Abstract
Peripheral neuropathy (PN) is a serious condition affecting the nerves that is commonly seen in patients with tuberculosis (TB). Causes of PN in patients with TB are multiple, and can include TB itself, other co-morbid conditions, such as Human Immune-deficiency virus (HIV) disease, malnutrition, or diabetes mellitus (DM), and several anti-tuberculous medications. The condition can manifest with a variety of symptoms, and a diagnosis can usually be made on a clinical basis. Treatment and prognosis of PN vary depending on the underlying cause, but often the condition can lead to permanent disability in individuals with TB. For this reason, primary prevention is key as is early identification and management of symptoms. Treatment can include withdrawal of possible offending agents, vitamin supplementation, physical therapy, analgesics, and targeted agents, including tricyclic antidepressants, selective serotonin reuptake inhibitors, and gabapentin. Additional research is needed to better describe the morbidity and disability associated with PN in persons with TB and to improve management strategies for persons at risk for and affected by this condition. Case review: RM is a 47 year-old man who is in his third month of treatment for drug-resistant TB (DR-TB). His treatment regimen consists of kanamycin (1 gm intramuscular daily), levofloxacin (1000 mg by mouth daily), cycloserine (750 mg by mouth daily), ethionamide (750 mg by mouth daily), pyrazinamide (1500 mg by mouth daily), and Para-Amino Salicylate (12 gm by mouth daily). He is HIV-infected with a CD4 count of 470 cell/µl and on a stable antiretroviral therapy regimen of tenofovir, lamivudine, and efavirenz, which he started 8 weeks ago. He works in a platinum mine, denies smoking, reports drinking beer “on the weekend” and denies other drugs. He presents for his 3 month clinical visit for his DR-TB follow-up and states he is doing well, but he does report some “burning” in the bottom of his feet.
Introduction
Peripheral nerves are parts of the nervous system responsible for transmitting information from all parts of the body to the spinal cord and brain [1]. Peripheral neuropathy (PN) is a condition in which the nerves are affected, compromising the relay of information from different parts of the body. It can affect sensory nerves, motor nerves, or autonomic nerves and cause a variety of symptoms and complications. It is estimated that as many as 500 million people in the world suffer from PN [2], and the problem is commonly seen among individuals with tuberculosis (TB) [3]. The pathophysiology of PN results from an insult to the body of the nerve or to the myelin sheath, leading to loss of normal function and development of deficits in the affected individual [4]. With TB, there are a number of factors that can lead to damage of the peripheral nerves and the development of neuropathy, including TB itself, other co-morbid conditions, and the medications used to treat the disease [5].
Epidemiology
Rates of peripheral neuropathy in the general population have been noted to be around 1.1% but as high as 6% in the elderly, although most of this data come from western countries and there is a need for more general baseline data in high-burden TB settings [6]. Several studies have assessed the prevalence of neuropathy in persons with TB. Among persons with drug-susceptible TB (DS-TB), rates between 0 and 10% have been reported in the literature [7]. In Swaziland, a cohort of 250 patients from one hospital with drug sensitive tuberculosis revealed a rate of 12%; however male patients had a higher rate 18% compared to females 7% (p < 0.03); the older the patient, the higher the rate of neuropathy (20% in those older than 45 years old compared to 9.6% in those less than 45 years old (p = 0.01) but no significant difference was demonstrated between those with HIV infection and those without (13% vs 11%, p = 0.12) probably because most of the HIV infected patients were already on ART prior to TB disease and treatment [8].
For those with drug-resistant TB (DR-TB) much higher rates have been seen, with studies reporting rates between 13 and 17% [9] Some of the variation in rates may be due to the different drugs used and the longer duration of treatment for DR-TB, but a recent study found that as many as 25% of patients have PN at the time they start treatment for DR-TB [10].
Etiology
There are multiple potential causes of PN in persons with all forms of TB. First, TB in and of itself has been associated with PN. This is felt to be due to immune-mediated mechanisms, although there may be more specific reasons, including granulomas in the nerves and deposition disease (although to date this has only been reported in rare cases of optic neuritis) [11], [12]. In general, other causes of neuropathy are sought before attributing causality to TB, and when TB is the cause of neuropathy, the symptoms usually improve with treatment, although this may take time [13]. There may also be some neurologic syndromes caused by TB that mimic signs and symptoms of peripheral neuropathy, including spinal tuberculomas and meningeal TB [14]. Of note, persons with TB may also experience other types of inflammatory neuropathies from the disease or treatment, including carpal tunnel syndrome and tenosynovitis [15]
Another common cause of PN in persons with TB is the existence of co-morbid conditions that in and of themselves are associated with PN [16]. Perhaps the most common co-morbid condition that is associated with PN and frequently seen in patients with TB is Human immune-deficiency virus (HIV) disease [17], and in some populations of TB patients, an HIV co-infection rate of upwards of 90% has been reported [18]. Nutritional deficiencies—most notably of the B family of vitamins—have also been associated with PN and are more commonly seen in individuals with all types of TB [19]. There is a global epidemic of diabetes mellitus (DM) [20], and there is also emerging evidence that diabetics are at increased risk for TB [21]. Given that PN is commonly associated with DM and the growing associations between TB and DM, there may be additional morbidity due to PN in patients in this co-morbid population [22]. Finally, in some settings, patients with high rates of substance abuse—especially alcohol abuse—are at increased risk of TB [23], and these same populations also have higher rates of PN as well [24]. Table 1 lists conditions commonly associated with TB that can also be associated with PN.
Table 1.
Common co-morbid conditions seen with TB and PN.
| Disease | Comment |
|---|---|
| HIV | Disorders of peripheral nerves are among the most frequent neurological complications of HIV infection. Antiretroviral toxic neuropathy is also associated with HIV treatment. |
| Malnutrition | Most notably the B vitamin deficiencies |
| DM | Diabetic neuropathy affects all peripheral nerves, which means it can affect all organs and systems. |
| Heavy metal toxicity | More common in mining populations and in farm and factory workers |
| Compressive adenopathy | A form of entrapment neuropathies where enlarged lymph nodes can cause isolated peripheral nerve injuries at specific locations via mechanical constriction |
| Hypothyroidism | A result of edematous infiltration of the endo- and perineurium, and also degeneration of the myelin sheaths and axis cylinders |
| Substance abuse | Most notably alcoholism |
The third factor that is associated with TB and PN are the medications used to treat TB. DS-TB is usually treated with a combination of four drugs given for a 6 month period. These drugs are isoniazid (INH), rifampin (RIF), pyrazinamide (PZA) and ethambutol (EMB), and of these medications, both INH and EMB have been associated with neuropathy [25]. EMB is usually associated with the development of optic neuritis/optic neuropathy but can be a rare cause of reversible, distal sensory neuropathy. Streptomycin and the other injectable agents may cause cranial nerve VIII toxicity but have not been associated with distal peripheral neuropathies. INH is one of the medications most commonly associated with PN, and there are estimates that as many as 10 % of patients receiving INH will develop some form of PN [26]. The drug's mechanism of action against Mycobacterium tuberculosis (MTB) leads to depletion of pyridoxine (vitamin B6), and it is the loss of pyridoxine that is toxic to the nerve [27]. Neurotoxicity of INH can be ameliorated by giving pyridoxine supplementation, although high doses (>50 mg per day) of pyridoxine have themselves been shown to be toxic to the nerves [28]. Pyridoxine-associated neuropathy tends to present with loss of vibration and proprioception, and strict adherence to dosing recommendations of pyridoxine should be followed to avoid this iatrogenic complication. Of note, pyridoxine is only effective when given alone and not as part of B complex vitamin therapy and should be discontinued after TB therapy is complete.
The treatment of DR-TB is more complicated than that of pan-susceptible disease and usually requires the use of multiple “second-line” agents given for a period of 18–24 months [29]. Many of these second-line agents have been associated with the development of PN, including cycloserine (CS), ethionamide (Ethio), and the fluoroquinolones [30]. With highly-resistant forms of TB emerging, new drugs-such as bedaquiline (BDQ) and delamanid (DEL)-and re-purposed drugs-such as clofazimine (CFZ) and linezolid (LZD)-are being used with increasing frequency [31]. The impact of new chemical entities on PN may not yet be apparent, since the drugs are often evaluated in shorter trials (i.e. 8–24 weeks) prior to introduction into the market, and such short administration and evaluation periods may not be sufficient to document any true effects on PN.
In terms of re-purposed agents, several of the more commonly used drugs have been associated with PN. One such agent is high-dose INH, which may be associated with a higher rate of PN compared with conventional doses [32]. CFZ has also be associated with PN is some case series reports, largely due to accumulation of the chemical substance in the peripheral nerves [33]. LZD is rapidly becoming a staple in the treatment of highly resistant forms of TB, and one of the most common adverse events associated with this drug is the development of PN [34]. In fact, the development of PN is often the limiting factor in the dose and duration of LZD therapy. Multiple observational studies have shown high rates of PN in persons receiving LZD for DR-TB, with rates of 10–40% reported in the literature [35], [36]. Increasing rates of neuropathy are seen when doses above 600 mg per day are given, and some alleviation of symptoms has been reported when the daily dose is lowered to 300 mg daily [37], [38]. The effect of pyridoxine supplementation on reducing LZD-associated PN has not been formally studied, but most patients being treated with LZD receive pyridoxine as part of standard of care [39].
Because so many of the medications used for DR-TB treatment can cause peripheral neuropathy, it is important that, whenever possible, treatment for DR-TB be based on drug-susceptibility testing with a goal of maximizing effectiveness while limiting toxicity. This may be especially important in patients on treatment for DR-TB, given that they may have also have malnutrition both from their underlying disease as well as from anorexia, vomiting, and diarrhea associated with the medications used in DR-TB treatment.
Finally, some of the medications used to treat co-morbid conditions in persons with TB can also lead to the development of PN [40]. This is most commonly seen with certain antiretroviral (ARV) therapies, especially the nucleoside reverse transcriptase inhibitors (NRTIs) [41]. Another common co-morbid condition in HIV-infected patients is Kaposi's Sarcoma which is treated with vinca alkaloids and this treatment can also cause PN [42]. Table 2 lists the medications used for treating TB and its common co-morbid conditions that have been associated with the development of PN
Table 2.
Medications associated with the development of PN in patients with TB.
| Medication Class | Medication | Comment |
|---|---|---|
| DS-TB | ||
| INH | The combination of INH and pyridoxine to form a hydrazone which is excreted in the urine, results in a relative deficiency of biologically active pyridoxine. | |
| EMB | Optic nerve toxicity resulting from the administration of ETH is a well-recognized complication of therapy | |
| DR-TB | ||
| CS | A structural analogue of alanine, a central neurotransmitter. Interestingly d-cycloserine may help lessen pain and other symptoms of PN caused by chemotherapy | |
| Ethio | A member of the thioamide family and structurally related to INH. Causes pyridoxine deficiency | |
| High dose-INH | ||
| LZD | May be a result of disrupted mitochondrial function in neurons. | |
| ARVs | ||
| Stavudine (D4T) | NRTI-associated mitochondrial dysfunction, inflammation and nutritional factors are implicated in the pathogenesis PN among ART patients. | |
| Didaonsine (ddI) | ||
| Zidovudine (AZT) |
For most patients with TB, the cause of PN will be multifactorial [43], resulting from the disease itself, common co-morbid conditions, and the medications used to bring about cure.
In the case of RM, there are multiple potential factors that could be causing his signs and symptoms. These include his TB, his HIV, or his use of alcohol. Several of his TB medications could be associated with PN, including his CS or his Ethio, and the patient is not receiving any pyridoxine. He has some exposure to alcohol and he may have exposure to heavy metals or other chemicals through his job. His nutritional status could be contributing if he has a low body mass index, and he could have other undiagnosed pathology—including hypothyroidism given that he is being treated with Ethio and PAS—that might be contributing to his symptoms.
Presenting symptoms
Most TB patients who are experiencing nerve damage will show no early signs or symptoms. Thus, routine evaluation and screening at all clinical encounters is crucial, as damage may be irreversible once symptoms begin. Routine screening questions should assess any neurologic symptoms in the extremities (i.e. burning, tingling, etc.), any problems with walking or tripping, and the development of any sores or injuries to the feet. Patients should be asked if the symptoms are bilateral or unilateral.
When patients do present with symptoms, they are usually localized to the feet and/or the hands in what is described as a classic “stocking and glove” distribution and they are often bilateral. Patients may note that the sensation is worse at night and may describe pain, burning, numbing, cold, or restless sensations. Typically, the development of these sensory symptoms precedes the development of any muscular or proprioceptive symptoms, but not always, and patients may complain of tripping or losing their balance as well.
In the case of RM, he is complaining of the most typical, early symptoms of PN, burning on the bottoms of his feet. The precise terms used by patients to describe the sensation may vary depending on regional variation, but they generally involve an uncomfortable temperature or tingling sensation on the bottoms of the feet and on the soles.
Diagnosis and evaluation
All patients with TB should undergo routine evaluation for PN at each clinical encounter. This includes the symptom screening described above but also a formal assessment of strength and reflexes in the extremities as well as an inspection of the hands and feet to assess for any signs of injury, sores, or ulceration. Formal testing of the peripheral nervous system is likely beyond the standard practice for most busy TB clinics. However, a targeted exam of the ankle reflexes and visual inspection of the feet and soles can be done quickly for all patients being treated for TB, with more in-depth testing being done in patients who report symptoms or having physical findings upon screening [44].
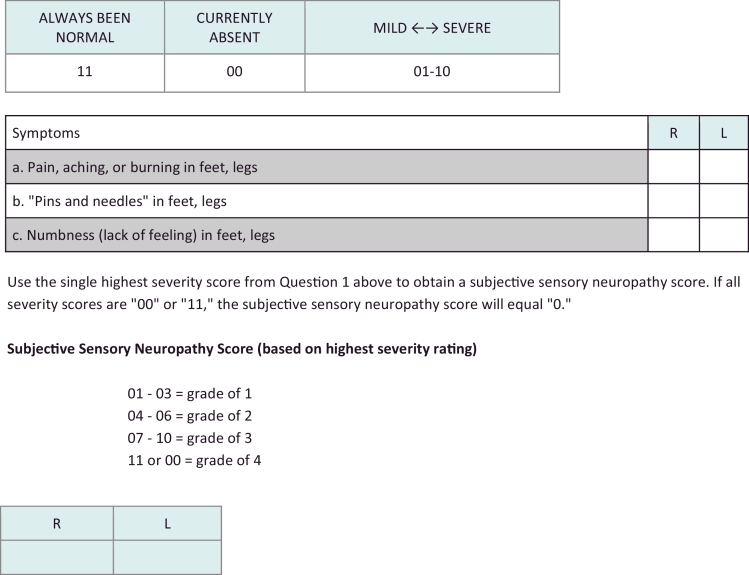
A formal diagnosis on PN is usually made using neurophysiologic tests such as electromyography (EMG), nerve conduction studies (NCS), and quantitative sensory tests (QST).In most TB endemic settings, however, such objective quantitative assessments of the function of the peripheral nervous system may not be readily accessible or may only be available at high cost in specialized centers [45]. For this reason, it is recommended that a clinical diagnosis of PN be used to guide management. While such a diagnosis may seem somewhat subjective, there are emerging scales that can be used to make the diagnosis of PN in persons with TB [46]. One of these is known as the Subjective Peripheral Neuropathy Score, where patients are asked about a set of symptoms, and, if present, asked to rate them on a scale of 1-10 [47]. The numbers are then used to given and overall score to determine the severity of the PN. A version of the subjective portion of the scale is included in Fig. 1 below, although this scale has not been validated for use in persons with TB.
Fig. 1.
Subjective Peripheral Neuropathy Scale [63]
Instructions: Ask the subject to rate the severity of each symptom listed in Question 1 on a scale of 01 (mild) to 10 (most severe) for right and left feet and legs. Enter the score for each symptom in the columns marked R (right lower limb) and L (left lower limb). If a symptom has been present in the past, but not since the last visit, enter “00 – Currently Absent”. If the symptom has never been present, enter “11 – Always Been Normal”.
An algorithmic approach to diagnosing PN in persons with TB is presented in Fig. 2.
Fig. 2.
Algorithmic Approach to Peripheral Neuropathy (PN) in Patients with TB
All patients with TB should have the following assessments and interventions done to minimize the impact of PN on their health.
Persons presenting with signs and symptoms of neuropathy who also have TB should be assessed for other co-morbid conditions that could impact the development of neuropathy. Thus all patients with PN should also undergo assessment for HIV, DM, hypothyroidism, malnutrition, and alcohol use [48].
In the case of RM, he should have his history carefully reviewed for any additional signs or symptoms of neuropathy and to determine if he has any other exposures to alcohol, additional medications (including herbal supplements), and to see if he has any signs of DM or hypothyroidism. He should undergo an assessment for neuropathy using the subjective neuropathy scale and should also have documentation of his strength and reflexes in the lower extremities bilaterally. His feet should be carefully examined for any lesions or sores. He should have a serum glucose, thyroid stimulating hormone and hemoglobin tested.
Management
A two-phased approach to the management of PN is recommended for all patients with TB [49]. The first phase focuses on prevention of the development or worsening of PN in all patients diagnosed with TB. The second focuses on treating the symptoms and consequences of PN
Prevention
In terms of prevention, all patients with TB should be started on pyridoxine supplementation at a dose of 50 mg daily. This drug should be provided free of charge as part of routine management for all persons with TB throughout the course of treatment. In addition, other nutritional deficiencies should be corrected as part of optimal TB management. Patients who are using alcohol or other drugs should be offered treatment or counseled in harm reduction to minimize their use of substances that could contribute to PN. All comorbid conditions should be optimally managed, and, when possible, use of other medications that can cause PN should be avoided.
Treatment
Once symptoms have developed, the management of PN can be complicated and depend on the type of problems the patient is having as well as the severity of the symptoms [50]. Often PN is irreversible, and can lead to significant morbidity and lifelong disability, and cessation of ongoing nerve damage is a priority.
All medication doses and durations should be assessed, and, if possible without compromising the integrity of the TB treatment regimen, adjustments should be made. In many cases, however, especially among patients with DR-TB, drug dose reduction or substitution may not be possible and management must then focus on preserving function and minimizing symptoms [51].
In terms of functional preservation, physical therapy with simple, home-based exercises can be successful in many ways [52]. Such exercises can focus on building compensatory muscle strength, preservation of range of motion and prevention of the development of contractures at joints, and adaptive motions to carry out daily activities [53]. Patients with PN should be provided with shoes that have thick soles and are able to protect their extremities from injury or ulceration. Massage and acupuncture may also help preserve function [54]. Walking aids—including crutches or canes—may be necessary in severe cases. Patients with severe neuropathy may need counseling and emotional support [55].
In terms of medical management of PN symptoms, a number of agents have been assessed with varying outcomes, although data from clinical trials in managing PN in persons with other medical issues (i.e. cancer, DM) do not support the use of any of these agents with the exception of the selective serotonin reuptake inhibitors [56] and the gaba-eric agents [57]. These include topical agents such as capsacin creams and lidocaine/lignocaine, but the effectiveness of these agents is questionable, and the cost and availability can be limiting in many TB endemic settings. The goal of systemic therapy is to target symptoms, as, to date, there have been no systemic therapies that have been shown to reverse nerve damage or symptoms [58]. Systemic therapies tend to fall into four classes: 1) tricyclic antidepressants; 2) anticonvulsants; 3) selective serotonin reuptake inhibitors (SSRIs) and 4) GABA-ergic compounds [59] and are reviewed in Table 3.
Table 3.
| Drug class | Agents | Dosing | Adverse events | Comments |
|---|---|---|---|---|
| Topical | ||||
| Capsacin | No clinical trials evidence to support its use | |||
| Lidocaine (5% patch) | 3 patches per day | Rash or erythema | No clinical trials evidence to support its use | |
| Tricyclics | ||||
| Amytriptyline | 10–25 mg every night | Cardiovascular disease (needs screening for QTc prolongation), anticholinergic effects, interact with drugs metabolized by cytochrome P450 2D6 (e.g., cimetidine, phenothiazine) | Avoid with LZD given possible serotonin syndrome; No clinical trials evidence to support its use | |
| Anticonvulsants | ||||
| Carbamazepine | 100–200 mg twice per day | Skin rashes | No clinical trials evidence to support its use | |
| SSRIs | ||||
| Duloxetine | 60–120 mg per day | Serotonin syndrome, weight gain | Clinical trials from oncology support its use; Avoid with LZD given possible serotonin syndrome | |
| GABA-eric | ||||
| Gabapentin | 100–300 mg every night or 100–300 mg three times daily | Somnolence, dizziness, GI symptoms, mild edema, cognitive impairment (elderly), exacerbation of gait problems | Clinical trials support its use at doses of 1800–3600 mg per day. | |
| Pregabalin | 25–50 mg 3 times per day | Dizziness, somnolence, xerostomia, edema, blurred vision, decreased concentration | Limited clinical trials evidence to support its use | |
Table 4 provides a summary of the recommendations reviewed in this section, with management strategies based on the severity of symptoms and physical findings.
Table 4.
Recommended management of peripheral neuropathy.
| Grade of disease | Interventions | Comments |
|---|---|---|
| Not yet present | Assessment at every visit | Preventive measures |
| Prevention with pyridoxine | ||
| Treatment of co-morbidities | ||
| alcohol and smoking cessation counseling | ||
| Mild | Treatment of co-morbidities alcohol and smoking cessation counseling | |
| increase dose of pyridoxine assessment of function at every visit | ||
| Moderate | Treatment of co-morbidities | |
| alcohol and smoking cessation counseling | ||
| increase dose of pyridoxine | ||
| assessment of function at every visit | ||
| reduce or alter dosage of neurotoxic drugs | ||
| symptomatic treatment | ||
| Severe | Treatment of co-morbidities alcohol and smoking cessation counseling | |
| Maximum dose of pyridoxine | ||
| Assessment of function at every visit | ||
| reduce or alter dosage of neurotoxic drugs – consider stopping drug if there is alternative | ||
| Symptomatic treatment with amitriptyline, duloxetine, gabapentin etc. |
In the case of RM, his PN is mild but he has multiple risk factors for PN that should be addressed in order to ensure his symptoms do not progress. He is started on pyridoxine and he is counseled to stop consuming alcohol. His body mass index is normal and he does not have any signs or symptoms of nutritional deficiencies. His lab tests are within normal limits. The suspected cause is his TB regimen but his clinicians determine that starting pyridoxine at a dose of 50 mg by mouth daily is the best initial management step. He is also counseled on further signs and symptoms that could indicate a worsening of neuropathy and told to report to clinic should he note any of these problems.
Prognosis and impact
Although PN is a common problem seen in persons with TB, there is little known about the impact of this problem on the long-term health and functionality of persons who develop the condition [60]. Although early recognition of signs or symptoms may allow for progression of PN to be halted, most PN is permanent and irreversible [61]. Thus, its ability to have long-term consequences for TB patients is high. In addition to these long-term functional consequences, the development of PN can be distressing for patients and may risk adherence to long-term TB treatment [62].
Future research
In spite of the fact that PN is one of the most common neurologic sequelae seen in persons with TB, there is little know about its prevalence, its optimal management, and the overall health and quality of life impact it has, even on those patients who are ultimately cured of their TB disease. There is a need for targeted research on PN in TB patients, with a focus on effective prevention, early and systematic diagnosis at the primary level, and effective treatment and mitigation strategies.
Conclusion
PN is a common condition, affecting many people with TB disease. The cause is likely multifactorial, presenting a complex clinical scenario, but the consequences can be severe and permanent hence a need for vigilance in management. Prevention strategies are key, and all persons being treated for TB should receive concomitant pyridoxine supplementation, and correction of any modifiable risk factors. All TB patients should be routinely screened for PN and have reflexes tested as well as a visual inspection of the feet at each exam. The presence of neuropathic symptoms, decreased ankle reflexes, and decreased distal sensations, regardless of distal muscle weakness and atrophy, makes the diagnosis of PN likely. Management should focus on halting damage to the nerve—although care should be taken not to compromise the TB regimen—and alleviating symptoms. Counseling and emotional support may be needed in those with severe forms of disease. Better research is needed to determine ideal management strategies and to contribute to better health and quality of life for all persons who are survivors of TB.
References
- 1.Torpy J., Kincaid J., Glass M. Peripheral neuropathy. JAMA. 2010;303(15):1556. doi: 10.1001/jama.303.15.1556. [DOI] [PubMed] [Google Scholar]
- 2.Smith B., Torrance N. Epidemiology of neuropathic pain and its impact of quality of life. Curr. Pain Headache Rep. 2012 doi: 10.1007/s11916-012-0256-0. [DOI] [PubMed] [Google Scholar]
- 3.Marks D.J., Dheda K., Dawson R., Ainslie G., Miller R.F. Adverse events to antituberculosis therapy: influence of HIV and antiretroviral drugs. Int J STD AIDS. 2009;20(5):339–345. doi: 10.1258/ijsa.2008.008361. [DOI] [PubMed] [Google Scholar]
- 4.Azhary H., Farooq M., Bhanushali M. Peripheral neuropathy: differential diagnosis and management. Am Fam Phys. 2010;81(7):887–892. [PubMed] [Google Scholar]
- 5.Blain P.G., Lane R.J. Neurological disorders. In: Davies D.M., Ferner R.E., de Glanville H., editors. Davies's Textbook of Adverse Drug Reactions. 5th ed. Chapman and Hall Medical; London, UK: 1998. pp. 585–629. [Google Scholar]
- 6.Hoffman E., Staff N., Robb J. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84(16):1644–1651. doi: 10.1212/WNL.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kass J.S., Shandera W.X. Nervous system effects of antituberculosis therapy. CNS Drugs. 2010;24(8):655–667. doi: 10.2165/11534340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Piggs Peak Hospital Annual TB report 2013. Piggs Peak, Swaziland.
- 9.Furin J.J., Mitnick C.D., Shin S.S., Bayona J., Becerra M.C., Singler J.M., Alcantara F., Castanieda C., Sanchez E., Acha J., Farmer P.E., Kim J.Y. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tubercul Lung Disease. 2001;5(7):648–655. [PubMed] [Google Scholar]
- 10.Conradie F., Mabiletsa T., Sefoka M., Mabaso S., Louw R., Evans D., Van Rie A. Prevalence and incidence of symmetrical symptomatic peripheral neuropathy in patients with multidrug-resistant TB. South Afr Med J. Suid-Afrik. Tydskr Vir Geneeskd. 2014;104(1):24–26. doi: 10.7196/samj.6455. [DOI] [PubMed] [Google Scholar]
- 11.Warpe B.M., Poflee S.V., Pande N.P., Shrikhande A.V. Tuberculous neuritis: a rare sequel of a common disease. Indian J Pathol Microbiol. 2014;57(1):69–71. doi: 10.4103/0377-4929.130902. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqi SA, Hashmi M, Azmat Z, Mustafa S, Siddiqui KA. Pulmonary tuberculosis with neuromyelitis optica: an uncommon association of a common disease. JCPSP, J Coll Physicians Surg – Pak. 2012;22(8):527–528. [PubMed] [Google Scholar]
- 13.Yeh S., Cunningham M.A., Patronas N., Foroozan R. Optic neuropathy and perichiasmal tuberculomas associated with Mycobacterium tuberculosis meningitis in pregnancy. Can J Ophthalmol. 2009;44(6):713–715. doi: 10.3129/i09-167. [DOI] [PubMed] [Google Scholar]
- 14.Park H.S., Song Y.J. Mutliple tuberculomas involving the brain and spinal cord in a person with military pulmonary tuberculosis. J Korean Neurosurg Soc. 2008;44(1):36–39. doi: 10.3340/jkns.2008.44.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques V., Vieira H., Alcantara A. Tenosynovitis and carpal tunnel syndrome from mycobacterium tuberculosis – a rare manifestation of extrapulmonary tuberculosis. Acta Reumatol Port. 2010;35(1):82–84. Jan-Mar. [PubMed] [Google Scholar]
- 16.Dai L., Mahajan S.D., Guo C., Zhang T., Wang W., Li T., Jiang T., Wu H., Li N. Spectrum of central nervous system disorders in hospitalized HIV/AIDS patients (2009-2011) at a major HIV/AIDS referral center in Beijing, China. J Neurol Sci. 2014;342(1--2):88–92. doi: 10.1016/j.jns.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Naidoo A., Naidoo K., Yende-Zuma N., Gengiah T.N., Padayatchi N., Gray A.L., Bamber S., Nair G., Karim S.S. Changes to antiretroviral drug regimens during integrated TB-HIV treatment: results of the SAPiT trial. Antivir Therapy. 2014;19(2):161–169. doi: 10.3851/IMP2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: Epidemiology, Diagnosis & Management. Indian J Med Res 2005; 121(4): 550–567. ISSN 0971-5916. [PubMed]
- 19.Ghavanini A.A., Kimpinski K. Revisiting the evidence for neuropathy caused by pyridoxine deficiency and excess. J Clin Neuromuscul Dis. 2014;16(1):25–31. doi: 10.1097/CND.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 20.Wild S., Roglic G., Green A., Sicree R., King H. Global Prevalence of Diabetes Estimates for the year 2000 and projections for 2030. Diabet Care. May 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 21.Dooley K., Chaisson R. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009 Dec;9(12):737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall V., Thomsen R.W., Henriksen O., Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. Syst Rev. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonnroth K., Williams B., Stadlin S., Jaramillo E., Dye C. Alcohol use as a risk factor for tuberculosis – a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin S.S., Pasechnikov A.D., Gelmanova I.Y. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11(12):1314–1320. [PubMed] [Google Scholar]
- 25.Vilholm O.J., Christensen A.A., Zedan A.H., Itani M. Drug-induced peripheral neuropathy. Basic Clin Pharmacol Toxicol. 2014;115(2):185–192. doi: 10.1111/bcpt.12261. [DOI] [PubMed] [Google Scholar]
- 26.Tuberculosis Chemotherapy Centre The prevention and treatment of isoniazid toxicity in the therapy of pulmonary tuberculosis 2. An assessment of the prophylactic effect of pyridoxine in low dosage. Bull World Health Organ. 1963;29(4):457–481. [PMC free article] [PubMed] [Google Scholar]
- 27.Sanfeliu C., Wright J.M., Kim S.U. Neurotoxicity of isoniazid and its metabolites in cultures of mouse dorsal root ganglion neurons and hybrid neuronal cell line. Neurotoxicology. 1999;20(6):935–944. [PubMed] [Google Scholar]
- 28.Nisar M., Watkin S., Bucknall R., Agnew R. Exacerbation of isoniazid induced peripheral neuropathy by pyridoxine. Thorax. 1990;45(5):419–420. doi: 10.1136/thx.45.5.419. PMCID: PMC462500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee J.S., Rich M.L., Socci A.R. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004 Feb 7;363(9407):474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 30.Shin S.S., Hyson A.M., Castaneda C. Peripheral neuropathy associated with treatment for multidrug-resistant tuberculosis. Int J Tubercul Lung Dis. 2003;7(4):347–353. [PubMed] [Google Scholar]
- 31.Zumla A., Gillespie S., Hoelscher M. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances and future prospects. Lancet Infect Dis. 2014;14:49–62. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 32.Steichen O., Martinez-Almoyna L., De Broucker T. [Isoniazid induced neuropathy: consider prevention] Rev Mal Respir. 2006;23(2 Pt 1):157–160. doi: 10.1016/s0761-8425(06)71480-2. [DOI] [PubMed] [Google Scholar]
- 33.Cholol M., Steel H., Fournie B. Clofazimine: current status and future prospects. J Antimicrob Chemother. 2011 doi: 10.1093/jac/dkr444. First published online: October 20, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Roongruangpitayakul C., Chuchottaworn C. Outcomes of MDR/XDR-TB patients treated with linezolid: experience in Thailand. J Med Assoc Thail. 2013;96(10):1273–1282. [PubMed] [Google Scholar]
- 35.Rose P.C., Hallbauer U.M., Seddon J.A., Hesseling A.C., Schaaf H.S. Linezolid-containing regimens for the treatment of drug-resistant tuberculosis in South African children. Int J Tubercul Lung Dis. 2012;16(12):1588–1593. doi: 10.5588/ijtld.12.0322. [DOI] [PubMed] [Google Scholar]
- 36.Anger H.A., Dworkin F., Sharma S., Munsiff S.S., Nilsen D.M., Ahuja S.D. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000-06. J Antimicrob Chemother. 2010;65(4):775–783. doi: 10.1093/jac/dkq017. [DOI] [PubMed] [Google Scholar]
- 37.Alffenaar J.W., van Altena R., Harmelink I.M., Filguera P., Molenaar E., Wessels A.M., van Soolingen D., Kosterink J.G., Uges D.R., van der Werf T.S. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin Pharmacokinet. 2010;49(8):559–565. doi: 10.2165/11532080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Koh W.J., Kang Y.R., Jeon K., Kwon O.J., Lyu J., Kim W.S., Shim T.S. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J Antimicrob Chemother. 2012;67(6):1503–1507. doi: 10.1093/jac/dks078. [DOI] [PubMed] [Google Scholar]
- 39.Schecter G.F., Scott C., True L., Raftery A., Flood J., Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50(1):49–55. doi: 10.1086/648675. [DOI] [PubMed] [Google Scholar]
- 40.Vilholm O.J., Christensen A.A., Zedan A.H., Itani M. Drug-induced peripheral neuropathy. Basic Clin Pharmacol Toxicol. 2014;115(2):185–192. doi: 10.1111/bcpt.12261. [DOI] [PubMed] [Google Scholar]
- 41.Dalakas M.C. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst. 2001;6(1):14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- 42.Mlombe Y. Management of HIV-associated Kaposi's Sarcoma in Malawi. Malawi Med J. 2008;20(4):129–132. doi: 10.4314/mmj.v20i4.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stavros K., Simpson D.M. Understanding the etiology and management of HIV-associated peripheral neuropathy. Current HIV/AIDS Rep. 2014;11(3):195–201. doi: 10.1007/s11904-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 44.Said G. Examination and clinical care of the patient with neuropathy. Handb Clin Neurol. 2013;115:235–244. doi: 10.1016/B978-0-444-52902-2.00013-8. [DOI] [PubMed] [Google Scholar]
- 45.Ross M.A. Electrodiagnosis of peripheral neuropathy. Neurol Clin. 2012;30(2):529–549. doi: 10.1016/j.ncl.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Cherry C.L., Wesselingh S.L., Lal L., McArthur J.C. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–1781. doi: 10.1212/01.wnl.0000187119.33075.41. [DOI] [PubMed] [Google Scholar]
- 47.Mehta S., Ahmed A., Kariuki B. Implementation of a Validated Peripheral Neuropathy Screening Tool in Patients Receiving Antiretroviral Therapy in Mombasa, Kenya. Am J Trop Med Hyg. 2010;83(3):565–570. doi: 10.4269/ajtmh.2010.09-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine T.D., Saperstein D.S. Laboratory evaluation of peripheral neuropathy. Neurol Clin. 2013;31(2):363–376. doi: 10.1016/j.ncl.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Hershman D.L., Lacchetti C., Dworkin R.H. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 50.Brannagan TH 3rd Current issues in peripheral neuropathy. J Peripher Nerv Syst. 2012;17 doi: 10.1111/j.1529-8027.2012.00387.x. Suppl 2:1--3. [DOI] [PubMed] [Google Scholar]
- 51.Pachman D.R., Watson J.C., Lustberg M.B. Management options for established chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2014;22(8):2281–2295. doi: 10.1007/s00520-014-2289-x. [DOI] [PubMed] [Google Scholar]
- 52.Tofthagen C., Visovsky C., Berry D.L. Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research. Oncol Nurs Forum. 2012;39(5):E416–E424. doi: 10.1188/12.ONF.E416-E424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki M. Peripheral neuropathy in the elderly. Handb Clin Neurol. 2013;115:803–813. doi: 10.1016/B978-0-444-52902-2.00046-1. [DOI] [PubMed] [Google Scholar]
- 54.Schröder S., Liepert J., Remppis A., Greten J.H. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. 2007;14(3):276–281. doi: 10.1111/j.1468-1331.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 55.Anastasi J.K., Capili B., Chang M. HIV peripheral neuropathy and foot care management: a review of assessment and relevant guidelines. Am J Nurs. 2013;113(12):34–40. doi: 10.1097/01.NAJ.0000438867.67777.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griebeler M.L., Morey-Vargas O.L., Brito J.P. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Annal Intern Med. 2014;161(9):639–649. doi: 10.7326/M14-0511. [DOI] [PubMed] [Google Scholar]
- 57.Backonia M., Glanzman R. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo controlled trials. Clin Ther. 2003;25(1):81–104. doi: 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 58.Kim J.H., Dougherty P.M., Abdi S. Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol Oncol. 2015;136(3):453–459. doi: 10.1016/j.ygyno.2015.01.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aziz M.T., Good B.L., Lowe D.K. Serotonin-norepinephrine reuptake inhibitors for the management of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2014;48(5):626–632. doi: 10.1177/1060028014525033. [DOI] [PubMed] [Google Scholar]
- 60.Mols F., Beijers T., Vreugdenhil G., van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22(8):2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 61.Li L., Hondzinski J.M. Select exercise modalities may reverse movement dysfunction because of peripheral neuropathy. Exerc Sport Sci Rev. 2012;40(3):133–137. doi: 10.1097/JES.0b013e31825f7483. [DOI] [PubMed] [Google Scholar]
- 62.Smith S.C., Lamping D.L., Maclaine G.D. Measuring health-related quality of life in diabetic peripheral neuropathy: a systematic review. Diabet Res Clin Pract. 2012;96(3):261–270. doi: 10.1016/j.diabres.2011.11.013. [Review] [Review] [DOI] [PubMed] [Google Scholar]
- 63.Simpson D.M., Kitch D., Evans S.R., McArthur J.C., Asmuth D.M., Cohen B., Goodkin K., Gerschenson M., So Y., Marra C.M., Diaz-Arrastia R., Shriver S., Millar L., Clifford D.B. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 64.Gilbert M.P. Screening and treatment by the primary care provider of common diabetes complications. Med Clin North Am. 2015;99(1):201–219. doi: 10.1016/j.mcna.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Aziz M.T., Good B.L., Lowe D.K. Serotonin-norepinephrine reuptake inhibitors for the management of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2014;48(5):626–632. doi: 10.1177/1060028014525033. [DOI] [PubMed] [Google Scholar]
- 66.Evans M., Manji H. Progress in peripheral nerve disease research in the last two years. J Neurol. 2013;260(12):3188–3192. doi: 10.1007/s00415-013-7121-x. [DOI] [PubMed] [Google Scholar]
- 67.Dworkin R.H., O'Connor A.B., Kent J. International Association for the Study of Pain Neuropathic Pain Special Interest Group. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154(11):2249–2261. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]