Abstract
Objectives
Evidence of accelerated brain aging among HIV-infected adults argues for the increased risk of developing cerebral β-amyloid (Aβ) plaques. We compared the frequency of Aβ plaque-bearing cases in our HIV cohort with that in a general cohort reported by Braak et al. (2011). We explored post-translationally modified Aβ forms (N3pE, E22P, phospho-Ser8) in plaques and E22P-Aβ in the postmortem cerebrospinal fluid (CSF) in the HIV cohort.
Design
Clinicopathological study of HIV-infected adults.
Methods
To assess frontal Aβ plaque deposition, we conducted immunohistochemistry for generic Aβ (4G8) and three modified Aβ forms. We determined CSF E22P-Aβ levels by ELISA.
Results
We found 4G8-Aβ plaques in 29% of 279 HIV-infected cases. Within the age range of 31–70 years, the frequency of 4G8-Aβ plaque-bearing cases was higher in our HIV cohort (n=273) compared with the general cohort (n=1110) overall (29.3% vs. 25.8%) and across four age groups by decade (odds ratio 2.35, P<0.0001). In HIV-infected cases with (n=37) and without (n=12) 4G8-Aβ plaques, modified Aβ forms occurred in order: N3pE, E22P, and phospho-Ser8. In CSF assays of HIV-infected cases with (n=27; 17 focal, 10 widespread) and without (n=11) 4G8-Aβ plaques, the median E22P-Aβ/Aβ40 ratio was higher among cases with widespread plaques than in cases with focal or absent plaques (P=0.047).
Conclusions
Our findings suggest HIV-infected adults are at increased risk of developing cerebral Aβ plaques. The occurrence of modified Aβ forms in order suggests the progression stages of Aβ plaque deposition. The potential for E22P-Aβ as a CSF biomarker of cerebral Aβ plaques should be investigated.
Keywords: Alzheimer’s disease, biomarker, cerebrospinal fluid, neuroHIV, post-translational modification
Introduction
Cerebral β-amyloid (Aβ) plaque deposition is one of the neuropathologic hallmarks of Alzheimer’s disease (AD) [1] and constitutes a biological construct in the research framework of AD continuum [2]. Aβ plaques first appear in the cerebral neocortex of adults in their fourth decade and progress with age to involve the allocortex, striatum, and other brain regions [3]. The frequency of persons having cerebral Aβ plaques increases in older age groups [4]. In studies of preclinical and symptomatic AD brains [3, 5, 6], Aβ plaques contained two major non-modified Aβ forms, Aβ1−40 (Aβ40) and Aβ1−42 (Aβ42). In the later stages of development, Aβ plaques also contained a variety of post-translationally modified Aβ forms, including N-terminal truncated forms with pyroglutamate modification (N3pE-Aβ, N11pE-Aβ) and phosphorylated forms (phospho-Ser8-Aβ, phospho-Ser26-Aβ). Whereas N3pE-Aβ was found in both preclinical and symptomatic AD, phospho-Ser8-Aβ was more strongly associated with symptomatic AD [3, 5]. Moreover, an Aβ42 conformer with a turn at positions Glu22 and Asp23, detectable by conformation-specific anti-E22P-Aβ antibodies, was found to preferably form stable low-molecular-weight oligomers and induce neurotoxicity in vitro and was present in plaques in AD brains [7–10].
Among HIV-infected individuals, evidence of accelerated brain aging was observed in structural [11] and functional [12] magnetic resonance imaging studies and in DNA methylation epigenetic analyses [13]. These findings argue for the increased risk of developing cerebral Aβ plaques in the HIV-infected population. Several HIV autopsy studies described cerebral Aβ plaque deposition [14–21]. Nonetheless, it remains debatable whether HIV-infected persons carry a higher risk of developing cerebral Aβ plaques. For instance, Esiri et al. [16] reported the frequency of cases showing neocortical Aβ plaques in the HIV-infected group (n=97, age range 30–69 years) prior to the era of highly active antiretroviral therapy (HAART) rose from 18% in the fourth decade to 50% in the seventh decade, as compared with a rise in frequency from 0% to 36% in the age-matched non-HIV group (n=125). In contrast, Gelman et al. [14] found no evidence of increased risk of developing hippocampal Aβ plaques in pre-HAART HIV-infected cases (n=25, age range 21–75 years) compared with age-matched non-HIV controls (n=25). Anthony et al. [15] observed hippocampal Aβ plaques in 55% of 20 pre-HAART and 22.2% of 9 HAART-treated HIV-infected cases (age range 32–60 years) and in 14.3% of 7 non-HIV controls (age range 30–48 years). In these studies [14–16], however, the frequency of apolipoprotein-E (APOE) ε4 allele, the strongest genetic risk factor for cerebral Aβ plaque deposition and AD [22], was not taken into account.
In the present study, we asked whether HIV-infected adults were at increased risk of developing cerebral Aβ plaques. We compared the frequencies of Aβ plaque-bearing cases across age groups in our HIV autopsy cohort in USA with those in a large-scale general autopsy cohort reported by Braak et al. from Germany [4] while accounting for the APOE ε4 allele frequency in the HIV cohort. Further, we asked whether cerebral Aβ plaques in HIV-infected persons contained post-translationally modified Aβ forms [3]. In line with the AD research framework focusing on biomarkers of cerebral Aβ deposition [2], we explored the relationship between E22P-Aβ levels in the postmortem cerebrospinal fluid (CSF) and cerebral Aβ plaque deposition in HIV-infected persons.
Methods
HIV autopsy cohort
We studied 279 autopsy HIV-infected cases from the National NeuroAIDS Tissue Consortium (NNTC) in USA that had frontal neocortex sections available. All study participants provided written informed consent including consent to autopsy at four operating sites in the NNTC. The University of California San Diego Human Research Protections Program approved the project as part of the California NeuroAIDS Tissue Network (CNTN) in the NNTC (Request # R432 and # R458). The participants died between 1999 and 2014 and ranged in age at death from 26 to 70 years [median (interquartile range) = 46 (40–55) years, n=279]. There were 231 men (82.8%) and 48 women (17.2%, compared with 19.4% in the NNTC tissue bank cohort [23]). For race/ethnicity, 148 participants (53.0%) were white, 59 (21.1%) black, 61 (21.9%) Hispanic, and 11 (3.9%) Asian or other. HAART was defined as regimens containing three or more antiretroviral drugs from at least two drug classes. The antiretroviral regimens recorded at the last clinical assessment [median (interquartile range) = 18.29 (7.21–36.14) weeks before death, n=274] were grouped into HAART (n=159, 58.0%), non-HAART (n=26, 9.5%), and no antiretroviral treatment [n=89, 32.5%, i.e. either discontinuation of (n=65) or never receiving (n=24) antiretroviral treatment]. At autopsy, 116 (41.9%) of 277 cases had postmortem delay (>12 hours), data not available in the remaining two cases. The 279 cases came from four operating sites in the NNTC: 112 (40.1%) CNTN, 79 (28.3%) National Neurological AIDS Bank, 48 (17.2%) Texas NeuroAIDS Research Center, and 40 (14.3%) Manhattan HIV Brain Bank.
Apolipoprotein-E genotyping
APOE genotypes (ε2, ε3, ε4 alleles) were determined at the University of California Los Angeles Biological Samples Processing Core. DNA was extracted from frozen brain tissue samples. Single nucleotide polymorphism genotyping (rs429358 and rs7412) was conducted on the Sequenom MassARRAY iPLEX platform (Agena Bioscience, San Diego, California, USA), as previously described [24].
Immunohistochemistry for β-amyloid plaques
We immuno-labeled 5-μm-thick paraffin-embedded formalin-fixed frontal neocortex sections with primary antibodies directed against Aβ17−24 (mouse monoclonal, clone 4G8; SIG-39220; Covance, Princeton, New Jersey, USA; 1:20000 dilution), N3pE-Aβ (rabbit polyclonal; 18591; Immuno-Biological Laboratories, Minneapolis, Minnesota, USA; 1:100), E22P-Aβ (mouse monoclonal, clone 11A1; 10379; Immuno-Biological Laboratories; 1:200) [10], and phospho-Ser8-Aβ (mouse monoclonal, clone 1E4E11; MABN878; EMD Millipore, Temecula, California, USA; 1:1000) [5].
Tissue sections were deparaffinized with xylene and rehydrated through graded ethanol series and water. For 4G8-Aβ, N3pE-Aβ, and E22P-Aβ, tissue sections were pretreated with 88% formic acid (5 min). For phospho-Ser8-Aβ, tissue sections were placed in 121°C autoclave (20 min) with 10-mM sodium citrate/0.05% Tween-20 buffer (pH 6) and then pretreated with 88% formic acid (3 min). The tissue sections were treated with 0.3% hydrogen peroxide/PBS (30 min), rinsed in PBS, and incubated with 2.5% normal horse serum (30 min; Vector Laboratories, Burlingame, California, USA). Following 24-hour incubation with primary antibodies at 4°C, the tissue sections were rinsed in 0.1% Tween-20/PBS and PBS and then incubated at room temperature (40 min) with horse anti-mouse or -rabbit IgG secondary antibody [ImmPRESS HRP anti-IgG (peroxidase) polymer detection kits; MP-7402 and MP-7401; Vector Laboratories]. Following washing with 0.1% Tween-20/PBS and PBS, the signals were developed with 3,3’-diaminobenzidine [ImmPACT DAB peroxidase (HRP) substrate; SK-4105; Vector Laboratories] at room temperature (5 min). Following water wash, the tissue sections were counterstained with Mayer hematoxylin, dehydrated through graded ethanol series, cleared in xylene, and mounted with Cytoseal 60 (Richard-Allan Scientific, Waltham, Massachusetts, USA). For the negative reagent control, the primary antibody was omitted, as previously described [25]. Neocortex sections from an AD brain were used as the positive tissue control.
On light microscopy, Aβ plaque deposition was designated as present when extracellular Aβ-immunoreactive plaques were noted, regardless of their density or type [26]. The density of frontal Aβ plaques was qualitatively graded as absent, focal, or widespread, as previously described [21].
Assays of β-amyloid forms in postmortem cerebrospinal fluid
The Aβ42, Aβ40, and Aβ38 levels in CSF samples were assayed by using V-PLEX Plus Aβ Peptide Panel 1 (4G8) Kit (K15199G; Meso Scale Discovery, Rockville, Maryland, USA). The E22P-Aβ levels in CSF samples were measured by using solid-phase sandwich enzyme-linked immunosorbent assay (ELISA; clone 24B3; 27709; Immuno-Biological Laboratories) with E22P-Aβ40 dimer used as standard [7]. The average of two technical replicates was used for data analysis. We used CSF Aβ42/Aβ38 and Aβ42/Aβ40 ratios for data analysis because the ratios were shown to be better than CSF Aβ42 levels in predicting cerebral Aβ deposition in preclinical AD (i.e. an inverse correlation between CSF Aβ42 levels and cerebral Aβ deposition) [27, 28]. Similarly, CSF E22P-Aβ/Aβ38 and E22P-Aβ/Aβ40 ratios were analyzed.
Statistical analysis
To test associations of frontal Aβ plaque deposition with relevant biological factors, we used logistic regression models. The odds ratio (OR) [95% confidence interval (CI)] measured the effect size. The Spearman’s rho was used to test linear correlations between CSF levels of Aβ forms. The Mann-Whitney U-test was used to test differences in median CSF levels of Aβ forms between two independent groups. Two-tailed P values of less than 0.05 were considered statistically significant. The statistical analyses were performed using IBM SPSS Statistics Version 25 and GraphPad Prism Version 6.0h (GraphPad Software, San Diego, California, USA).
Results
Apolipoprotein-E ε4 allele frequency in HIV cohort
Of the 279 cases (age range 26–70 years), 250 cases had frozen tissue available for APOE genotyping. The distribution of APOE genotypes was as follows: ε2/ε2: 1 (0.4%), ε2/ε3: 25 (10.0%), ε2/ε4: 9 (3.6%), ε3/ε3: 157 (62.8%), ε3/ε4: 54 (21.6%), and ε4/ε4: 4 (1.6%). Overall, the APOE ε2 allele frequency was 0.072 and the APOE ε4 allele frequency was 0.142.
Among 245 of the 273 cases (age range 31–70 years), the distribution of APOE genotypes was as follows: ε2/ε2: 1 (0.4%), ε2/ε3: 25 (10.2%), ε2/ε4: 9 (3.7%), ε3/ε3: 152 (62.0%), ε3/ε4: 54 (22.0%), and ε4/ε4: 4 (1.6%). Overall, the APOE ε2 allele frequency was 0.073 and the APOE ε4 allele frequency was 0.145. By race/ethnicity groups, the APOE ε4 allele frequency was 0.143 among white participants (n=129, 52.7%), 0.189 among black (n=53, 21.6%), 0.118 among Hispanic (n=55, 22.4%), and 0.063 among Asian or other (n=8, 3.3%).
Cerebral β-amyloid plaques in HIV cohort
By immunohistochemistry with generic 4G8 pan-Aβ antibody to detect most Aβ forms [10], we found frontal Aβ plaques in 81 (29%; 60 focal, 21 widespread) of 279 cases (Table 1). In affected cases, the vast majority of Aβ plaques were of diffuse type [26]. Cored Aβ plaques were occasionally seen, particularly among cases having Aβ plaques of widespread density.
Table 1.
The number of cases having neocortical β-amyloid plaques across age groups.
| Age groups (years) | Number of cases | ||
|---|---|---|---|
| Total | Women | With β-amyloid plaques: focal, widespreada | |
| HIV cohortb | |||
| 26–30 | 6 | 2 (33.3%) | 1 (16.7%): 1, 0 |
| 31–40 | 65 | 12 (18.5%) | 8 (12.3%): 8, 0 |
| 41–50 | 108 | 18 (16.7%) | 32 (29.6%): 27, 5 |
| 51–60 | 76 | 14 (18.4%) | 26 (34.2%): 15, 11 |
| 61–70 | 24 | 2 (8.3%) | 14 (58.3%): 9, 5 |
| General cohortc | |||
| 21–30 | 61 | 28 (45.9%) | 0 (0%) |
| 31–40 | 100 | 47 (47.0%) | 4 (4.0%) |
| 41–50 | 188 | 90 (47.9%) | 20 (10.6%) |
| 51–60 | 330 | 112 (33.9%) | 81 (24.5%) |
| 61–70 | 492 | 182 (37.0%) | 181 (36.8%) |
The density of β-amyloid plaques is graded as absent, focal, or widespread only in the HIV cohort.
In the HIV cohort, all cases (n=279) range in age from 26 to 70 years. Cases with frontal β-amyloid plaques (n=81) range in age from 30 to 68 years, and cases without plaques (n=198) from 26 to 70 years.
Already existing data are obtained from Braak et al. [4]. Not shown are data from age groups younger than 21 or older than 70 years in the general cohort.
In the original binary logistic regression model, age (one-year increase) and APOE ε4 carriage predicted Aβ plaque deposition [OR (95% CI) = 1.08 (1.04–1.12) and 3.28 (1.74–6.18), P<0.0001 and =0.0003, respectively, n=250]. When an age by APOE ε4 carriage interaction term was added to the original model, no significant interaction effect was observed (P=0.83, n=250). When added to the original model, neither sex alone (P=0.14, n=250) nor both sex and a sex by APOE ε4 carriage interaction term (P=0.19 and P=0.74, respectively, n=250) showed significant effects. When added to the original model, neither race/ethnicity alone (P=0.94, n=250) nor both race/ethnicity and a race/ethnicity by APOE ε4 carriage interaction term (P=0.79 and P=0.96, respectively, n=250) showed significant effects. When added to the original model, neither HAART status alone (P=0.91, n=248) nor both HAART status and a HAART status by APOE ε4 carriage interaction term (P=0.94 and P=0.84, respectively, n=248) showed significant effects. When added together to the original model, both postmortem delay and NNTC site showed no significant effects (P=0.53 and P=0.52, respectively, n=248). In all of the additional models that included sex, race/ethnicity, HAART status, or both postmortem delay and NNTC site, the main effects of age and APOE ε4 carriage remained significant (P<0.0001 and <0.041, respectively).
Regarding the density grades of Aβ plaque deposition (relative to absent), age (one-year increase) and APOE ε4 carriage predicted focal Aβ plaques [OR (95% CI) = 1.06 (1.02–1.10) and 2.27 (1.13–4.58), P=0.003 and =0.022, respectively] and widespread Aβ plaques [OR (95% CI) = 1.19 (1.11–1.29) and 14.08 (4.23–46.83), respectively, both P<0.0001, n=250, multinomial logistic regression].
Increased risk of developing cerebral β-amyloid plaques among HIV-infected adults
We chose to compare the frequency of Aβ plaque-bearing cases in our HIV autopsy cohort with that in the already existing general autopsy cohort reported by Braak et al. from Germany [4] because of the large scale and non-selected characteristics of this general cohort and critical similarities between the two cohorts. Similar to the HIV cohort, the general cohort focused on AD-related neuropathologic changes and hence excluded Niemann-Pick disease type C, subacute sclerosing panencephalitis, progressive supranuclear palsy, Pick disease, and corticobasal degeneration. Tissue-related parameters were also similar, including non-selected obtainment of autopsied human brains from affiliated university hospitals, immersion fixation with 4% buffered formaldehyde, and Aβ immunohistochemistry with formic acid pretreatment and the 4G8 antibody (shown to be effective in brain samples fixed in formalin for up to 14 years [29]). Nonetheless, 5-μm-thick paraffin-embedded frontal neocortex sections were used in the HIV cohort, whereas 100-μm-thick polyethylene glycol-embedded medial temporal lobe sections were used in the general cohort.
To compare the HIV cohort (age range 26–70 years) with the general cohort (age range 1–100 years) [4], we limited our statistical analysis to cases ranging in age from 31 to 70 years [HIV cohort: median (interquartile range) = 46 (41–55) years, n=273; general cohort: median age fell in the range of 51–60 years, n=1110; Table 1]. The proportion of women was lower in the HIV cohort compared with the general cohort (16.8% of 273 cases vs. 38.8% of 1110 cases, Table 1) [OR (95% CI) = 0.27 (0.19–0.39), P<0.0001, binary logistic regression weighted by count] when controlling for age groups.
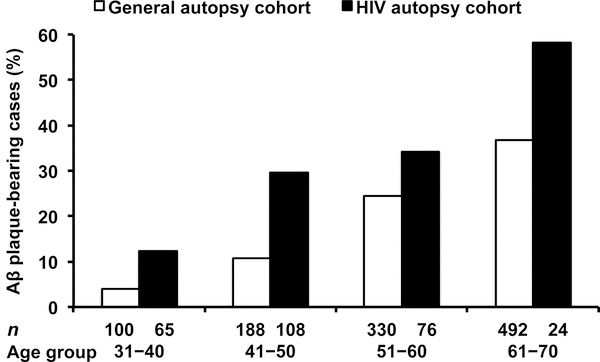
Within the age range of 31–70 years, the overall frequency of 4G8-Aβ plaque-bearing cases was 29.3% of 273 cases in the HIV cohort and 25.8% of 1110 cases in the general cohort (Table 1). Across four age groups by decade (Fig. 1), the frequencies of 4G8-Aβ plaque-bearing cases were higher in the HIV cohort than in the general cohort [OR (95% CI) = 2.35 (1.67–3.31), P<0.0001, binary logistic regression weighted by count] when controlling for age groups. In the HIV cohort, compared with the youngest age group (31–40 years), the frequencies of 4G8-Aβ plaque-bearing cases were higher in the older age groups (41–50, 51–60, and 61–70 years) [OR (95% CI) = 3.00 (1.29–7.00), 3.71 (1.54–8.92), and 9.98 (3.33–29.91); P=0.011, =0.003, and <0.0001, respectively].
Fig. 1. The frequency distribution of cerebral β-amyloid (Aβ) plaque deposition by age groups (years).
The frequencies of Aβ plaque-bearing cases are higher in our HIV autopsy cohort (n=273) than those frequencies in a general autopsy cohort reported by Braak et al. [4] [n=1110; odds ratio (95% confidence interval) = 2.35 (1.67–3.31), P<0.0001, binary logistic regression weighted by count] when controlling for age groups.
Occurrence of post-translationally modified β-amyloid forms in plaques
To explore the occurrence of post-translationally modified Aβ forms (i.e. N3pE, E22P, and phospho-Ser8) in plaques, we examined by immunohistochemistry a subset of cases with frontal 4G8-Aβ plaques (n=37; 17 focal, 20 widespread; Table 2) from the HIV cohort (n=81; 60 focal, 21 widespread; Table 1) and a subset of cases without plaques (n=12; Table 2) from the HIV cohort (n=198; Table 1). The rationale for selecting these 37 cases was to include as many as possible cases that showed 4G8-Aβ plaques of widespread density from the limited availability of paraffin-embedded frontal neocortex sections from the NNTC. Representative Aβ immunoreactivity patterns are shown in Fig. 2.
Table 2.
The occurrence of post-translationally modified β-amyloid forms in plaques in frontal neocortex sections.
| Number of cases positive/negative for | Plaques immunoreactive for | |||
|---|---|---|---|---|
| 4G8 | N3pE | E22P | Phospho-Ser8 | |
| With 4G8 plaques (n=37, age range 34–68 years) | 37/0 | 31/6 | 11/26 | 2/35 |
| Non-modified only (16.2%) | 6/0 | 0/6 | 0/6 | 0/6 |
| N3pE added (54.1%) | 20/0 | 20/0 | 0/20 | 0/20 |
| E22P added (24.3%) | 9/0 | 9/0 | 9/0 | 0/9 |
| Phospho-Ser8 added (5.4%) | 2/0 | 2/0 | 2/0 | 2/0 |
| Without 4G8 plaques (n=12, age range 26–54 years) | 0/12 | 0/12 | 0/12 | 0/12 |
Fig. 2. Representative β-amyloid (Aβ) immunoreactivity patterns by diaminobenzidine immunohistochemistry with hematoxylin counterstaining.
The Aβ immunoreactivity patterns are shown in four adjacent sections of the same neocortex blocks obtained from an HIV-infected person (a−d) and a symptomatic Alzheimer’s disease patient (e−h). Plaque deposits of generic 4G8 (a and e, widespread density), N3pE (b and f, widespread), E22P (c and g, focal), and phospho-Ser8 (d and h, focal) Aβ forms are depicted; scale bar, 300 μm for (a−h). High-magnification images of Aβ plaques (arrows) are shown in the corresponding insets. Diffuse and cored Aβ plaques are illustrated in (a) and (b) insets, respectively.
Of 37 4G8-Aβ-positive cases, N3pE-Aβ was found in 31 (83.8%; 18 focal, 13 widespread). E22P-Aβ was observed in 11 (35.5%; 7 focal, 4 widespread) of 31 N3pE-Aβ-positive cases but not in 6 N3pE-Aβ-negative cases. Phospho-Ser8-Aβ was seen in 2 (18.2%; 2 focal) of 11 E22P-Aβ-positive cases but not in 26 E22P-Aβ-negative cases. Among 12 4G8-Aβ-negative cases, none of modified Aβ forms examined were present. In other words, 4G8-Aβ-negative cases were always negative for N3pE, E22P, and phospho-Ser8 Aβ forms (Table 2). Among 4G8-Aβ-positive cases, N3pE-Aβ-negative cases were always negative for E22P and phospho-Ser8 Aβ forms. E22P-Aβ-negative cases were always negative for phospho-Ser8 Aβ form. Collectively, the post-translational modification of Aβ forms in plaques occurred in order: non-modified, N3pE, E22P, and phospho-Ser8.
Relationship between E22P-β-amyloid in cerebrospinal fluid and cerebral β-amyloid plaque deposition
To explore whether CSF E22P-Aβ levels were related to cerebral Aβ plaque deposition, we included HIV-infected cases across all three grades of frontal 4G8-Aβ plaque deposition (i.e. absent, focal, and widespread). From the limited availability of postmortem CSF samples from the NNTC, we examined subsets of cases with 4G8-Aβ plaques (n=27; 17 focal, 10 widespread; age range 34–68 years) and without plaques (n=11; age range 26–54 years) from the HIV cohort. We conducted CSF assays for E22P-Aβ, Aβ42, Aβ40, and Aβ38.
We found inverse correlations between E22P-Aβ/Aβ38 and Aβ42/Aβ38 ratios, and between E22P-Aβ/Aβ40 and Aβ42/Aβ40 ratios (Spearman’s rho = –0.56 and –0.33, P=0.0003 and =0.046, respectively, n=38; see Figure, Supplemental Digital Content 1, which illustrates the scatter plots with trend lines). The median CSF E22P-Aβ/Aβ40 ratio was higher among cases with widespread 4G8-Aβ plaques (n=10) than in cases with focal or absent plaques (n=28; P=0.047, U-test; Fig. 3a), and the median CSF E22P-Aβ/Aβ38 ratio showed a similar trend (P=0.051, U-test; Fig. 3b). The median CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios were lower among cases with 4G8-Aβ plaques (n=27) than in cases without plaques (n=11; P=0.038 and =0.041, respectively, U-test; Fig. 3c and d).
Fig. 3. Scatter plots of E22P-β-amyloid (Aβ)/Aβ40, E22P-Aβ/Aβ38, Aβ42/Aβ40, and Aβ42/Aβ38 ratios in the postmortem cerebrospinal fluid (CSF) of HIV-infected persons, categorized by frontal 4G8-Aβ plaque deposition.
The median E22P-Aβ/Aβ40 ratio (a) is higher among cases with widespread plaques (n=10) than in cases with focal or absent plaques (n=28; U=80, P=0.047, Mann-Whitney U-test), and the median E22P-Aβ/Aβ38 ratio (b) shows a similar trend (U=81, P=0.051). The median Aβ42/Aβ40 (c) and Aβ42/Aβ38 (d) ratios are lower among cases with plaques (n=27) than in cases without plaques (n=11; U=84 and =85, P=0.038 and =0.041, respectively, U-test). Horizontal bars represent median and interquartile range values; *, P<0.05.
DISCUSSION
We found that diffuse Aβ plaques, regarded to indicate the early stages of Aβ plaque development [26], constituted the vast majority of frontal Aβ plaques in our HIV autopsy cohort, in agreement with prior HIV autopsy studies [14–16, 18–20]. In our study, both increasing age and APOE ε4 carriage were associated with the presence and relative abundance of frontal Aβ plaque deposition. The overall frequency of Aβ plaque-bearing cases in our HIV cohort (29% of 279 cases, age range 26–70 years) was comparable to that reported by Esiri et al. [16] (29% of 97 HIV-infected cases, age range 30–69 years).
In the age range of 31–70 years, the overall frequency of 4G8-Aβ plaque-bearing cases was 29.3% of 273 cases in our HIV cohort and 25.8% of 1110 cases in the general autopsy cohort reported by Braak et al. [4]. Across age groups (31–40, 41–50, 51–60, 61–70 years), the frequencies of Aβ plaque-bearing cases in our HIV cohort were higher than those in the general cohort. The higher frequency of Aβ plaque-bearing cases in the HIV cohort was likely an underestimate of the true difference because the sensitivity for detecting Aβ plaques might be higher for the general cohort in which neocortex sections used (100-μm) were thicker than those used in the HIV cohort (5-μm). Our findings of increased risk of developing neocortical Aβ plaques in HIV-infected adults are consistent with those reported by Esiri et al. [16]. On the other hand, Gelman et al. [14] did not find the increased likelihood of developing hippocampal Aβ plaques among HIV-infected cases. This discrepancy may be explained by the characteristic sequence of Aβ plaque progression, where the involvement of neocortex precedes that of allocortex (e.g. hippocampus) [3].
Our autopsy findings contrast with the findings from a clinical study conducted by Ances et al. [30] showing no evidence of cerebral Aβ deposition in HIV-infected participants (n=16, age range 38–67 years) by positron emission tomography (PET) with Pittsburgh Compound B (PiB). One potential explanation for this discrepancy is the lower sensitivity of PiB for detecting diffuse plaques (containing small amounts of fibrillar Aβ [31]) as compared with cored plaques (composed predominantly of fibrillar Aβ) [28, 32]. In this clinical study [30], however, CSF Aβ42 levels were reduced (<500 pg/ml cutoff) in 5 of 13 HIV-infected participants. Given a well-documented inverse relationship between CSF Aβ42 and cerebral Aβ deposition [28], this CSF finding [30] suggests that some HIV-infected persons have cerebral Aβ deposition that is not detectable by PiB PET due to the subthreshold burden of fibrillar Aβ.
Although information on the APOE ε4 allele frequency was not available in the general cohort from Germany [4], the overall frequency in our HIV cohort (0.145) was comparable to that in the general population (estimated at 0.15) [33]. Among white participants, who constituted the majority of our HIV cohort (52.7%), the APOE ε4 allele frequency in the HIV cohort (0.143) was also similar to that in the general population (estimated at 0.15) [34]. Thus, a difference in the APOE ε4 allele frequency could not account for the higher likelihood of cerebral Aβ plaques in the HIV cohort compared with the general cohort, although even a small difference in the APOE ε4 allele frequency between the HIV and general cohorts could contribute to the finding. The age-dependent increase in cerebral Aβ plaque burden was greater in women than men in the general cohort [4]. Additionally, in a meta-analysis of 40 AD studies [35], Farrer et al. found that women were more likely than men to develop AD among white APOE ε4 carriers (age range 40–90 years). In comparison with the general cohort, we found the higher frequency of Aβ plaque-bearing cases in the HIV cohort even with the lower proportion of women. We did not find any significant effect of sex or sex by APOE ε4 carriage interaction on frontal Aβ plaque deposition when controlling for age and APOE ε4 carriage. Together, our findings suggest that HIV-infected adults between 31 and 70 years of age are at increased risk of developing cerebral Aβ plaques when compared with the general population. Our findings may be confirmed in future studies comparing HIV-infected cases with non-HIV controls from the same autopsy cohort and under the same neuropathological protocols.
Cerebral Aβ plaque deposition in HIV-infected adults may be mechanistically related to accelerated brain aging observed in this population [11–13], e.g. regarding deficiencies in Aβ clearance [36, 37]. Clearance of soluble Aβ forms from the brain parenchyma may be mediated by enzymatic degradation, perivascular macrophages, receptor-mediated transcytosis across the blood-brain barrier [37], bulk flow of the interstitial fluid into the ventricular CSF compartment [38], and drainage of the interstitial fluid along the brain vasculature, including the paravascular glymphatic pathway [39] and the intramural vascular basement membrane pathway [40]. Accordingly, age-related degeneration of cerebral blood vessels may play a role in the development of cerebral Aβ plaques [40, 41]. In our present study, however, data on cerebral vascular disease were not available in either of the HIV and general autopsy cohorts. In addition to deficiencies in Aβ clearance, it is possible that cerebral Aβ plaques develop in response to chronic microbial infection, with the potential interaction with the APOE ε4 isoform [42] since Aβ can function as an antimicrobial peptide in innate immunity [42, 43]. In HIV disease, HIV-1 derived proteins or other microbes [44] may enhance neuronal production of Aβ. Future studies are warranted to explore whether there are specific profiles of brain microbiota that lead to the development of cerebral Aβ plaques.
In the HIV cohort, we found that post-translational Aβ modification in plaques occurred in order: non-modified, N3pE, E22P, and phospho-Ser8. Our finding suggests the existence of progression stages of Aβ plaque deposition, in agreement with prior studies in the general population [3, 5] in which N3pE-Aβ was observed in both preclinical and symptomatic AD brains, whereas phospho-Ser8-Aβ was present mainly in symptomatic AD brains. The significance of modified Aβ forms was also supported by experimental results. Both N3pE-Aβ and phospho-Ser8-Aβ were found in AD-model mouse brains [45, 46] and to have an increased propensity to form oligomeric and fibrillar assemblies in vitro [47, 48].
Along with its predisposition to form neurotoxic oligomers in vitro [7–10], E22P-Aβ was associated with cognitive impairment in vivo. In AD-model mice, the chronic intraperitoneal injection of anti-E22P-Aβ antibody (clone 24B3) ameliorated impairments in spatial memory and executive function [9]. In our CSF assays, we found inverse correlations between E22P-Aβ/Aβ38 and Aβ42/Aβ38 ratios, and between E22P-Aβ/Aβ40 and Aβ42/Aβ40 ratios. These findings suggest that CSF E22P-Aβ/Aβ38 and E22P-Aβ/Aβ40 ratios directly reflect cerebral Aβ deposition because a reduction in the CSF Aβ42/Aβ38 or Aβ42/Aβ40 ratio is predictive of cerebral Aβ deposition [27, 28]. This interpretation is further supported by our findings that the median CSF E22P-Aβ/Aβ40 ratio was higher among HIV-infected cases with widespread frontal 4G8-Aβ plaques compared with those with focal or absent plaques. The median CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios were lower among HIV-infected cases with frontal 4G8-Aβ plaques compared with those without plaques, in agreement with the observations in the general population [27, 28].
Postmortem studies are naturally biased toward advanced stages of disease conditions. The profiles of our HIV autopsy cohort might not fully reflect those of living HIV-infected persons with systemic viral suppression in the HAART era. Not every case had all tissue or fluid samples of interest available for examination. Our findings on post-translational Aβ modification in plaques were based on the limited availability of paraffin-embedded frontal neocortex sections from the NNTC and thereby might be subject to a selection bias. In addition, there was a degree of subjectivity in our qualitative assessment of the density of frontal Aβ plaques (i.e. absent, focal, or widespread). Regarding our CSF assays, the sample size was relatively small due to the necessary inclusion of all three grades of frontal 4G8-Aβ plaque deposition, together with the limited availability of CSF samples from the NNTC. Therefore, our findings on CSF E22P-Aβ may be seen as preliminary to further systematic investigations.
In conclusion, our findings suggest that HIV-infected adults between 31 and 70 years of age are at increased risk of developing cerebral Aβ plaques when compared with the general population. It remains to be determined whether the Aβ plaque progression stages based on post-translational Aβ modification denote the progression toward more advanced neurodegeneration [3, 5]. The potential for CSF E22P-Aβ as a biomarker of cerebral Aβ deposition should be further investigated [7]. Future studies are warranted to explore the implications of cerebral Aβ plaque deposition for the development of neurocognitive decline among HIV-infected persons.
Supplementary Material
Supplemental Digital Content 1. Figure that illustrates the scatter plots with trend lines. pdf
Acknowledgements
We thank Dr. Eliezer Masliah (Departments of Pathology and Neurosciences, University of California San Diego) for providing Alzheimer’s disease neocortex sections.
Sources of funding: Research reported in this publication was supported by the United States National Institutes of Health (NIH) under Award Number R56AG059437 (V.S., A.U., B.G., D.J.M.), R01MH096648 (A.J.L., D.J.M., B.G., V.S.), RF1AG061070 (D.J.M., E.E.S., B.G., V.S.), U24MH100928 (D.J.M., R.J.E., A.U., B.G., V.S.), and P50DA026306 (V.S., R.J.E., B.S.).
This publication was made possible from NIH funding through the NIMH and NINDS Institutes by the following grants: Manhattan HIV Brain Bank (MHBB): U24MH100931, Texas NeuroAIDS Research Center (TNRC): U24MH100930, National Neurological AIDS Bank (NNAB): U24MH100929, California NeuroAIDS Tissue Network (CNTN): U24MH100928, Data Coordinating Center (DCC): U24MH100925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National NeuroAIDS Tissue Consortium (NNTC) or NIH.
Conflicts of Interest and Source of funding: Research reported in this publication was supported by the United States National Institutes of Health (NIH) under Award Number R56AG059437 (V.S., A.U., B.G., D.J.M.), R01MH096648 (A.J.L., D.J.M., B.G., V.S.), RF1AG061070 (D.J.M., E.E.S., B.G., V.S.), U24MH100928 (D.J.M., R.J.E., A.U., B.G., V.S.), and P50DA026306 (V.S., R.J.E., B.S.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 2012; 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thal DR, Walter J, Saido TC, Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol 2015; 129:167–182. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70:960–969. [DOI] [PubMed] [Google Scholar]
- 5.Rijal Upadhaya A, Kosterin I, Kumar S, von Arnim CA, Yamaguchi H, Fandrich M, et al. Biochemical stages of amyloid-beta peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer’s disease. Brain 2014; 137:887–903. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Wirths O, Stuber K, Wunderlich P, Koch P, Theil S, et al. Phosphorylation of the amyloid beta-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol 2016; 131:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami K, Tokuda M, Suzuki T, Irie Y, Hanaki M, Izuo N, et al. Monoclonal antibody with conformational specificity for a toxic conformer of amyloid beta42 and its application toward the Alzheimer’s disease diagnosis. Sci Rep 2016; 6:29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda Y, Uemura S, Ohashi R, Nakanishi A, Takegoshi K, Shimizu T, et al. Identification of physiological and toxic conformations in Abeta42 aggregates. Chembiochem 2009; 10:287–295. [DOI] [PubMed] [Google Scholar]
- 9.Izuo N, Kasahara C, Murakami K, Kume T, Maeda M, Irie K, et al. A toxic conformer of Abeta42 with a turn at 22–23 is a novel therapeutic target for Alzheimer’s disease. Sci Rep 2017; 7:11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami K, Horikoshi-Sakuraba Y, Murata N, Noda Y, Masuda Y, Kinoshita N, et al. Monoclonal antibody against the turn of the 42-residue amyloid beta-protein at positions 22 and 23. ACS Chem Neurosci 2010; 1:747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging 2014; 35:1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, et al. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 2010; 201:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis 2015; 212:1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol 2004; 10:98–108. [DOI] [PubMed] [Google Scholar]
- 15.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 2006; 111:529–538. [DOI] [PubMed] [Google Scholar]
- 16.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry 1998; 65:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005; 19:407–411. [DOI] [PubMed] [Google Scholar]
- 18.Izycka-Swieszewska E, Zółtowska A, Rzepko R, Gross M, Borowska-Lehman J. Vasculopathy and amyloid beta reactivity in brains of patients with acquired immune deficiency (AIDS). Folia Neuropathol 2000; 38:175–182. [PubMed] [Google Scholar]
- 19.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 2005; 19:127–135. [DOI] [PubMed] [Google Scholar]
- 20.Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol 2014; 20:28–38. [DOI] [PubMed] [Google Scholar]
- 21.Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS 2012; 26:2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010; 67:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heithoff AJ, Totusek SA, Le D, Barwick L, Gensler G, Franklin DR, et al. The integrated National NeuroAIDS Tissue Consortium database: a rich platform for neuroHIV research. Database (Oxford) 2019; 2019:bay134. doi: 10.1093/database/bay134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine AJ, Soontornniyomkij V, Achim CL, Masliah E, Gelman BB, Sinsheimer JS, et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol 2016; 22:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soontornniyomkij V, Soontornniyomkij B, Moore DJ, Gouaux B, Masliah E, Tung S, et al. Antioxidant sestrin-2 redistribution to neuronal soma in human immunodeficiency virus-associated neurocognitive disorders. J Neuroimmune Pharmacol 2012; 7:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol 2009; 118:5–36. [DOI] [PubMed] [Google Scholar]
- 27.Janelidze S, Zetterberg H, Mattsson N, Palmqvist S, Vanderstichele H, Lindberg O, et al. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016; 3:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blennow K, Mattsson N, Scholl M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci 2015; 36:297–309. [DOI] [PubMed] [Google Scholar]
- 29.Pikkarainen M, Martikainen P, Alafuzoff I. The effect of prolonged fixation time on immunohistochemical staining of common neurodegenerative disease markers. J Neuropathol Exp Neurol 2010; 69:40–52. [DOI] [PubMed] [Google Scholar]
- 30.Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol 2012; 69:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi H, Nakazato Y, Hirai S, Shoji M, Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol 1989; 135:593–597. [PMC free article] [PubMed] [Google Scholar]
- 32.Ikonomovic M, Klunk W, Abrahamson E, Mathis C, Price J, Tsopelas N, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 2008; 131:1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz F, Springer SA, Altheide TK, Varki NM, Gagneux P, Varki A. Human-specific derived alleles of CD33 and other genes protect against postreproductive cognitive decline. Proc Natl Acad Sci U S A 2016; 113:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 2000; 1:507–537. [DOI] [PubMed] [Google Scholar]
- 35.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278:1349–1356. [PubMed] [Google Scholar]
- 36.Thal DR. Clearance of amyloid β-protein and its role in the spreading of Alzheimer’s disease pathology. Front Aging Neurosci 2015; 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soontornniyomkij V, Achim CL. Aging In: The Neurology of AIDS 3E. Gendelman HE (editor). New York: Oxford University Press; 2012. pp. 567–580. [Google Scholar]
- 38.Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, et al. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 2015; 12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris AW, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol 2016; 131:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014; 76:845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement 2018; 14:1602–1614. [DOI] [PubMed] [Google Scholar]
- 43.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 2016; 8:340ra372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Branton WG, Ellestad KK, Maingat F, Wheatley BM, Rud E, Warren RL, et al. Brain microbial populations in HIV/AIDS: alpha-proteobacteria predominate independent of host immune status. PLoS One 2013; 8:e54673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S, Wirths O, Theil S, Gerth J, Bayer TA, Walter J. Early intraneuronal accumulation and increased aggregation of phosphorylated Abeta in a mouse model of Alzheimer’s disease. Acta Neuropathol 2013; 125:699–709. [DOI] [PubMed] [Google Scholar]
- 46.Bayer TA, Wirths O. Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol 2014; 127:787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Rezaei-Ghaleh N, Terwel D, Thal DR, Richard M, Hoch M, et al. Extracellular phosphorylation of the amyloid beta-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer’s disease. EMBO J 2011; 30:2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlenzig D, Manhart S, Cinar Y, Kleinschmidt M, Hause G, Willbold D, et al. Pyroglutamate formation influences solubility and amyloidogenicity of amyloid peptides. Biochemistry 2009; 48:7072–7078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure that illustrates the scatter plots with trend lines. pdf