Abstract
Background
Both periodontal disease and benign prostatic hyperplasia are age-related diseases that affect millions of people worldwide. Hence, this study aimed to investigate the association between periodontal disease and the risk of benign prostatic hyperplasia.
Methods
A total of 4930 participants were selected from an available health examination that was carried out in 2017, only males were considered for further analysis. All eligible males were divided into benign prostatic hyperplasia and normal groups, the benign prostatic hyperplasia group was then divided into prostate volume ≤ 60 g and > 60 g subgroups; all their periodontal status was extracted and then into normal (CPI score of 0), periodontal disease (CPI score between 1 and 4), and periodontitis (CPI score between 3 and 4) groups. The correlation between periodontal disease and benign prostatic hyperplasia was investigated using logistic regression analyses and greedy matching case-control analysis. Subgroup analysis based on prostate volume was also performed. All analyses were conducted with SAS 9.4 software.
Results
A total of 2171 males were selected for this analysis. The presence of periodontal disease significantly increased the risk of benign prostatic hyperplasia by 1.68 times (OR = 1.68, 95% CI: 1.26–2.24), and individuals with periodontitis showed a higher risk (OR = 4.18, 95% CI: 2.75–6.35). In addition, among matched cases and controls, this association remained robust (periodontal disease: OR = 1.85, 95% CI: 1.30–2.64; periodontitis: OR = 4.83, 95% CI: 2.57–9.07). Subgroup analysis revealed that periodontal disease significantly increased benign prostate hyperplasia risk as well (for prostate volume ≤ 60 g: OR = 1.64, 95% CI: 1.22–2.20; for volume > 60 g: OR = 2.17, 95% CI: 1.04–4.53), and there was a higher risk in the group with a prostate volume greater than 60 g.
Conclusion
Periodontal disease is significantly and positively associated with an increased risk of benign prostatic hyperplasia. Further validation studies should be performed to explore the relationship between periodontal treatment and benign prostate hyperplasia.
Keywords: Benign prostate hyperplasia, Periodontal disease, Periodontitis, Risk factor, Inflammatory disease
Background
Periodontal disease is a complex polymicrobial inflammation and a global burden disease (GBD), and periodontal disease mainly includes gingivitis and periodontitis. In 2015, the incidence of severe chronic periodontitis reached 616 million cases around the world [1]. In China, the standardized disability-adjusted life years (DALYs) rate for this disease has risen from 24.7 in 1990 to 25.7 in 2013 according to the 2013 GBD study [2]. Moreover, periodontal disease is involved in increasing the risk of various systematic diseases, such as atherosclerotic complications (cardiovascular disease [3, 4], arterial stiffness [5], carotid intima-media thickness [6], carotid atherosclerosis [7], stroke [8], coronary heart disease [9], erectile dysfunction [10], hypertension [11, 12], etc.), cancers (head and neck cancer [13], breast cancer [14], lung cancer [15], pancreatic cancer [16], etc.), and metabolic diseases (diabetes [17], overweight/obesity [18], gestational diabetes mellitus [19], etc.).
Benign prostatic hyperplasia (BPH) is defined as unregulated proliferation of connective tissue, smooth muscle and glandular epithelium within the prostate transition zone and is one of the most common diseases in humans. It is estimated that the doubling time of BPH growth is 4.5 years among individuals between the ages of 31 and 50 and 10 years among those between 51 and 70 years old [20]. A meta-analysis including data from 25 countries showed a lifetime prevalence of BPH of 26.2% [95% confidence interval (CI): 22.8–29.6%] without regional or ethnic discrepancies [21]. BPH treatment imposes heavy social and economic burdens on individuals, families, communities and countries. In addition, the estimated cost for annual BPH treatment is approximately $4 billion in the United States alone [22]. Hence, identifying risk factors for BPH to improve early prevention is meaningful.
Periodontal disease and BPH shared common risk factors, such as age, smoking, obesity, diabetes, physical activity, socioeconomic status, and inflammation. Accordingly, it has been hypothesized that periodontal disease may be a marker for BPH. In 2013, Boland et al. performed a case-control study and, for the first time, found that periodontal disease could significantly increase the risk of BPH by 1.50 times [odds ratio (OR) = 1.50, 95% CI, 1.05–2.10] [23]. In 2017, Estemalik et al. demonstrated a marked positive association between oral pathogens and BPH onset; that is, at least one oral pathogen could be detected in 9 out of 10 BPH patients in their prostatic secretions [24]. Therefore, we performed the current study to explore whether there exists an association between periodontal disease and BPH in the Chinese Han population.
Methods
We used the Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) [25] statement to report this cross-sectional study.
Study design and data extraction
With a retrospective design, this study adopted all available data from a health examination at Henan University in 2017 [12]. This study was reviewed and approved by the Committee for Ethical Affairs of the Huaihe Hospital of Henan University, Henan Province. We first identified 4930 records with full information on name, gender, date of birth, relevant physical examinations and laboratory examinations on February 12, 2019, and finished analyses on April 25, 2019. The records would be included for further analysis when information on gender, age, periodontal status, weight, height and prostate status was all included. Only data for males were selected for further screening. However, the health examination did not refer to the history of alcohol or tobacco use, and the absence of these data could result in insufficient capability to assess confounding factors. All eligible data were finally divided into BPH Yes and No groups according to clinical diagnosis results accompanied by relevant examination information, including physical examination and prostate ultrasonography [26]. Patients with records of receiving prostatectomy were automatically enrolled into the BPH group.
Assessment of variables
The largest anteroposterior (height, H), transverse (width, W), and cephalocaudal (length, L) prostate diameters were detected for BPH patients using transabdominal B-ultrasonography. A normal prostate was directly recorded without information on those measurements. Prostate volume (PV) was obtained using the prostate ellipsoid formula: PV = π/6 × [H (cm) × W (cm) × L (cm)]. Then, the BPH group was divided into PV ≤60 g and > 60 g subgroups. Clinical oral examinations of the participants in the dental chair were performed by dentists using a headlamp, a mouth mirror, and a periodontal probe. Periodontal status was assessed using the Community Periodontal Index (CPI), and maximal CPI score of sextants ranged from 0 to 4: 0 referred to healthy periodontal tissue, 1 to the appearance of bleeding, 2 to the existence of calculus, 3 to pocket depth of 4–5 mm, and 4 to pocket depth ≥ 4 mm [27, 28]. A CPI score between 1 and 4 denoted an unhealthy periodontium, while a score of 0 referred to a healthy periodontium. For data analysis, based on periodontal status, individuals were divided into normal (CPI score of 0), periodontal disease (CPI score between 1 and 4), and periodontitis (CPI score between 3 and 4) groups.
Covariates were selected based on originally recorded data and the current knowledge of potential influencers of BPH and periodontal disease. The weight and height of each participant were measured, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Systolic blood pressure (SBP; mmHg) and diastolic blood pressure (DBP; mmHg) were measured, and hypertension referred to SBP ≥140 mmHg, DBP ≥90 mmHg or use of medicine for hypertension. Covariates for the analyses included age, BMI, hypertension status, fasting blood glucose (FBG, ng/ml), serum lipid composition [total cholesterol (TC; mmol/L), triglycerides (TGs; mmol/L), high-density lipoprotein cholesterol (HDL-C; mmol/L), low-density lipoprotein cholesterol (LDL-C; mmol/L)], erythrocyte sedimentation rate (ESR; mm/h), C-reactive protein (CRP; mg/L), urea nitrogen (UN; mmol/L), uric acid (UA; μmol/L), creatinine (μmol/L), and proteinuria.
Statistical analysis
Basic characteristics were summarized for the overall sample as well as for the normal and BPH groups. Categorical variables are shown as counts (percentages), while continuous variables are shown as the means ± standard deviations or medians (interquartile ranges) based on results from the normal distribution test. Comparisons between the normal and BPH groups were conducted using two independent sample t tests (or Wilcoxon rank sum tests) for continuous variables and chi-squared tests (or Fisher exact tests) for categorical variables.
The association between periodontal diseases and BPH was examined with univariable and multivariable logistic regressions. Further and detailed evaluations were performed according to the CPI scores individually. Three models were established stepwise. Specifically, model 1 was constructed without adjustment; model 2 was adjusted for age, BMI, and blood pressure status; and model 3 adopted additional adjustments for FBG, TC, TGs, LDL-C, HDL-C, ESR, UN, UA, creatinine, and proteinuria in addition to the variables adjusted in model 2. Statistical presentation includes OR, 95% CI and corresponding P value for simplification.
Sensitivity analysis was performed to detect whether differences in age, BMI, or blood pressure status between the normal and BPH groups influenced the final results. We matched cases and controls using greedy matching age ± 5 years, BMI ±2, and the same blood pressure status (hypertension and normotension). If the results of the sensitivity analysis were similar to the original results, associations were robust; otherwise, we adopted matched results. Subgroup analysis was conducted on the basis of PV to explore specific effects in groups with different PV sizes. All statistical analyses were calculated with SAS 9.4 software. Tests on statistical significance were two-sided, and P < 0.05 was considered significant.
Results
General characteristics
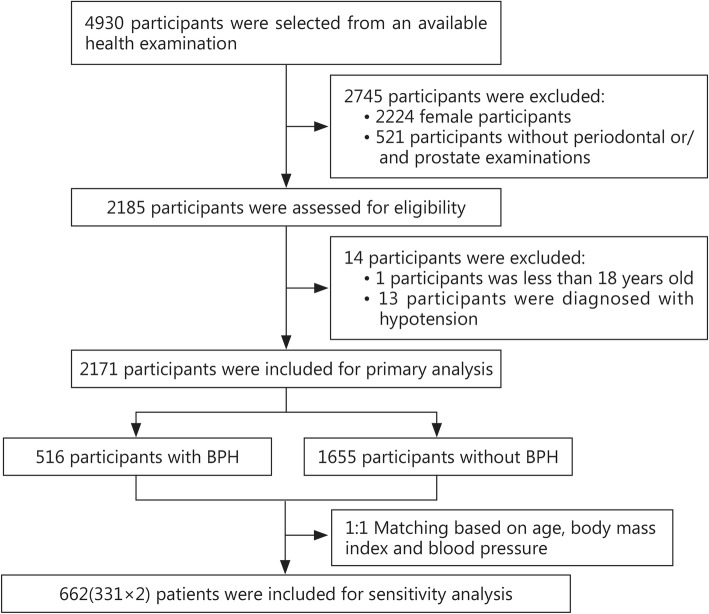
Finally, 2171 participants were included in this analysis after excluding females and those not undergoing periodontal or prostate status examinations. The selection process is shown in Fig. 1. Of the participants, 516 were classified into the BPH group, while 1655 were classified into the normal group. Moreover, 1405 subjects were free from periodontal disease, while 766 had periodontal disease, accounting for 35.3% of all participants. The mean age was 51.09 ± 15.25 years in the overall sample, 67.02 ± 12.83 years in the BPH group and 46.13 ± 12.25 years in the normal group. The mean BMI was 24.73 ± 3.08 kg/m2 in the BPH group and 25.20 ± 3.00 kg/m2 in the normal group; the mean PV was 41.03 ± 18.11 g in the BPH group. Age, BMI and blood pressure status showed statistically significant differences between the two groups (P < 0.05). The baseline characteristics of all of these participants are presented in Table 1.
Fig. 1.
Summary the selection process. A total of 2171 participants were included for primary analysis, from which 516 were classified into the BPH group. About 662 patients were included for sensitivity analysis
Table 1.
Baseline characteristics of the participants (n = 2171)
| Characteristics | Sample (n = 2171) | Benign prostatic hyperplasia | P | |
|---|---|---|---|---|
| Yes (n = 516) | No (n = 1655) | |||
| Age (year, mean ± SD) | 51.09 ± 15.25 | 67.02 ± 12.83 | 46.13 ± 12.25 | 0.00 |
| BMI (kg/m2, mean ± SD) | 25.09 ± 3.03 | 24.73 ± 3.08 | 25.20 ± 3.00 | 0.00 |
| Blood pressure [n(%)] | 0.00 | |||
| Hypertension | 794 (37.4) | 249 (49.3) | 545 (33.7) | |
| Normotension | 1327 (62.6) | 256 (50.7) | 1071 (66.3) | |
| Periodontal disease [n(%)] | 0.40 | |||
| No | 1405 (64.7) | 342 (66.3) | 1063 (64.2) | |
| Yes | 766 (35.3) | 174 (33.7) | 592 (35.8) | |
| FBG (ng/ml, mean ± SD) | 5.64 ± 1.45 | 5.96 ± 1.62 | 5.54 ± 1.37 | 0.00 |
| LDL-C (mmol/L, mean ± SD) | 2.77 ± 0.70 | 2.75 ± 0.72 | 2.78 ± 0.69 | 0.43 |
| Triglycerides (mmol/L, mean ± SD) | 1.55 ± 1.01 | 1.39 ± 0.92 | 1.61 ± 1.04 | 0.00 |
| HDL-C (mmol/L, mean ± SD) | 1.23 ± 0.25 | 1.23 ± 0.25 | 1.23 ± 0.26 | 0.86 |
| TC (mmol/L, mean ± SD) | 4.63 ± 0.86 | 4.63 ± 0.91 | 4.63 ± 0.85 | 0.89 |
| ESR (mm/h, mean ± SD) | 5.51 ± 6.01 | 8.24 ± 8.36 | 4.66 ± 4.74 | 0.00 |
| Uric acid (μmol/L, mean ± SD) | 320.44 ± 72.01 | 314.48 ± 71.63 | 322.31 ± 72.06 | 0.03 |
| Creatinine (μmol/L, mean ± SD) | 81.25 ± 13.04 | 80.03 ± 14.13 | 81.64 ± 12.65 | 0.02 |
| Urea nitrogen (mmol/L, mean ± SD) | 5.13 ± 1.21 | 5.20 ± 1.39 | 5.10 ± 1.14 | 0.13 |
| Prostate volume (g, mean ± SD) | 41.03 ± 18.11 | NR | NA | |
| ≤ 60 g (n = 444) | 35.93 ± 9.24 | NR | ||
| > 60 g (n = 62) | 77.92 ± 22.79 | NR | ||
BMI Body mass index, FBG Fasting blood glucose, TC Total cholesterol, LDL-C Low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol, ESR Erythrocyte sedimentation rate, NR Not reported, NA Not available
Overall analysis
Table 2 shows the overall results from the univariable and multivariable logistic regression analyses. In the univariable analysis, periodontal disease was not related to BPH risk (OR = 0.92, 95% CI: 0.74–1.13), but it was shown that periodontitis might increase the risk of BPH (OR = 4.97, 95% CI: 3.59–6.90). Adjusted analysis using model 2 revealed that periodontal disease (OR = 1.40, 95% CI: 1.15–1.69) and periodontitis (OR = 4.10, 95% CI: 2.75–6.09) were both significantly associated with an increased risk of BPH (P < 0.05). Adjusted analysis adopting model 3 also achieved similar results (periodontal disease: OR = 1.68, 95% CI: 1.26–2.24; periodontitis: OR = 4.18, 95% CI: 2.75–6.35; P < 0.05).
Table 2.
Multivariable analysis results for the relationship between periodontal disease and benign prostatic hyperplasia
| Category | Periodontal disease | Periodontitis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Overall | ||||
| Model 1 | 0.92 (0.74–1.13) | 0.43 | 4.95 (3.59–6.90) | < 0.00 |
| Model 2 | 1.75 (1.34–2.33) | < 0.00 | 4.10 (2.75–6.09) | < 0.00 |
| Model 3 | 1.68 (1.26–2.24) | < 0.00 | 4.18 (2.75–6.35) | < 0.00 |
| Prostate volume ≤ 60 g | ||||
| Model 1 | 0.94 (0.75–1.18) | 0.59 | 5.08 (3.62–7.13) | < 0.00 |
| Model 2 | 1.73 (1.30–2.31) | < 0.00 | 4.13 (2.75–6.19) | < 0.00 |
| Model 3 | 1.64 (1.22–2.20) | < 0.01 | 4.20 (2.74–6.43) | < 0.00 |
| Prostate volume > 60 g | ||||
| Model 1 | 0.74 (0.42–1.29) | 0.29 | 4.18 (2.04–8.57) | < 0.00 |
| Model 2 | 2.26 (1.10–4.65) | 0.03 | 7.32 (2.90–18.48) | < 0.00 |
| Model 3 | 2.17 (1.04–4.53) | 0.04 | 8.36 (3.21–21.78) | < 0.00 |
OR Odds ratio, CI Confidence interval, Model 1: Without adjustment; Model 2: Adjusted for age, body mass index and blood pressure status; Model 3: Further adjusted for fasting blood glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, total cholesterol, erythrocyte sedimentation rate, uric acid, creatinine, and urea nitrogen
Subgroup analysis
Table 2 also demonstrates the subgroup results from univariable and multivariable logistic regression analyses. Periodontal disease was not associated with BPH risk in either the PV ≤60 g group or the PV > 60 g group (P > 0.05). However, periodontitis was significantly associated with an increased risk of BPH in both groups (P < 0.05). After adjustment, both model 2 and model 3 analyses revealed a significant positive relationship of periodontal disease with BPH onset (P < 0.05). However, the disease risk in the PV > 60 g group was higher than that in the PV ≤60 g group.
Sensitivity analysis
We matched cases and controls using greedy matching age ± 5 years, BMI ±2, and the same blood pressure status (hypertension and normotension). Finally, 662 participants were enrolled in the analysis, involving 331 cases and 331 controls. Table 3 presents basic information for overall subjects as well as for cases and controls. The results from the model 3 analysis indicated that periodontitis (OR = 4.83, 95% CI: 2.57–9.07) and periodontal disease (OR = 1.85, 95% CI: 1.30–2.64) significantly increased BPH susceptibility. Compared to the overall analysis findings, these results demonstrated that the association of periodontal disease with BPH was robust.
Table 3.
Baseline characteristics of the matched participants (n = 662)a
| Characteristics | Samples (n = 662) | Benign prostatic hyperplasia | P | |
|---|---|---|---|---|
| Yes (n = 331) | No (n = 331) | |||
| Age (year, mean ± SD) | 61.66 ± 11.41 | 61.92 ± 11.57 | 61.41 ± 11.25 | 0.57 |
| BMI (kg/m2) | 24.82 ± 2.97 | 24.85 ± 2.96 | 24.79 ± 2.99 | 0.80 |
| Blood pressure [n(%)] | 1.00 | |||
| Hypertension | 320 (48.3) | 160 (48.3) | 160 (48.3) | |
| Normotension | 342 (51.7) | 171 (51.7) | 171 (51.7) | |
| Periodontal disease [n(%)] | 0.00 | |||
| No | 459 (69.3) | 208 (62.8) | 251 (75.8) | |
| Yes | 203 (30.7) | 123 (37.2) | 80 (24.2) | |
| FBG (ng/ml, mean ± SD) | 5.92 ± 1.63 | 5.84 ± 1.48 | 5.99 ± 1.76 | 0.25 |
| LDL-C (mmol/L, mean ± SD) | 2.83 ± 0.73 | 2.80 ± 0.72 | 2.85 ± 0.73 | 0.40 |
| Triglycerides (mmol/L, mean ± SD) | 1.46 ± 0.90 | 1.45 ± 0.98 | 1.47 ± 0.80 | 0.69 |
| HDL-C (mmol/L, mean ± SD) | 1.24 ± 0.25 | 1.22 ± 0.23 | 1.26 ± 0.27 | 0.09 |
| TC (mmol/L, mean ± SD) | 4.73 ± 0.91 | 4.67 ± 0.90 | 4.78 ± 0.93 | 0.13 |
| ESR (mm/h, mean ± SD) | 6.75 ± 6.53 | 6.48 ± 6.29 | 7.02 ± 6.77 | 0.29 |
| Uric acid (μmol/L, mean ± SD) | 313.41 ± 73.87 | 313.15 ± 68.88 | 313.68 ± 78.68 | 0.93 |
| Creatinine (μmol/L, mean ± SD) | 79.85 ± 14.16 | 79.74 ± 13.15 | 79.97 ± 15.13 | 0.83 |
| Urea nitrogen (mmol/L, mean ± SD) | 5.19 ± 1.41 | 5.11 ± 1.34 | 5.26 ± 1.48 | 0.17 |
| Prostate volume (g, mean ± SD) | 39.56 ± 17.21 | NR | NA | |
| ≤ 60 g (n = 296) | 35.53 ± 9.19 | NR | ||
| > 60 g (n = 32) | 77.40 ± 26.20 | NR | ||
BMI Body mass index, FBG Fasting blood glucose, TC Total cholesterol, LDL-C Low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol, ESR Erythrocyte sedimentation rate, NR Not reported, NA Not available
aWe matched cases and controls using greedy matching age ± 5 years, BMI ±2, and same blood pressure status (hypertension and normotension)
Discussion
In our study, periodontitis was associated with an increased risk of BPH in both crude and adjusted analyses; periodontal disease had no independent relationship with BPH in crude analysis, but statistical significance appeared after adjustments and subgroup analysis. Moreover, when we performed sensitivity analyses using the greedy matching method (matched age, BMI, and blood pressure status), the association of periodontal disease with BPH remained the same.
Investigating the association between periodontal disease and BPH risk is an interesting topic. In 2013, Boland et al. [23] performed a case-control study based on USA patients and found that periodontitis (diagnosed according to the ICD-9, including gingivitis) can increase the risk of BPH (with or without urinary obstruction) by 1.50 times (OR = 1.50, 95% CI: 1.05–2.10) in males younger than 70 years old after adjustments. Then, they reported subgroup results for Asians (OR = 2.90, 95% CI: 2.35–3.71), individuals of European descent (OR = 1.20, 95% CI: 1.02–1.30), a Hispanic population (OR = 2.20, 95% CI: 2.04–2.42), an unknown ethnicity group (OR = 1.40, 95% CI: 1.29–1.63), and others (OR = 3.80, 95% CI: 3.03–4.67). Our results, based on Asians, confirmed their findings, revealing a significant positive association between the two diseases (OR = 1.68, 95% CI: 1.26–2.24).
Our study revealed that periodontal disease was probably an independent risk factor for BPH. BPH is strongly associated with age [29], as are the increased prevalence and severity of periodontal disease [30]. Hypertension and diabetes were also associated with BPH in 1966 [31]; accordingly, metabolic syndrome (MetS) patients had significantly larger total PV (+ 1.8 ml, 95% CI: 0.74–2.87, P < 0.001) than those without MetS. Boland and colleagues [23] also reported the relationship between BPH and diabetes (OR = 1.4, 95% CI: 1.20–1.55), hypertension (OR = 1.3, 95% CI: 1.10–1.60), obesity (OR = 1.3, 95% CI: 0.91–1.89), and lipid conditions (OR = 1.2, 95% CI: 0.96–1.57). These comorbidities might increase the risk of BPH among people in the United States [31]. In our study, the sensitivity analysis matched for age, BMI, and hypertension status revealed that periodontal disease was associated with an increased risk of BPH. Hence, we concluded that the association between periodontal disease and BPH was not influenced by age, hypertension status, BMI or ethnicity. MetS is a cluster of medical conditions, including obesity, impaired glucose metabolism (abnormal glucose metabolism), hypertriglyceridemia, low HDL-C and hypertension [32]. Hyperlipidemia is also shown to increase the risk of BPH [33], and ESR [34] and BMI [35] might increase PV. Hence, we also adjusted for LDL-C, HDL-C, TGs, TC, and ESR after original adjustments for FBG, hypertension status and BMI. As a result, the main findings were unchanged, indicating their robustness. In addition, we also investigated the relationship between periodontal disease and PV. We divided BPH patients into two groups: PV greater than and equal to or less than 60 g, and the results obtained for the two groups were similar to those for overall cases. However, the risk was higher in the group with PV greater than 60 g. This finding indicated a potential dose-response relationship between periodontal disease and PV in BPH patients. Unfortunately, we failed to perform further analysis on this aspect due to limited data.
The primary mechanism of periodontal disease promoting BPH origination and progression may be associated with inflammation and accompanied by possible influences from oral bacteria. The oral cavity presents a potential reservoir for pathogens, including bacteria and viruses [36, 37], and dental plaque may play an important role in this reservoir, possibly also contributing to periodontal disease [38]. Plausible mechanisms may involve the movement of pathogens and dental plaques from the mouth to the prostate through water drinking or/or capillary vessels. Pathogens and dental plaques can enter capillary vessels and thus trigger systemic inflammatory responses. The prostate is an immune-competent organ with a complex immune system, and tissue damage and chronic tissue healing could result in BPH nodules [39, 40]. Hence, when pathogens and dental plaques travel to a normal prostate, prostatic inflammation may occur; later, BPH would develop. In 2017, Estemalik et al. demonstrated an association between oral pathogens and BPH. In that study, they investigated Porphyromonasgingivalis (Pg), Prevotella intermedia (Pi), Treponema denticola (Td), and Escherichia coli (E. coli) in expressed prostatic secretions from patients with both periodontal disease and chronic prostatitis or BPH and found at least one oral pathogen in 9 out of 10 BPH patients in their prostatic secretions [24]. This finding suggested that periodontal scaling should be covered in routine healthcare for old males and that BPH therapy should be accompanied by periodontal treatment. In addition, asymptomatic prostate inflammation can worsen lower urinary tract symptoms and urinary flow rate in patients with BPH [41], while periodontal treatment can effectively reduce such risks. Additionally, basic laboratory studies have demonstrated that infections by periodontal pathogens can accelerate some systemic conditions by inciting inflammatory responses and further affecting apoptosis, and the mechanisms have been corroborated in atheroma deposition [42], preterm low birth weight among infants [43], cognitive decline [44] and respiratory infection [45] but not in BPH until now. Consequently, experimental studies should be conducted to further explain the potential mechanisms underlying this association.
Neither smoking nor alcohol consumption status was included in our data, so we could not analyze their potential influences in this study. However, a recent systematic review and meta-analysis based on 44,100 subjects showed no significant association between cigarette smoking and BPH risk for either ex-smokers or current smokers [46]. Another systematic review in 2017 showed marked associations of modest alcohol intake with decreased BPH diagnosis and lower urinary tract symptoms [46]. Hence, we can conclude that our results might not be substantially affected by the lack of data on smoking and alcohol consumption. Nevertheless, our study adopted a retrospective design based on existing data, so fragmentary data might affect final results. For example, the information on prostate H/W/L was not recorded for individuals with normal prostates, leading to the missing data on PV for normal subjects. Other potentially relevant information was also not included, such as tooth loss, denture use and decay missing filling tooth (DMFT). Therefore, the associations of denture use and DMFT with BPH were not analyzed to further explore the potential relationship between dental body defect and BPH. Nonetheless, the sensitivity analysis in our study revealed that dental body defects independently affected BPH susceptibility, which might be attributed to the lack of precise information on the above-mentioned indexes. These limitations should be avoided in further studies.
Conclusions
To our knowledge, this was the first study on the association between periodontal disease and BPH risk in Chinese people. Our study revealed a significant robust relationship between increased BPH risk and periodontal disease. This relationship may be a consequence of periodontal and prostatic inflammation. According to these results, we recommend that old males pay more attention to their oral health, especially BPH patients developing relevant symptoms. Periodontal treatment, which is easy and inexpensive, should be included in algorithms to predict BPH surgery risk in future studies.
Acknowledgements
The authors thank all the doctors, coordinators, and technicians for their hard field work and all participants for their cooperation.
Abbreviations
- BMI
Body mass index
- BPH
Benign prostatic hyperplasia
- CI
Confidence interval
- CPI
Community Periodontal Index
- CRP
C-reactive protein
- DALYs
Disability-adjusted life years
- DBP
Diastolic blood pressure
- DMFT
Decay missing filling tooth
- E: coli
Escherichia coli
- ESR
Erythrocyte sedimentation rate
- FBG
Fasting blood glucose
- GBD
Global burden disease
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- MetS
Metabolic syndrome
- OR
Odds ratio
- Pg
Porphyromonasingivalis
- Pi
Prevotella intermedia
- PV
Prostate volume
- SBP
Systolic blood pressure
- TC
Total cholesterol
- Td
Treponema denticola
- TG
Triglycerides
- UA
Uric acid
- UN
Urea nitrogen
Authors’ contributions
XTZ and YJZ are responsible for the design of the study and review the manuscript; LW, BHL, and CYW contributed to the data collection; YYW and QH contributed to the statistical analysis of the data and preparing the article; LW, HZ, and HW contributed to the preparing the article. All authors read and approved the final manuscript.
Funding
This work was supported (in part) by the Nature Science Foundation of Hubei Province (2019CFB760), the Health Commission of Hubei Province Scientific Research Project (WJ2019H035), the Technical Innovation Major Program of Hubei province (Grant NO. 2016ACA152), and the National Key Research and Development Plan of China (2016YFC0106300). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that there are no conflicts of interest in this study.
Availability of data and materials
The datasets used and/ or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The protocol of this study was approved by the Committee for Ethical Affairs of the Huaihe Hospital of Henan University, Henan Province (reference 2018068).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Lan Wu, Email: wulanlalala@163.com.
Bing-Hui Li, Email: dclibinghui@126.com.
Yun-Yun Wang, Email: 13545027094@163.com.
Chao-Yang Wang, Email: wangchaoyang10475@163.com.
Hao Zi, Email: zihao0828@126.com.
Hong Weng, Email: wengh92@163.com.
Qiao Huang, Email: stat_bigdata@163.com.
You-Jia Zhu, Email: Zhuyoujia1954@sina.com.
Xian-Tao Zeng, Email: zengxiantao1128@163.com, Email: zengxiantao1128@gmail.com.
References
- 1.Kassebaum NJ, Smith AGC, Bernabe E, Fleming TD, Reynolds AE, Vos T, et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990-2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. 2017;96(4):380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, Li Z, Wang C, Liu Y, Yang Y, Bussell S, et al. A comparison of DALYs for periodontal disease in China between 1990 and 2013: insights from the 2013 global burden of disease study. BMC Oral Health. 2017;17(1):74. doi: 10.1186/s12903-017-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustapha IZ, Sarah D, Michael O, Richard U. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol. 2007;78(12):2289–2302. doi: 10.1902/jop.2007.070140. [DOI] [PubMed] [Google Scholar]
- 4.Johar N, Dhodapkar SV, Kumar R, Verma T, Jajoo A. Association of relationship between periodontal disease and cardiovascular disease. Mymensingh Med J. 2017;26(2):439–447. [PubMed] [Google Scholar]
- 5.Schmitt A, Carra MC, Boutouyrie P, Bouchard P. Periodontitis and arterial stiffness: a systematic review and meta-analysis. J Clin Periodontol. 2016;42(11):977–987. doi: 10.1111/jcpe.12467. [DOI] [PubMed] [Google Scholar]
- 6.Orlandi M, Suvan J, Petrie A, Donos N, Masi S, Hingorani A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Zeng XT, Leng WD, Lam YY, Yan BP, Wei XM, Weng H, et al. Periodontal disease and carotid atherosclerosis: a meta-analysis of 17,330 participants. Int J Cardiol. 2016;203:1044–1051. doi: 10.1016/j.ijcard.2015.11.092. [DOI] [PubMed] [Google Scholar]
- 8.Lafon A, Pereira B, Dufour T, Rigouby V, Giroud M, Béjot Y, et al. Periodontal disease and stroke: a meta-analysis of cohort studies. Eur J Neurol. 2014;21(9):1155–61.e66-7. doi: 10.1111/ene.12415. [DOI] [PubMed] [Google Scholar]
- 9.Leng WD, Zeng XT, Kwong JS, Hua XP. Periodontal disease and risk of coronary heart disease: an updated meta-analysis of prospective cohort studies. Int J Cardiol. 2015;201:469–472. doi: 10.1016/j.ijcard.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 10.Doostiirani A, Cheraghi Z. Chronic periodontitis and the risk of erectile dysfunction: a systematic review and meta-analysis: methodological issues. Int J Impot Res. 2017;29(6):262. doi: 10.1038/ijir.2017.33. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Cabezas R, Seelam N, Petit C, Agossa K, Gaertner S, Tenenbaum H, et al. Association between periodontitis and arterial hypertension: a systematic review and meta-analysis. Am Heart J. 2016;180:98–112. doi: 10.1016/j.ahj.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhao MJ, Qiao YX, Wu L, Huang Q, Li BH, Zeng XT. Periodontal disease is associated with increased risk of hypertension: a cross-sectional study. Front Physiol. 2019;10:440. doi: 10.3389/fphys.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng XT, Deng AP, Li C, Xia LY, Niu YM, Leng WD. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8(10):e79017. doi: 10.1371/journal.pone.0079017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao J, Wu L, Leng WD, Fang C, Zhu YJ, Jin YH, et al. Periodontal disease and breast cancer: a meta-analysis of 1,73,162 participants. Front Oncol. 2018;8:601. doi: 10.3389/fonc.2018.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal disease and incident lung cancer risk: a meta-analysis of cohort studies. J Periodontol. 2016;87(10):1158–1164. doi: 10.1902/jop.2016.150597. [DOI] [PubMed] [Google Scholar]
- 16.Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann Oncol. 2017;28(5):985–995. doi: 10.1093/annonc/mdx019. [DOI] [PubMed] [Google Scholar]
- 17.Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J Clin Periodontol. 2018;45(2):167–187. doi: 10.1111/jcpe.12837. [DOI] [PubMed] [Google Scholar]
- 18.Papageorgiou SN, Christoph R, Andreas JG, James D. Effect of overweight/obesity on response to periodontal treatment: systematic review and a meta-analysis. J Clin Periodontol. 2015;42(3):247–261. doi: 10.1111/jcpe.12365. [DOI] [PubMed] [Google Scholar]
- 19.Abariga SA, Whitcomb BW. Periodontitis and gestational diabetes mellitus: a systematic review and meta-analysis of observational studies. BMC Pregnancy Childbirth. 2016;16(1):344. doi: 10.1186/s12884-016-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camille V, Loughlin KR. Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol. 2015;22(1):1–6. [PubMed] [Google Scholar]
- 21.Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Sci Rep. 2017;7(1):7984. doi: 10.1038/s41598-017-06628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taub DA, Wei JT. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr Urol Rep. 2006;7(4):272. doi: 10.1007/s11934-996-0006-0. [DOI] [PubMed] [Google Scholar]
- 23.Boland MR, Hripcsak G, Albers DJ, Wei Y, Wilcox AB, Wei J, et al. Discovering medical conditions associated with periodontitis using linked electronic health records. J Clin Periodontol. 2013;40(5):474–482. doi: 10.1111/jcpe.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estemalik J, Demko C, Bissada NF, Joshi N, Bodner D, Shankar E, et al. Simultaneous detection of oral pathogens in subgingival plaque and prostatic fluid of men with periodontal and prostatic diseases. J Periodontol. 2017;88(9):823–829. doi: 10.1902/jop.2017.160477. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.CRHA, CPAM, The Project Team for Minimally Invasive Plasmakinetic System and Cloud Planning Solution of The National Key Research and Development Program of China Transurethral bipolar plasmakinetic prostatectomy treatment for benign prostatic hyperplasia in Chinese: development of a national evidence-based clinical practice guideline (2018 standard version) Natl Med J Chin. 2018;98(20):1549–1560. [Google Scholar]
- 27.Holborow DW. The community periodontal index of treatment needs-uses and abuses? N Z Dent J. 1998;94(417):120–121. [PubMed] [Google Scholar]
- 28.Cutress TW, Ainamo J, Sardo-Infirri J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. Int Dent J. 1987;37(4):222–233. [PubMed] [Google Scholar]
- 29.Martin SA, Haren MT, Marshall VR, Lange K, Wittert GA, Members of the Florey Adelaide Male Ageing Study Prevalence and factors associated with uncomplicated storage and voiding lower urinary tract symptoms in community-dwelling Australian men. World J Urol. 2011;29(2):179–184. doi: 10.1007/s00345-010-0605-8. [DOI] [PubMed] [Google Scholar]
- 30.Ebersole JL, Graves CL, Gonzalez OA, Morford LA, Huja PE, Jr HJ, et al. Aging, inflammation, immunity and periodontal disease. Periodontology. 2016;72(1):54–75. doi: 10.1111/prd.12135. [DOI] [PubMed] [Google Scholar]
- 31.Bourke JB, Griffin JP. Hypertension, diabetes mellitus, and blood groups in benign prostatic hypertrophy. BJU Int. 2010;38(1):18–23. doi: 10.1111/j.1464-410X.1966.tb09675.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao MJ, Huang Q, Wang XH, Ren XY, Jin YH, Zeng XT. Comparing clinical parameters of abnormal and normal fasting blood glucose in benign prostatic hyperplasia patients. Aging Male. 2019:1–8. 10.1080/13685538.2019.1570493. [DOI] [PubMed]
- 33.Shih HJ, Huang CJ, Lin JA, Kao MC, Fan YC, Tsai PS. Hyperlipidemia is associated with an increased risk of clinical benign prostatic hyperplasia. Prostate. 2018;78(2):113–120. doi: 10.1002/pros.23451. [DOI] [PubMed] [Google Scholar]
- 34.Zorba OÜ, Uzun H, Önem K, Çetinkaya M, Rifaioğlu M, Akça N. Association between prostate volume and red cell distribution width. Low Urin Tract Symptoms. 2014;6(1):52–56. doi: 10.1111/luts.12019. [DOI] [PubMed] [Google Scholar]
- 35.Li BH, Deng T, Huang Q, Zi H, Weng H, Zeng XT. Body mass index and risk of prostate volume, international prostate symptom score, maximum urinary flow rate, and post-void residual in benign prostatic hyperplasia patients. Am J Mens Health. 2019;13(4):1557988319870382. doi: 10.1177/1557988319870382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mojon P. Oral health and respiratory infection. J Can Dent Assoc. 2002;68(6):340–345. [PubMed] [Google Scholar]
- 37.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 38.Coulthwaite L, Verran J. Potential pathogenic aspects of denture plaque. Br J Biomed Sci. 2007;64(4):180–189. doi: 10.1080/09674845.2007.11732784. [DOI] [PubMed] [Google Scholar]
- 39.De Nunzio C, Presicce F, Tubaro A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol. 2016;13(10):613–626. doi: 10.1038/nrurol.2016.168. [DOI] [PubMed] [Google Scholar]
- 40.Norström MM, Rådestad E, Sundberg B, Mattsson J, Henningsohn L, Levitsky V, et al. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget. 2016;7(17):23581–23593. doi: 10.18632/oncotarget.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cakir SS, Polat EC, Ozcan L, Besiroglu H, Ötunctemur A, Ozbek E. The effect of prostatic inflammation on clinical outcomes in patients with benign prostate hyperplasia. Prostate Int. 2018;6(2):71–74. doi: 10.1016/j.prnil.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maekawa T, Takahashi N, Tabeta K, Aoki Y, Miyashita H, Miyauchi S, et al. Chronic oral infection with Porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PLoS One. 2011;6(5):e20240. doi: 10.1371/journal.pone.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo H, Ren H, Liang S, Ji Y, Jiang H, Zhang P, et al. Phosphatidylinositol 3-Kinase/Akt signal pathway resists the apoptosis and inflammation in human extravillous trophoblasts induced by Porphyromonas gingivalis. Mol Immunol. 2018;104:100–107. doi: 10.1016/j.molimm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Ni J, Liu Y, Teeling JL, Takayama F, Collcutt A, et al. Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav Immun. 2017;65:350–361. doi: 10.1016/j.bbi.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Zhou R, Yi Z, Li Y, Fu Y, Zhang Y, et al. Porphyromonas gingivalisinduced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/Caspase-3 signaling pathway. Mol Med Rep. 2018;18(1):97–104. doi: 10.3892/mmr.2018.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu H, Fu S, Chen Y, Chen Q, Gu M, Wang Z. Smoking habits and benign prostatic hyperplasia: a systematic review and meta-analysis of observational studies. Medicine (Baltimore) 2016;95(32):e4565. doi: 10.1097/MD.0000000000004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/ or analyzed during the current study are available from the corresponding author upon reasonable request.