Abstract
Objective:
The present study aimed to investigate the possible role of IL-6 and 1α,25-dihydroxyvitamin D3 (1,25D) signaling in epithelial-mesenchymal transition (EMT) and stemness in triple-negative breast cancer (TNBC) cell line.
Methods:
TNBC cell line, HCC 1806, was treated with IL-6 and 1,25D for three and six days. Also, the role of vitamin D receptor (VDR) was studied by transfection of TNBC cell line with VDR gene and transfection efficiency was assessed using Human VDR enzyme-linked immunosorbent assay (ELISA). Changes in E-cadherin gene expression were analyzed by quantitative real-time PCR (qRT-PCR). Also, changes in CD44+ cells were analyzed by flow cytometry. Finally, morphological changes were investigated by light microscopy after 6 days.
Results:
Treatment of HCC1806 cells with IL-6 has no significant effect either on E-cadherin gene expression or CD44+ cells, (p > 0.05). However, E-cadherin gene expression was significantly up-regulated after treatment with 1,25D for 6 days, (p < 0.05). Also, CD44+ cells were significantly reduced after treatment with 1,25D either for 3 or 6 days, (p < 0.05). Transfection of TNBC cell line with VDR gene significantly up-regulated VDR protein expression, (p < 0.05). In addition, overexpression of VDR in TNBC cells and treatment with 1,25D significantly up-regulated E-cadherin gene expression, (p < 0.05) and reduced CD44+ cells, (p < 0.05). Moreover, transfection with VDR and treatment with a combination of 1,25D and IL-6 significantly down-regulated E-cadherin gene expression and increased CD44+ cells compared with transfected cells with VDR treated with 1,25D alone, (p < 0.05). No significant morphological changes were observed in treated cells, 6 days post-treatment.
Conclusion:
The presence of IL-6 in the breast tumor microenvironment may impair the activity of vitamin D3 signaling, limiting its anti-tumor effects in TNBC.
Key Words: Epithelial, mesenchymal transition, IL-6, Vitamin D3, Vitamin D receptor, CD44, E-cadherin
Introduction
Breast cancer is the most common female malignancy accounting for about 22.9% of all female cancers worldwide (Ferlay et al., 2015) and 37.7% of all female cancers in Egypt (Ibrahim et al., 2014). Besides being the 2nd most common cancer, breast cancer is the 5th cause of death from cancer worldwide (Ferlay et al., 2015). As a subtype of breast cancer, triple negative (ER- PR-HER2-) breast cancer (TNBC) is characterized by its aggressive nature, distinct metastatic patterns and lack of targeted therapies (Yao et al., 2017). Metastasis is considered as the main cause of death in breast cancer patients as it is responsible for about 90% of cancer-related mortality (Guan, 2015). Pathologically, metastasis is a multistep complex process that involves the dissemination and metastatic outgrowth of cancer cells (Lambert et al., 2017). In this aspect, growing evidence suggests that epithelial-mesenchymal transition (EMT) plays a crucial role in promoting metastasis in epithelium-derived carcinoma (Chou and Yang, 2015). EMT process is associated with a number of morphological and biochemical changes whereby polarized and basal-membrane anchored epithelial cells acquire a mesenchymal phenotype (Corallino et al., 2015). The hallmark of the EMT process is the downregulation of E-cadherin, a glycoprotein which belongs to the superfamily of classical cadherins and provides a tight connection between epithelial cells (Lecuit and Yap, 2015). A reduced level of E-cadherin influences the overall and disease-free survival and could be utilized as a useful biomarker for evaluating the metastatic risk in breast cancer patients (Vergara et al., 2016). Moreover, an essential link between passage through EMT and the acquisition of molecular and functional properties of stemness has been approved in breast cancer (Sikandar et al., 2017). Stemness involves the ability of cells to self-renew and differentiate into multiple lineages (Wu and Izpisua Belmonte, 2016). Although these properties are displayed by adult stem cells to maintain tissue homeostasis, Cancer Stem Cells (CSCs), a small population of cells within tumors holding stemness properties, are responsible for tumor recurrence, metastasis, and drug resistance (Aponte and Caicedo, 2017). A transmembrane glycoprotein, CD44, has been recognized as one of the key markers for stemness in breast cancer (Shima et al., 2017). CD44 is an important receptor for several extracellular matrix ligands, promoting migration in normal cells and is highly expressed in cancer cells (Senbanjo and Chellaiah, 2017).
Evidently, the tumor microenvironment plays a fundamental role in tumor development, metastasis, and response to therapy (Mittal et al., 2018). Being clinically relevant to the breast tumor microenvironment, interleukin-6 (IL-6) plays an important role in the pathophysiology of cancer (Gyamfi et al., 2018). Thus, aberrant IL-6 expression has been positively associated with the development and progression of carcinomas in human, particularly with an increased metastatic burden and diminished overall survival (Johnson et al., 2018). It has been suggested that IL-6 is capable of inducing EMT phenotype with the enrichment of CD44+ cells in some breast cancer cell lines (Xie et al., 2012).
On the other hand, 1α,25-dihydroxyvitamin D3 (1,25D), the active metabolite of vitamin D3, has been found to inhibit a number of critical processes for tumor survival and progression including EMT (Jeon and Shin, 2018). Also, in vitro and xenograft studies indicated that 1,25D decreased tumor growth and reduced CD44 expression, and this may reflect the role of 1,25D in targeting breast cancer stem cells (So et al., 2013; Wahler et al., 2015).
Accordingly, the present study aimed to shed more lights to the crosstalk between IL-6 and 1,25D in the regulation of E-cadherin gene expression, as EMT marker and CD44+ cells, as a marker for stemness, in TNBC cells. To approach the aim of the present study, the HCC1806 TNBC cell line was treated with IL-6 and 1,25D for 3 and 6 days. It should be noted that treatment was also carried out in transfected cells with vitamin D receptor (VDR). E-cadherin gene fold change and CD44+ cells were assessed three and six days post-treatment. Also, morphological changes were investigated 6 days post-treatment.
Materials and Methods
Cell culture
Triple Negative human breast cancer cell line HCC1806 was obtained from American type culture collection (ATCC, Manassas, VA, USA). HCC1806 cells were maintained in DMEM high glucose with L-glutamine (Lonza, Verviers, Belgium) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St Louis, MO, USA). For EMT induction, HCC1806 cells were cultured in complete growth medium containing 50 ng/ml IL-6 (R and D systems, Minneapolis, MN, USA). To investigate the role of 1,25D, HCC1806 cells were cultured in complete growth medium containing 100 nM 1,25D (Sigma-Aldrich, St Louis, MO, USA).
Over-expression of VDR in HCC1806 cells
VDR was over-expressed in HCC1806 cells by transient transfection using Myc-DDK-tagged human VDR ORF clone (NM_001017535) (Cat# RC219628) which was obtained from Origene Technologies (Rockville, MD) using standard techniques. Transfection was carried out with Lipofectamine 3000® and Opti-MEM® I reduced serum medium (Invitrogen™, Carlsbad, CA, USA). The day before transfection, cells (2 x 105 cells /well) were plated in 0.5 ml of complete growth medium on a 24-well plate. On the day of transfection, cell density was 70% confluent and the growth medium was removed from cells and replaced with 0.5 ml of complete growth medium. For each well of cells to be transfected, 1 μg of DNA was diluted in 50 μl of Opti-MEM® I reduced serum media and 2 μl P3000™ Reagent was added directly to the diluted DNA. Lipofectamine 3000® reagent (1.5 μl) was diluted with 25ul Opti-MEM® I reduced serum media and then added to 25 ul diluted DNA (with P3000™ Reagent), mixed gently and incubated 5 min at room temperature to form DNA - Lipofectamine 3000® reagent complexes. After 5 min incubation, 50 μl of the DNA-Lipofectamine 3000® Reagent complex was added directly to each well containing cells and mixed gently. After incubation for 3 days at 37oC in a CO2 incubator, the medium was replaced with fresh complete growth medium. VDR protein expression was analyzed by ELISA to confirm the transgene expression.
Analysis of VDR protein expression
Three days post-transfection with VDR ORF clone, cells were diluted with PBS (PH 7.2- 7.4) to 1 million/ml. Cells were damaged with repeated freeze-thaw to release intracellular components. The cell suspension was centrifuged 20 min at speed of 3,000 rpm. Supernatants were assayed for VDR protein expression using Human VDR ELISA kit obtained from Shanghai Sunred Biological Technology (Shanghai, China). VDR protein expression was significantly up-regulated (25%), in VDR overexpressed (VDR OE.) groups (p = 0.008), Figure (1). Transfected cells were treated either by 1,25D alone or a combination of 1,25D and IL-6.
Figure 1.
Compare the Expression of VDR Proteins in the Control Cells with Transfected Cells Alone without Treatment. VDR protein expression was significantly increased in transfected cells with VDR (VDR OE.) compared to untransfected cells (control). Student's t-test was applied, (* p ≤ 0.05, ** p ≤ 0.01)
RNA extraction and reverse transcription
Cells were harvested, and RNA was extracted using RNA-spinTM Total RNA Extraction Kit obtained from iNtRON Biotechnology, INC. (Kyungki-Do, KOREA) following the manufacturer’s protocol. One microgram of total RNA was reverse transcribed into cDNA using TIANScript RT Kit obtained from TIANGEN (Shanghai, China) following the manufacturer’s protocol.
Quantitative Real-time PCR
Quantitative Real-time PCR (qRT-PCR) was performed using QuantiFast® SYBR® Green PCR Master obtained from Qiagen (Germantown, MD, USA) using an ABI PRISM 7500 Sequence Detection System (Perkin-Elmer/Applied Biosystems, Rotkreuz, Switzerland). Data were analyzed and normalized to the expression of the housekeeping gene GAPDH. The fold change of target mRNA expression was calculated based on the threshold cycle (Ct) using the 2-ΔΔCt method.
Primer sequences are as follow:
E-cadherin: GenBank Accession number: NM_004360
Forward, 5’-CCCACCACGTACAAGGGTC-3’,
Reverse, 5’-TGGGGTATTGGGGGCATC-3’;
GAPDH: GenBank Accession number: NC_000012
Forward: 5’-GATGCTGGCGCTGAGTACG-3’,
Reverse: 5’- CTAAGCAGTTGGTGGTGC-3’
Flow cytometric analysis
Cells were trypsinized, suspended into single-cell mixtures and washed with PBS. Next, cells (1 x 106 cells) were incubated at room temperature in the dark for 30 min with monoclonal antibodies specific for human cell surface marker CD44-FITC (Immunostep, Scientific Park of the University of Salamanca, Spain) and analyzed on BD FACS Calibur flow cytometer (BD Biosciences).
Light microscopy
HCC1806 cells (2 X 105 cells) were maintained in DMEM high glucose with L-glutamine supplemented with 10% fetal bovine serum. The cells were left untreated or treated with 50 ng/ml IL-6 or 100 nM 1,25D or transfected with VDR ORF clone and treated with either 100 nM 1,25D or combination of 50 ng/ml IL-6 and 100 nM 1,25D. The media was replenished every 48 hours for 6 days. The effect of treatment on cell morphology was investigated by light microscopy (Magnification x 200).
Statistical analysis
Differences among groups were analyzed using a chi-squared or variance (ANOVA) method using SPSS (20.0) software (SPSS, Chicago, IL, USA). For normally distributed data, a comparison between more than two populations was analyzed by ANOVA with a post-hoc Tukey’s multiple comparison test. A probability level of 0.05 was chosen for statistical significance.
Results
Effect of IL-6 on E-cadherin gene expression and CD44+ cells in the TNBC cell line
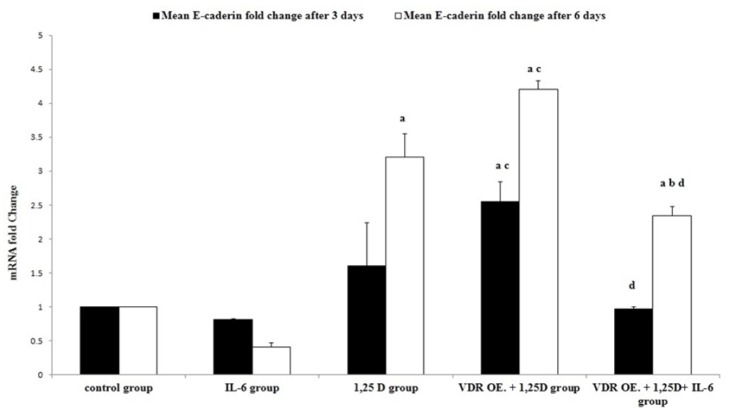
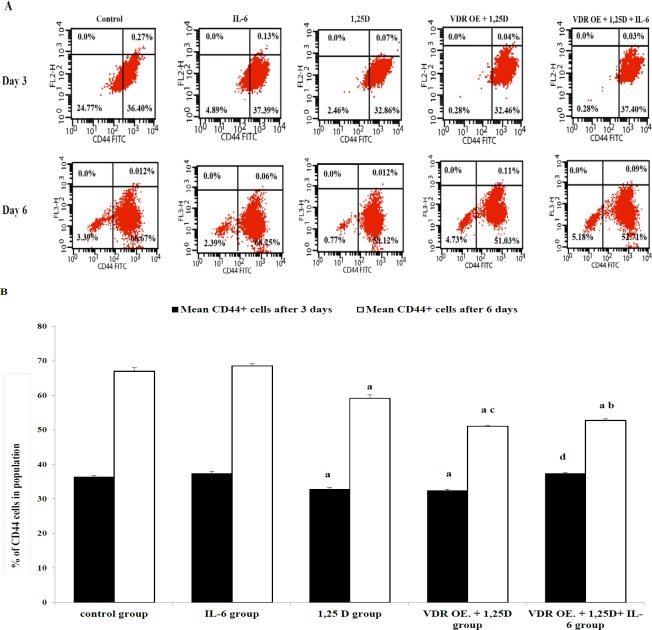
As a hallmark of EMT, E-cadherin gene fold change was measured by qRT-PCR. Treatment of HCC1806 cells with IL-6 insignificantly down-regulated E-cadherin gene expression after 3 days, (p = 0.857). While the down-regulation of E-cadherin gene expression was more pronounced after 6 days, it has not attained a significant level, (p = 0.197), Figure 2. Being one of the key markers of stemness in TNBC, CD44+ cells were assessed by flow cytometer. CD44+ cells were insignificantly modified after treatment with IL-6 either for 3 or 6 days, (p = 0.58; p = 0.741, respectively), Figure 3.
Figure 2.
Effect of IL-6, 1,25D, and VDR on E-cadherin Gene Expression in HCC1806 Cells, Three and Six Days Post-treatment. Quantitative Real-time PCR was used to measure E-cadherin gene expression and the data were normalized to GAPDH gene expression. Experiments were performed in triplicate and the data are presented as the mean ± SD. post-hocTukey's multiple comparison test was applied (a significance vs control group, b significance vs IL-6 group, c significance vs 1,25D group, d significance vs VDR OE.+ 1,25D group,* p ≤ 0.05)
Figure 3.
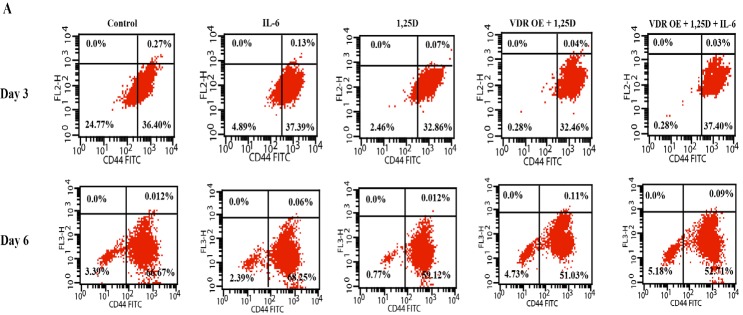
Effect of IL-6, 1,25D, and VDR on CD44+ Cells in HCC1806 Cells, Three and Six Days Post-Treatment. A . Representative histogram from flow cytometry in control and treated cells after 3 and 6 days are shown. B. The average percentage of CD44+ cells from triplicate measurements are represented as bar graphs to show the difference between control and treated groups. The data are presented as the mean ± SD. post-hoc Tukey's multiple comparison test was applied (a significance vs control group, b significance vs IL-6 group, c significance vs 1,25D group, d significance vs VDR OE.+ 1,25D group,* p ≤ 0.05)
Regulation of E-cadherin gene expression and CD44+ cells by 1,25D in TNBC cell line
E-cadherin gene expression was significantly up-regulated after treatment with 1,25D for 6 days, (p = 0.0001), Figure 1. Meanwhile, CD44+ cells were significantly reduced after treatment with 1,25D either for 3 or 6 days, (p = 0.002; p = 0.0001, respectively), Figure 2.
Genetic Manipulation of VDR expression in the TNBC cell line
VDR protein expression was significantly up-regulated (25%), in VDR OE. (VDR overexpressed) groups, (p = 0.008), Figure (1). Overexpression of VDR in HCC1806 cells and treatment with 1,25D significantly up-regulated E-cadherin gene expression compared to untransfected cells treated with 1,25D after 3 or 6 days, (p = 0.004; p = 0.016, respectively), Figure (2). Besides, overexpression of VDR resulted in a significant reduction in CD44+ cells compared to untransfected cells after treatment with 1,25D for 6 days, (p = 0.0001), Figure 3.
Effect of a combination of IL-6 and 1,25D in TNBC cells transfected with VDR
Overexpression of VDR and treatment with a combination of 1,25D and IL-6 significantly up-regulated E-cadherin gene expression in transfected cells treated with the combination compared to untransfected cells treated with IL-6 alone after 6 days, (p = 0.0001), Figure (2). Meanwhile, CD44+ cells were significantly reduced in transfected cells treated with the combination compared to untransfected cells treated with IL-6 alone after 6 days,(p = 0.0001), Figure 3.
On the other hand, overexpression of VDR and treatment with combination of 1,25D and IL-6 significantly down-regulated E-cadherin gene expression compared to transfected cells treated with 1,25D alone either for 3 or 6 days, (p = 0.0001), Figure (2). Moreover, CD44+ cells were significantly increased in transfected cells treated with the combination compared to transfected cells treated with 1,25D alone after 3 days, (p = 0.0001), Figure 3.
Morphological changes after treatment
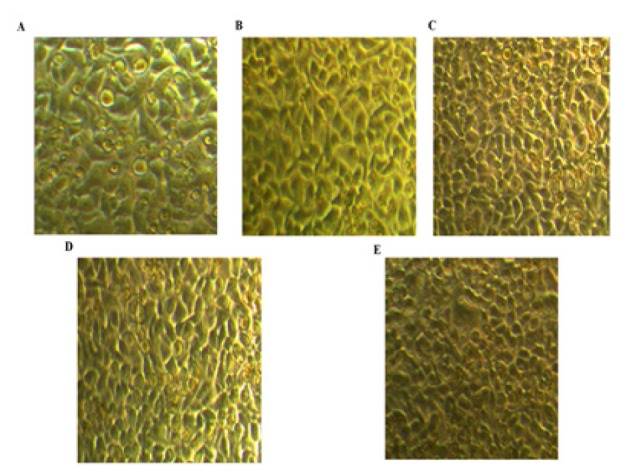
No significant morphological changes were seen in treated cells after 6 days compared to control, Figure 4.
Figure 4.
Representative Photographs of Treated Cells Six Days Post-Treatment. A. Control group: untreated cells. B. IL-6 group: HCC1806 cells were treated with 50 ng/ml IL-6 for 6 days. C. 1,25 D group: HCC1806 cells were treated with 100 nM 1,25D. D. VDR OE. + 1,25D group: HCC1806 were transfected with VDR and treated with 100 nM 1,25D. E. VDR OE. + 1,25D + IL-6 group: HCC1806 were transfected with VDR and treated with 100 nM 1,25D and 50 ng/ml IL-6. The media was replenished every 2 days for 6 days. The effect of treatment on cell morphology was investigated by light microscopy (Magnification x 200)
Discussion
Previously, it has been suggested that E-cadherin could be an independent prognostic marker in TNBC (Shen et al., 2016). Thus, the frequent lymph node metastasis associated with TNBC was explained by the loss of cell-cell adhesion due to E-cadherin down-regulation (Yang et al., 2017). Sullivan et al., (2009) have reported that IL-6 induced EMT and decreased E-cadherin in different breast cancer cell lines through activation of JAK2-STAT3 signaling pathway. Thus, IL-6 has been shown to regulate breast cancer stemness and the maintenance of a stable equilibrium between cancer stem cells and non-stem cancer cells (Iliopoulos et al., 2011). Also, the Induction of EMT by IL-6 was associated with the generation of CD44+ cells in the epithelial-like T47D breast cancer cells (Xie et al., 2012). However, Asgeirsson et al., (1998) demonstrated the anti-adhesive effects of IL-6 in the ER+ breast cancer cell lines, but not the ER- breast cancer cell line MDA-MB-231. Thus, responsiveness to IL-6 is closely related to ER and PR expression with hormone-sensitive breast cancer cells being more responsive than hormone insensitive cells (Espinoza et al., 2016). Also, Hartman et al., (2013) suggested that the growth of TNBC cells depends on the autocrine production of IL-6 and IL-8 in these cells. The present study showed that exogenous recombinant IL-6 has no significant effect either on E-cadherin gene expression or CD44+ cells in the TNBC HCC1806 cell line. Also, no morphological changes were observed after treatment with IL-6. This may be explained by the suggestion that exogenous IL-6 has a low effect on ER- breast cancer cell lines due to the autocrine production of IL-6 in these cells (Chavey et al., 2007).
A compelling body of evidence supports reciprocal regulation between the active vitamin D derivative, 1,25D and EMT (Larriba et al., 2016). Lopes et al., (2012) demonstrated that 1,25D can promote de novo E-cadherin re-expression in TNBC cells. Also, several studies have reported that 1,25D can inhibit TGFβ mediated EMT in different cancer types (Upadhyay et al., 2013; Fischer and Agrawal, 2014). This may explain the significant up-regulation of E-cadherin gene expression in HCC1806 cell line treated with 1,25D which may be through demethylation of E-cadherin promotor, inhibition of EMT-inducers or inhibition of the expression of EMT-transcription factors (Larriba et al., 2016). However, no morphological changes were observed after treatment with 1,25D. This may be explained by the suggestion that cadherin switching isn’t essential for the morphological changes associated with EMT (Maeda et al., 2005). Additionally, So et al., (2013) showed that 1,25D repressed CD44+ cells with inhibition of invasion in basal-like breast cancer. Besides, Wahler et al., (2015) found that 1,25D can reduce mammosphere formation and down-regulate the expression of breast cancer stem cell phenotype CD44+/CD24-. The results of the present study may support that 1,25D can repress CD44+ cells which may result in inhibition of breast cancer invasion.
It is well known that 1,25D exerts the majority of its biological activities by binding to VDR (Ryan et al., 2015). However, Costa et al., (2009) found that the growth-inhibitory effect of 1,25D was not significantly reduced after complete knockdown of VDR in MCF-7 cells by VDR siRNA. Conversely, Pervin et al., (2013) showed that over-expression of VDR gene expression was associated with a significant increase in E-cadherin expression in mammosphere culture. Moreover, LaPorta et al., (2014) conducted a genomic study in a mouse mammary tumor model of TNBC which showed that 1,25D failed to alter gene expression after knockdown of VDR. Besides, So et al., (2013) showed that the repression of CD44 expression induced by 1,25D was blocked by siRNA VDR. The present study provides a direct effect of manipulation of VDR expression by over-expression of VDR in HCC1806 breast cancer cell line which was associated with more up-regulation of E-cadherin gene expression and reduction in CD44+ cells. Collectively, this may point out that the regulation of E-cadherin and CD44 expression by 1,25D is apparently a VDR-dependent event.
In addition, the role of 1,25D and VDR was studied in the presence of the pro-inflammatory cytokine, IL-6. The significant up-regulation of E-cadherin gene expression and the reduction in CD44+ cells in transfected cells with VDR treated with a combination of 1,25D and IL-6 compared to untransfected cells treated with IL-6 alone may support the role of 1,25D and VDR in the regulation of E-cadherin and CD44 expression.
Surprisingly, there was significant down-regulation in E-cadherin gene expression and a significant increase in CD44+ cells in transfected cells treated with the combination compared to transfected cells treated with 1,25D alone. Although IL-6 alone has no significant effect in HCC1806 cells, it significantly suppressed the effects of 1,25D and VDR in these cells. This may support the suggestion that the presence of pro-inflammatory cytokines as IL-6 in the tumor microenvironment has a negative feedback effect on the antitumor activity of 1,25D (Hummel et al., 2014).
In conclusion, IL-6 insignificantly induced EMT and has no effect on stemness in TNBC cells. In contrast, 1,25D may have an inhibitory effect on EMT and stemness in TNBC cells, which is enhanced by overexpression of VDR. The present study may point out the possible role of IL-6 in the breast tumor microenvironment in inhibiting the antitumor activity of 1,25D through the suppression of 1,25D signaling pathway in EMT and stemness.
Acknowledgments
The research wasn’t supported from any agencies.
Statement of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Statement conflict of Interest
The authors declare no conflict of interest.
References
- Aponte PM, Caicedo A. Stemness in cancer: Stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017;2017:5619472. doi: 10.1155/2017/5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgeirsson KS, Olafsdottir K, Jonasson JG, et al. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 1998;10:720–8. doi: 10.1006/cyto.1998.0349. [DOI] [PubMed] [Google Scholar]
- Chavey C, Bibeau F, Gourgou-Bourgade S, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y-S, Yang M-H. Epithelial-mesenchymal transition-related factors in solid tumor and hematological malignancy. J Chin Med Assoc. 2015;78:438–45. doi: 10.1016/j.jcma.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Corallino S, Malabarba MG, Zobel M, et al. Epithelial-to-mesenchymal plasticity harnesses endocytic circuitries. Front Oncol. 2015;5:45. doi: 10.3389/fonc.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JL, Eijk PP, van de Wiel MA, et al. Anti-proliferative action of vitamin D in MCF7 is still active after siRNA-VDR knock-down. BMC Genomics. 2009;10:499. doi: 10.1186/1471-2164-10-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza JA, Jabeen S, Batra R, et al. Cytokine profiling of tumor interstitial fluid of the breast and its relationship with lymphocyte infiltration and clinicopathological characteristics. Oncoimmunology. 2016;5:e1248015. doi: 10.1080/2162402X.2016.1248015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Agrawal DK. Vitamin D regulating TGF-beta induced epithelial-mesenchymal transition. Respir Res. 2014;15:146. doi: 10.1186/s12931-014-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–18. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi J, Eom M, Koo J-S, et al. Multifaceted roles of interleukin-6 in adipocyte-breast cancer cell interaction. Transl Oncol. 2018;11:275–85. doi: 10.1016/j.tranon.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Poage GM, den Hollander P, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel DM, Fetahu IS, Groschel C, et al. Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon cancer cells. J Steroid Biochem Mol Biol. 2014;144:91–5. doi: 10.1016/j.jsbmb.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Khaled HM, Mikhail NN, et al. Cancer incidence in egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I Iliopoulos D, Hirsch HA, Wang G, et al. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S-M, Shin E-A. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorta E, Welsh J. Modeling vitamin D actions in triple negative/basal-like breast cancer. J Steroid Biochem Mol Biol. 2014;144:65–73. doi: 10.1016/j.jsbmb.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriba MJ, Garcia de Herreros A, Munoz A. Vitamin D and the epithelial to mesenchymal transition. Stem Cells Int. 2016;2016:6213872. doi: 10.1155/2016/6213872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–9. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- Lopes N, Carvalho J, Duraes C, et al. 1Alpha, 25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res. 2012;32:249–57. [PubMed] [Google Scholar]
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–87. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- Mittal S, Brown NJ, Holen I. The breast tumor microenvironment: role in cancer development, progression and response to therapy. Expert Rev Mol Diagn. 2018;18:227–43. doi: 10.1080/14737159.2018.1439382. [DOI] [PubMed] [Google Scholar]
- P Pervin S, Hewison M, Braga M, et al. Down-regulation of vitamin D receptor in mammospheres: implications for vitamin D resistance in breast cancer and potential for combination therapy. PLoS One. 2013;8:e53287–e. doi: 10.1371/journal.pone.0053287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JW, Anderson PH, Morris HA. Pleiotropic activities of Vitamin D receptors - adequate activation for multiple health outcomes. Clin Biochem Rev. 2015;36:53–61. [PMC free article] [PubMed] [Google Scholar]
- Senbanjo LT, Chellaiah MA. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Zhang K, Siegal GP, et al. Prognostic value of E-Cadherin and beta-Catenin in triple-negative breast cancer. Am J Clin Pathol. 2016;146:603–10. doi: 10.1093/ajcp/aqw183. [DOI] [PubMed] [Google Scholar]
- Shima H, Yamada A, Ishikawa T, et al. Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy? Gland Surg. 2017;6:82–8. doi: 10.21037/gs.2016.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar SS, Kuo AH, Kalisky T, et al. Role of epithelial to mesenchymal transition associated genes in mammary gland regeneration and breast tumorigenesis. Nat Commun. 2017;8:1669. doi: 10.1038/s41467-017-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So JY, Smolarek AK, Salerno DM, et al. Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS One. 2013;8:e54020–e. doi: 10.1371/journal.pone.0054020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–7. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay SK, Verone A, Shoemaker S, et al. 1,25-Dihydroxyvitamin D3 (1,25(OH)2D3) Signaling Capacity and the Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer (NSCLC): Implications for Use of 1,25(OH)2D3 in NSCLC Treatment. Cancers (Basel) 2013;5:1504–21. doi: 10.3390/cancers5041504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara D, Simeone P, Franck J, et al. Translating epithelial mesenchymal transition markers into the clinic: Novel insights from proteomics. EuPA Open Proteom. 2016;10:31–41. doi: 10.1016/j.euprot.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahler J, So JY, Cheng LC, et al. Vitamin D compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. J Steroid Biochem Mol Biol. 2015;148:148–55. doi: 10.1016/j.jsbmb.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Izpisua Belmonte JC. Stem cells: A renaissance in human biology research. Cell. 2016;165:1572–85. doi: 10.1016/j.cell.2016.05.043. [DOI] [PubMed] [Google Scholar]
- Xie G, Yao Q, Liu Y, et al. IL-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int J Oncol. 2012;40:1171–9. doi: 10.3892/ijo.2011.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhao X, Cui N, et al. Cadherins associate with distinct stem cell-related transcription factors to coordinate the maintenance of stemness in triple-negative breast cancer. Stem Cells Int. 2017;2017:5091541. doi: 10.1155/2017/5091541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, He G, Yan S, et al. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017;8:1913–24. doi: 10.18632/oncotarget.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]