Abstract
Objective:
Cancer is one of the common diseases in the world, and cervical cancer is the fourth one. In this type of cancer, many risk factors, especially infectious diseases, such as human papilloma virus (HPV) and gram-negative bacteria can have important effects on the expression of epithelial to mesenchymal transition related genes like Snail, E-cadherin, and ZEB-1, responsible for connecting cell tissues. In this study, we have investigated the effect of Escherichia coli O111:B4 Lipopolysaccharide (LPS) on HPV positive cell line (HeLa), the expression level of the (Snail, E-cadherin, and ZEB-1), HPV oncogenes (E6, E7) and also microRNA-9, 192.
Materials and Methods:
HeLa cell line was treated with LPS to analyze Snail, E-cadherin, ZEB-1, E6, E7 and also microRNA-9, 192 expression by quantitative real-time PCR in 24, 48 and 72 hours.
Results:
Quantitative real-time PCR revealed a significant reduction in E-cadherin mRNA level at 10ug/L of LPS in three time-points and after 24 hours at 5ug/L of LPS; however, ZEB-1 at 10ug/L of LPS and Snail at 5, 10ug/L of LPS are up-regulated. E7 also illustrated a slight increase, but we did not find any relationship between E7 and LPS treatment. Additionally, there are upward trends in microRNA-9, 192 levels.
Conclusion:
The result of this study, LPS is able to reduce E-cadherin expression, caused by increase in repressor E-cadherin protein expression and some microRNAs, probably. Since bacterial infection can be in cervical site, it is likely to be effective in reducing the E-cadherin expression in the EMT and enhance cancer process, therefore; removing these infections by using the appropriate antibiotics may result in slowing down this process, which requires more research.
Key Words: HPV, LPS, Snail, E-cadherin, ZEB-1
Introduction
Cancer is one of the most causes of death, and there are multiple types of cancer in the world. Among different types of this disease, cervical cancer causes more than 200,000 deaths, annually (Pisani et al., 2002). This cancer is the fourth common cancer in the word (Lai et al., 2019), and the diagnosis of more than 75,000 new cases of invasive cervical malignancy is estimated in the United States (Louie et al., 2002).
Many risk factors seem to be involved in the progression of cervical cancer, including radiation, environmental pollution, (Scheurer et al., 2014; Eslamizadeh et al., 2018) alcohol consumption, smoking (Kapeu et al., 2009), and even infectious diseases (Salimi et al., 2017; Faghihloo et al., 2014; Vaezjalali et al., 2013). Nowadays, it has been accepted that human papilloma virus (HPV) has a strong relationship with cervical cancer (Franco, 1995; Faghihloo et al., 2014; Mirzaei et al., 2018) HPV is a non-enveloped virus, and has double stranded DNA (Favre, 1995). E6 and E7 are the HPV oncoproteins with significant roles in cervical cancer development, and with disrupting tumor suppression like P53, E6 is able to accelerate the progression of cancer (Thomas et al., 1999); in addition, binding E7 to Rb promotes the degradation of tumor suppressor protein proteasome (Syrjänen and Syrjänen, 1999).
Furthermore, many bacteria are able to live in vaginal site, especially gram- negative ones. When the balance of immune system and this flora destroyed, bacteria can replicate; therefore, bacteria tend to produce some inflmatory mediators, probably (Donders et al., 2014). One of the common agents in structure of flora, especially in gram-negative bacteria is cell wall, called Lipopolysaccharide (LPS) (Sharma et al., 2014) LPS can induce NF-kB and TLR-4 expression, which call immune cells to inflmatory site.
Metastasis stage is a hardly curable disease in all kind of cancers, and cervical metastasis is no exception. In invasive cervical cancer, connecting adhesive proteins are reduced between cells; hence, tumor cells are able to spread in all parts of body, replicate, and create tumor cell (Granados López and López, 2014; Insinga et al., 2014) This process has been defined as epithelial to mesenchyme transition (EMT), related to the low expression of connecting inter cellular proteins (Kalluri and Neilson, 2003) E-cadherin is one of the important EMT related protein families, which is involved in the adhesion between cells. Some other EMT related proteins like ZEB-1 and Snail may also regulate the expression of E-cadherin (Grooteclaes and Frisch, 2000), and reduce the expression of adhesive cell proteins; therefore, metastasis can occur (Vaure and Liu, 2014).
It is supposed that, the level of EMT related genes and HPV oncoproteins (E6, E7) and some microRNAs such as mir-9, mir-192 expression can have an effect on cancer progression. Virtually, it has been reported, there is a strong relationship between cervical cancer and HPV; however, the effect of bacterial LPS on cervical cancer, and the relationship between HPV and LPS on EMT related genes expression in cervical cancer is not clear. In this study, human cervical cancer cell line (Hela) was treated with LPS, and the expression of EMT-related genes was evaluated.
Materials and Methods
Cell culture and treatment
HeLa cell line (cervical carcinoma), which is HPV positive, cultured in Dulbecco’s Modified Eagle Medium (DMEM, gibco ) with 2 mM/ L-glutamine (Sigma-Aldrich, MO, USA), supplemented with 10% fetal bovine serum (Life Technologies, Camarillo, CA, USA), and 100 units/ml penicillin/streptomycin (Invitrogen, Life Technologies, Camarillo, CA, USA). Cells were incubated in 37oC and 5% CO2 ( IR.SBMU.MSP.REC.1396.879.).
Lipopolysaccharide preparation
Escherichia coli (E.coli) O111:B4 Lipopolysaccharide (LPS) was prepared from (Sigma-Aldrich, MO, USA). In order to make five different concentrations (1, 2, 5, 10, and 20 ug/l), LPS was dissolved in phosphate buffered saline (PBS).
Proliferation assay
Escherichia coli O111:B4 Lipopolysaccharide (LPS) was evaluated by MTT assay with using the 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). Briefly, HeLa cell line was incubated in 96 well-plates with a condition of 37oC and 5% CO2 until cells reach sufficient population. Medium containing LPS at concentrations of 0, 1, 2, 5, 10 and 20 ug/l was added into 96-well plates. Then, 20-μl MTT solution (0.25 g/ml) was added in 24, 48 and 72 hours, and incubated for 4 hours in incubator with previous condition. DMSO was added, and the absorbance was measured at 570 nm and 625 nm. HeLa cell line without treatment was employed as control.
RNA extraction
HeLa cell line was seeded in 12 well-plates. After filling the 80 percent of plate , HeLa cell line was treated with medium containing various concentrations of LPS (1 ug/l, 5 ug/l, 10 ug/l) in 24 , 48 and 72 hours . To RNA extraction, RNX-plus solution (Cinnagen, Tehran, Iran) and chloroform were employed to discharge proteins. Then, RNA was precipitated with propanol, and washed with 70% alcohol. Finally, the RNA quality and quantity was evaluated at the wavelength of 260 nm by nanodrop spectrophotometry (Eppendrof, Humburg, Germany). The purity of RNA was also analyzed by running with 1% agarose gel electrophoresis.
cDNA synthesis
cDNA synthesis kit was purchased from BioFACT (Daejeon, South Korea). Cellular gene cDNA was synthesized by reverse transcriptase and random hexamers. We have added 10 μl of RNA, 1 μl of random hexamer and 9 μl reverse transcriptase in a sterile micro centrifuge tube to make a 20 μl final volume.
microRNA cDNA was prepared by 10 μl of RNA, and 10μl of reverse transcriptase, and 0.5 μl of microRNA-9 primer TCCGAGGTATTCGCACTGGATACGACTCATAC-3’), and 0.5 μl of microRNA-192 primer (5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTC GCACTGGATACGACGGCTGT-3’) to make cDNA, respectively.
All samples were incubated in 50oC for 40 minutes, and after that incubated in 95oC for 5 minutes. All cDNAs were diluted (2 times in sterile water), and as template for quantitative real-time PCR analysis.
Quantitative real-time PCR
The expression of cellular genes (Snail, E-cadherin, ZEB-1, and GAPDH) and microRNA (microRNAs -9,192) were analyzed by using the BIOFACT™ 2X real-time PCR master mix (for SYBR Green I; BIOFACT, South Korea); Furthermore, in this study, the expression of E6 and E7 genes in HPV positive cell line (HeLa cell line) were evaluated.
SYBR master mix, 1 μl of forward primer, 1 μl of reverse primer, 2 μl of 1/2 diluted cDNA and 6 μl of sterile water were combined. To confirm our results, all samples were run in triplicate at the same time. The primers of all genes were documented in Table 1.
Table 1.
the Primer of Cellular Genes and microRNA, Evaluated with Real-Time PCR
| Primer name | Sequence |
|---|---|
| ZEB-1 (Forward) | 5'-GATGATGAATGCGAGTCAGATGC-3' |
| ZEB-1 (Reverse) | 5'-CTGGTCCTCTTCAGGTGCC-3' |
| Snail 1 (Forward) | 5'-CAGACCCACTCAGATGTCAA-3' |
| Snail 1 (Reverse) | 5'-CATAGTTAGTCACACCTCGT-3' |
| E7 (Forward) | CCGCTCGAGATGCATGGACCTAAGGCAAC |
| E7 (Reverse) | CGCGGATCCTTACTGCTGGGATGCACACC |
| E-cadherin (Forward) |
AGGGGTTAAGCACAACAGCA |
| E-cadherin (Reverse) |
GGTATTGGGGGCATCAGCAT |
| GAPDH (Forward) | ATGTTCGTCATGGGTGTGAA |
| GAPDH (Reverse) | GGTGCTAAGCAGTTGGTGGT |
| U6 (Forward) | 5'-GAGAAGATTAGCATGGCCCCT- 3' |
| U6 (Reverse) | 5'-ATATGGAACGCTTCACGAATTTGC- 3' |
| miRNA -9-5P (Forward) | 5'-CTTTGGTTATCTAGCTGTATGAGTCGT-3' |
| Forward miRNA -192-5P (Forward) | 5'-CTGACCTATGAATTGACAGCCGT-3' |
| Universal Reverse | 5'-ATCCAGTGCAGGGTCCGA-3' |
We have two temperature profiles for cellular and microRNA genes: 95oC for 10 minutes; 40 cycles of 95 oC for 30 Seconds, 55oC for 30 Seconds, and 72oC for 30 Seconds for cellular genes, and 95oC for 10 minutes;40 cycles of 95oC for 20 Seconds, 60oC for 20 Seconds, and 72oC for 20 Seconds for microRNA. Melting curve program was between 60 and 95oC.
The threshold cycle (ct) of all genes in treated cell line was compared with the ct of control cell line ,then all cellular and microRNA were normalized with housekeeping gene (GAPDH and u6), respectively, by the 2–ΔΔct method.
Statistical analysis
Graph-Pad Prism software was used to analyze the difference between groups, and ANOVA test shows the comparison between mRNA levels. We consider the P-value less than 0.05 (P<0.05) as statistically significant for the differences.
Results
Optimization of the LPS concentration
The best LPS concentrations to measure the expression of cellular and microRNA are 1, 5 and 10 ug/L of LPS in 24, 48 and 72 hours. The data was not shown.
LPS down-regulates the expression of E-cadherin
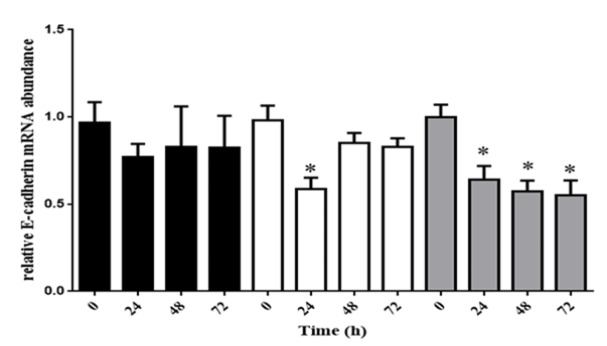
After treating with LPS in 24, 48 and 72 hours, RNA was extracted by RNX-plus, and cDNA was synthesized. Quantitative real-time was employed to analyze EMT related genes. The E-cadherin has revealed a noticeable down-regulation in all times of 10ug/L of LPS and 24 hours after treating with 5ug/L of LPS (Figure 1).
Figure 1.
HeLa Cell Line was Treated with LPS by 1, 5 and 10 mmol/L, when the Cells Reached 80% Confluency. In all times of 10 mmol/L of LPS and 24 hours after treating with 5 mmol/L of LPS, E-cadherin expression have been raised
LPS up-regulates the expression of Snail
In our study, 5 and 10ug/L of LPS can have a strong influence on expression of Snail. We have found in this period of time Snail has an up-ward trend expression, but 1ug/L of LPS ,Snail has not a meaningful increase (Figure 2).
Figure 2.
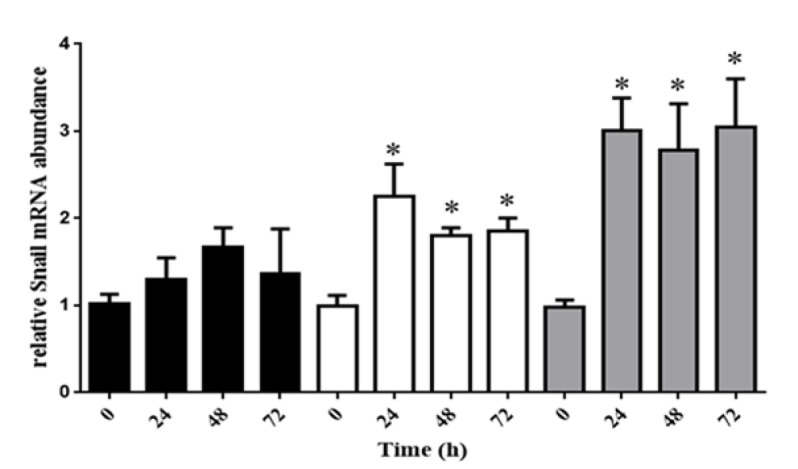
HeLa Cell Line have been Cultured with DMEM Containing LPS at Concentration of 1, 5 and 10 mmol/L. Snail Expression was Revealed a Remarkable Growth in 5 and 10 mmol/L of LPS
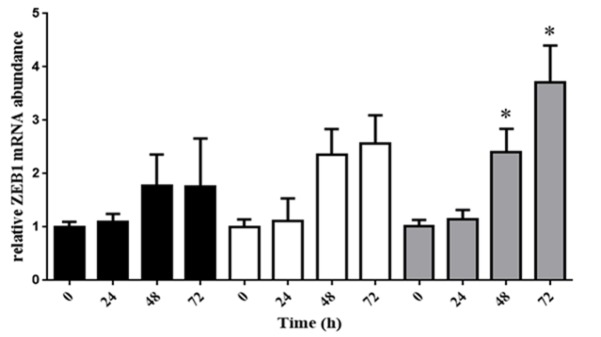
ZEB-1 is over expressed by treating LPS
Analysis of another gene (ZEB-1) has demonstrated a meaningful increase in 48 and 72 hours after treating 10ug/L of LPS in HeLa cell line. Generally, LPS induces an increase in ZEB-1 expression at 1 and 5ug/L of LPS, but it was not meaningful, statistically (Figure 3).
Figure 3.
LPS is Able to Increase Expression of ZEB-1, in HeLa Cell Line, after 48 and 72 hours in 10 mmol/L
LPS increases E7 HPV expression
LPS is able to increase the level of E7 HPV expression, but in our study, we have not found any meaningful relationship between LPS and the expression of E7 HPV (Figure 4).
Figure 4.
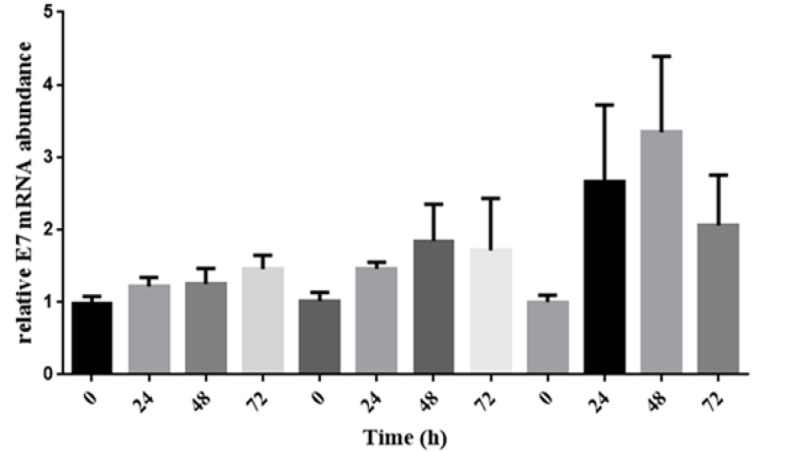
The Level of E7 HPV Expression after Treating HeLa Cell Line with LPS .The bar graph have demonstrated a meaningful increase in amount of E7 HPV expression, however it is not meaningful relation
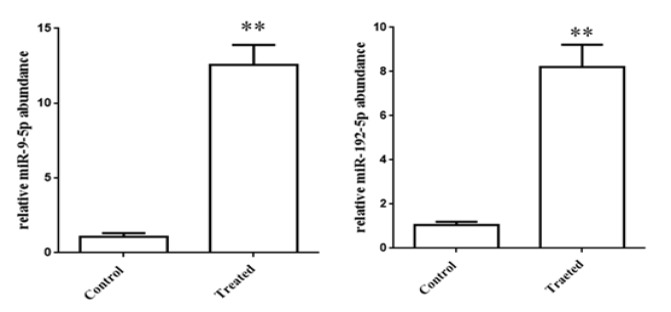
MicroRNA-9 and 192 expression is up-regulated by LPS
We analyzed two microRNAs (microRNA-9 and 192) expression .There are an increase in amount of microRNA-9 (almost 12 times) and 192 (almost 8 times) expression (Figure 5).
Figure 5.
There are a Remarkable Increase in the amount of microRNA-9 and 192 Expressions after Treating LPS to HeLa Cell Line
Discussion
Epigenetic changes may result in changes in gene expression. EMT related genes, such as Snail, ZEB-1 are important gene groups help cells in different organs to connect each other, especially Snail, E-cadherin, and ZEB-1. Down, or up-regulation of these genes can have some effects on cancer progression and invasive metastasis (Adhya and Basu, 2010).
Cervical cancer is the second most important cancer in women, all around the world (Ferlay et al., 2012), and risk factors, ranging from pollution , radiation, smoking and consumption of alcohol to infectious diseases can affect the EMT related genes expression (Scheurer et al., 2014). We studied on the effect of gram- negative bacterial cell wall.
LPS, which is known endotoxin, is a vital structure in all kinds of bacteria, composed of lipid and polysaccharide. This molecule has a complex structure, which can be divided in 3 parts: outer hydrophilic O-antigenic polysaccharide side chain, a hydrophilic core polysaccharide and a hydrophilic lipid, and this hydrophilic lipid acts as an endotoxin (Kilár et al., 2013). We have purchased E.coli O111:B4 LPS to find what might be the relation between bacterial flora and HPV is, and how EMT related genes expression changes.
Our result shown, 10 ug/L of LPS is able to decrease 1.5 times E-cadherin expression .Other studies have the same results for example, Porphyromonas gingivalis-LPS was examined in human gingival epithelial cells, and E-cadherin expression destruct epithelial barrier function (Abe-Yutori et al., 2017) LPS on some cells like macrophages can activate TNF and IL-6 secretion by Raw264.7 pathway, which induces a decrease in E-cadherin expression (Van den Bossche et al., 2015). Other studies have used some LPS antagonism, for example it has studied the effects of Shikonin, an antagonism of LPS, and observed that it can prevent down-regulation of E-cadherin from the effect of LPS in breast cancer cell line (Hong et al., 2015).
Another important EMT related genes is Snail, after treating HeLa cell line with LPS, considerable increase in amount of Snail is observed. Briefly, LPS at concentration of 5 and 10 ug/l is able to increase the expression of Snail, in HeLa cell line, almost duplicate and triplicate, respectively. This result is similar to some other studies, for example in study of Jing, when human hepatocellular carcinoma cell line (HCC) is treated with LPS. In this case in HCC, LPS is able to activate TLR-4 receptor, promoted NF-κB signaling, and Snail increases, subsequently (Jing et al., 2012), furthermore; activation of TLR-4 by LPS can also induce VEGF, Therefore; VEGF causes increase in Snail expression and metastasis progress (Zhu et al., 2016). The study has offered to use Curcumin as an antagonism of LPS. Curcumin can regulate the snail over-expression, induced by LPS (Huang et al., 2013).
We have supposed, LPS with effecting on Snail can lose E-cadherin expression. It seems, Snail is a repressor binding to the E-cadherin promoter, and leads to lose of E-cadherin expression (Cano et al., 2000; Takagi et al., 1998). Actually, in order to repress E-cadherin promoter, Snail requires histone deacetylase activity (Jiang et al., 2013) Peinado has suggested, trichostatin a can inhibit histone deacetylase activity ,therefore ; snail is not able to reduce E-cadherin expression (Peinado et al., 2014).
In this study, we examined the ZEB-1 expression by treating HeLa cell line with LPS. 10ug/L of LPS is able to increase in ZEB-1 expression. It seems, ZEB-1 with the same mechanism of snail can inhibit the expression of E-cadherin (Comijn et al., 2001). We have supposed, LPS by increasing ZEB-1 level can down-regulate the E-cadherin expression. Recently, it has been suggested that Claudin-1, like LPS can up-regulate ZEB-1 expression, consequently E-cadherin is repressed (Singh et al., 2011).
HeLa is a HPV positive cell line, and E7 integrated in HeLa genome. Our result is demonstrated, LPS can cause a slight increase in amount of E7 expression, but there is no meaningful correlate between LPS and E7. Probably, E7 may repress the E-cadherin by DNA methyltransferase 1 (DNMT1) expression. Laurson et al., (2010) Caberg also has supposed, decrease in E-cadherin expression perhaps result from repressing RB by E7 (Caberg et al., 2008). One of other mechanisms has reported that E7 may be over-express cdc6, which is one of key factors to reduce E-cadherin expression (Faghihloo et al., 2016). We suppose, LPS by over-expression of E7, reduce E-cadherin. It has shown valproic acid has the same effect on E7, and E-cadherin down-regulate, subsequently. (Faghihloo et al., 2016)
MicroRNA is able to regulate EMT genes (Abba et al., 2016) Observations have shown, different microRNAs can mediate the expression of E-cadherin (Wong et al., 2014). Furthermore, in this study, LPS treatment was able to increase in amount of microRNA-9 and 192 expressions almost 12 and 8 times, respectively. The result of other studies confirm our experiment for example, Flavia Bazzoni has suggested, LPS activate TLR-4, therefore NF-κB signaling initiate, and microRNA-9 increase (Bazzoni et al., 2009). Wu et al., (2012) also found after treating LPS, microRNA-192 over-expressed. Probably, microRNA-9 is able to suppress E-cadherin (Zhou et al., 2017) LPS over-expresses microRNA-9 and 192 , and these microRNAs can down-regulate E-cadherin expression, probably.
In conclusion, LPS is able to have effects on EMT related genes, such as E-cadherin, Snail and ZEB-1; therefore, gram-negative bacterial infectious diseases in cervix probably can have the same mechanism, and the cervical cancer progression can occur due to LPS in gram- negative bacteria in this site. To prevent cervical cancer progression in early stages, using appropriate antibiotics, is suggested.
Acknowledgements
The present article is financially supported by Research Department of the School of Medicine Shahid Beheshti University of Medical Sciences (grant No.12577).
Ethical Considerations
IR.SBMU.MSP.REC.1396.879.
Conflict of Interest
The authors declare no conflicts of interests.
Authors’ Contributions
E.F., SH.T, assisted in the study design and performed the cell culture and molecular experiments. H.G., G.E performed the statistical Analyses. All authors read and approved the final version of the manuscript.
References
- Abba ML, Patil N, Leupold JH, Allgayer H. MicroRNA regulation of epithelial to mesenchymal transition. J Clin Med. 2016;14:pii: E8. doi: 10.3390/jcm5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe-Yutori M, Chikazawa T, Shibasaki K, Murakami S. Decreased expression of E-cadherin by Porphyromonas gingivalis-lipopolysaccharide attenuates epithelial barrier function. J Periodontal Res. 2017;52:42–50. doi: 10.1111/jre.12367. [DOI] [PubMed] [Google Scholar]
- Adhya D, Basu A. Epigenetic modulation of host: new insights into immune evasion by viruses. J Biosci. 2010;35:647–63. doi: 10.1007/s12038-010-0072-9. [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Rossato M, Fabbri M, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106:5282–7. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberg JH, Hubert PM, Begon DY, et al. Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes. Carcinogenesis. 2008;29:1441–7. doi: 10.1093/carcin/bgn145. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–78. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Donders GG, Zodzika J, Rezeberga D. Treatment of bacterial vaginosis: what we have and what we miss. Exp Opin Pharmacother. 2014;15:645–57. doi: 10.1517/14656566.2014.881800. [DOI] [PubMed] [Google Scholar]
- Eslamizadeh S, Heidari M, Agah S, et al. The role of MicroRNA signature as diagnostic biomarkers in different clinical stages of colorectal cancer. Cell J. 2018;20:220–30. doi: 10.22074/cellj.2018.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihloo E, Saremi MR, Mahabadi M, Akbari H, Saberfar E. Prevalence and characteristics of Epstein-Barr virus-associated gastric cancer in Iran. Arch Iran Med. 2014;17:767–70. [PubMed] [Google Scholar]
- Faghihloo E, Saremi MR, Mahabadi M, Akbari H, Saberfar E. Prevalence and characteristics of Epstein-Barr virus-associated gastric cancer in Iran. Arch Iran Med. 2014;17:767–70. [PubMed] [Google Scholar]
- Faghihloo E, Sadeghizadeh M, Shahmahmoodi S, Mokhtari-Azad T. Cdc6 expression is induced by HPV16 E6 and E7 oncogenes and represses E-cadherin expression. Cancer Gene Ther. 2016;2016 doi: 10.1038/cgt.2016.51. Nov 11. [DOI] [PubMed] [Google Scholar]
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975;15:1239–47. doi: 10.1128/jvi.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 . 2012. [Google Scholar]
- Franco EL. Cancer causes revisited:human papillomavirus and cervical neoplasia. J Natl Cancer Inst. 1995;87:779–80. doi: 10.1093/jnci/87.11.779. [DOI] [PubMed] [Google Scholar]
- Granados López AJ, López JA. Multistep model of cervical cancer: Participation of miRNAs and coding genes. Int J Mol Sci. 2014;15:15700–33. doi: 10.3390/ijms150915700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooteclaes ML, Frisch SM. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene. 2000;19:3823–8. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- Hong D, Jang SY, Jang EH, et al. Shikonin as an inhibitor of the LPS-induced epithelial-tomesenchymal transition in human breast cancer cells. Int J Mol Med. 2015;36:1601–6. doi: 10.3892/ijmm.2015.2373. [DOI] [PubMed] [Google Scholar]
- Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT related through down regulation of NF-κB-Snail signaling in breast cancer cells. Oncol Rep. 2013;29:117–24. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: a population-based study. Am J Obstet Gynecol. 2004;191:105–13. doi: 10.1016/j.ajog.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Jiang GM, Wang HS, Zhang F, et al. Histone deacetylase inhibitor induction of epithelial–mesenchymal transitions via up-regulation of Snail facilitates cancer progression. Biochim Biophys Acta. 2013;1833:663–71. doi: 10.1016/j.bbamcr.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Jing YY, Han ZP, Sun K, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98. doi: 10.1186/1741-7015-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeu AS, Luostarinen T, Jellum E, et al. Is smoking an independent risk factor for invasive cervical cancer? A nested case-control study within Nordic Biobanks. Am J Epidemiol. 2009;169:480–8. doi: 10.1093/aje/kwn354. [DOI] [PubMed] [Google Scholar]
- Kilár A, Dörnyei Á, Kocsis B. Structural characterization of bacterial lipopolysaccharides with mass spectrometry and on- and off-line separation techniques. Mass Spectrom Rev. 2013;32:90–117. doi: 10.1002/mas.21352. [DOI] [PubMed] [Google Scholar]
- Lai TO, Boon SS, Law PT, et al. Oncogenicitiy Comparison of Human Papillomavirus Type 52 E6 Variants. J Gen Virol. 2019;100:484–96. doi: 10.1099/jgv.0.001222. [DOI] [PubMed] [Google Scholar]
- Laurson J, Khan S, Chung R, Cross K, Raj K. Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein. Carcinogenesis. 2010;31:918–26. doi: 10.1093/carcin/bgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie KS, de Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: A comprehensive review. Trop Med Int Health. 2009;14:1287–302. doi: 10.1111/j.1365-3156.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Goudarzi H, Eslami G, Faghihloo E. Role of viruses in gastrointestinal cancer. J Cell Physiol. 2018;233:4000–14. doi: 10.1002/jcp.26194. [DOI] [PubMed] [Google Scholar]
- Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-Cadherin repression by the recruitment of the Sin3A/Histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–19. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani P, Bray F, Parkin DM. Estimates of the worldwide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- Salimi V, Ramezani A, Mirzaei H, et al. Evaluation of the expression level of 12/15 lipoxygenase and the related inflammatory factors (CCL5, CCL3) in respiratory syncytial virus infection in mice model. Microb Pathog. 2017;109:209–13. doi: 10.1016/j.micpath.2017.05.045. [DOI] [PubMed] [Google Scholar]
- Scheurer ME, Danysh HE, Follen M, Lupo PJ. Association of traffic-related hazardous air pollutants and cervical dysplasia in an urban multiethnic population: a cross-sectional study. Environ Health. 2014;13:52. doi: 10.1186/1476-069X-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H, Tal R, Clark NA, Segars JH. Microbiota and pelvic inflammatory disease. Semin Reprod Med. 2014;32:43–9. doi: 10.1055/s-0033-1361822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AB, Sharma A, Smith JJ, et al. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-Cadherin expression in colon cancer cells. Gastroenterology. 2011;141:2140–53. doi: 10.1053/j.gastro.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjänen SM, Syrjänen KJ. New concepts on the role of human papillomavirus in cell cycle regulation. Ann Med. 1999;31:175–87. doi: 10.3109/07853899909115976. [DOI] [PubMed] [Google Scholar]
- Takagi T, Moribe H, Kondoh H, Higashi Y. A zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development. 1998;125:21–31. doi: 10.1242/dev.125.1.21. [DOI] [PubMed] [Google Scholar]
- Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- Vaezjalali M, Rashidpour S, Rezaee H, et al. Hepatitis B viral DNA among HBs antigen negative healthy blood donors. Hepat Mon. 2013;13:e6590. doi: 10.5812/hepatmon.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J, Laoui D, Naessens T, et al. E-cadherin expression in macrophages dampens their inflammatory responsiveness in vitro, but does not modulate M2-regulated pathologies in vivo. Sci Rep. 2015;5:12599. doi: 10.1038/srep12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;10:316. doi: 10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TS, Gao W, Chan JY. Interactions between E-Cadherin and MicroRNA Deregulation in Head and Neck Cancers: The Potential Interplay. Biomed Res Int. 2014;2014:126038. doi: 10.1155/2014/126038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TH, Pan CY, Lin MC, et al. In vivo screening of zebrafish microRNA responses to bacterial infection and their possible roles in regulating immune response genes after lipopolysaccharide stimulation. Fish Physiol Biochem. 2012;38:1299–1310. doi: 10.1007/s10695-012-9617-1. [DOI] [PubMed] [Google Scholar]
- Zhou B, Xu H, Xia M, et al. Overexpressed miR-9 promotes tumor metastasis via targeting E-cadherin in serous ovarian cancer. Front Med. 2017;11:214–22. doi: 10.1007/s11684-017-0518-7. [DOI] [PubMed] [Google Scholar]
- Zhu G, Huang Q, Huang Y, et al. Lipopolysaccharide increases the release of VEGF-C that enhances cell motility and promotes lymphangiogenesis and lymphatic metastasis through the TLR4- NF-κB/JNK pathways in colorectal cancer. Oncotarget. 2016;7:73711–24. doi: 10.18632/oncotarget.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]