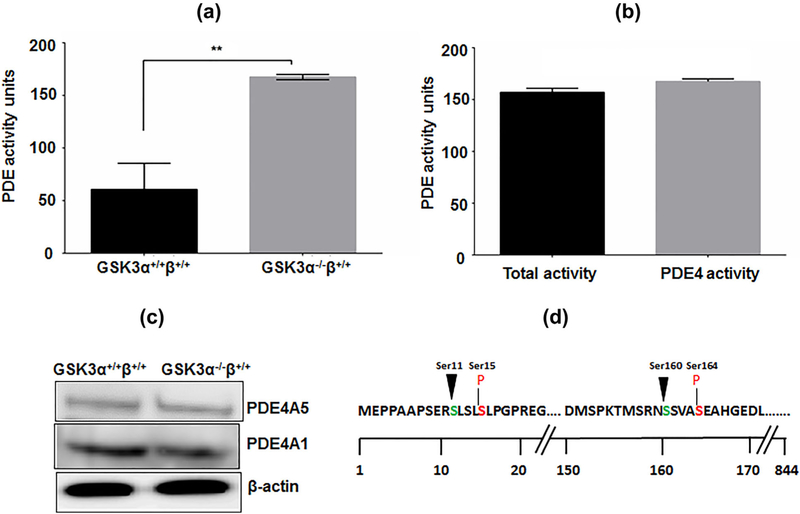
The levels of cAMP in GSK3 depleted spermatozoa increased when sperm were treated with the PDE inhibitor IBMX (Figure 2a). Cyclic AMP levels in sperm from mice heterozygous for Gsk3α and β or in sperm from mice lacking GSK3α, were increased in the presence of IBMX to levels comparable to that of normal wild-type sperm. In an effort to identify the PDE responsible for the decrease in cAMP we used several putative PDE isoform specific inhibitors. Experiments with the isoform specific PDE inhibitors revealed that the compound RS24344, considered a selective PDE4 antagonist (Maurice et al., 2014) could restore cAMP to levels comparable to the isoform-nonspecific PDE inhibitor IBMX. Thus PDE4 activity was likely elevated in sperm with reduced GSK3 levels (Figure 2b, Table 1). Next we determined PDE activity in sperm from wild-type and Gsk3α knockout mice. Figure 3 shows that net PDE activity was higher in extracts of sperm from GSK3α−/−β+/+compared to wild-type mice. It also suggests that PDE4 contributes almost entirely to the total PDE catalytic activity in Gsk3α knockout mouse sperm extracts (Figures 3a and 3b). This increased PDE activity in knockout sperm could be due to increased enzyme activity or due to higher protein levels of the enzyme. Levels of the PDE4 sub-isoforms A1 and A5 as seen in Western blots were not different in sperm extracts from knockout compared to wild-type mice (Figure 3c), indicating the protein levels was not the reason for the altered activity. Figure 3d shows possible phosphorylation sites of PDE4 at the GSK3 consensus sites; thus suggesting a relationship between GSK3 and PDE4.
FIGURE 2.
Effect of PDE antagonists in restoring intracellular cAMP status in GSK3 knocked down spermatozoa. (a) Effect of non-specific PDE inhibitor, IBMX (0.5 mM) after global deletion of copies of α and β alleles of GSK3 gene from mice. (b) Status of [cAMP]i after treating sperm cells with PDE isoform specific inhibitors: PDE1 inhibitor, Vinpocetine (100 μM); PDE4 inhibitor, RS25344 (5 μM); PDE10 inhibitor, Papaverine HCl (50 μM); PDE11 inhibitor, BC 11–38 (20 μM). Incubation was done at 37 °C for 30 min for all of the above treatments. Data represent mean ± SEM of at least n = 3 for all the values. *p ≤ 0.05, **p ≤ 0.01 versus control or untreated values
TABLE 1.
Effect of different isoform specific PDE antagonists in regulation of intracellular cAMP content in GSK3α knockout spermatozoa
| cAMP as % of control | ||
|---|---|---|
| GSK3α+/+β+/+ | GSK3α+/−β+/− | |
| Untreated | 100.0 | 100.0 |
| IBMX (0.5 mM) | 179.0 | 358.0 |
| Vinpocetine (100 μM) | 110.5 | 114.4 |
| RS25344 (5 μM) | 131 | 314.7 |
| Papaverine HCl (50 μM) | 93 | 185.2 |
| BC 11–38 (20 μM) | 98 | 164.4 |
Data represent mean ± SEM of n = 3 for all the values. Percentage values are in comparison to corresponding untreated values of GSK3α+/+β+/+ and GSK3α−/−β+/+.
FIGURE 3.
Verification of involvement of PDE4 for reduced cAMP titer in GSK3α knockout mice spermatozoa. (a) Comparison of profile of PDE4 activity in GSK3α+/+β+/+and GSK3α−/−β+/+ mice sperm cells. (b) Comparison of profile of total PDE activity and PDE4 activity in GSK3α−/−β+/+ mice sperm cells. Incubation was done at 30 °C for 30 min. Data represent mean ± SEM of at least n = 3 for all the values. **p ≤ 0.01, versus GSK3α+/+β+/+ values. (c) Comparison of levels of different PDE4 isoforms in whole cell lysates of GSK3α+/+β+/+and GSK3α−/ −β+/+ mice sperm cells. (d) Consensus sequence for GSK3 phosphorylating sites on PDE4A; arrow indicates Ser residue to be phosphorylated by GSK3 (nth residue), while n+ 4th pre-phosphorylated Ser residue is indicated in red
3.3 |. Cyclic AMP increases GSK3 phosphorylation
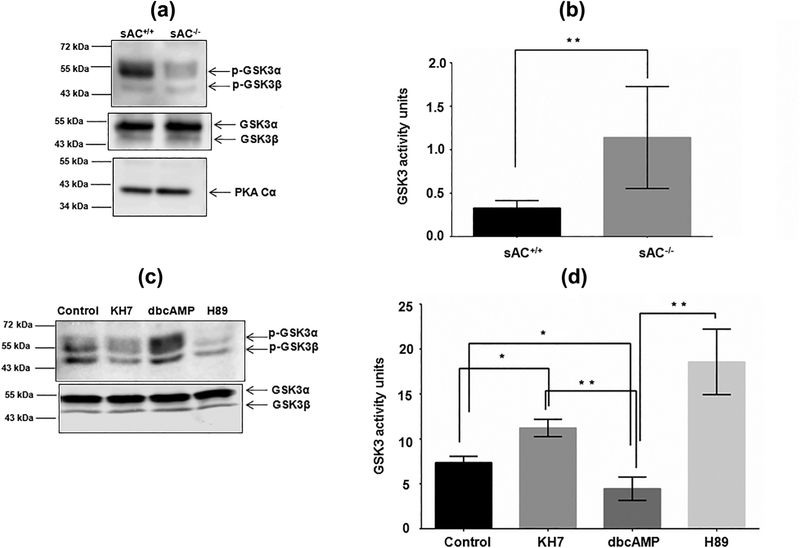
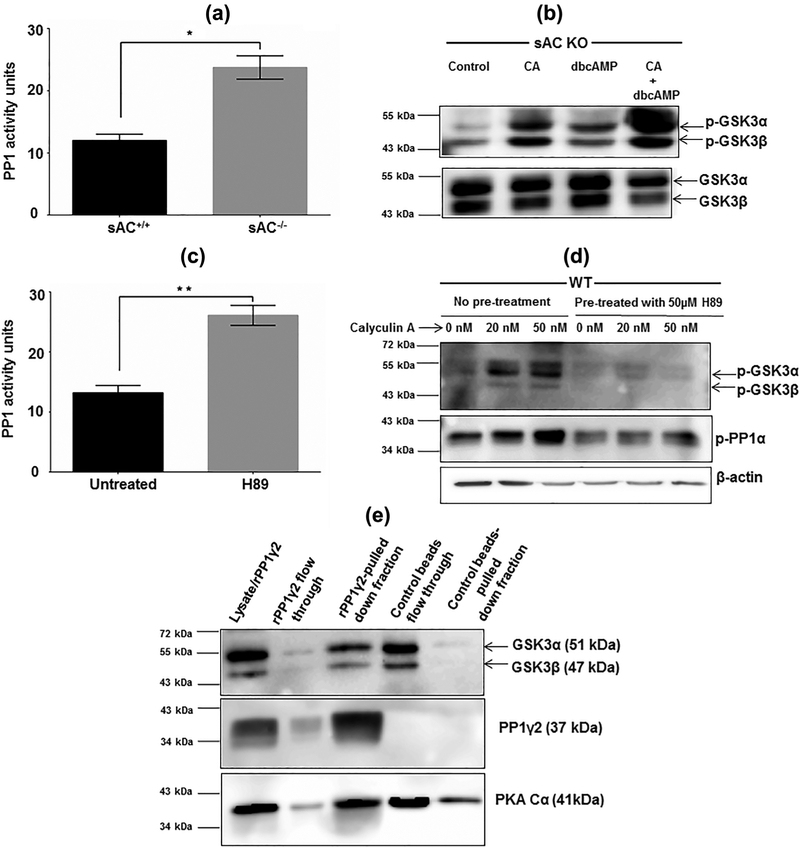
We have previously shown in bovine sperm that increased cAMP increased GSK3 phosphorylation levels (Vijayaraghavan et al., 2000). Here we wished to investigate in greater detail whether the relationship between cAMP and GSK3 is also present in mouse sperm. We used sAC knockout mice which lack cAMP due to the absence of soluble adenylyl cyclase (Hess et al., 2005). Figure 4a provides proof that GSK3 phosphorylation is significantly lower in sperm lacking sAC compared to wild-type sperm. Lowered phosphorylation is reflected in higher GSK3 activity in sperm lacking sAC. Catalytic activity of GSK3 was at least 2–3 fold higher in sAC null compared to wild type sperm (Figure 4b). Figure 4c shows that GSK3 phosphorylation is reduced in sperm treated with the sAC inhibitor KH7 and also by the PKA inhibitor H89. Conversely exogenously added cell permeant cAMP analog, dibutryl cAMP, increased GSK3 phosphorylation. As anticipated, treatments that reduced GSK3 phosphorylation increased its catalytic activity while treatments that increased GSK3 phosphorylation decreased it catalytic activity (Figure 4d).
FIGURE 4.
Contribution of cAMP in regulation of GSK3 activity in mouse spermatozoa. (a) GSK3 phosphorylation profile in sAC knockout mice sperm lysate. Lower panels indicate the corresponding levels of GSK3αβ and PKA in those mouse sperm. (b) Comparison of GSK3 activities in soluble protein fraction of the sAC knockout to wild-type mice sperm cells. Incubation was done at 30 °C for 15 min. Data represent mean ± SEM of at least n = 3 for all the values. **p ≤ 0.01 versus sAC+/+ values (c) Modulatory role of cAMP in Ser-phosphorylation status of GSK3 in vitro. Incubation was done at 37 °C for 30 min for all the treatments. The corresponding Western blot data for GSK3αβ in the lower panel was obtained by running samples from the same experiment in a different gel. (d) Regulatory effect of cAMP on GSK3 activity of wild-type C57/BL6 mice sperm protein. Data represent mean ± SEM of at least n = 3 for all the values. *p ≤ 0.05, **p ≤ 0.01 versus corresponding set as indicated in the figure
3.4 |. Regulation of GSK3 function by cAMP in mouse sperm is mediated by PP1
We have previously shown that PP1 inhibition increased GSK3 phosphorylation in bovine sperm (Vijayaraghavan et al., 2000). Here we investigated this interrelationship between cAMP, PP1, and GSK3 using sperm from sAC knockout mice. Figure 5a shows that PP1 activity is more than 2-fold higher in extracts of sperm lacking sAC compared to normal wild-type sperm. Supplementing sperm from sAC knockout mice with dbcAMP resulted in a nominal increase in GSK3 phosphorylation. However inhibition of PP1 with calyculin A in the absence or presence of dbcAMP significantly increased GSK3 phosphorylation suggesting that low cAMP levels and increased phosphatase activity were responsible for low GSK3 phosphorylation in sperm from sAC null mice (Figure 5b). Next we examined if the relationship between PP1 activity and cAMP action through PKA could also be observed in sperm from wild type mice (Figure 5c). Figure 5d shows that inhibition of PP1 by calyculin A elevated GSK3 phosphorylation and this increase was ablated in the presence of the PKA inhibitor H89. Thus PP1 and PKA seem to work in concert to contribute to the net phosphorylation level of GSK3.
FIGURE 5.
Involvement of PP1 in manifestation of cAMP/PKA axis for regulation of GSK3 function. (a) Comparison of phosphatase activity in soluble protein fraction of the sAC knockout to wild-type mice sperm cells; PP1 activity was determined using both I2 and okadaic acid (2 nM), separately. Data represent mean ± SEM of n = 5 for all the values. **p ≤ 0.01 vs sAC+/+ values. (b) In vitro regulation of Ser 21/9-phosphorylation profile of GSK3 by PP1 under influence of PKA in the sAC knockout mice sperm. (c) Effect of PKA inhibition in phosphatase activity in WT mouse sperm extract. (d) In vitro regulation of phospho-Ser-GSK3 profile by PP1 under influence of PKA in WT sperm. Pre-treatment using H89 was done for 30 min for the designated sets followed by incubation with calyculin A for another 30 min at 37 °C. (e) Pull down assay to check interactions among GSK3, PP1 and PKA using mouse rPP1γ2. All the probing were done using corresponding rabbit raised antibodies except for GSK3 where anti-mouse antibody was used
3.5 |. Interaction between PKA, PP1, GSK3, and PDE4
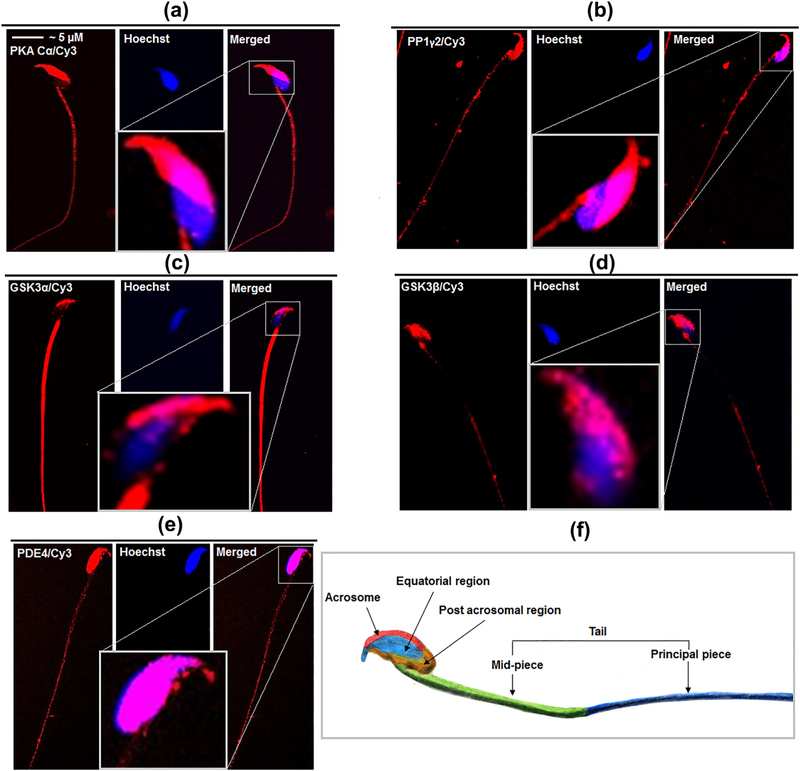
Figure 5e shows that recombinant PP1γ2 can pull down both its upstream and downstream regulator, PKA Cα, and GSK3α/β, respectively. Immuno-cytochemical study for the presence of these molecules including PDE4 provides significant evidence for the points of interactions among these signaling molecules (Figure 6a–e). Table 2 summarizes these interaction points; PKA and PP1γ2 can interact at equatorial as well as at tail regions of mouse sperm. PP1γ2 and GSK3α share common contact points in sperm acrosome and tail region while GSK3β co-localizes with PP1γ2 in the equatorial region of sperm head; PDE4 and GSK3α/β share localization in different portions of mouse sperm head.
FIGURE 6.
Immuno-cytochemical study for the localization of PKA, PP1, GSK3α/β, and PDE4 in mouse spermatozoa. Mouse spermatozoa were labeled rabbit anti-PKA C α (a), rabbit anti-PP1γ2 (b), rabbit anti-GSK3α (c) rabbit anti-GSK3 β (d) and rabbit anti-PDE4 (e) antibodies. Specificity of the primary antibodies was previously confirmed. Specific secondary antibodies conjugated with Cyanine 3 fluorophores, were used. Sperm nucleus, was stained with Hoechst. Details of the antibodies and dilutions used for them have been provided in the section 2. The inset figures provided magnified view of the sperm head stained with corresponding antibodies. (f) An image of mouse sperm taken by bright field microscope was manually colored to display different regions in a sperm to enable better understanding of the immunocytofluorescence data
TABLE 2.
Point of interaction among PKA, PP1, GSK3 and PDE4
| Acrosome | Equatorial region | Post acrosomal | Mid-piece | Principal piece | |
|---|---|---|---|---|---|
| PKA Cα | − | + | − | + | + |
| PP1γ2 | + | + | − | + | + |
| GSK3α | + | − | − | + | + |
| GSK3β | − | + | − | − | − |
| PDE4 | + | + | + | + (low) | + (low) |
At least three sets of sperm cells were investigated by immune-cytochemistry technique to verify the localization of these signaling molecules.
3.6 |. Effect of pharmacological inhibitors of GSK3 on mouse IVF
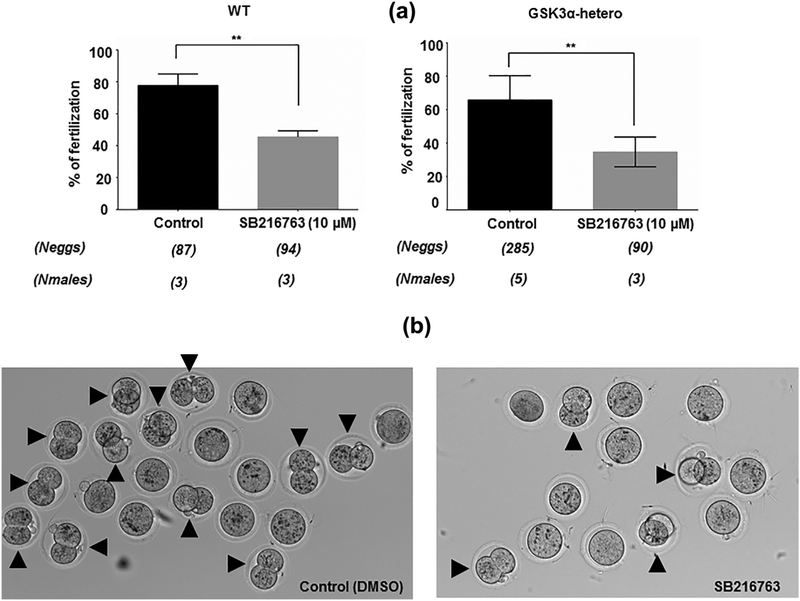
While we have shown that GSK3 is essential in sperm, the role of the enzyme during fertilization is not known. Figure 7a suggests that GSK3 activity may be required in sperm for fertilization, as pharmacological inhibition of this enzyme caused significant reduction in percent of fertilized eggs observed by counting numbers of two-cell staged embryo (Figure 7b). A more potent inhibition was observed when one copy Gsk3α was already deleted where net IVF was reduced from 70% in control to 30% in inhibitor-treated sperm.
FIGURE 7.
Physiologic relevance of residual GSK3 activity in fertilization process. (a) Bar diagrams showing the percent of two-cell stage formation after SB216763 (10 μM) treatment of WT and GSK3α-hetero sperm. Untreated set contained equivalent amount of DMSO used for solubilizing SB216763 in the treated set. **p ≤ 0.01 versus control observations. (b) A set of representative light microscopic image showing the reduced number of two-cell staged embryo; black arrow showing the oocytes that formed two-cell stage
4 |. DISCUSSION
GSK3 is a key signaling enzyme in both somatic and germ cells. The role of the second messenger cAMP in GSK3 signaling is not well investigated. There has been some progress made to determine its status in GSK3 signaling in cardiomyocytes and renal cells (Rao, Patel, Hao, Woodgett, & Harris, 2010; Tao et al., 2015; Zhou et al., 2010). The present study, shows that GSK3 regulates sperm cAMP levels by modulating PDE4 function in mouse sperm. Cyclic AMP/PKA in turn plays a role in regulating GSK3 activity through modifying sperm PP1γ2 activity. Previously, studies in cells in culture show that a similar regulatory relationship between GSK, cAMP and PP1 exists (Fang et al., 2000; Hernandez et al., 2010; Tanji et al., 2002). This is the first study to show that an interrelationship between GSK3, cAMP, and PP1 is present in sperm.
Cardiomyocytes from GSK3α knockout mouse contain lowered cAMP levels (Zhou et al., 2010). Targeted deletion of Gsk3β in renal epithelial cells also causes reduction in intracellular cAMP levels (Rao et al., 2010; Tao et al., 2015). If two copies of any of the two GSK3 isoforms are absent in mouse sperm, [cAMP]i levels are reduced to almost half of that in wild-type sperm (Figure 1a). The reason behind this depletion of cAMP titer was not due to changes in sperm ATP levels, because only the absence GSK3α, but not the β-isoform, results in altered hexokinase activity and lowered ATP levels (Bhattacharjee et al., 2018). Changes in sAC activity in sperm lacking GSK3α sperm is unlikely because intracellular Ca2+ titer of these cells were unaltered (Supplementary Figure S2) and because PDE inhibitors could restore cAMP levels. A likely target of GSK3 is sperm cAMP phosphodiesterase. Sperm lacking GSK3 possessed significantly higher PDE activity compared to WT sperm; a major proportion of the total sperm PDE activity was contributed by PDE4 in those mice (Figures 3a and 3b). PDE4 contains at least two GSK3 phosphorylating motifs (Figure 3d). Interaction between GSK3 and PDE4 has been shown to occur (Carlyle, Mackie, Christie, Millar, & Porteous, 2011; Soares, Carlyle, Bradshaw, & Porteous, 2011). GSK3β has also been found to be involved in phosphorylation and proteosomal degradation of PDE4 (Zhu et al., 2010). However, due to the unavailability of a phospho-PDE4 antibody we could not compare the phosphorylation status of sperm PDE4 from WT and knockout mice. But as Figure 3c shows, alteration in PDE4 protein levels was not responsible for the changes in cAMP in sperm lacking GSK3. Taken together our data suggest that GSK3 is involved in regulating sperm cAMP levels. Further studies with purified PDE4 are required to determine whether it is a target for GSK3 phosphorylation. The effect of GSK3 on sperm cAMP breakdown will be consistent with the notion that GSK3 is generally considered a negative regulator in cells.
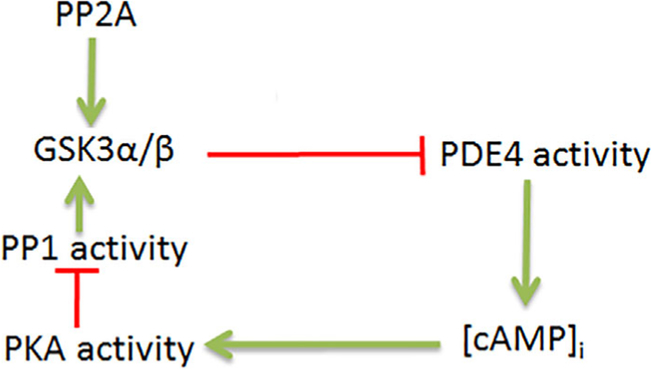
In immature caput epididymal sperm, the phosphatases PP2A and PP1 contribute to lower GSK3 phosphorylation and consequently higher catalytic activity. In mature caudal epididymal spermatozoa, activities of these phosphatases are significantly lower and perhaps as a result GSK3 phosphorylation is higher leading to its lower catalytic activity. The observation that sperm lacking sAC have lower GSK3 phosphorylation and increased GSK3 activity (Figures 4a and 4b) compared to normal sperm, clearly suggests a role for cAMP/PKA in either directly or indirectly in regulating GSK3 phosphorylation. It appears that increased PKA activity is associated with decreased protein phosphatase activity. This suggestion is also consistent with the observation that sperm treated with the cAMP-analogue, dbcAMP, have lower GSK3 activity (Figures 4c and 4d). It has been shown that the absence of GSK3α results in high PP1γ2 activity in caudal sperm, thus making them similar to immature caput sperm (Bhattacharjee et al., 2015). Data in this report suggest that altered PP1 activity in sperm lacking GSK3 could be due to decreased cAMP levels and PKA activity (Figures 1a and 1c). This relationship between cAMP, PP1 and GSK3 activity was also confirmed in sperm lacking sAC (Figures 5a and 5b). It is possible that PKA phosphorylates Thr320 of the carboxy terminus of PP1 and inhibits its activity. Alternatively, PKA could be responsible for phosphorylating the protein regulators of sperm PP1γ2 (Figure 5c). Direct interaction between PKA and GSK3 and between PP1 and GSK3 have been shown in previous studies in somatic while a possible interaction between PKA and PP1γ2 has been suggested in mouse spermatozoa (Fang et al., 2000; Goto & Harayama, 2009; Hernandez et al., 2010; Tanji et al., 2002). Whether these three signaling enzymes form a trimeric complex or whether they form a complex mediated by one or more scaffolding proteins requires further investigation. Figure 8 depicts a likely mechanistic scheme for regulation of GSK3 by cAMP.
FIGURE 8.
Schematic diagram for regulation of GSK3 function by PDE4-cAMP-PKA axis. Green and red arrows indicate stimulatory and inhibitory actions, respectively
It appears that an active GSK3 in immature sperm and its inhibition in mature sperm are essential for normal epididymal sperm maturation. Deletion of Gsk3α (but not the beta isoform) during sperm differentiation leads to abnormal motility patterns that result in infertility (Bhattacharjee et al., 2015). Thus GSK3α isoform appears to be essential for normal epididymal sperm maturation. Whether there is a role for GSK3 in sperm following their maturation in the epididymis is an intriguing question. We have found that IVF by wild-type sperm or sperm from Gsk3α-heterozygous mice are impaired when treated with the GSK3 inhibitor, SB21673. A 50–60% reduction in IVF is observed in inhibitor treated sperm. Thus loss of GSK3 in sperm or inhibition of GSK3 in wild-type both result in impaired fertilization. Thus it is likely that GSK3 may not only have a role during sperm development in the epididymis but also during fertilization. Changes in Ser-phosphorylation of GSK3 during human sperm capacitation has been reported (Ando & Aquila, 2005; Aquila et al., 2005, 2009).
The link between GSK3 activities with cAMP levels appears to be at odds with the long held belief that an increase in cAMP levels is responsible for epididymal sperm maturation and initiation of motility (Bedford & Hoskins, 1990; Carr & Acott, 1989). Our data would appear to suggest that lower GSK3 activity, as seen in mature caudal epididymal sperm, should be associated with decreased, rather than increased, cAMP levels. A simple correlation between cAMP levels and sperm function is not possible because the signaling pathways operating during epididymal sperm are more complex than previously recognized. For example, it is known that elimination of cAMP action, by sAC or PKA catalytic subunit knockout, does not abolish sperm motility: sperm from these knockout mice are not immotile but do have basal motility (Esposito et al., 2004; Nolan et al., 2004). We and others have already shown that high protein phosphatase activity limits sperm motility (Fardilha et al., 2011; Vijayaraghavan et al., 1996). Furthermore, disruption of GSK3α either by knockout of the gene or by knockout of Wnt signaling impairs epididymal sperm maturation possibly due to elevated protein phosphatase activity (Bhattacharjee et al., 2015; Koch, Acebron, Herbst, Hatiboglu, & Niehrs, 2015). Yet another signaling enzyme involved in epididymal sperm maturation is the calcium regulated phosphatase calcineurin (Miyata et al., 2015). Determination of the mechanistic details of the interrelationships between these enzymes and how changes in their activities are triggered in developing sperm requires additional studies. In summary data in this report for the first time show that the signaling enzymes GSK3, PKA, and PP1γ2 are interrelated in sperm.
Supplementary Material
Funding information
National Institute of Health (NIH), USA, Grant number: R21HD086839
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose and declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- Ando S, & Aquila S (2005). Arguments raised by the recent discovery that insulin and leptin are expressed in and secreted by human ejaculated spermatozoa. Molecular and Cellular Endocrinology, 245(1–2), 1–6. [DOI] [PubMed] [Google Scholar]
- Aquila S, Gentile M, Middea E, Catalano S, Morelli C, Pezzi V, & Ando S (2005). Leptin secretion by human ejaculated spermatozoa. The Journal of Clinical Endocrinology and Metabolism, 90(8), 4753–4761. [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, & Ando S (2009). Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reproductive Biology and Endocrinology, 7, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JM, & Hoskins DD, (1990). The mammalian spermatozoon: Morphology, biochemistry and physiology In Lamming GE, (Ed.), Marshall’s physiology of reproduction. Edinburgh, London, Melbourne, New York: Churchill Livingstone; Vol. 379. [Google Scholar]
- Bender AT, & Beavo JA (2006). Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacological Reviews, 58(3), 488–520. [DOI] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, & Jope RS (2015). Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacology & Therapeutics, 148, 114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R, Goswami S, Dudiki T, Popkie AP, Phiel CJ, Kline D, & Vijayaraghavan S (2015). Targeted disruption of glycogen synthase kinase 3A (GSK3A) in mice affects sperm motility resulting in male infertility. Biology of Reproduction, 92(3), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R, Goswami S, Dey S, Gangoda M, Brothag C, Eisa A, … Vijayaraghavan S (2018). Isoform specific requirement for GSK3α in sperm for male fertility. Biology of Reproduction, 10.1093/biolre/ioy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Wertheimer EV, Visconti PE, & Krapf D (2014). Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochimica Et Biophysica Acta, 1842(12 Pt B), 2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle BC, Mackie S, Christie S, Millar JK, & Porteous DJ (2011). Co-ordinated action of DISC1, PDE4B and GSK3beta in modulation of cAMP signalling. Molecular Psychiatry, 16(7), 693–694. [DOI] [PubMed] [Google Scholar]
- Carr DW, & Acott TS (1989). Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biology of Reproduction, 41(5), 907–920. [DOI] [PubMed] [Google Scholar]
- Dacheux JL, & Dacheux F (2014). New insights into epididymal function in relation to sperm maturation. Reproduction, 147(2), R27–R42. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, & Dedhar S (1998). Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proceedings of the National Academy of Sciences of the United States of America, 95(19), 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudiki T, Kadunganattil S, Ferrara JK, Kline DW, & Vijayaraghavan S (2015). Changes in carboxy methylation and tyrosine phosphorylation of protein phosphatase PP2A are associated with epididymal sperm maturation and motility. PLoS ONE, 10(11), e0141961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essayan DM (2001). Cyclic nucleotide phosphodiesterases. Journal of Allergy and Clinical Immunology, 108(5), 671–680. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, … Gossen JA (2004). Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC Jr., Woodgett JR, & Mills GB (2000). Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proceedings of the National Academy of Sciences of the United States of America, 97(22), 11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardilha M, Esteves SL, Korrodi-Gregório L, Pelech S, da Cruz E, Silva OA, … Silva E (2011). Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility. Molecular Human Reproduction, 17(8), 466–477. [DOI] [PubMed] [Google Scholar]
- Freitas MJ, Vijayaraghavan S, & Fardilha M (2017). Signaling mechanisms in mammalian sperm motility. Biology of Reproduction, 96(1), 2–12. [DOI] [PubMed] [Google Scholar]
- Goto N, & Harayama H (2009). Calyculin A-Sensitive protein phosphatases are involved in maintenance of progressive movement in mouse spermatozoa In vitro by suppression of autophosphorylation of protein kinase A. The Journal of Reproduction and Development, 55(3), 327–334. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, & Tsien RY (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry, 260, 3440–3450. [PubMed] [Google Scholar]
- Hernandez F, Langa E, Cuadros R, Avila J, & Villanueva N (2010). Regulation of GSK3 isoforms by phosphatases PP1 and PP2A. Molecular and Cellular Biochemistry, 344(1–2), 211–215. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, … Moss SB (2005). The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental Cell, 9(2), 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, & Woodgett JR (2000). Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature, 406(6791), 86–90. [DOI] [PubMed] [Google Scholar]
- Huang Z, & Vijayaraghavan S (2004). Increased phosphorylation of a distinct subcellular pool of protein phosphatase, PP1gamma2, during epididymal sperm maturation. Biology of Reproduction, 70(2), 439–447. [DOI] [PubMed] [Google Scholar]
- Keravis T, & Lugnier C (2012). Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. British Journal of Pharmacology, 165(5), 1288–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Acebron SP, Herbst J, Hatiboglu G, & Niehrs C (2015). Post-transcriptional wnt signaling governs epididymal sperm maturation. Cell, 163(5), 1225–1236. [DOI] [PubMed] [Google Scholar]
- Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, & Manganiello VC (2014). Advances in targeting cyclic nucleotide phosphodiesterases. Nature Reviews Drug Discovery, 13(4), 290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M, & Wandosell F (2011). Deconstructing GSK-3: the fine regulation of its activity. International Journal of Alzheimer’s Disease, 2011, 479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Satouh Y, Mashiko D, Muto M, Nozawa K, Shiba K, … Ikawa M (2015). Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science, 350(6259), 442–445. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, & Behringer R (2003). Manipulating the mouse embryo. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Noel S, Dhooghe B, & Leal T (2012). PDE5 inhibitors as potential tools in the treatment of cystic fibrosis. Frontiers in Pharmacology, 3(167), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, & McKnight GS (2004). Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proceedings of the National Academy of Sciences of the United States of America, 101(37), 13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K, & Kotera J (2007). Overview of PDEs and their regulation. Circulation Research, 100(3), 309–327. [DOI] [PubMed] [Google Scholar]
- Rao R, Patel S, Hao CM, Woodgett J, & Harris R (2010). GSK3 beta mediates renal response to vasopressin by modulating adenylate cyclase activity. Journal of the American Society of Nephrology, 21(3), 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryves WJ, Fryer L, Dale T, & Harwood AJ (1998). An assay for glycogen synthase kinase 3 (GSK-3) for use in crude cell extracts. Analytical Biochemistry, 264(1), 124–127. [DOI] [PubMed] [Google Scholar]
- Sinha N, Puri P, Nairn AC, & Vijayaraghavan S (2013). Selective ablation of Ppp1cc gene in testicular germ cells causes oligoteratozoospermia and infertility in mice. Biology of Reproduction, 89(5), 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DC, Carlyle BC, Bradshaw NJ, & Porteous DJ (2011). DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chemical Neuroscience, 2(11), 609–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, Jack SL, & Vijayaraghavan S (2004). Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. Journal of Andrology, 25(4), 605–617. [DOI] [PubMed] [Google Scholar]
- Tanji C, Yamamoto H, Yorioka N, Kohno N, Kikuchi K, & Kikuchi A (2002). A-kinase anchoring protein AKAP220 binds to glycogen synthase kinase-3beta (GSK-3beta) and mediates protein kinase A-dependent inhibition of GSK-3beta. Journal of Biological Chemistry, 277(40), 36955–36961. [DOI] [PubMed] [Google Scholar]
- Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, & Rao R (2015). Glycogen synthase kinase-3beta promotes cyst expansion in polycystic kidney disease. Kidney International, 87(6), 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torremans A, Ahnaou A, Van Hemelrijck A, Straetemans R, Geys H, Vanhoof G, … Drinkenburg WH (2010). Effects of phosphodiesterase 10 inhibition on striatal cyclic AMP and peripheral physiology in rats. Acta Neurobiologiae Experimentalis, 70(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Vadnais ML, Aghajanian HK, Lin A, & Gerton GL (2013). Signaling in sperm: Toward a molecular understanding of the acquisition of sperm motility in the mouse epididymis. Biology of Reproduction, 89(5), 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Mohan J, Gray F, Khatra B, & Carr D (2000). A role for phosphoryaltion of glycogen synthase kinase-3alpha in bovine sperm motility regulation. Biology of Reproduction, 62, 1647–1654. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, & Hoskins DD (1986). Regulation of bovine sperm motility and cyclic adenosine 3′,5′-monophosphate by adenosine and its analogues. Biology of Reproduction, 34(3), 468–477. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, & Greengard P (1996). Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biology of Reproduction, 54(3), 709–718. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Trautman KD, Goueli SA, & Carr DW (1997). A tyrosine-phosphorylated 55-kilodalton motility-associated bovine sperm protein is regulated by cyclic adenosine 3′,5′-monophosphates and calcium. Biology of Reproduction, 56(6), 1450–1457. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, … Force T (2010). GSK-3alpha directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. The Journal of Clinical Investigation, 120(7), 2280–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Suk HY, Yu RYL, Brancho D, Olabisi O, Yang TTC, … Chow CW (2010). Evolutionarily conserved role of calcineurin in phosphodegron-Dependent degradation of phosphodiesterase 4D. Molecular and Cellular Biology, 30(18), 4379–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.