Abstract
Improvements in health care depend on research involving health-care providers (HCPs) and health-care organizations (HCOs). Existing research suggests that involvement in research studies is still much lower than it could be. This study investigates factors that may impede or facilitate research involvement. A standardized online questionnaire was used to carry out a survey, in 3 countries, of key informants in colorectal cancer centers that hold certification in accordance with the requirements of the German Cancer Society. A total of 184 individuals responded (response rate 65%). The respondents found it difficult to identify studies suitable for their patients (40% agreement), criticized the small overall number of studies available (48%), and found that many studies are not worthwhile financially (56%). Among respondents who were not involved in studies as the principal investigators (PIs), 66% agreed they lacked the research infrastructure needed and 81% that they did not have enough staff. Among respondents who were involved as PIs, only 22% indicated that their hospital management encouraged them to initiate and conduct clinical trials. Eighty-five percent of the respondents agreed that the general population lacks information about the importance of studies. Five recommendations for health policy makers are derived from these findings for ways of increasing the involvement of HCPs and HCOs in research, and in cancer research in particular.
Keywords: clinical trial, recruitment, research subject, data collection, cancer, patient selection, sample size, consent
Introduction
The involvement of health-care providers (HCPs) and health-care organizations (HCOs) in research studies is crucial for the further development of patient care—whether preventive or acute or related to surgery, drug therapy, or psychosocial care. There have been many campaigns aiming to encourage both HCPs and HCOs to take part in research and provide some financial and also reputational support, among which both large programs like the Cancer Moonshot Initiative as well as incentives to increase trial participation like the one launched by the German Cancer Society (details below) can be subsumed. However, the existing research suggests that the extent to which patients are included in clinical trials is still much lower than it could be.1,2
Involvement in research is one way in which physicians can make a difference in clinical practice, and it has long been regarded as offering a high level of intrinsic motivation—for example, through the intellectual stimulation and challenge that research involves.3 For many, doing research is regarded as part of a doctor’s job description, and it has even been argued that there is a moral obligation for HCPs to engage in research.4,5 Both research findings and expert opinion statements have drawn attention time and again to the small numbers of patients who are being included in trials and the slowness of recruitment, with many trials not reaching their recruitment targets.1,2 The need to conduct more research into this problem and find ways of supporting HCPs with recruitment has also been emphasized.6,7 There is a strong body of research describing the barriers that prevent HCPs and HCOs from becoming involved in research. Reviews of research involvement suggest that a wide range of barriers exist in addition to time restrictions—most notably, no interest in research and a lack of research skills, but also issues involving the complexity of study protocols, lack of staff, fear of negatively affecting relationships with patients, and the administrative burden associated with involvement in trials.8-10 The barriers reported have changed little in recent decades. Once a study has started, difficulties in identifying patients become another important issue.9 Besides barriers reported by doctors, interesting insights into the organizational factors that are associated with patient recruitment into trials have recently been provided by Williams et al11 and Rigal et al12 who describe wide variation in recruitment rates between sites and recommend that attention should be given to the way in which sites are structured and what types of patient population they serve before a study is set up.
In addition to obstacles that exist for providers, there are of course also barriers that prevent patients from participating in research. Although this is not the focus of the present report, we may briefly mention here some of the issues that have been described in the literature, in order to provide a more comprehensive picture. Systematic reviews of patient-reported barriers to participation in (cancer) trials have reported a wide variety of patient-reported concerns, among them concerns with the trial design, dislike of randomization, issues with the information provided, and the additional demands on them associated with participation in the trial.10,13 Qualitative research suggests that there is a strong connection between the patients’ knowledge of a disease and its treatment, as well as the research process, and their willingness to take part.14 Reasons motivating patients to participate include expectations of personal health benefits and an opportunity to be of help to other patients.15-17 In addition, there appears to be evidence that financial incentives for patients are effective.18 Other authors have investigated the effects of ethnicity, sex, age, and socioeconomic position on trial participation.19,20 It was found that minority groups, women, and the elderly population, as well as groups with lower socioeconomic status, are underrepresented in cancer trials. Similarly, the study by Williams et al mentioned above, using data for the social environment, reported that there were higher recruitment rates in family physician practices located in areas with higher socioeconomic status.11
Factors that actively facilitate research participation for physicians (apart from simply an absence of obstructive factors such as having patients of higher socioeconomic status) include motivational elements such as financial incentives, a feeling that one is able to “make a difference,” and reputation among peers—that is, scientific merit and opinion leadership that may ultimately lead to more prestigious academic positions. One way to increase study involvement on a regional or national basis is to formulate laws or implicit standards that make participation in studies mandatory. One such initiative is the cancer center certification program organized by the German Cancer Society (Deutsche Krebsgesellschaft [DKG]). Cancer centers in Germany and abroad can apply for certification as an organ cancer center (such as breast cancer center, prostate cancer center, colorectal cancer center [CRCC]) within this voluntary program. In order to be awarded the certificate centers have to fulfill a set of criteria that include, for example, treatment according to the clinical guidelines, staffing, technical infrastructure, minimum caseloads, and multidisciplinary care. Fulfillment of these standards is checked based on data provided and during on-site audits.21 As of January 2018, over 450 hospitals in Germany, Austria, Italy, and Switzerland hold one or more certificate. One of the requirements for certification of cancer centers is a research study participation quota of 5% of primary patients—a requirement that makes all certified centers research hospitals but is difficult to achieve for many of them. Studies counted for the quota are not limited to randomized trials but include weaker designs as well. If noncompliance with the requirements is found during the audit, the center has 3 months to remedy this deviation, with positive remedying being the precondition for the award of the certificate. Thus, centers may fall short of 5% recruitment in one, but not the following year.
Since most work on barriers and facilitators to research involvement has been conducted in the United States and the United Kingdom, this article investigates the point of view of key individuals working in CRCCs in Germany, Austria, and Switzerland to identify issues that may be specific for these countries and to develop recommendations to increase study participation.
Methods
We included all CRCC sites certified in accordance with DKG requirements at the time of the study. CRCC provides all services related to colorectal cancer care including—but not limited to—screening, surgery, radiotherapy, systemic therapy, psychosocial, and palliative care in multidisciplinary teams including office-based physicians to ensure continuity of care. The minimum annual caseload requirement for CRCC sites is ≥30 operative patients with primary colon cancer and ≥20 operative patients with primary rectum cancer. The CRCCs can be funded privately (for profit), publicly, or charitably and include academic as well as nonacademic sites. The coordinators of all 284 CRCC sites certified at the time of the study were asked to participate in a survey using a standardized online questionnaire. The coordinators were able to delegate the survey to a deputy if they felt they lacked the necessary expertise to answer it. The survey was developed using the “SoSci Survey” tool, which is free of charge for academic and noncommercial users.22 The coordinators were contacted with one e-mail announcing the survey and a second one 4 days later that contained a personalized link to the survey. The data were collected anonymously, in that the answers could not be linked to the respondents. The software kept a record of who had responded to the questionnaire, and nonresponders were reminded up to 2 times (after 10 and 23 days). The survey period was November 21, 2016, through December 30, 2016. The survey was developed as part of a research study (ESDa) to evaluate the “StudyBox” (note 1), a trial registry for certified CRCCs.23,24 This was the second survey of CRCC coordinators conducted in the ESDa study, following an earlier one in June and July 2015—that is, 18 months earlier—partly containing the same items.24 The study was funded by the German Ministry of Health and was approved by the ethics committee of the Medical Association of Berlin (Eth-08/15).
The survey comprised items on factors that facilitate and obstruct study involvement, as well as site characteristics (eg, patient volume, ownership, teaching status). The selection of items was based on a literature review, adaptation of existing items,9,25 and qualitative testing. Details are reported by Kowalski et al,24 Agree/disagree scales were applied, ranging from 0 = “strongly disagree” to 4 = “strongly agree.” The questionnaire comprised items on the identification of studies, factors obstructing and facilitating their initiation, and issues with patient recruitment, most of which had been used in the survey of coordinators in the same centers in 2015 and are reported in the earlier article.24 The respondents were also asked about items in the StudyBox and the accreditation process, as well as 2 open-ended questions that are not reported here. In addition to the items used in 2015, and following the respondents’ comments, items on financial and academic rewards, about the “scientific infrastructure,” and about the centers’ primary experience with setting up studies were added to the 2016 survey in order to illuminate specific issues that the centers had. Overall, the respondents were asked 29 to 44 items, depending on filtering. The data were analyzed using descriptive statistics. Some of the results were compared with the results from the earlier survey in order to detect changes over time. Analyses were carried out using IBM SPSS Statistics, version 23.0.
Results
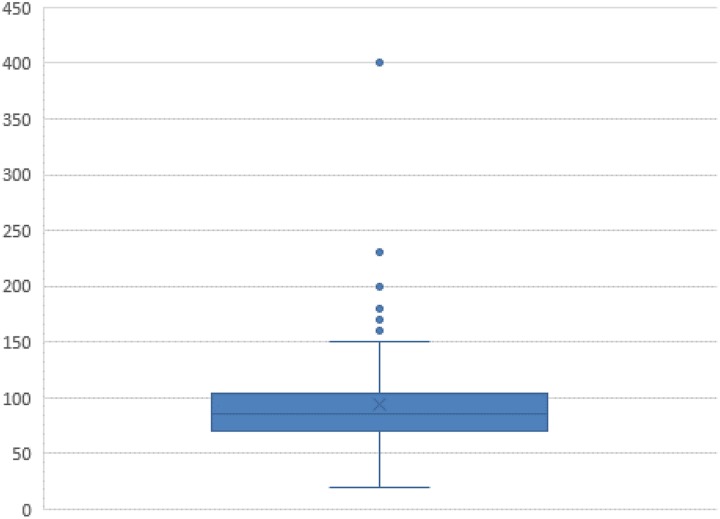
A total of 184 individuals responded (response rate 64.8%). On average, the completers spent 6.5 minutes answering the survey. Most of the participants were from public hospitals, one-third from charitable ones, and 13.7% from privately owned institutions (Table 1). The vast majority were teaching hospitals (86.3%), and 54.1% were located in cities with more than 100 000 inhabitants. A little over one-third had at least one study principal investigator (PI) on site. The participants’ indication of the number of patients with primary colorectal cancer treated in 2015 (Table 2 and Figure 1) yielded a mean of 93 and a median of 85. These figures are very similar to the numbers found for all sites in the same year as reported in the annual report, which includes validated data from the centers that provide the basis for certification (mean: 92.4; median: 87, range: 42-233). However, some survey responses were implausible, possibly due to typing errors (eg, 20 primary cases is well below the minimum requirement of 50). The numbers of patients recruited for studies (mean: 17.4; median: 11, range: 0-125) are very close to those documented for certification by all sites as published in the annual report (mean: 19.5; median: 13, range: 0-127), with a mean of 18.3% of patients recruited for studies (median: 13, range: 0%-88%).
Table 1.
Characteristics of the Sample: Hospital Ownership, Teaching Status, and Urban Population Size.
| Site characteristic | % (n) |
|---|---|
| Hospital ownership (missing responses: 2) | |
| Charitable | 31.9 (58) |
| Public | 54.4 (99) |
| Private | 13.7 (25) |
| Teaching status (missing responses: 2) | |
| Yes | 86.3 (157) |
| No | 13.7 (25) |
| Urban population (missing responses: 3) | |
| <20 000 | 3.3 (6) |
| 20 000-100 000 | 42.5 (77) |
| >100 000 | 54.1 (98) |
| Principal investigator at the site (missing responses: 0) | |
| Yes | 39.7 (73) |
| No | 60.3 (111) |
Table 2.
Hospital Sample: Patient Volume, Study Patients.
| Site characteristic | Mean (Annual Report26) | Median (Annual Report26) | Minimum– Maximum (Annual Report26) |
|---|---|---|---|
| Primary cases of colorectal cancer in 2015 (estimate; missing data: 10) | 93.0 (92.4) | 85 (87) | 20-400 (42-233) |
| Patients with colorectal cancer recruited for studies in 2015 (estimate; missing data: 8) | 17.4 (19.5) | 11 (13) | 0-125 (0-127) |
Figure 1.
Number of patients with primary colorectal cancer treated in 2015.
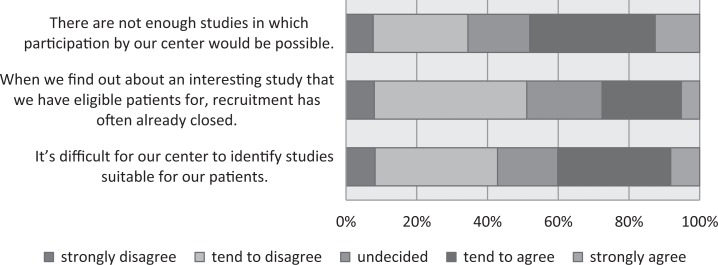
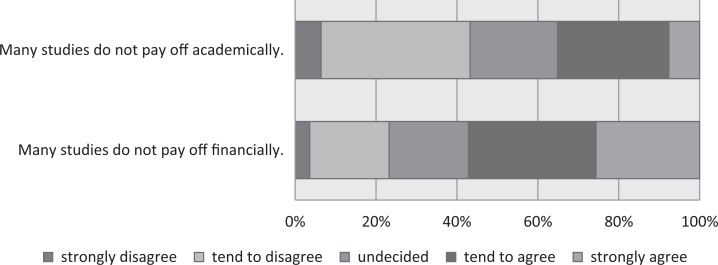
In a first step, we asked the site coordinators about issues concerning the identification of studies in which they might participate. Forty-eight percent of the respondents (the top 2 in the 5 categories, “strongly agree” and “tend to agree”) agreed with the statement that “There are not enough studies in which participation by our center would be possible” and 40% agreed that “It’s difficult for our center to identify studies suitable for our patients” (Figure 2). In comparison with 2015, there was a notable increase in the proportion of respondents agreeing with the item “When we find out about an interesting study that we have eligible patients for, recruitment has often already closed” (2015: 17%; 2016: 28%; 2015 survey results in Appendix A). Another major barrier appears to be the anticipated reward: The majority of respondents agreed that many studies are not worthwhile for them financially or even academically and over one-third agreed that many studies are not worthwhile (Figure 3).
Figure 2.
Identifying studies.
Figure 3.
Worthwhileness of conducting research studies.
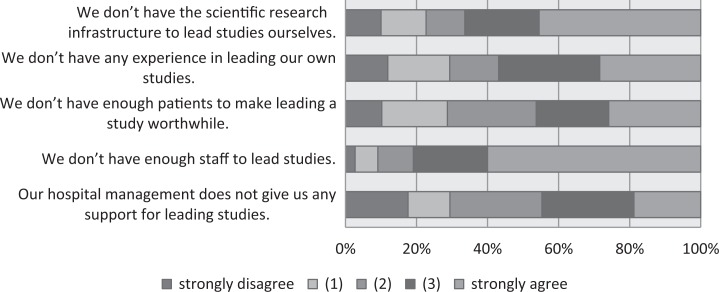
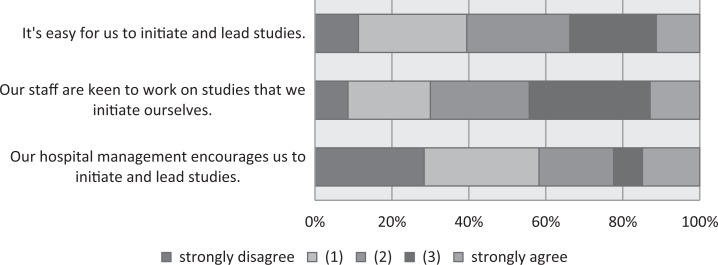
In a second step, we focused on the on-site initiation of studies. Respondents who indicated that their site was one that does not initiate any clinical trials (non-PI sites, n = 111) were asked to respond to the items shown in Figure 4. The highest proportions of agreement (3 and 4 on the 0-4 agree/disagree scale) were found for the items “We don’t have enough staff to lead studies” (80%) and “We don’t have the scientific research infrastructure to lead studies ourselves” (66%). Proportions of 57%, 46%, and 44% agreement were noted for the items “We don’t have any experience in leading our own studies,” “We don’t have enough patients to make leading a study worthwhile,” and “hospital management does not give us any support for leading studies”. The questions were already asked in 2015 (Appendix A), and no notable changes were seen in 2016. The respondents from sites that lead studies (PI sites, Figure 5) also reported a lack of support from hospital management, with 58% disagreeing that hospital management encouraged them to initiate and lead studies. Forty-four percent were in agreement that their “staff are keen to work on studies that we initiate ourselves” and 35% agreed that for them it was “easy to initiate and lead studies.” No notable changes were observed in comparison with 2015.
Figure 4.
Non-principal investigator sites: barriers to initiating studies.
Figure 5.
Principal investigator sites: reasons for conducting studies.
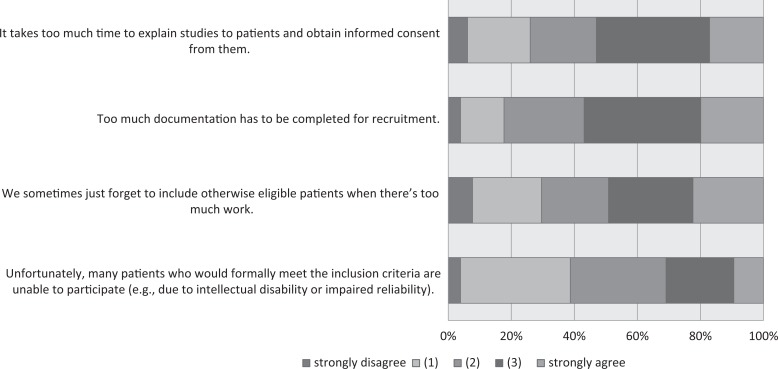
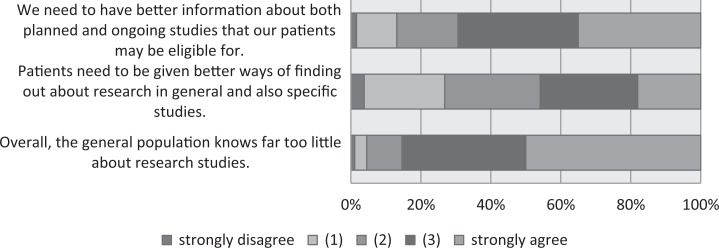
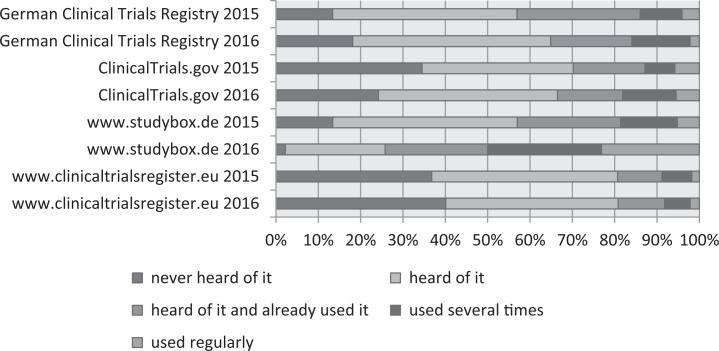
When the participants were asked about difficulties with patient recruitment (Figure 6), over 50% thought that “Too much documentation has to be completed for recruitment” and that “It takes too much time to explain studies to patients.” Forty-nine percent agreed that they “sometimes just forget to include otherwise eligible patients when there’s too much work.” When asked about information issues related to studies, 85% agreed that “the general population knows far too little about research studies” and 69% agreed that centers “need to have better information about both planned and ongoing studies that our patients may be eligible for” (Figure 7). In comparison with 2015, the proportion of respondents in agreement with the item “patients need to be given better ways of finding out about research in general and also specific studies” decreased from 57% to 46%. One means by which HCPs (and less so patients) can collect information about ongoing and completed studies are trial registries. We therefore asked respondents whether they had heard of and/or used clinicaltrialsregister.eu and clinicaltrials.gov, both of which are aimed at an international audience; the German Clinical Trials Registry (Deutsches Register Klinischer Studien [DRKS]), which is the German World Health Organization (WHO) primary registry; and the StudyBox, the registry for German colorectal studies mentioned above. The responses for 2015 are compared with those for 2016, as shown in Figure 8. Over 20% of the respondents had not heard of clinicaltrials.gov or clinicaltrialsregister.eu. Over time, regular utilization remained stable for clinicaltrialsregister.eu and increased for the DRKS, clinicaltrials.gov, and the StudyBox. In 2016, the registry with the highest proportion of users in the sample was the StudyBox, followed by the DRKS and clinicaltrials.gov.
Figure 6.
Patient recruitment.
Figure 7.
Need for better information about research studies.
Figure 8.
Awareness and usage of registries.
Discussion
This survey describes factors obstructing and facilitating participation in research studies from the point of view of a sample of cancer center coordinators that is generally difficult to assess. From the point of view of the CRCC coordinators, resource limitations and limited study availability are major obstacles to participating in and initiating research studies. Although this finding supports the results from the literature over the last 2 decades, we also identified other barriers that ought to be highlighted—most notably the respondents’ impression that there is scarce knowledge about trials among the general public. The relatively low overall rate of participation in research studies may be due to the fact that there is little understanding of studies and their relevance among the population (in Germany). As yet, there has been little comprehensive research on this issue for the situation in Germany, but a survey among patients with primary colorectal cancer (n = 132) and patients with breast cancer (n = 566) conducted in 2014 showed that, when asked whether they were taking part in a clinical trial, 12% (colorectal) and 15% (breast) indicated that they were, while at the same time 15% in each sample stated that they didn’t know whether they were taking part in any trials.27 This strongly suggests that there is a lot of work that still needs to be done to provide better information for patients and the public.
Although few changes were identified in comparison with the previous survey, the slight increase in the usage of study registries suggests that greater research involvement may be expected in the near future. Part of this increase, and the increase in the use of the StudyBox in particular, may be explained by the change in CRCC certification criteria. The StudyBox was launched in early 2015, and from 2017 onward, only patients in studies listed in the StudyBox count for the study quota criterion. For the other 3 registries, however, changes have been relatively small. Other issues with study involvement identified in this survey that have not been the focus of previous research include problems that centers have in identifying ongoing studies for their patients. Another alarming result is the respondents’ views regarding the academic rewards of carrying out research studies. This suggests that many existing studies may have quality issues. Clearly, further research is needed to discover whether this only holds true for certain kinds of studies—for example, industry-driven observational studies or small-scale studies—or if it also applies to some randomized controlled trials on approved drugs. To summarize these findings: the high barriers to the conduct of clinical trials, raised both by government and industry, involve procedures that are time-consuming and require a substantial training effort. Most of these have been established for good reasons, but they are not as yet adequately compensated for, either academically or financially.
Future Directions
These findings and those of earlier studies have led us to formulate 5 recommendations for policy and practice in order to increase research involvement and improve recruitment. Denhoff et al emphasize the role of “recruitment in person” for reaching or at least coming close to target enrollment,28 and Adams et al highlight “the tension between clinical and clinical research workloads” and the low recruitment skills of staff.29 The present findings suggest that informing patients and the paperwork it involves, which can often only be done by a physician, is a burden in combination with existing time constraints. Like Ross et al,10 we therefore suggest:
1. The (expensive) solution of establishing (nonindustry) funding for physician members of staff who can commit work hours exclusively to patient recruitment. It is imperative for these funds to be used exclusively for recruitment purposes; they must not trickle away or compensate for a lack of resources for everyday patient care.
Previous research14,30 suggests that the extent of patients’ knowledge about the disease and about the way in which research is conducted is associated with their willingness to take part in trials. The present survey shows that doctors generally regard patients as having little knowledge about medical research. We therefore suggest that
2. Public awareness of the significance of health research needs to be raised and the public needs to be informed about the way in which research is conducted. One approach might be a conventional campaign such as those conducted in the past on issues such as sexually transmitted diseases or organ donation. It should be emphasized that such campaigns should not be funded by industry. We suggest that a campaign of this type should be linked to recommendation no. 3.
Our findings show that hospital management provides very little support for research. Making high-quality research more visible might lead to better reputation effects and attract patients, which in turn might convince hospital management to invest in research. We would therefore suggest that
3. Quality labels (like a “certificate”) should be established to give high-value research a recognizable identity and serve as a recommendation for patients, as well as for physicians, to take part in specific studies. They would also incentivize the initiation of better studies.
The present survey, like previous reports, discusses selection bias and difficulties in recruiting specific patient groups,12,20,21,31 irrespective of whether the patient groups concerned are dismissive of research studies or the recruiters prefer to take advantage of “low-hanging fruit.” Selection bias may have a tremendous impact on the generalizability of results. Similarly, restrictive eligibility criteria reduce the generalizability of findings and make it difficult to identify patients.32 We therefore suggest that
4. The generalizability of results should be increased by broadening the eligibility criteria, when possible, and tailoring recruitment procedures toward patients who are difficult to access for research. This also links back to the necessity of suggestion 1 as tailoring recruitment toward patients that are difficult to access increases research costs.
The paperwork associated with conducting trials is a significant burden for those involved. Although informing patients and obtaining informed consent from them are necessary for each specific trial, patient/clinical information is then often documented more than once—for example, both in a clinical registry and for the specific trial database. To avoid multiple documentation of the same data, we—following examples like33,34—suggest that
5. It should be made easier for existing databases, particularly clinical registries, to be used in connection with clinical trials.
Limitations
Finally, the limitations of the present survey need to be carefully considered when interpreting the results. The study sample consists of coordinators of CRCCs or their deputies, and it is not clear to what extent the findings can be generalized to certified CRCCs that did not respond, noncertified hospitals treating patients with colorectal cancer, or even to other cancer entities. We believe that due to the study quota criterion, research studies are a higher priority topic in certified CRCCs than they are in noncertified units. Due to the relatively high response rate and the consistency of survey data and certification documentation, we also believe that the sample represents the certified CRCC sufficiently well. It should be emphasized that the study populations in the 2 surveys discussed here consisted of the coordinators of the CRCCs that were certified at the 2 time points. The individuals responding and the institutions included in the 2015 and 2016 samples are thus not necessarily identical (since centers may lose their certificate or may have been certified for the first time in the interval), and this needs to be taken into consideration when considering changes over time. As with the limitations discussed by Fayter et al,30 we would emphasize that this study relies solely on the information collected by the survey and does not necessarily describe the actual behavior of the participants, nor can the respondents’ views necessarily be associated with what happens in the centers they work for. In addition, the numbers presented may suggest greater objectivity than they actually have, since respondents may rate “lack of time” as a major concern, although the underlying issue is much more complex—for example, a mixture of insufficient rewards, suboptimal priority setting, and staff who already have a high workload. Qualitative studies may help illuminate the underlying complexities and offer solutions. One such approach has been undertaken by McCann et al, who carried out a meta-ethnographic synthesis on patients’ reasons for participating in clinical trials.35 They found that the specific patient’s situation is extremely important for the decision to participate and that this should influence recruitment design.
In conclusion, we would like to emphasize that efforts need to be undertaken to increase the numbers of research studies being conducted across the cancer continuum. We would therefore urge health policy makers to put (cancer) research high on the agenda and to assist HCPs and HCOs to contribute to the generation of medical knowledge.
Appendix A
Items Asked in Both Surveys
| Item | Strongly Disagree | Tend to Disagree | Undecided | Tend to Agree | Strongly Agree | Missing | |
|---|---|---|---|---|---|---|---|
| There are not enough studies in which participation by our center would be possible (176/184 sites) | 2015: % (n) | 7.0 (12) | 29.1 (50) | 15.1 (26) | 33.1 (57) | 15.7 (27) | 4 |
| 2016: % (n) | 7.7 (14) | 26.8 (49) | 17.5 (32) | 35.5 (65) | 12.6 (23) | 1 | |
| When we find out about an interesting study that we have eligible patients for, recruitment has often already closed (176/184 sites) | 2015: % (n) | 15.6 (27) | 45.1 (78) | 22.0 (38) | 14.5 (25) | 2.9 (5) | 3 |
| 2016: % (n) | 8.0 (14) | 43.1 (75) | 21.3 (37) | 22.4 (39) | 5.2 (9) | 10 | |
| It’s difficult for our center to identify studies suitable for our patients (176/184 sites) | 2015: % (n) | 5.1 (9) | 41.7 (73) | 16.6 (29) | 27.4 (48) | 9.1 (16) | 1 |
| 2016: % (n) | 8.2 (15) | 34.6 (63) | 17.0 (31) | 31.9 (58) | 8.2 (15) | 2 | |
| We don’t have the scientific research infrastructure to lead studies ourselves (99/111 non-PI sites) | 2015: % (n) | 10.2 (10) | 11.2 (11) | 9.2 (9) | 30.6 (30) | 38.8 (38) | 1 |
| 2016: % (n) | 10.0 (11) | 12.7 (14) | 10.9 (12) | 20.9 (23) | 45.5 (50) | 1 | |
| We don’t have any experience in leading our own studies (99/111 non-PI sites) | 2015: % (n) | 11.5 (11) | 17.7 (17) | 16.7 (16) | 22.9 (22) | 31.3 (30) | 3 |
| 2016: % (n) | 11.9 (13) | 17.4 (19) | 13.8 (15) | 28.4 (31) | 28.4 (31) | 2 | |
| We don’t have enough patients to make leading a study worthwhile (99/111 non-PI sites) | 2015: % (n) | 7.3 (7) | 19.8 (19) | 22.9 (22) | 33.3 (32) | 16.7 (16) | 3 |
| 2016: % (n) | 10.2 (11) | 18.5 (20) | 25.0 (27) | 20.4 (22) | 25.9 (28) | 3 | |
| We don’t have enough staff to lead studies (99/111 non-PI sites) | 2015: % (n) | 5.1 (5) | 6.1 (6) | 12.1 (12) | 19.2 (19) | 57.6 (57) | - |
| 2016: % (n) | 2.7 (3) | 6.4 (7) | 10.0 (11) | 20.9 (23) | 60.0 (66) | 1 | |
| Our hospital management does not give us any support for leading studies (99/111 non-PI sites) | 2015: % (n) | 18.7 (14) | 16.0 (12) | 25.3 (19) | 21.3 (16) | 18.7 (14) | 24 |
| 2016: % (n) | 17.6 (15) | 11.8 (10) | 25.9 (22) | 25.9 (22) | 18.8 (16) | 26 | |
| It’s easy for us to initiate and lead studies (77/73 PI sites) | 2015: % (n) | 8.0 (6) | 36.0 (27) | 22.7 (17) | 22.7 (17) | 10.7 (8) | 2 |
| 2016: % (n) | 11.3 (8) | 28.2 (20) | 26.8 (19) | 22.5 (16) | 11.3 (8) | 2 | |
| Our staff are keen to work on studies that we initiate ourselves (77/73 PI sites) | 2015: % (n) | 12.5 (9) | 16.7 (12) | 30.6 (22) | 26.4 (19) | 13.9 (10) | 5 |
| 2016: % (n) | 8.6 (6) | 21.4 (15) | 25.7 (18) | 31.4 (22) | 12.9 (9) | 3 | |
| Our hospital management encourages us to initiate and lead studies (77/73 PI sites) | 2015: % (n) | 26.8 (19) | 32.4 (23) | 16.9 (12) | 16.9 (12) | 7.0 (5) | 6 |
| 2016: % (n) | 28.4 (19) | 29.9 (20) | 19.4 (13) | 7.5 (5) | 14.9 (10) | 6 | |
| It takes too much time to explain studies to patients and obtain informed consent from them (176/184 sites) | 2015: % (n) | 2.9 (5) | 23.4 (41) | 30.9 (54) | 29.7 (52) | 13.1 (23) | 1 |
| 2016: % (n) | 6.1 (11) | 19.9 (36) | 21.0 (38) | 35.9 (65) | 17.1 (31) | 3 | |
| Too much documentation has to be completed for recruitment (176/184 sites) | 2015: % (n) | 2.9 (5) | 15.4 (27) | 29.1 (51) | 33.7 (59) | 18.9 (33) | 1 |
| 2016: % (n) | 3.9 (7) | 13.8 (25) | 25.4 (46) | 37.0 (67) | 19.9 (36) | 3 | |
| We sometimes just forget to include otherwise eligible patients when there’s too much work (176/184 sites) | 2015: % (n) | 12.5 (22) | 25.6 (45) | 21.6 (38) | 26.1 (46) | 14.2 (25) | - |
| 2016: % (n) | 7.7 (14) | 21.9 (40) | 21.3 (39) | 26.8 (49) | 22.4 (41) | 1 | |
| Unfortunately, many patients who would formally meet the inclusion criteria are unable to participate (eg, due to intellectual disability or impaired reliability; 176/184 sites) | 2015: % (n) | 4.6 (8) | 38.3 (67) | 36.0 (63) | 16.0 (28) | 5.1 (9) | 1 |
| 2016: % (n) | 3.9 (7) | 34.8 (63) | 30.4 (55) | 21.5 (39) | 9.4 (17) | 3 | |
| We need to have better information about both planned and ongoing studies that our patients may be eligible for (176/184 sites) | 2015: % (n) | 4.0 (7) | 9.7 (17) | 11.9 (21) | 38.6 (68) | 35.8 (63) | - |
| 2016: % (n) | 1.6 (3) | 11.5 (21) | 17.5 (32) | 34.4 (63) | 35.0 (64) | 1 | |
| Patients need to be given better ways of finding out about research in general and also specific studies (176/184 sites) | 2015: % (n) | 6.3 (11) | 14.9 (26) | 21.7 (38) | 35.4 (62) | 21.7 (38) | 1 |
| 2016: % (n) | 3.8 (7) | 23.0 (42) | 27.3 (50) | 27.9 (51) | 18.0 (33) | 1 | |
| Overall, the general population knows far too little about research studies (176/184 sites) | 2015: % (n) | 1.1 (2) | 4.0 (7) | 12.1 (21) | 29.9 (52) | 52.9 (92) | 2 |
| 2016: % (n) | 1.1 (2) | 3.4 (6) | 10.1 (18) | 35.4 (63) | 50.0 (89) | 6 |
Abbreviation: PI, principal investigator.
Note
The StudyBox lists colorectal cancer studies that meet quality criteria, and it should therefore help centers to find high-quality studies for their patients and encourage study initiators to improve the overall quality of studies. Details of the StudyBox are available at http://www.studybox.de (in German).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.W. and C.K. are employees of the German Cancer Society and J.F. is an employee of the OnkoZert certification institute. S.P. and T.S. are the chairpersons of the colorectal cancer certification committee of the German Cancer Society. S.R.B. is chair of the German Association of Certified Colorectal Cancer Centers (addz). S.R.B., S.P., and T.S. are heads of certified centers. S.B., S.P., and T.S. have received research funding or hold other financial relationships with (pharmaceutical) companies that are not in conflict with the research presented here. A full list will be provided by the corresponding author upon request.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was done as part of a study funded by the German Ministry of Health (grant no. ZMVI5-2515FSB003). We would like to thank the cancer center coordinators who participated in the survey.
References
- 1. McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Briel M, Olu KK, von Elm E, et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol. 2016;80:8–15. [DOI] [PubMed] [Google Scholar]
- 3. Straus SE, Straus C, Tzanetos K. Career choice in academic medicine: systematic review. J Gen Intern Med. 2006;21(12):1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris J. Scientific research is a moral duty. J Med Ethics. 2005;31(4):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell MK, Weijer C, Goldstein CE, Edwards SJL. Do doctors have a duty to take part in pragmatic randomised trials? BMJ. 2017;357:j2817. [DOI] [PubMed] [Google Scholar]
- 6. Stein MA, Shaffer M, Echo-Hawk A, Smith J, Stapleton A, Melvin A. Research START: a multimethod study of barriers and accelerators of recruiting research participants. Clin Transl Sci. 2015;8(6):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patterson S, Duhig M, Connell M, Scott J. Successful recruitment to a study of first-episode psychosis by clinicians: a qualitative account of outcomes and influences on process. J Ment Heal. 2014;23(5):225–230. [DOI] [PubMed] [Google Scholar]
- 8. Sahin D, Yaffe MJ, Sussman T, McCusker J. A mixed studies literature review of family physicians’ participation in research. Fam Med. 2014;46(7):503–514. [PubMed] [Google Scholar]
- 9. Spaar A, Frey M, Turk A, Karrer W, Puhan MA. Recruitment barriers in a randomized controlled trial from the physicians’ perspective: a postal survey. BMC Med Res Methodol. 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials. J Clin Epidemiol. 1999;52(12):1143–1156. [DOI] [PubMed] [Google Scholar]
- 11. Williams CM, Maher CG, Hancock MJ, McAuley JH, Lin CW, Latimer J. Recruitment rate for a clinical trial was associated with particular operational procedures and clinician characteristics. J Clin Epidemiol. 2014;67(2):169–175. [DOI] [PubMed] [Google Scholar]
- 12. Rigal L, Saurel-Cubizolles MJ, Falcoff H, Bouyer J, Ringa V. The organization of the health care provider’s practice influenced patient participation in research: a multilevel analysis. J Clin Epidemiol. 2013;66(4):426–435. [DOI] [PubMed] [Google Scholar]
- 13. Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141–148. [DOI] [PubMed] [Google Scholar]
- 14. Leighton P, Lonsdale AJ, Tildsley J, King AJ. The willingness of patients presenting with advanced glaucoma to participate in a trial comparing primary medical vs primary surgical treatment. Eye (Lond). 2012;26(2):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halpern SD, Karlawish JH, Casarett D, Berlin JA, Townsend RR, Asch DA. Hypertensive patients’ willingness to participate in placebo-controlled trials: implications for recruitment efficiency. Am Heart J. 2003;146(6):985–992. [DOI] [PubMed] [Google Scholar]
- 16. Gaul C, Malcherczyk A, Schmidt T, Helm J, Haerting J. Bereitschaft von patienten zum einschluss in klinische studien. Med Klin (Munich). 2010;105(2):73–79. [DOI] [PubMed] [Google Scholar]
- 17. Guedj M, Ballester S, Kamar N, et al. Patients’ motives for consenting or refusing to participate in a clinical trial in organ transplantation. Clin Transplant. 2013;27(5):724–731. [DOI] [PubMed] [Google Scholar]
- 18. Caldwell PHY, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS Med. 2010;7(11):e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 20. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kowalski C, Graeven U, von Kalle C, et al. Shifting cancer care towards multidisciplinarity: the cancer center certification program of the German cancer society. BMC Cancer. 2017;17(1):850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. SoSci Survey [computer program]. Version 2.5.00-i. München, Germany: SoSci Survey GmbH; 2014. [Google Scholar]
- 23. Kowalski C, Jena S, Kliemann D, Antes G. Zur Diskussion: studienregister für die onkologie nutzen: studybox und deutsches register klinischer studien. Z Evid Fortbild Qual Gesundhwes. 2015;109(6):431–436. [DOI] [PubMed] [Google Scholar]
- 24. Kowalski C, Ferencz J, Benz S, et al. Hemmende und fördernde Faktoren bei der Durchführung von Studien: Die Sicht von Darmkrebszentrumskoordinatorinnen und -koordinatoren. Z Gastroenterol. 2016;54(5):409–415. [DOI] [PubMed] [Google Scholar]
- 25. Kowalski C, Wesselmann S, Ansmann L, Kreienberg R, Pfaff H. Key informants’ perspectives on accredited breast cancer centres: results of a survey. Geburtshilfe Frauenheilkd. 2012;72(3):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deutsche Krebsgesellschaft. Jahresbericht der zertifizierten Darmkrebszentren. Berlin, Germany; 2016. https://www.krebsgesellschaft.de/gcs/german-cancer-society/certification/documents.html?file=files/dkg/deutsche-krebsgesellschaft/content/pdf/Zertifizierung/Jahresberichte%20mit%20DOI%20und%20ISBN/colorectal_annual%20report-2016-A3%28160721%29.pdf. Accessed March 9, 2018. [Google Scholar]
- 27. Blettenberg LM. Aufklärung in Brust- und Darmkrebszentren über die Teilnahme an klinischen Studien: Wissensstand der Patienten über die Teilnahme an einer klinischen Studie [Unpublished bachelor’s thesis] Köln, Germany: Universität zu Köln; 2016. [Google Scholar]
- 28. Denhoff ER, Milliren CE, de Ferranti SD, Steltz SK, Osganian SK. Factors associated with clinical research recruitment in a pediatric academic medical center: a web-based survey. PLoS One. 2015;10(10):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams M, Caffrey L, McKevitt C. Barriers and opportunities for enhancing patient recruitment and retention in clinical research: findings from an interview study in an NHS academic health science centre. Health Res Policy Syst. 2015;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol. 2007;60(10):990–1001. [DOI] [PubMed] [Google Scholar]
- 31. Crocker JC, Beecham E, Kelly P, et al. Inviting parents to take part in paediatric palliative care research: a mixed-methods examination of selection bias. Palliat Med. 2015;29(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benz SE, Stricker I, Tam Y, Tannapfel A. CME or traditional surgery for right-sided colon cancer? Coloproctology. 2017;39(3):184–189. [Google Scholar]
- 34. Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371(12):1111–1120. [DOI] [PubMed] [Google Scholar]
- 35. McCann S, Campbell M, Entwistle V. Recruitment to clinical trials: a meta-ethnographic synthesis of studies of reasons for participation. J Health Serv Res Policy. 2013;18(4):233–241. [DOI] [PubMed] [Google Scholar]