Abstract
A growing body of evidence suggests that only a small subpopulation of malignant glioma cells have true tumorigenic potential. A study by Peñuelas et al. in this issue of Cancer Cell demonstrates that TGF-β can stimulate self-renewal and inhibit differentiation in a proportion of these glioma-initiating cells.
Primary central nervous system (CNS) malignancies represent an outstanding model system to explore the cancer stem cell hypothesis in that tumor- and glioblastoma-initiating/stem cells (GICs/GSCs) have been readily identified in both medulloblastomas and glioblastomas (GBMs) (Hemmati et al., 2003; Singh et al., 2004) and more is known about the normal development of embryonic and adult normal neural stem cells (NSCs) than any other tissue-specific stem cell except hematopoietic stem cells. In fact, GICs/GSCs have been shown to have significant similarities to NSCs through their ability to self-renew, expression of similar transcriptome profiles, and capability of differentiating along both glial and neuronal lines (Lee et al., 2006). Comparing the cellular, molecular, genetic, and epigenetic mechanisms that underlie basic stem cell properties such as self-renewal and differentiation should further elucidate the similarities and differences between NSCs and GICs/GSCs.
With this as a backdrop, Peñuelas et al. (2009) report in this issue of Cancer Cell on the effects of transforming growth factor beta (TGF-β) on GICs/GSCs. TGF-β has long been considered a potentially promising therapeutic target in malignant gliomas, as much through guilt by association as because of clear experimental and/or clinical evidence (Massagué, 2008). The overexpression of TGF-β commonly seen in malignant glioma has been variously implicated in glioma cell proliferation, migration, decreased apoptosis, and/or tumor-specific immunosuppression. In a new twist, Peñuelas and coworkers demonstrate that TGF-β induces GIC/GSC self-renewal in vitro and enhanced tumorigenicity in vivo. TGF-β mediates this activity through activation of and subsequent binding of a Smad2/3/4 complex to the promoter region of the leukemia inhibitory factor (LIF) gene. LIF then activates the JAK-STAT pathway, as demonstrated by phosphorylation of STAT3, leading to increased GIC/GSC tumorigenesis secondary to their increased self-renewal and decreased differentiation. Peñuelas et al. find that although LIF enhances NSC self-renewal and partially inhibits differentiation, TGF-β in contrast does not induce LIF expression and thus has no discernable effect on NSCs.
So does the different response of GICs/GSCs versus NSCs to TGF-β represent a corrupted TGF-β signaling pathway, as is often seen in other epithelial cancers? Although TGF-β signals through the Smad2/3/4 complex in both the GICs/GSCs and NSCs evaluated by Peñuelas et al., it is known that various cofactors associated with the Smad complex result in differential gene expression and repression in a cell context-dependent manner. It is therefore plausible that a different set of tumor-related Smad complex-associated cofactors accounts for the differential effects of TGF-β induction on the LIF promoter in GICs/GSCs compared to NSCs.
Alternately, it is possible that this TGFβ-mediated pro-self-renewal phenotype may not be tumor specific but may rather be representative of normal signaling within a particular NSC subtype not utilized in the Peñuelas et al. study. It is well known that the effects of TGF-β and TGF-β family members (BMPs, activins, inhibins) on CNS development are cell intrinsic, cell extrinsic, and context dependent. For example, TGF-β inhibits Wnt signalling-mediated NSC self-renewal/proliferation and promotes neuronal differentiation in midbrain-derived NSCs but has no effects on forebrain-derived NSCs (Falk et al., 2008). Unlike hematopoiesis, CNS and NSC development occurs in an intricately choreographed pattern highly specified in terms of both anterior-posterior/dorsal-ventral anatomical positioning and developmental stage timing. The same transcription factor or signaling pathway can generate very different biological consequences depending on the spatial and chronology-restricted domains of that particular NSC. It has been shown that even in adults, NSCs in the subventricular zone less than 1 mm apart are spatially restricted relative to fate determination. The control NSCs used by Peñuelas and coworkers came from a stage of fetal CNS development that is largely postproliferative and represented a collection of total cerebral NSCs. Thus, the phenotype of a unique TGF-β-responsive anatomically and temporally specified subpopulation of NSCs may have been obscured by the use of this heterogeneous group of NSCs.
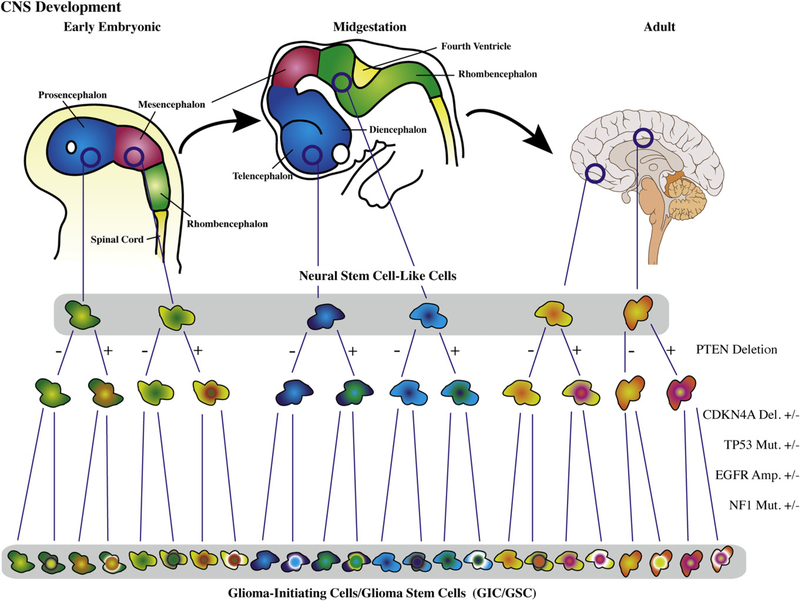
The study by Peñuelas et al. raises important questions regarding the therapeutic potential of TGF-β inhibition through the demonstration that inhibition of TGF-βand JAK-STAT signaling resulted in decreased self-renewal and diminished tumorigenicity of their GICs/GSCs. It is unclear how prevalent this phenotype will prove to be among GICs/GSCs from different GBMs, but since less than half of the GBMs evaluated by Peñuelas et al. demonstrated increased expression of TGF-β and/or LIF, it is reasonable to assume that a large percentage of GBMs may not have a TGF-β-responsive GIC/GSC phenotype. This raises the issue of tumor-specific GIC/GSC heterogeneity, for if GICs/GSCs are indeed derived from precursor cells from various stages of development and different anatomical domains, one might expect significant GIC/GSC heterogeneity from one tumor to another, a complexity compounded by the unique genetic and epigenetic alterations found within any given GBM (TCGA Research Network, 2008) (Figure 1).
Figure 1. Potential for Glioma-Initiating Cell/Glioma Stem Cell Heterogeneity.
Neural stem cell-like cells can have different biological outcomes to the activation of the same signaling pathway in a contextually dependent manner based on both the target cell’s spatial domain and its relative stage of neural stem cell/central nervous system (CNS) development. This complexity, compounded by the diverse genomic and epigenetic aberrations found in malignant gliomas, could result in biologically different glioma-initiating cells/glioma stem cells from one tumor to another. Del, deletion; Mut, mutation; Amp, amplification. Developmental stage cartoons and brain image courtesy of Wikimedia Commons; brain image by Patrick J. Lynch and C. Carl Jaffe.
The potential for divergent biology from one tumor-derived GIC/GSC population to another has profound therapeutic impli-cations. For example, it was demonstrated that BMP2/4 induces terminal differentia-tion in GICs/GSCs, leading to the sugges-tion that BMP2/4 and/or their agonist might represent a therapeutic strategy for gliomas (Piccirillo et al., 2006). When GICs/GSCs from a larger set of GBMs were eval-uated, however, it was found that about 20% of GBMs harbor GICs/GSCs with ab-errant methylation of the BMP receptor 1b (BMPR1b) promoter converting BMP2/4 from a differentiation agent to a mitogenic agent (Lee et al., 2008). Thus, not only would a BMP2/4 agonist fail as a therapeutic strategy in these tumors, it might actually promote GIC/GSC expansion and tumor growth. In fact, these same BMPR1b-methylated GICs/GSCs are LIF unresponsive secondary to the failure of LIF to induce phosphorylation of STAT3 in these cells. These GICs/GSCs (and their parental tumors) are therefore likely to be refractory to TGF-β and thus are unlikely to benefit from therapeutic inhibition of TGF-β and/or the JAK-STAT pathway. Indeed, in the context of a very early embryonic NSC phenotype, STAT3 inhibition is required for cells to exit symmetric cell division and terminally differentiate. Thus, any therapeutic strategy aimed at blocking the JAK-STAT pathway in GICs/GSCs derived from, or with a phenotype similar to, a very early NSC could actually cause expansion of the GIC/GSC pool.
Targeting stem cell pathways may ultimately prove to be an effective therapeutic strategy against malignant gliomas; however, such pathways may have dramatically divergent roles in GIC/GSC populations from different GBMs. Thus, regardless of whether GICs/GSCs come from a true NSC or from the dedifferentiation of a mature somatic cell (e.g., astrocyte), understanding the divergent signaling mechanisms and phenotypes of developmentally and anatomically specified normal NSCs will be vital for understanding the unique biology of GIC/GSC populations from different malignant tumors (Figure 1). Once those pathways have been elucidated and validated as potentially useful therapeutic targets, we will ultimately need to identify easily assessed surrogate molecular markers of stem cell pathway activation within each specific tumor (e.g., BMPR1b, TGF-β/LIF expression) if we want to use such information to guide therapeutic decision-making for individual patients. The promise of patient-specific stem cell pathway therapeutic targeting will therefore be best realized through an ever greater collaborative effort between neuroscientists, developmental biologists, cancer researchers, and clinical scientists.
REFERENCES
- Cancer Genome Atlas Research Network. (2008). Nature 455, 1061–1068.18772890 [Google Scholar]
- Falk S, Wurdak H, Ittner LM, Ille F, Sumara G, Schmid MT, Draganova K, Lang KS, Paratore C, Leveen P, et al. (2008). Cell Stem Cell 2, 472–483. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, and Kornblum HI (2003). Proc. Natl. Acad. Sci. USA 100, 15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. (2006). Cancer Cell 9, 391–403. [DOI] [PubMed] [Google Scholar]
- Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, et al. (2008). Cancer Cell 13, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. (2008). Cell 134, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas S, Anido J, Prieto-Sánchez R, Folch G, Barba I, Cuartas I, García-Dorado D, Poca MA, Sahuquillo J, Baselga J, and Seoane J. (2009). Cancer Cell 15, this issue, 315–327. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, and Vescovi AL (2006). Nature 444, 761–765. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, and Dirks PB (2004). Nature 432, 396–401. [DOI] [PubMed] [Google Scholar]