Abstract
Background
With the progress in surgical techniques and management of complications, pancreatic resection can be safely performed in experienced hospitals. Pancreatic resection enables surgeons to assess the effect of surgery for metastatic cases, even when there is limited information. In the present study we evaluated the role of primary tumor resection for metastatic pancreatic cancer (mPC) by using the Surveillance, Epidemiology and End Results (SEER) database.
Material/Methods
Metastatic pancreatic cancer patients treated at our hospital from 2004 to 2015 were identified. The effect of surgery on cancer-specific survival was assessed by restricted mean survival time (RMST) and stabilized inverse probability of treatment weight-adjusted analysis after propensity score matching (PSM).
Results
A total of 2694 mPC patients were included. Of this population, 365 adults underwent primary tumor resection. After propensity matching, postsurgical patients had longer RMST than non-surgery patients (1: 1 PSM 11.60 months vs. 8.98 months; 1: 2 PSM 11.61 months vs. 9.10 months; p<0.01). Stabilized inverse probability of treatment weight-adjusted analysis yielded similar results (p<0.01).
Conclusions
Our study supports the hypothesis that patients with mPC can benefit from primary tumor surgery. However, the surgical inclusion criteria and the appropriate role of surgery, such as its effect on symptom control, quality of life, and the extent to which it prolongs survival for metastatic pancreatic cancer, remain to be completely assessed by well-designed, prospective, randomized clinical trials.
MeSH Keywords: Neoplasm Metastasis, Pancreatic Neoplasms, SEER Program
Background
Pancreatic cancer (PC) is among the most frustrating diseases for clinicians, and it has an extremely poor prognosis [1]. By the year 2030, it is predicted to become the second leading cause of cancer-associated mortality in the USA [2]. Because of the late onset of symptoms and early metastasis, over 50% of patients present with metastatic disease. Surgical resection, which is the only way to achieve long-term survival, is commonly unavailable for these people [3,4].
Regarding the choice of surgical resection, several classifications primarily focus on the vascular attachment status of the primary pancreatic tumor [5,6]. According to the National Comprehensive Cancer Network guideline, local resectability is generally classified as resectable, borderline resectable, or locally advanced (irresectable), whereas metastatic disease is not fully included in this definition. During the last decade, with the regionalization of pancreatic surgery into high-volume medical centers, the resection rate has increased to about 60% and the indications for surgery have been extended [7]. Therefore, dilemmas often arise in daily clinical practice: patients develop metastatic disease, and primary tumors appear to be resectable. In this setting, surgery is occasionally performed according to the surgeon’s experience and individual wishes, but its impact on survival has not been clearly elucidated.
Evidence from some other solid malignancies, such as metastatic breast cancer [8], metastatic renal-cell cancer [9], and metastatic colorectal cancer [10], has shown favorable outcomes for primary tumor resection, whereas minimal data exist for metastatic pancreatic cancer. This may be due to a skeptical attitude towards the safety and efficacy of surgery for pancreatic cancer [11]. Recent progress allows pancreatic surgery to be safely performed, with low morbidity and mortality rates [12]. At present, postoperative mortality has fallen to well below 5% in experienced hospitals, giving surgeons impetus to assess the effectiveness of surgery in metastatic cases [13–16]. However, the existing recommendations are controversial. Some case studies reported that resection is beneficial in well-selected patients [17–22]. By contrast, a few surgical series demonstrated that resection of the main tumor and its metastatic lesions conveyed no survival benefit, and resection could not be recommended [23,24]. No credible conclusion can be drawn from these studies, as they are all small and nonrandomized, and are selected cohorts from single institutions. Thus, the exact role of this unconventional therapy merits more systematic valuation.
Therefore, we conducted this study with a large population-based cohort based on SEER data. The prognostic value of primary tumor resection for metastatic pancreatic cancer was evaluated after minimizing possible selection bias by propensity score matching.
Material and Methods
Data source
By using de-identified data exempt from supervision by the Institutional Review Board, we conducted a retrospective analysis using the Surveillance, Epidemiology and End Results (SEER) program. The SEER program is a population-based cancer registry covering about 28% of the US population. It primarily collects data on patient demographics, tumor characteristics, therapies, and end result [25].
Study population
Based on the SEER database submitted in November 2017, we obtained a total of 60 229 pancreatic patients aged 18+ years with clinical stage IV (anyTanyNM1) between the years 2004 and 2015. We set the following inclusion criteria: (a) active follow-up case (exclude “autopsy only” or “death certificate only” case); (b) histology codes: 8000, 8010, 8020, 8050, 8140, 8144, 8141, 8210, 8211, 8255, 8260, 8261, 8262, 8263, 8490, 8500, or 8560 according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) (exclude enterochromaffin tumors, neuroendocrine tumors, and lymphomas); (c) primary pancreatic cancer in the patient’s lifetime; (d) survival time between 3 and 60 months; (e) no surgery of distant site; and (f) clear information on tumor characteristics and therapies (surgery type, T stage, N stage, tumor size, and grade). The included patients were partitioned were divided into 2 groups according to whether they had undergone primary tumor resection.
Statistical analysis
Baseline differences between the 2 groups were analyzed utilizing the Pearson chi-square test or Fisher exact test, as appropriate. Since patients were not randomly assigned to get every treatment, propensity score matching (PSM) was performed to help limit selection bias [26,27]. Firstly, a measure assessing the degree to which a covariate confounds the treatment impact on result was proposed [28]. Covariates with a vast degree (relative effect >0.1) are potential candidates for inclusion in the PSM model. Then, greedy different proportional algorithms were used to match patients who underwent primary tumor surgery to those that did not, based on a range of ±0.05 of the propensity score. The matching range of ±0.05 was selected because it offers balance of the included covariates, and does not lose many treated people as unmatchable. After PSM, the standardized differences were calculated for balance checks of covariate distributions between the treatment groups. The cut-off point at which a decision about balance is made is set to 10 [29,30].
Within the matched patient group, scaled Schoenfeld residuals analyses were conducted to test proportional hazards assumptions after using Cox regression models. In case of non-proportionality, the restricted mean survival time (RMST) was conducted to estimate cancer-specific survival differences during a 20-month period [31–33].
In addition, because propensity score matching can eliminate many patients and reduce power, a stabilized inverse probability of treatment weight-adjusted analysis (IPTW) based on the propensity score was performed [34–36]. The log-rank test was used to compare cancer-specific survival between treatment groups.
All statistical analyses were performed with R statistical software (www.r-project.org). Two-sided p values were considered statistically significant at p<0.01.
Results
Patient characteristics
After screening, a total of 2694 metastatic pancreatic cancer patients were enrolled in the formal analysis (Supplementary Figure 1). Of this population, 365 adults (median age 63 years, range 30–92 years) underwent tumor resection as part of first-course therapy. The major surgery type was Whipple (194, 53.2%). Patients were divided into 2 groups: a primary tumor surgery group and a no primary tumor surgery group. The clinicopathological characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients with metastatic pancreatic cancer.
| Patient characteristics in raw data | ||||||
|---|---|---|---|---|---|---|
| Total (n=2694) | No primary tumor surgery (n=2329) | Primary tumor surgery (n=365) | p Value | Relative effect | Standardized difference | |
| Era of diagnosis | 0.105 | 0.334 | 9.436 | |||
| 2004–2006 | 511 (19.0) | 442 (19.0) | 69 (18.9) | |||
| 2007–2009 | 694 (25.8) | 584 (25.1) | 110 (30.1) | |||
| 2010–2012 | 742 (27.5) | 641 (27.5) | 101 (27.7) | |||
| 2013–2015 | 747 (27.7) | 662 (28.4) | 85 (23.3) | |||
| Sex | 0.478 | 0.083 | 4.313 | |||
| Male | 1423 (52.8) | 1237 (53.1) | 186 (51.0) | |||
| Female | 1271 (47.2) | 1092 (46.9) | 179 (49.0) | |||
| Age (years) | 0.271 | 1.861 | 9.327 | |||
| Up to 55 | 578 (21.5) | 494 (21.2) | 84 (23.0) | |||
| 56–65 | 836 (31.0) | 711 (30.5) | 125 (34.2) | |||
| 66–75 | 803 (29.8) | 705 (30.3) | 98 (26.8) | |||
| 76+ | 477 (17.7) | 419 (18.0) | 58 (15.9) | |||
| Race/ethnicity | 0.001 | 0.002 | 0.138 | |||
| White | 2143 (79.5) | 1843 (79.1) | 300 (82.2) | |||
| Black | 325 (12.1) | 300 (12.9) | 25 (6.8) | |||
| Others | 226 (8.4) | 186 (8.0) | 40 (11.0) | |||
| Marital status | 0.002 | 1.176 | 9.753 | |||
| Single | 317 (11.8) | 285 (12.2) | 32 (8.8) | |||
| Married | 1681 (62.4) | 1421 (61.0) | 260 (71.2) | |||
| Others | 413 (15.3) | 372 (16.0) | 41 (11.2) | |||
| Tumor location | <0.001 | 0.265 | 11.574 | |||
| Body/tail | 965 (35.8) | 878 (37.7) | 87 (23.8) | |||
| Head | 1259 (46.7) | 1025 (44.0) | 234 (64.1) | |||
| Others | 470 (17.4) | 426 (18.3) | 44 (12.1) | |||
| Grade | 0.003 | 2.775 | 14.217 | |||
| G1 | 247 (9.2) | 216 (9.3) | 31 (8.5) | |||
| G2 | 1064 (39.5) | 891 (38.3) | 173 (47.4) | |||
| G3 | 1305 (48.4) | 1148 (49.3) | 157 (43.0) | |||
| G4 | 78 (2.9) | 74 (3.2) | 4 (1.1) | |||
| N stage | <0.001 | 1.351 | 72.936 | |||
| N0 | 1467 (54.5) | 1375 (59.0) | 92 (25.2) | |||
| N1 | 1227 (45.5) | 954 (41.0) | 273 (74.8) | |||
| T stage | <0.001 | 0.312 | 11.128 | |||
| T1 | 83 (3.1) | 76 (3.3) | 7 (1.9) | |||
| T2 | 744 (27.6) | 710 (30.5) | 34 (9.3) | |||
| T3 | 1156 (42.9) | 878 (37.7) | 278 (76.2) | |||
| T4 | 711 (26.4) | 665 (28.6) | 46 (12.6) | |||
| Tumor size (mm) | <0.001 | 1.463 | 25.706 | |||
| ≤30 | 697 (25.8) | 572 (24.6) | 125 (34.2) | |||
| 31–40 | 695 (25.8) | 590 (25.3) | 105 (28.8) | |||
| 41–50 | 557 (20.7) | 502 (21.6) | 55 (15.1) | |||
| >50 | 745 (27.7) | 665 (28.6) | 80 (21.9) | |||
| Chemotherapy | <0.001 | 6.275 | 23.147 | |||
| None/unknown | 606 (22.5) | 492 (21.1) | 114 (31.2) | |||
| Yes | 2088 (77.5) | 1837 (78.9) | 251 (68.8) | |||
| Radiotherapy | 0.033 | 0.808 | 11.914 | |||
| None/unknown | 2388 (88.6) | 2077 (89.2) | 311 (85.2) | |||
| Yes | 306 (11.4) | 252 (10.8) | 54 (14.8) | |||
Propensity score matching
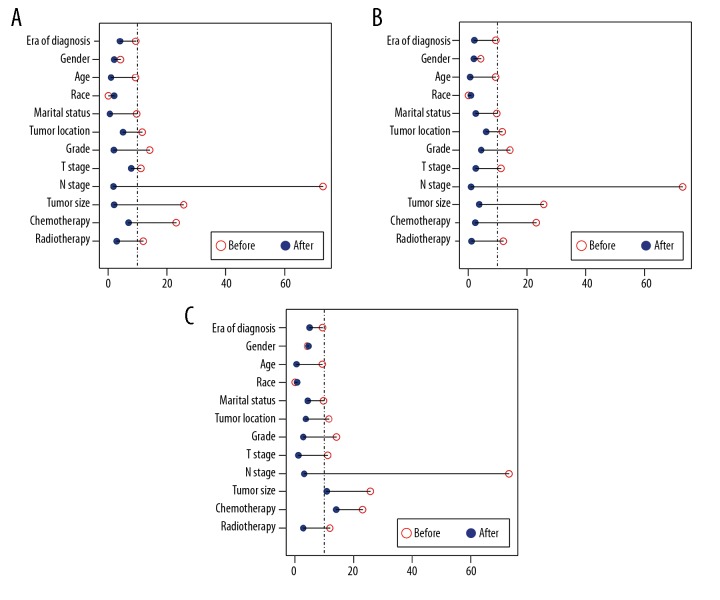
Because of differences between the 2 groups (p<0.05), propensity score matching (PSM) was performed to minimize the bias. After assessing the extent to which a covariate confounded the outcomes, the following variables were included in PSM (relative effect >0.1, Table 1): era of diagnosis, age at diagnosis, tumor location, marital status, grade, T stage, N stage, tumor size, chemotherapy, and radiotherapy. After 1: 1 and 1: 2 PSM, all covariates were well balanced by standardized differences (Figure 1, Table 2). In addition, 1: 3 PSM was also tried in order to make full use of cases, which resulted in 2 imbalance covariates: chemotherapy and tumor size (Figure 1). In this context, 1: 1 and 1: 2 PSM were determined as the basis of subsequent survival analysis.
Figure 1.
Illustration of standardized differences in original data and after (A) 1: 1 propensity score matching (PSM), (B) 1: 2 PSM, and (C) 1: 3 PSM.
Table 2.
Comparison of baseline variables between 2 groups in the matched dataset.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |||||||
|---|---|---|---|---|---|---|---|---|
| No surgery No. (%) |
Surgery No. (%) |
p Value | Standardized differences | No surgery No. (%) |
Surgery No. (%) |
p Value | Standardized differences | |
| Era of diagnosis | 0.553 | 4.125 | 0.306 | 2.045 | ||||
| 2004–2006 | 74 (20.3) | 69 (18.9) | 156 (21.4) | 69 (19.0) | ||||
| 2007–2009 | 93 (25.5) | 110 (30.1) | 192 (26.4) | 109 (29.9) | ||||
| 2010–2012 | 104 (28.5) | 101 (27.7) | 184 (25.3) | 101 (27.7) | ||||
| 2013–2015 | 94 (25.8) | 85 (23.3) | 196 (26.9) | 85 (23.4) | ||||
| Sex | 0.824 | 2.193 | 0.814 | 1.924 | ||||
| Male | 190 (52.1) | 186 (51.0) | 379 (52.1) | 186 (51.1) | ||||
| Female | 175 (47.9) | 179 (49.0) | 349 (47.9) | 178 (48.9) | ||||
| Age (years) | 0.724 | 1.071 | 0.826 | 0.676 | ||||
| Up to 55 | 94 (25.8) | 84 (23.0) | 180 (24.7) | 83 (22.8) | ||||
| 56–65 | 113 (31.0) | 125 (34.2) | 232 (31.9) | 125 (34.3) | ||||
| 66–75 | 96 (26.3) | 98 (26.8) | 195 (26.8) | 98 (26.9) | ||||
| 76+ | 62 (17.0) | 58 (15.9) | 121 (16.6) | 58 (15.9) | ||||
| Race/ethnicity | 0.001 | 2.179 | 0.002 | 0.888 | ||||
| White | 283 (77.5) | 300 (82.2) | 579 (79.5) | 300 (82.4) | ||||
| Black | 54 (14.8) | 25 (6.8) | 96 (13.2) | 25 (6.9) | ||||
| Others | 28 (7.7) | 40 (11.0) | 53 (7.3) | 39 (10.7) | ||||
| Marital status | 0.032 | 0.683 | 0.013 | 2.562 | ||||
| Single | 51 (14.0) | 32 (8.8) | 98 (13.5) | 32 (8.8) | ||||
| Married | 225 (61.6) | 260 (71.2) | 448 (61.5) | 259 (71.2) | ||||
| Divorced | 43 (11.8) | 32 (8.8) | 87 (12.0) | 32 (8.8) | ||||
| Others | 46 (12.6) | 41 (11.2) | 95 (13.0) | 41 (11.3) | ||||
| Tumor location | 0.001 | 5.116 | <0.001 | 6.202 | ||||
| Body/tail | 119 (32.6) | 87 (23.8) | 243 (33.4) | 87 (23.9) | ||||
| Head | 182 (49.9) | 234 (64.1) | 359 (49.3) | 234 (64.3) | ||||
| Others | 64 (17.5) | 44 (12.1) | 126 (17.3) | 43 (11.8) | ||||
| Grade | 0.794 | 2.053 | 0.160 | 4.430 | ||||
| G1 | 37 (10.1) | 31 (8.5) | 92 (12.6) | 31 (8.5) | ||||
| G2 | 168 (46.0) | 173 (47.4) | 308 (42.3) | 172 (47.3) | ||||
| G3 | 154 (42.2) | 157 (43.0) | 318 (43.7) | 157 (43.1) | ||||
| G4 | 6 (1.6) | 4 (1.1) | 10 (1.4) | 4 (1.1) | ||||
| N stage | 0.865 | 1.883 | 0.941 | 0.946 | ||||
| N0 | 95 (26.0) | 92 (25.2) | 187 (25.7) | 92 (25.3) | ||||
| N1 | 270 (74.0) | 273 (74.8) | 541 (74.3) | 272 (74.7) | ||||
| T stage | <0.001 | 7.918 | <0.001 | 2.543 | ||||
| T1 | 14 (3.8) | 7 (1.9) | 25 (3.4) | 7 (1.9) | ||||
| T2 | 90 (24.7) | 34 (9.3) | 173 (23.8) | 34 (9.3) | ||||
| T2 | 90 (24.7) | 34 (9.3) | 173 (23.8) | 34 (9.3) | ||||
| T3 | 165 (45.2) | 278 (76.2) | 300 (41.2) | 278 (76.4) | ||||
| T4 | 96 (26.3) | 46 (12.6) | 230 (31.6) | 45 (12.4) | ||||
| Tumor size (mm) | 0.846 | 2.161 | 0.243 | 3.758 | ||||
| ≤30 | 121 (33.2) | 125 (34.2) | 232 (31.9) | 124 (34.1) | ||||
| 41–50 | 64 (17.5) | 55 (15.1) | 147 (20.2) | 55 (15.1) | ||||
| 31–40 | 102 (27.9) | 105 (28.8) | 200 (27.5) | 105 (28.8) | ||||
| 41–50 | 64 (17.5) | 55 (15.1) | 147 (20.2) | 55 (15.1) | ||||
| >50 | 78 (21.4) | 80 (21.9) | 149 (20.5) | 80 (22.0) | ||||
| Chemotherapy | 0.386 | 7.003 | 0.762 | 2.387 | ||||
| None/unknown | 126 (34.5) | 114 (31.2) | 218 (29.9) | 113 (31.0) | ||||
| Yes | 239 (65.5) | 251 (68.8) | 510 (70.1) | 251 (69.0) | ||||
| Radiotherapy | 0.758 | 3.041 | 0.929 | 1.153 | ||||
| None/unknown | 307 (84.1) | 311 (85.2) | 617 (84.8) | 310 (85.2) | ||||
| Yes | 58 (15.9) | 54 (14.8) | 111 (15.2) | 54 (14.8) | ||||
Survival analysis
The median follow-up was 7 months. The median cancer-specific survival (CSS) of patients after resection of primary tumor was 9 months (range 3–59 months), which was longer than in patients without surgery (7 months, range 3–54 months). On univariate survival analysis, primary tumor surgery, era of diagnosis, age at diagnosis, grade, and chemotherapy were associated with CSS (p<0.001) in 2 PSM cohorts (Supplementary Table 1). The following known prognostic factors variables were included in the multivariable analysis by the Cox proportional hazards model: primary tumor surgery, era of diagnosis, tumor location, T stage, N stage, tumor size, grade, marital status, chemotherapy, age at diagnosis, and radiotherapy. Then, the proportional hazards assumption for the Cox regression model fit was tested using scaled Schoenfeld residuals analysis. The results showed that P values of the overall model and some variables were less than 0.05 (Supplementary Table 2). Thus, the proportional hazards assumption was violated. In this case, restricted mean survival time (RMST) was used to estimate the survival differences during a 20-month period, as over two-thirds of the population had died by this time-point after their diagnosis. The RMST differed significantly between the primary tumor surgery group and the non-surgery group (1: 1 PSM: 11.60 months vs. 8.98 months, p<0.01; 1: 2 PSM: 11.61 months vs. 9.10 months, p<0.01) (Figure 2, Supplementary Table 3). The difference in RMST between the 2 groups was 2.6 months (95%CI 1.7–3.5) and 2.5 months (95%CI 1.7–3.3) for the 2 PSM cohorts. In addition, after adjusting for important prognostic factors using a ANCOVA type adjusted analysis [32], patients who underwent surgery still had longer survival on average than those in the non-surgery group (p<0.01). A stabilized inverse probability of treatment weight-adjusted analysis yielded similar results (IPTW) (p<0.01) (Figure 3).
Figure 2.
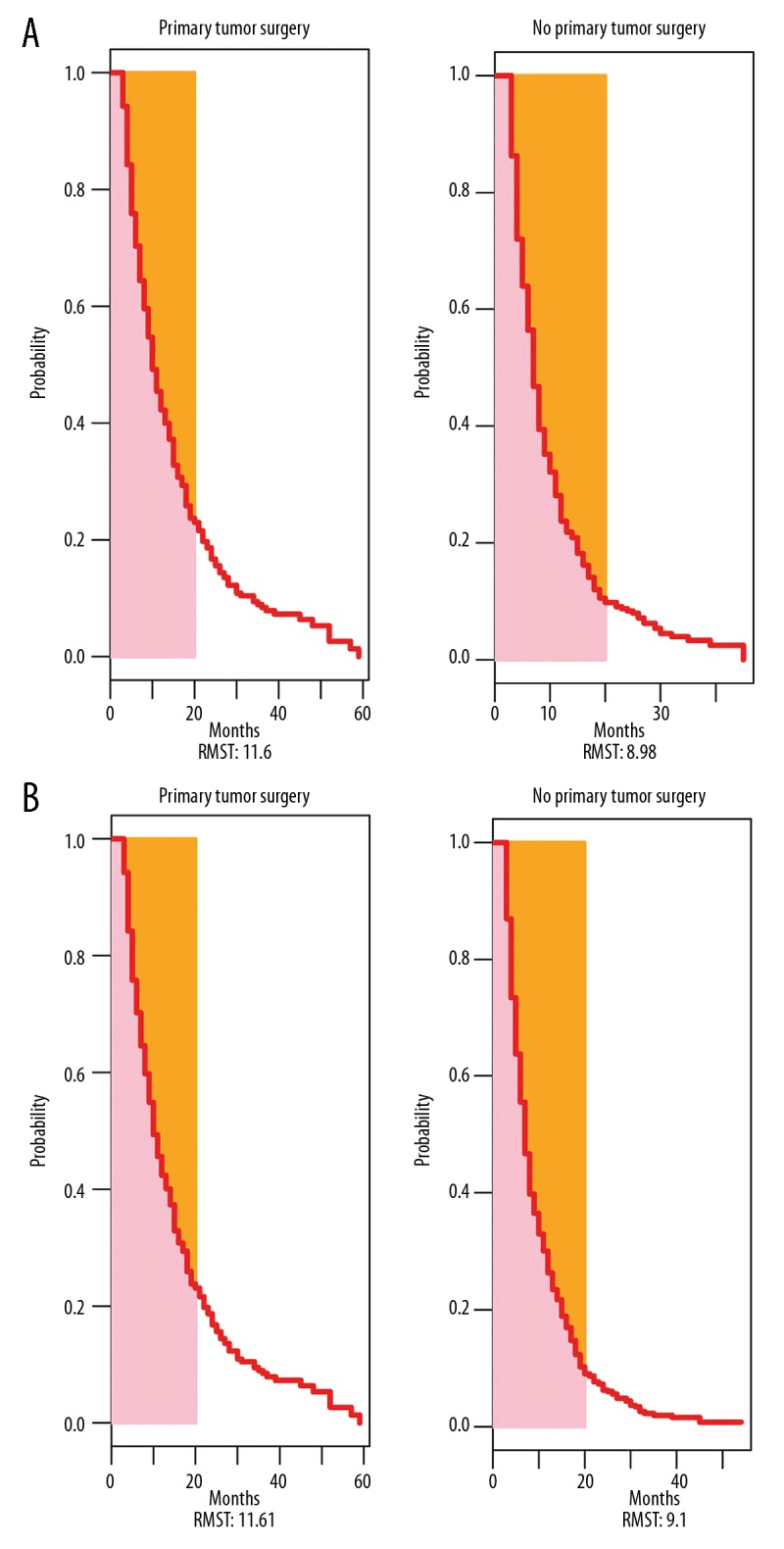
Restricted mean survival time (RMST) in the 2 groups after (A) 1: 1 propensity score matching (PSM), and (B) 1: 2 PSM. RMST – restricted mean survival time.
Figure 3.
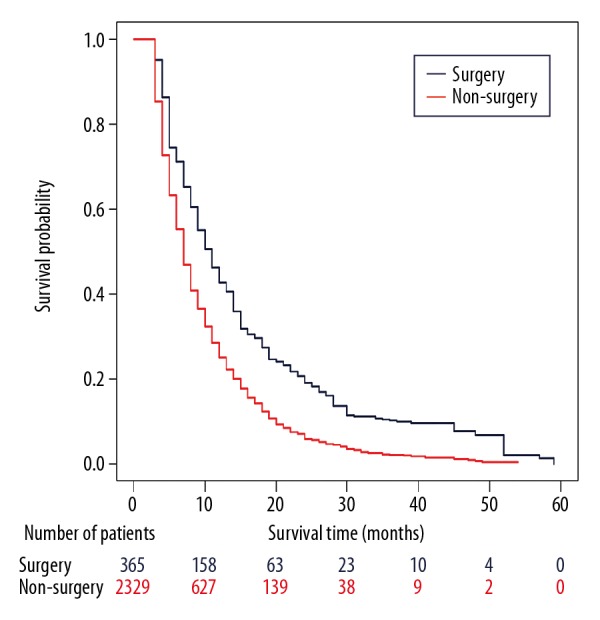
Cancer-specific survival by stable inverse probability of treatment weight-adjusted analysis in patients with metastatic pancreatic cancer.
Discussion
To the best of our knowledge, this study is the first to use propensity score matching to assess the effect of primary tumor resection in patients with mPC. We found a clear association of primary tumor resection with prolonged cancer-specific survival. One of the greatest potential strengths of our study is that it was performed in a real-world, large-scale cohort, and thus provides more powerful evidence than previous publications. Propensity scores were calculated and restricted mean survival time was estimated in the absence of proportional hazards assumptions. Moreover, a stabilized inverse probability of treatment weight-adjusted analysis also showed the benefit of resection.
Chemotherapy is the cornerstone of management for metastatic PC. During the last 17 years, new intensive chemotherapeutic combinations (FOLFIRINOX and gemcitabine plus nab-paclitaxel) have been the most commonly administered first-line therapies. They not only prolong overall survival, but also offer the possibility of good tumor response [37,38]. Consequently, some previously unresectable patients were being reconsidered for surgery after chemotherapy. In a study conducted at the University of Heidelberg, 575 patients with locally advanced and unresectable PC received neoadjuvant treatment. After re-staging, 292 patients underwent resection (including 51 of the 135 patients with metastatic disease), and the survival rate of patients who underwent resection was higher than that of patients who only underwent exploration (15.3 months vs. 8.5 months, P<0.0001) [39]. In another retrospective study of 22 metastatic PC patients, primary tumor size decreased from 31 to 19 mm after chemotherapy and R0 resection was achieved in 88% of cases. The results showed an overall survival of 56 months and a progression-free survival of 27 months for patients after surgery [40]. By comparison, our investigation found only modest benefits for surgical patients (9 months vs. 7 months, p<0.01). This was partly due to the differences in cohort size, chemotherapy regimen, and inclusion criteria. We were not sure all surgical patients had a good treatment response, and those diagnosed before 2011 were less likely to have received the new intensive regimens. However, our study showed that a large proportion of patients who underwent resection presented with head tumors (64.1%), which become symptomatic earlier than malignancies in other locations, and these patients were relatively easy to diagnose and treat early.
From the perspective of tumor pathophysiology, the practice of primary tumor resection also has a theoretical basis. Pancreatic cancer is a highly stroma-abundant, tough tumor [41]. The dense fibrotic stroma obstructs entry of chemotherapeutic drugs into the tumor, causing a poor treatment response [42]. Moreover, the stroma has important biochemical and physical effects in promoting tumor survival, proliferation, and metastasis [43,44]. Differences in stromal density between primary and metastatic lesions may contribute to a discrepancy in therapeutic response. Therefore, it seems logical that low tumor burden increases the success of chemotherapy for mPC.
In other cases, detecting the existence of micrometastasis is difficult. Metastases are often founded in the operating room. This leaves surgeons with a tough choice: the primary tumor seems to be resectable, the patient’s performance status is good, the surgeon has confidence in the low risk of complications, and the patient’s relatives strongly support the removal despite having been informed of the danger. In a report published by Kim et al. [45], 115 patients were confirmed as having mPC during surgery, and 35 of them underwent primary tumor resection. The results showed that the survival rate of the resected patients was significantly better than that of unresected patients (p<0.001). Although surgery is a passive decision in this condition, favorable outcomes cannot be overlooked.
Apart from primary tumor resection, surgery for micrometastasis has drawn increased attention in recent years [22,23,46–48]. With the increasing use of contrast enhancement and rapid multislice computed tomography, a growing number of pancreatic cancer patients presented with oligometastases and a resectable primary lesion [49]. In this setting, synchronous or metachronous metastasectomy may be appropriate, together with pancreatic resection. Tachezy et al. reported that patients who underwent simultaneous pancreas and liver metastasis resections had longer overall survival than non-resected mPC patients (median overall survival 14 months vs. 8 months, p<0.001). In subgroup analysis, they demonstrated that only patients with pancreatic head tumor benefit from surgery (median overall survival 13.6 vs. 7 months, P<0.001) [50]. In contrast, a study by Seelig et al. identified 20 metastatic pancreatic cancer patients and performed surgery and metastasectomy. The mean postoperative survival was 10.7 months, which was not significantly different from a matched-pair group (15.6 months; P=0.1) [17]. They concluded that synchronous resection remained an individual approach for super-selected patients only. Limited by data of the SEER database, our current analysis only evaluated the prognostic value of primary tumor resection. After propensity score matching, surgical patients were found to have longer RMST than non-surgery patients (1: 1 PSM 11.60 months vs. 8.98 months; 1: 2 PSM 11.61 months vs. 9.10 months; p<0.01). A stabilized inverse probability of treatment weight-adjusted analysis was conducted to make full use of this large cohort, and we found that the survival benefit was still driven by the resected patients (p<0.01).
Our study revealed primary tumor resection can be beneficial in patients with mPC. However, as patients were selected in the non-randomized setting, our findings cannot be used to suggest that primary tumor resection should be performed more frequently. Our central goal was to encourage the involved oncologists to critically revisit the impact of surgery in the treatment of metastatic PC.
In addition, we would like to acknowledge other limitations of this study. Firstly, inherent selection bias cannot be excluded due to its retrospective nature. Secondly, the SEER registry does not provide any data on performance status, volume, or location of the metastases, comorbidity, and other important factors. Thirdly, information about the time interval between resection and the onset of chemotherapy may affect prognosis and is crucial for clinical practice.
Conclusions
In conclusion, our study supports the hypothesis that patients with mPC can benefit from primary tumor surgery. We speculated that patients with oligometastasis after chemotherapy can be considered for resection. However, the surgical inclusion criteria and the appropriate role of surgery, such as its effect on symptom control, quality of life, and the extent to which it prolongs survival, still require thorough evaluation through well-designed, prospective, randomized clinical trials.
Supplementary Data
Flow chart for creation of the Surveillance, Epidemiology and End Results (SEER) patient dataset. AJCC – American Joint Committee on Cancer.
Supplementary Table 1.
Univariate analysis of cancer-specific survival in the matched dataset.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |
|---|---|---|
| Primary tumor surgery | <0.001 | <0.001 |
| Era of diagnosis | 0.001 | <0.001 |
| Sex | 0.768 | 0.862 |
| Age, y | 0.001 | 0.001 |
| Race/ethnicity | 0.449 | 0.911 |
| Marital status | 0.133 | 0.048 |
| Tumor location | 0.184 | 0.312 |
| Grade | 0.004 | <0.001 |
| T stage | 0.932 | 0.458 |
| N stage | 0.927 | 0.841 |
| Tumor size (mm) | 0.753 | 0.536 |
| Chemotherapy | <0.001 | <0.001 |
| Radiotherapy | 0.197 | 0.067 |
Supplementary Table 2.
Proportional hazards assumption test for the Cox regression model fit by scaled Schoenfeld residuals analyses.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |
|---|---|---|
| Global | <0.001 | <0.001 |
| Primary tumor surgery | 0.288 | 0.416 |
| Era of diagnosis | 0.325 | 0.066 |
| Sex | NA | NA |
| Age, y | 0.156 | 0.269 |
| Race/ethnicity | NAa | NAa |
| Marital status | 0.385 | 0.579 |
| Tumor location | 0.043 | 0.365 |
| Grade | 0.093 | 0.050 |
| T stage | 0.437 | 0.625 |
| N stage | 0.479 | 0.485 |
| Tumor size | 0.165 | 0.133 |
| Chemotherapy | <0.001 | <0.001 |
| Radiotherapy | 0.035 | 0.024 |
NA – not applicable.
Supplementary Table 3.
Restricted mean survival time in the matched dataset.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |||||
|---|---|---|---|---|---|---|
| No surgery | Surgery | p Value | No surgery | Surgery | p Value | |
| Months (95% CI) | Months (95% CI) | Months (95% CI) | Months (95% CI) | |||
| Restricted mean survival time | 8.98 (8.39–9.56) | 11.60 (10.94–12.26) | <0.01 | 9.10 (8.68–9.53) | 11.61 (10.95–12.27) | <0.01 |
| Restricted mean time lost | 11.03 (10.44–11.61) | 8.40 (7.74–9.06) | <0.01 | 10.90 (10.47–11.32) | 8.39 (7.73–9.05) | <0.01 |
Acknowledgements
All authors would like to thank the SEER program for providing open access to the database.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015;102(12):1459–72. doi: 10.1002/bjs.9892. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Recent updates to NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 1. 2019. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 5.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–33. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 6.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155(6):977–88. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 7.McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: A national perspective. Ann Surg. 2007;246(2):246–53. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warschkow R, Guller U, Tarantino I, et al. Improved survival after primary tumor surgery in metastatic breast cancer: A propensity-adjusted, population-based SEER trend analysis. Ann Surg. 2016;263(6):1188–98. doi: 10.1097/SLA.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 9.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–59. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 10.Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage IV colorectal cancer: How aggressive should we be? Arch Surg. 2000;135(5):530–34. doi: 10.1001/archsurg.135.5.530. discussion 534–35. [DOI] [PubMed] [Google Scholar]
- 11.Strobel O, Neoptolemos J, Jager D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 12.Hackert T, Buchler MW. Pancreatic cancer: Advances in treatment, results and limitations. Dig Dis. 2013;31(1):51–56. doi: 10.1159/000347178. [DOI] [PubMed] [Google Scholar]
- 13.Nentwich MF, Bockhorn M, Konig A, et al. Surgery for advanced and metastatic pancreatic cancer – current state and trends. Anticancer Res. 2012;32(5):1999–2002. [PubMed] [Google Scholar]
- 14.De Jong MC, Farnell MB, Sclabas G, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: A dual-center analysis. Ann Surg. 2010;252(1):142–48. doi: 10.1097/SLA.0b013e3181dbb7a7. [DOI] [PubMed] [Google Scholar]
- 15.Edwards J, Scoggins C, McMasters K, et al. Combined pancreas and liver therapies: Resection and ablation in hepato-pancreatico-biliary malignancies. J Surg Oncol. 2013;107(7):709–12. doi: 10.1002/jso.23318. [DOI] [PubMed] [Google Scholar]
- 16.Scally CP, Yin H, Birkmeyer JD, et al. Comparing perioperative processes of care in high and low mortality centers performing pancreatic surgery. J Surg Oncol. 2015;112(8):866–71. doi: 10.1002/jso.24085. [DOI] [PubMed] [Google Scholar]
- 17.Seelig SK, Burkert B, Chromik AM, et al. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg. 2010;2010 doi: 10.1155/2010/579672. 579672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Singh T, Chaudhary A. Synchronous resection of solitary liver metastases with pancreaticoduodenectomy. JOP. 2010;11(5):434–38. [PubMed] [Google Scholar]
- 19.Ko K, Fujioka S, Kato K, et al. Resection of liver metastasis after a pancreatoduodenectomy for pancreatic cancer: A case report. Hepatogastroenterology. 2001;48(38):375–77. [PubMed] [Google Scholar]
- 20.Ibusuki M, Hiraoka T, Kanemitsu K, et al. Complete remission of pancreatic cancer after multiple resections of locally pancreatic recurrent sites and liver metastasis: report of a case. Surg Today. 2008;38(6):563–66. doi: 10.1007/s00595-007-3642-1. [DOI] [PubMed] [Google Scholar]
- 21.Shimada K, Kosuge T, Yamamoto J, et al. Successful outcome after resection of pancreatic cancer with a solitary hepatic metastasis. Hepatogastroenterology. 2004;51(56):603–5. [PubMed] [Google Scholar]
- 22.Lemke J, Barth TF, Juchems M, et al. Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Res. 2011;31(12):4599–603. [PubMed] [Google Scholar]
- 23.Dunschede F, Will L, von Langsdorf C, et al. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res. 2010;44(3–4):209–13. doi: 10.1159/000313532. [DOI] [PubMed] [Google Scholar]
- 24.Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer. 2007;110(11):2484–92. doi: 10.1002/cncr.23074. [DOI] [PubMed] [Google Scholar]
- 25.Duggan MA, Anderson WF, Altekruse S, et al. The surveillance, epidemiology, and end results (SEER) program and pathology: Toward strengthening the critical relationship. Am J Surg Pathol. 2016;40(12):e94–102. doi: 10.1097/PAS.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Agostino RB, Jr, D’Agostino RB., Sr Estimating treatment effects using observational data. JAMA. 2007;297(3):314–16. doi: 10.1001/jama.297.3.314. [DOI] [PubMed] [Google Scholar]
- 27.Joffe MM, Rosenbaum PR. Invited commentary: Propensity scores. Am J Epidemiol. 1999;150(4):327–33. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 28.Lunt M, Solomon D, Rothman K, et al. Different methods of balancing covariates leading to different effect estimates in the presence of effect modification. Am J Epidemiol. 2009;169(7):909–17. doi: 10.1093/aje/kwn391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill J. Discussion of research using propensity-score matching: Comments on ‘A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003’ by Peter Austin, Statistics in Medicine. Stat Med. 2008;27(12):2055–61. doi: 10.1002/sim.3245. discussion 2066–69. [DOI] [PubMed] [Google Scholar]
- 30.Stuart EA. Developing practical recommendations for the use of propensity scores: Discussion of ‘A critical appraisal of propensity score matching in the medical literature between 1996 and 2003’ by Peter Austin, Statistics in Medicine. Stat Med. 2008;27(12):2062–65. doi: 10.1002/sim.3207. discussion 2066–69. [DOI] [PubMed] [Google Scholar]
- 31.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–85. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian L, Zhao L, Wei LJ. Predicting the restricted mean event time with the subject’s baseline covariates in survival analysis. Biostatistics. 2014;15(2):222–33. doi: 10.1093/biostatistics/kxt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A’Hern RP. Restricted mean survival time: An obligatory end point for time-to-event analysis in cancer trials? J Clin Oncol. 2016;34(28):3474–76. doi: 10.1200/JCO.2016.67.8045. [DOI] [PubMed] [Google Scholar]
- 34.Le Borgne F, Giraudeau B, Querard AH, et al. Comparisons of the performance of different statistical tests for time-to-event analysis with confounding factors: Practical illustrations in kidney transplantation. Stat Med. 2016;35(7):1103–16. doi: 10.1002/sim.6777. [DOI] [PubMed] [Google Scholar]
- 35.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 37.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 39.Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: Neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457–63. doi: 10.1097/SLA.0000000000001850. [DOI] [PubMed] [Google Scholar]
- 40.Frigerio I, Regi P, Giardino A, et al. Downstaging in stage IV pancreatic cancer: A new population eligible for surgery? Ann Surg Oncol. 2017;24(8):2397–403. doi: 10.1245/s10434-017-5885-4. [DOI] [PubMed] [Google Scholar]
- 41.Neesse A, Algul H, Tuveson DA, et al. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut. 2015;64(9):1476–84. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 42.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol. 2011;29(34):4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weniger M, Honselmann KC, Liss AS. The extracellular matrix and pancreatic cancer: A complex relationship. Cancers (Basel) 2018;10(9) doi: 10.3390/cancers10090316. pii: E316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veenstra VL, Damhofer H, Waasdorp C, et al. ADAM12 is a circulating marker for stromal activation in pancreatic cancer and predicts response to chemotherapy. Oncogenesis. 2018;7(11):87. doi: 10.1038/s41389-018-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y, Kim SC, Song KB, et al. Improved survival after palliative resection of unsuspected stage IV pancreatic ductal adenocarcinoma. HPB (Oxford) 2016;18(4):325–31. doi: 10.1016/j.hpb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa H, Takano K, Ando T, et al. [Long-term survival after reoperation for lung metastasis of resected pancreatic adenocarcinoma – a case report]. Gan To Kagaku Ryoho. 2016;43(12):2199–201. [in Japanese] [PubMed] [Google Scholar]
- 47.Zanini N, Lombardi R, Masetti M, et al. Surgery for isolated liver metastases from pancreatic cancer. Updates Surg. 2015;67(1):19–25. doi: 10.1007/s13304-015-0283-6. [DOI] [PubMed] [Google Scholar]
- 48.Matsuki R, Sugiyama M, Takei H, et al. Long-term survival with repeat resection for lung oligometastasis from pancreatic ductal adenocarcinoma: A case report. Surg Case Rep. 2018;4(1):26. doi: 10.1186/s40792-018-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 50.Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160(1):136–44. doi: 10.1016/j.surg.2016.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart for creation of the Surveillance, Epidemiology and End Results (SEER) patient dataset. AJCC – American Joint Committee on Cancer.
Supplementary Table 1.
Univariate analysis of cancer-specific survival in the matched dataset.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |
|---|---|---|
| Primary tumor surgery | <0.001 | <0.001 |
| Era of diagnosis | 0.001 | <0.001 |
| Sex | 0.768 | 0.862 |
| Age, y | 0.001 | 0.001 |
| Race/ethnicity | 0.449 | 0.911 |
| Marital status | 0.133 | 0.048 |
| Tumor location | 0.184 | 0.312 |
| Grade | 0.004 | <0.001 |
| T stage | 0.932 | 0.458 |
| N stage | 0.927 | 0.841 |
| Tumor size (mm) | 0.753 | 0.536 |
| Chemotherapy | <0.001 | <0.001 |
| Radiotherapy | 0.197 | 0.067 |
Supplementary Table 2.
Proportional hazards assumption test for the Cox regression model fit by scaled Schoenfeld residuals analyses.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |
|---|---|---|
| Global | <0.001 | <0.001 |
| Primary tumor surgery | 0.288 | 0.416 |
| Era of diagnosis | 0.325 | 0.066 |
| Sex | NA | NA |
| Age, y | 0.156 | 0.269 |
| Race/ethnicity | NAa | NAa |
| Marital status | 0.385 | 0.579 |
| Tumor location | 0.043 | 0.365 |
| Grade | 0.093 | 0.050 |
| T stage | 0.437 | 0.625 |
| N stage | 0.479 | 0.485 |
| Tumor size | 0.165 | 0.133 |
| Chemotherapy | <0.001 | <0.001 |
| Radiotherapy | 0.035 | 0.024 |
NA – not applicable.
Supplementary Table 3.
Restricted mean survival time in the matched dataset.
| 1: 1 Propensity score matched | 1: 2 Propensity score matched | |||||
|---|---|---|---|---|---|---|
| No surgery | Surgery | p Value | No surgery | Surgery | p Value | |
| Months (95% CI) | Months (95% CI) | Months (95% CI) | Months (95% CI) | |||
| Restricted mean survival time | 8.98 (8.39–9.56) | 11.60 (10.94–12.26) | <0.01 | 9.10 (8.68–9.53) | 11.61 (10.95–12.27) | <0.01 |
| Restricted mean time lost | 11.03 (10.44–11.61) | 8.40 (7.74–9.06) | <0.01 | 10.90 (10.47–11.32) | 8.39 (7.73–9.05) | <0.01 |