Abstract
In this study, semen seminal plasma contents and the motility of sperm cells were determined in Capoeta trutta via a computer-assisted sperm analysis system. In addition, we evaluated the relationship between semen seminal plasma ions and the velocities of sperm cells. Although the predominant ions were K ( mg L) and Na ( mg L) in the semen seminal plasma, Ca ( mg L) and Mg ( mg L) were not predominate according to our results. However, partially strong relationships between the curvilinear velocity value (VCL) and K (; ) were found, while it was moderate with Mg (; ). There was a weak relationship with Na (; ) and Ca (; ). In our results, while the trace metals were determined as Zn > Al > B > Li > Cu in semen seminal plasma, they are not correlated with sperm cell velocities. Finally, we hope that the present information on the motility parameters of Capoeta trutta in this paper will eventually help artificial insemination in reproduction practices.
1. Introduction
Capoeta trutta (Heckel, 1843) is a species in the Cyprinidae family. The fish species is distributed in the Euphrates and Tigris rivers in Turkey, Iran, Iraq, and Syria (Esmaeili et al., 2010; Demirsoy, 1993). Capoeta trutta has been listed as “least concern” on the IUCN Red List of Threatened Species since 2014, but its population has been decreasing in its natural habitat because of overfishing and water pollution (Freyhof, 2014).
The aquatic species should be domesticated because commercial aquaculture has developed very rapidly, and threatened populations have needed additional restoration in wild habitats (Lorenzen et al., 2012). However, the control of quality gametes is important for the performance of brood stocks in aquaculture systems. Therefore, the semen quality is very important for fish farming industry, commercial species, and biotechnological applications (Bobe and Labbé, 2010; Cabrita et al., 2014; Şahinöz et al., 2008). The motility, duration of motility and density, pH, spermatocrit, and seminal plasma contents are major parameters to determine the semen quality in fish (Billard and Cosson, 1992; Rurangwa et al., 2004). However, seminal plasma has very important ions that support the viability, motility, or immobilization of sperm cells. In teleost fish species, the sperm cells are immobilized in the testes and seminal fluid, and the start of motility depends on the conditions of the fertilization environment. The ion concentrations (, , , , etc.), osmolality, and pH in activator media are among the most important to regulate motility of sperm cells, in addition to other factors (Alavi and Cosson, 2006; Browne et al., 2015; Dzyuba and Cosson, 2014). For example, rainbow trout sperm cells were inactivated after being diluted with solutions containing high concentrations of the ion. However, the isotonic NaCl solution triggers immediate activation of sperm cell motility (Alavi and Cosson, 2006). For the motility of rainbow trout sperm cells, exchange may also be responsible for the participation of ions (Dzyuba and Cosson, 2014). However, researchers have found that intracellular Ca ion concentrations increase when the motility of sperm cells begins. Therefore, Ca ion concentration is a necessity for the motility of sperm cells (Alavi et al., 2011; Alavi and Cosson, 2006; Cosson et al., 1989; Tanimoto and Morisawa, 1988). However, some trace elements such as Zn, Mg, Cu, and Ca are important for the maintenance of normal spermatogenesis, sperm maturation, and DNA metabolism (Yuyan et al., 2008). There are some studies that associated trace elements with the parameters of oxidative stress and sperm cell motility in Acipenser ruthenus (Li et al., 2010); there are also associated relationships between trace elements and sperm cell motility in Cyprinus carpio (Kovacik et al., 2018), Barbus grypus (Ögretmen et al., 2014), the levels and effects of trace elements of semen seminal plasma in infertile men (Bassey et al., 2013; Vickram et al., 2013), and bovine semen (Tvrdá et al., 2013). Computer-assisted sperm analysis (CASA) systems are very popular for the practical analysis of sperm cell motility in various species and have combined the methods of predicting the analyzer's emotions with classical methods used in the past (Fauvel et al., 2010; Özgür et al., 2019).
The reproduction properties of Capoeta trutta can help to understand their management, conservation in nature, and reproduction status in artificial conditions. Thus, we studied some parameters of reproduction, including sperm cell motility, sperm cell density, and the contents of semen seminal plasma.
2. Material and methods
2.1. Semen sample collection
Wild males of Capoeta trutta (weight: g, total length: cm, ) were caught at Karakaya Dam Lake in the upper part of the Euphrates River, Malatya Province, Turkey, on May 2018. The semen samples were collected without hormone injection. The sexual maturity of the fish was confirmed by the urogenital opening and breeding tubercles on the nose. Therefore, semen samples collected in Eppendorf tubes with gentle pressure to the abdomen. Then, they were rapidly transported in Styrofoam boxes with ice to the laboratory and were analyzed. Care was taken to avoid any contamination by urine during the collection of semen samples.
2.2. The determination of sperm cell motility
Fresh semen samples from 10 individual fish were diluted at a ratio of with immotile solution (IMS) (128.4 mM NaCl, 2.7 mM KCl, 1.4 mM , 2.4 mM ; pH 8.1) (Linhart et al., 2000) and were stored at 4 C until the analysis was performed, 1 h. Then, semen samples were activated under the microscope to determine the parameters of sperm cell motility at a ratio of with activation solution (AS) (mM NaCl; 5 mM KCl; 20 mM Tris–HCl, pH 8) (Linhart et al., 2000) at room temperature. Sperm cells were analyzed without coverslips. All semen samples were kept on ice and examined under an Olympus CX31 microscope with 200 magnification lens and a Sony CCD camera with 30 fbs video recording capacity. The motility of sperm cells was analyzed via the computer-assisted sperm analysis system BASA-Sperm Aqua, produced by Merck Biotechnology Ltd. Co. in Turkey. The values of motility parameters such as VSL (straight line velocity, m s), VCL (curvilinear velocity, m s), VAP (angular path velocity, m s), LIN (linearity, %, (VSL/VCL) 100), BCF (beat cross frequency, Hz), ALH (amplitude of lateral displacement of the sperm cell head, m), and MAD (mean angular displacement, ()) (Fauvel et al., 2010; Özgür et al., 2019) are examined in the study. The following setting were used in the picture settings and parameters of BASA Sperm Aqua: acquired time delay of 0, an image field maximum of 60; images per record of 90, frame per second minimum of 30, track immotile level of 5, track motile level of 25, velocity maximum of 500.
The semen samples were diluted with 125 mM NaCl up to 16-fold and determined in a spectrophotometer with the absorbance at 505 nm for sperm cell concentration (Ciereszko and Dabrowski, 1993).
2.3. The analysis of semen seminal plasma ions
Semen seminal plasma was collected after centrifugation of the semen at 3400 g for 10 min in Beckman L-8-70M ultracentrifuge (Rotor SW-28, Munich, Germany). Seminal plasma was centrifuged twice to avoid possible contamination with sperm cell and stored at 20 C until analysis. The samples of semen were digested in the ultrasonic bath for 10 min by using 1 mL . Digested solutions were diluted with deionized water until adjusted to 10 mL. After dilution, they were analyzed for Na, K, Ca, Mg, Al, B, Cu, Li, and Zn (Massanyi et al., 2008; Özgür et al., 2015; Slivkova et al., 2009). Metal concentrations were determined by inductively coupled plasma optical emission spectrometry (ICP-OES VARIAN 725-ES, with CCD detector). All standard solutions (0.01, 0.05, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, and 100 mg L) were prepared by diluting 1 mg mL stock multielement standard solutions for ICP-OES. The optimum instrumental parameters of ICP-OES were, for example, radiofrequency power (1.2 kW), plasma gas flow rate (15.0 L min), auxiliary gas flow rate (1.5 L min), nebulizer flow rate (0.75 L min), replicate read time (3 s), delay (15 s), sample uptake delay (30 s), pump rate (15 rpm), rinse time (20 s), replicates (3), and torch (quartz for vertical view) for detection of multi-elements.
2.4. Statistical analysis
The relationships between all data were tested by using the bivariate correlation coefficients of Pearson. Then, linear and non-linear regression models were investigated between parameters. Descriptive analysis (means SD, ) and the normality test were performed between the data in the SPSS 17 program.
3. Results
Some motility parameters were evaluated characterizing velocities (VSL, VCL, and VAP) and movement styles (LIN, BCF, ALH and MAD) in sperm cells and semen seminal plasma contents of Capoeta trutta (Table 1). Sperm cell concentration ranged from 7.5 to 11.84 (mean SE: ) 10 sperm cell mL. The velocities of sperm cells were found: the straight line velocity (VSL, m s), the curvilinear velocity (VCL, m s), and the angular path velocity (VAP, m s). In the semen seminal plasma ions K ( mg L) and Na ( mg L) were the predominant ions. Ca and Mg were measured to be and mg L, respectively.
Table 1.
The motility parameters of sperm cells and semen seminal plasma contents in Capoeta trutta.
| Mean SD | Minimum | Maximum | |
|---|---|---|---|
| Motility parameters | |||
| VSL (m s) | 43.17 | 63.99 | |
| VCL (m s) | 64.62 | 135.30 | |
| VAP (m s) | 43.42 | 79.67 | |
| LIN (%) | 16.33 | 41.38 | |
| BCF (Hz) | 6.17 | 11.34 | |
| ALH (m) | 23.15 | 49.31 | |
| MAD () |
|
0.01 |
0.04 |
| Seminal plasma contents | |||
| Ca (mg L) | 8.82 | 22.06 | |
| K (mg L) | 151.36 | 265.00 | |
| Mg (mg L) | 2.66 | 4.20 | |
| Na (mg L) | 88.50 | 168.67 | |
| 0.38 | 0.82 | ||
| Al (mg L) | 4.68 | 6.78 | |
| B (mg L) | 0.69 | 11.82 | |
| Cu (mg L) | 0.36 | 0.54 | |
| Li (mg L) | 1.24 | 1.77 | |
| Zn (mg L) | 6.90 | 9.80 | |
Positive relationships were found between K, Na, Ca, and Mg in semen seminal plasma and the values of VSL, VCL, and VAP in sperm cell velocities. However, the partially strong relationship with the VCL value was with K (; ), while it was moderate with Mg (; ) and comparable with Na (; ) or Ca (; ) (Fig. 1, Table 3). The correlation between the contents of semen seminal plasma and motility parameters of sperm cells is shown in Table 2. According to our results, K and Mg ions correlated positively with the VCL value of sperm cells, and , , respectively. In this study, there was no correlation between the trace metals and sperm cell velocities according to our results (Table 2).
Table 2.
Pearson correlations between sperm cell motility and semen seminal plasma contents of Capoeta trutta.
| Pearson correlations |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VSL | VCL | VAP | LIN | BCF | ALH | MAD | Ca | K | Mg | Na | Al | B | Cu | Li | |
| VCL | 0.555 | ||||||||||||||
| VAP | 0.755 | 0.504 | |||||||||||||
| LIN | 0.357 | 0.580 | 0.154 | ||||||||||||
| BCF | 0.130 | 0.574 | 0.023 | 0.371 | |||||||||||
| ALH | 0.021 | 0.131 | 0.223 | 0.546 | 0.349 | ||||||||||
| MAD | 0.390 | 0.803 | 0.183 | 0.695 | 0.231 | 0.299 | |||||||||
| Ca | 0.242 | 0.587 | 0.009 | 0.590 | 0.394 | 0.537 | 0.575 | ||||||||
| K | 0.451 | 0.820 | 0.362 | 0.496 | 0.375 | 0.004 | 0.823 | 0.361 | |||||||
| Mg | 0.324 | 0.696 | 0.452 | 0.605 | 0.244 | 0.221 | 0.609 | 0.305 | 0.716 | ||||||
| Na | 0.313 | 0.410 | 0.135 | 0.043 | 0.204 | 0.002 | 0.471 | 0.279 | 0.459 | 0.321 | |||||
| Al | 0.268 | 0.423 | 0.355 | 0.644 | 0.056 | 0.103 | 0.537 | 0.063 | 0.608 | 0.820 | 0.075 | ||||
| B | 0.131 | 0.047 | 0.059 | 0.131 | 0.345 | 0.356 | 0.198 | 0.278 | 0.062 | 0.296 | 0.319 | 0.365 | |||
| Cu | 0.061 | 0.108 | 0.398 | 0.310 | 0.187 | 0.294 | 0.199 | 0.214 | 0.007 | 0.133 | 0.057 | 0.438 | 0.381 | ||
| Li | 0.005 | 0.342 | 0.170 | 0.377 | 0.369 | 0.075 | 0.253 | 0.328 | 0.337 | 0.468 | 0.021 | 0.691 | 0.385 | 0.508 | |
| Zn | 0.007 | 0.354 | 0.208 | 0.419 | 0.322 | 0.067 | 0.288 | 0.305 | 0.373 | 0.532 | 0.053 | 0.756 | -0.348 | 0.558 | 0.992 |
Correlation is significant at the 0.05 level (two-tailed). Correlation is significant at the 0.01 level (two-tailed).
Table 3.
Linear regression relationship between some ions of semen seminal plasma and the velocities of sperm cells in Capoeta trutta.
| Parameters | Regression equation | Regression square |
|---|---|---|
| Potassium–VSL | 0.20 | |
| Potassium–VAP | 0.13 | |
| Calcium–VSL | 0.06 | |
| Calcium–VAP | 0.00 | |
| Sodium–VSL | 0.10 | |
| Sodium–VAP | 0.02 | |
| Magnesium–VSL | 0.11 | |
| Magnesium–VAP | 0.20 |
Figure 1.
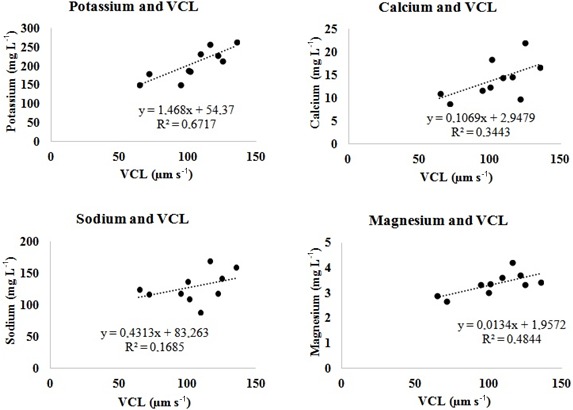
Linear regression graphics between some ions of semen seminal plasma and the VCL value of sperm cell in Capoeta trutta.
4. Discussion
The assessment semen quality can give great information for gamete management and the protocols of artificial reproduction of wild fish species in captivity (Alavi et al., 2006; Mylonas et al., 2010). Therefore, we aimed to determine gamete quality parameters on a domestic fish species, Capoeta trutta in the Euphrates River. In this study, we analyzed the relationships between sperm cell velocities and semen seminal plasma contents in Capoeta trutta. Thereby, the values of VSL, VCL, and VAP were higher than those for Barbus sharpeyi, Catla catla, and Labeo rohita (Kalbassi et al., 2013); they were similar to Labeo calbasu, Cirrhinus mrigala, Hypophthalmichthys molitrix, and Ctenopharyngodon idella (Verma et al., 2009). However, the sperm cell density of Capoeta trutta () was found to be more than the sperm cell density of Liza abu () (Şahinöz et al., 2008) and Capoeta damascina () (Zadmajid et al., 2018), while it was found to be less for Barbus grypus () (Khodadadi et al., 2016), Cyprinus carpio (16.96–) (Cejko et al., 2014), and Ctenopharyngodon idella () (Bozkurt et al., 2008). These differences can be caused by hormonal treatments which increase sperm cell density in some fish species (Zadmajid et al., 2018).
There are very important ions (, , , , etc.) in semen seminal plasma which support the viability, motility, or immobilization of sperm cells which are immobilized in the testes and semen seminal plasma in teleost fish species. The initial motility depends on the conditions of the fertilization environment (Alavi and Cosson, 2006; Browne et al., 2015; Dzyuba and Cosson, 2014). For example, the motility of rainbow trout sperm cells was inhibited with high concentrations of the ion (Alavi and Cosson, 2006). However, there is a positive correlation between sperm cell velocity and Ca ion content of semen seminal plasma (Alavi et al., 2011; Dziewulska and Domagała, 2013; Nynca et al., 2014). On the other hand, ions completely inhibited sperm cell activation (Alavi et al., 2011; Ögretmen et al., 2014).
In our study, and were seen as the main electrolytes involved in the maintenance of the osmolality of semen seminal plasma (Alavi and Cosson, 2006; Morisawa et al., 1983; Zadmajid, 2016). However, a positive correlation has been reported among osmolality, , and in the seminal plasma of Alburnus alburnus (Lahnsteiner et al., 1996). This status can occur due to the different species (Alavi and Cosson, 2006; Verma et al., 2009).
Positive linear regression relationships (weak in VSL and VAP in Table 3, moderately strong in VCL in Fig. 1) were found between velocities and , , Mg, and in the semen seminal plasma of Capoeta trutta. In some teleosts, some researchers have found a positive relationship between sperm cell motility and and of seminal plasma (Alavi and Cosson, 2006; Bozkurt et al., 2008; Lahnsteiner et al., 1996) and of northern pike Esox lucius (Siddique et al., 2016a). However, the ratio of in the semen seminal plasma is vital in understanding the characteristics of fish sperm cell motility (Alavi and Cosson, 2006). The ratio of in the semen seminal plasma of Capoeta trutta was determined to be about , and it was lower than that for northern pike (Siddique et al., 2016b), some species of Salmonidae and Acipenseridae (Alavi and Cosson, 2006), and Barbus grypus (Khodadadi et al., 2016). However, it was parallel with Barbus sharpeyi (Alavi et al., 2010), Ctenopharyngodon idella (Bozkurt et al., 2008), and Cyprinus carpio (Bozkurt et al., 2009). These differences were probably present due to the individual characteristics of the species (Verma et al., 2009).
In conclusion, sperm cells generally move on a straight or slightly curved route after activation, which was found to be similar to and/or closely comparable to the results of some authors according to the obtained parameters (Alavi et al., 2014; Fitzpatrick et al., 2008). Trace metals such as Zn, Al, B, Li, and Cu in semen seminal plasma were not correlated with sperm cell velocities. A similar status was confirmed for Zn in human semen (Bassey et al., 2013), while this was not the case for Zn in common carp semen (Kovacik et al., 2018) and human semen (Vickram et al., 2013), and for Zn and Cu in bovine semen (Tvrdá et al., 2013). These differences are probably due to the individual characteristics of the species (Verma et al., 2009), the activity of male reproductive accessory organs (Alavi et al., 2004), and the metal pollution of water (Acosta et al., 2016).
Finally, our results have shown that seminal plasma ions such as , , Mg, and were correlated with sperm cell velocities, while there is only the partially strong relationship between the VCL value and ion of sperm seminal plasma. However, we are hopeful that our data can lead to a better understanding of fertilization mechanisms of Capoeta trutta sperm cells because the knowledge of sperm cell motility and semen seminal plasma contents is a prerequisite for the successful assessment of the reproductive capacity of wild fish species.
Appendix A.
| Abbreviations and their definitions | |
|---|---|
| VSL | the straight line velocity of sperm cell |
| VCL | the curvilinear velocity of sperm cell |
| VAP | the angular path velocity of sperm cell |
| LIN | the linearity of sperm cell |
| BCF | the beat cross frequency of sperm cell |
| ALH | the amplitude of lateral displacement of the sperm cell head |
| MAD | the mean angular displacement of sperm cell |
| Ca | calcium |
| K | potassium |
| Mg | magnesium |
| Na | sodium |
| Al | aluminum |
| B | boron |
| Cu | copper |
| Li | lithium |
| Zn | zinc |
Data availability
The data used in the present study are confidential and therefore not publicly available.
Author contributions
MEÖ designed the experimental setup. MEÖ and SE carried out the samplings. Measurements and chemical analyses were conducted by MEÖ, SE, and ZM. The statistical and scientific assessment of data was processed by MEÖ and SE. The manuscript was written by MEÖ with contributions from all coauthors.
Review statement
This paper was edited by Manfred Mielenz and reviewed by two anonymous referees.
References
- Acosta IB, Junior ASV, e Silva EF, Cardoso TF, Caldas JS, Jardim RD, Corcini CD. Effects of exposure to cadmium in sperm cells of zebrafish, Danio rerio. Toxicol Reports . 2016;3:696–700. doi: 10.1016/j.toxrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi SMH, Cosson J. Sperm motility in fishes. (II) Effects of ions and osmolality: A review. Cell Biol Int. 2006;30:1–14. doi: 10.1016/j.cellbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Alavi SMH, Cosson J, Karami M, Abdolhay H, Amiri BM. Chemical composition and osmolality of seminal fluid of Acipenser persicus; their physiological relationship with sperm motility . Aquac Res. 2004;35:1238–1243. doi: 10.1111/j.1365-2109.2004.01132.x. [DOI] [Google Scholar]
- Alavi SMH, Jorfi E, Hatef A, Saheb Mortezavi SA. Sperm motility and seminal plasma characteristics in Barbus sharpeyi (Günther, 1874) Aquac Res. 2010;41:688–694. doi: 10.1111/j.1365-2109.2010.02600.x. [DOI] [Google Scholar]
- Alavi SMH, Gela D, Rodina M, Linhart O. Roles of osmolality, calcium – Potassium antagonist and calcium in activation and flagellar beating pattern of sturgeon sperm. Comp Biochem Physiol. 2011;160:166–174. doi: 10.1016/j.cbpa.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Alavi SMH, Matsumura N, Shiba K, Itoh N, Takahashi KG, Inaba K, Osada M. Roles of extracellular ions and pH in 5-HT-induced sperm motility in marine bivalve. Reproduction. 2014;147:331–345. doi: 10.1530/REP-13-0418. [DOI] [PubMed] [Google Scholar]
- Bassey IE, Essien OE, Udoh AE, Imo IU, Effiong IO. Seminal Plasma Selenium, Calcium, Magnesium and Zinc Levels in Infertile Men. J Med Sci. 2013;13:483–487. doi: 10.3923/jms.2013.483.487. [DOI] [Google Scholar]
- Billard R, Cosson MP. Some problems related to the assessment of sperm motility in freshwater fish. J Exp Zool. 1992;261:122–131. doi: 10.1002/jez.1402610203. [DOI] [Google Scholar]
- Bobe J, Labbé C. Egg and sperm quality in fish. Gen Comp Endocrinol. 2010;165:535–548. doi: 10.1016/j.ygcen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Bozkurt Y, Ögretmen F, Erçin U, Yildiz Ü. Seminal plasma composition and its relationship with physical spermatological parameters of Grass carp (Ctenopharyngodon idella) semen: With emphasis on sperm motility . Aquac Res. 2008;39:1666–1672. doi: 10.1111/j.1365-2109.2008.02041.x. [DOI] [Google Scholar]
- Bozkurt Y, Ogretmen F, Secer FS, Ercin U. Effects of seminal plasma composition on sperm motility in mirror carp (Cyprinus carpio) . Isr J Aquac-Bamidgeh. 2009;61:307–314. [Google Scholar]
- Browne RK, Kaurova SA, Uteshev VK, Shishova NV, McGinnity D, Figiel CR, Mansour N, Agnew D, Wu M, Gakhova EN, Dzyuba B, Cosson J. Sperm motility of externally fertilizing fish and amphibians. Theriogenology. 2015;83:1–13. doi: 10.1016/j.theriogenology.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Cabrita E, Martínez-Páramo S, Gavaia PJ, Riesco MF, Valcarce DG, Sarasquete C, Herráez MP, Robles V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture. 2014;432:389–401. doi: 10.1016/j.aquaculture.2014.04.034. [DOI] [Google Scholar]
- Cejko BI, Krejszeff S, Judycka S, Sarosiek B, Dietrich M, Kucharczyk D, Kowalski RK. Sperm quality and selected biochemical markers of seminal plasma at the beginning of the reproductive period of common carp, Cyprinus carpio L. Aquac Int . 2014;22:111–122. doi: 10.1007/s10499-013-9672-3. [DOI] [Google Scholar]
- Ciereszko A, Dabrowski K. Estimation of sperm concentration of rainbow trout, whitefish and yellow perch using a spectrophotometric technique. Aquaculture. 1993;109:367–373. doi: 10.1016/0044-8486(93)90175-X. [DOI] [Google Scholar]
- Cosson MP, Billard R, Letellier L. Rise of internal accompanies the initiation of trout sperm motility . Cell Motil Cytoskel. 1989;14:424–434. doi: 10.1002/cm.970140312. [DOI] [Google Scholar]
- Demirsoy A, editor. Basic Rules of Life, Vertebrates/Anamniyota, Volume III, Part I. Meteksan Publications; Ankara, Turkey: 1993. [Google Scholar]
- Dziewulska K, Domagała J. Effect of pH and cation concentrations on spermatozoan motility of sea trout (Salmo trutta m. trutta L.) . Theriogenology. 2013;79:48–58. doi: 10.1016/j.theriogenology.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Dzyuba V, Cosson J. Motility of fish spermatozoa: from external signaling to flagella response. Reprod Biol. 2014;14:165–175. doi: 10.1016/j.repbio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Esmaeili HR, Coad BW, Gholamifard A, Teimory A. Annotated checklist of the freshwater fishes of Iran. Zoosystematica Ross. 2010;19:361–386. [Google Scholar]
- Fauvel C, Suquet M, Cosson J. Evaluation of fish sperm quality. J Appl Ichthyol. 2010;26:636–643. doi: 10.1111/j.1439-0426.2010.01529.x. [DOI] [Google Scholar]
- Fitzpatrick JL, Nadella S, Bucking C, Balshine S, Wood CM. The relative sensitivity of sperm, eggs and embryos to copper in the blue mussel (Mytilus trossulus) Comp Biochem Physiol . 2008;147:441–449. doi: 10.1016/j.cbpc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Freyhof J. Capoeta trutta, The IUCN red list of threatened species, e.T19027513A19222908 . 2014. [last access: 24 September 2019]. available at: http://iucnredlist.org/details/19027513/0 .
- Kalbassi MR, Lorestani R, Ghafle MJ. Analysis of saline activator solution effects on sperm quality indices of Barbus sharpeyi by Image J software . Iran J Fish Sci. 2013;12:357–377. doi: 10.3168/jds.2017-12732. [DOI] [Google Scholar]
- Khodadadi M, Arab A, Jaferian A. A preliminary study on sperm morphology, motility and composition of seminal plasma of Shirbot, Barbus grypus. Turkish J Fish Aquat Sci . 2016;16:947–951. doi: 10.4194/1303-2712-v16_4_22. [DOI] [Google Scholar]
- Kovacik A, Tirpak F, Tomka M, Miskeje M, Tvrda E, Arvay J, Andreji J, Slanina T, Gabor M, Hleba L, Fik M, Jambor T, Cisarova M, Massanyi P. Trace elements content in semen and their interactions with sperm quality and RedOx status in freshwater fish Cyprinus carpio: A correlation study . J Trace Elem Med Biol. 2018;50:399–407. doi: 10.1016/j.jtemb.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F, Berger B, Weismann T, Patzner RA. Motility of spermatozoa of Alburnus alburnus (Cyprinidae) and its relationship to seminal plasma composition and sperm metabolism . Fish Physiol Biochem. 1996;15:167–179. doi: 10.1007/BF01875596. [DOI] [PubMed] [Google Scholar]
- Li ZH, Li P, Dzyuba B, Randak T. Influence of environmental related concentrations of heavy metals on motility parameters and antioxidant responses in sturgeon sperm. Chem Biol Interact. 2010;188:473–477. doi: 10.1016/j.cbi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Linhart O, Rodina M, Cosson J. Cryopreservation of sperm in common carp Cyprinus carpio: Sperm motility and hatching success of embryos . Cryobiology. 2000;41:241–250. doi: 10.1006/cryo.2000.2284. [DOI] [PubMed] [Google Scholar]
- Lorenzen K, Beveridge MCM, Mangel M. Cultured fish: integrative biology and management of domestication and interactions with wild fish. Biol Rev. 2012;87:639–660. doi: 10.1111/j.1469-185X.2011.00215.x. [DOI] [PubMed] [Google Scholar]
- Massanyi P, Weis J, Lukac N, Trandzik J, Bystricka J. Cadmium, zinc, copper, sodium and potassium concentrations in rooster and turkey semen and their correlation. J Environ Sci Heal Pt A. 2008;43:563–565. doi: 10.1080/10934520701796655. [DOI] [PubMed] [Google Scholar]
- Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K. Effects of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J Exp Biol. 1983;107:95–103. doi: 10.1016/j.jallcom.2014.06.123. [DOI] [PubMed] [Google Scholar]
- Mylonas CC, Fostier A, Zanuy S. Broodstock management and hormonal manipulations of fish reproduction. Gen Comp Endocrinol. 2010;165:516–534. doi: 10.1016/j.ygcen.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Nynca J, Dietrich GJ, Dobosz S, Grudniewska J, Ciereszko A. Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture. 2014;433:62–65. doi: 10.1016/j.aquaculture.2014.05.037. [DOI] [Google Scholar]
- Ögretmen F, Gölbaşi S, Inanan BE. Inhibitory effect of K and Ca2 concentrations, pH, and osmolality of activation solution on motility of shabut (Barbus grypus Heckel 1843) spermatozoa . Turkish J Vet Anim Sci . 2014;38:245–252. doi: 10.3906/vet-1308-9. [DOI] [Google Scholar]
- Özgür ME, Yumuşakbaş H, Dağli M, Erdoğan S. Comparison of some elements in sperm seminal plasma of rainbow trout (Oncorhynchus mykiss) and Brown trout (Salmo trutta fario) . J Appl Biol Sci. 2015;9:64–67. [Google Scholar]
- Özgür ME, Okumuş F, Kocamaz AF. A Novel Computer Assisted Sperm Analyzer for Assessment of Spermatozoa Motility in Fish; BASA-Sperm Aqua . El-Cezeri Fen ve Mühendislik Derg. 2019;6:208–219. doi: 10.31202/ecjse.486342. [DOI] [Google Scholar]
- Rurangwa E, Kime DE, Ollevier F, Nash JP. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 2004;234:1–28. doi: 10.1016/j.aquaculture.2003.12.006. [DOI] [Google Scholar]
- Şahinöz E, Aral F, Dogu Z. Determination of spermatological properties of male Liza abu (Heckel, 1843) in Atatürk Dam Lake, Şanliurfa . Fish Physiol Biochem. 2008;34:71–76. doi: 10.1007/s10695-007-9148-3. [DOI] [PubMed] [Google Scholar]
- Siddique M, Linhart O, Kujawa R, Krejszeff S, Butts I. Composition of seminal plasma and ovarian fluid in Ide Leuciscus idus and Northern pike Esox lucius . Reprod Domest Anim. 2016;51:960–969. doi: 10.1111/rda.12773. [DOI] [PubMed] [Google Scholar]
- Slivkova J, Popelkova M, Massanyi P, Toporcerova S, Stawarz R, Formicki G, Lukac N, Putała A, Guzik M. Concentration of trace elements in human semen and relation to spermatozoa quality. J Environ Sci Heal Pt A. 2009;44:370–375. doi: 10.1080/10934520802659729. [DOI] [PubMed] [Google Scholar]
- Tanimoto S, Morisawa M. Roles for Potassium and Calcium Channels in the Initiation of Sperm Motility in Rainbow Trout, (K /Ca /channel/sperm motility/rainbow trout) Dev Growth Differ . 1988;30:117–124. doi: 10.1111/j.1440-169X.1988.00117.x. [DOI] [PubMed] [Google Scholar]
- Tvrdá E, Lukáč N, Schneidgenová M, Lukáčová J, Szabó C, Goc Z, Greń A, Massányi P. Impact of Seminal Chemical Elements on the Oxidative Balance in Bovine Seminal Plasma and Spermatozoa. J Vet Med. 2013:1–8. doi: 10.1155/2013/125096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma DK, Routray P, Dash C, Dasgupta S, Jena JK. Physical and biochemical characteristics of semen and ultrastructure of spermatozoa in six carp species. Turkish J Fish Aquat Sci. 2009;9:67–76. [Google Scholar]
- Vickram S, Muthugadhalli S, Jayaraman G, Kamini R, Ramesh Pathy M, Sridharan B. Influence of trace elements and their correlation with semen quality in fertile and infertile subjects. Turkish J Med Sci. 2013;43:1000–1007. doi: 10.3906/sag-1211-54. [DOI] [Google Scholar]
- Yuyan L, Junqing W, Wei Y, Weijin Z, Ersheng G. Are serum zinc and copper levels related to semen quality? Fertil Steril. 2008;89:1008–1011. doi: 10.1016/j.fertnstert.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Zadmajid V. Comparative effects of human chorionic gonadotropin (hCG) and Ovaprim™ (sGnRHa + domperidone) on the reproductive characteristics of wild-caught male Longspine scraper, Capoeta trutta (Heckel, 1843) . Aquaculture . 2016;463:7–15. doi: 10.1016/j.aquaculture.2016.05.029. [DOI] [Google Scholar]
- Zadmajid V, Bashiri S, Sharafi N, Butts IAE. Effect of hCG and Ovaprim™ on reproductive characteristics of male Levantine scraper, Capoeta damascina (Valenciennes, 1842) . Theriogenology . 2018;115:45–56. doi: 10.1016/j.theriogenology.2018.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the present study are confidential and therefore not publicly available.


