Abstract
Bisphenol A is widely used as a material for the production of epoxy resins and polycarbonate plastics. It contaminates various food stuffs by getting leached out from their container lining. Limited information is available on its effects on the male reproductive system. The aim of the present study was to evaluate the extent to which bisphenol A can affect the reproductive system by measuring biochemical and histological changes in the epididymis. Inbred Swiss strain male albino mice were orally administered 80, 120 and 240 mg/kg body weight/day of BPA for 45 days. After completion of treatment, the animals were sacrificed; cauda epididymis was isolated, weighed, used for biochemical and histopathological studies. The results revealed that BPA administered for 45 days caused significant (p<0.05) and dose-dependent reduction in epididymis weight. There was significant (p<0.05) increase in lipid peroxidation and the acid phosphatase activity. Dose dependent reduction in protein, sialic acid contents, as well as the activity of enzymatic antioxidants and mitochondrial enzymes was recorded compared to vehicle treated group. The effect was dose-dependent. Histopathological alteration was observed. This study concludes that BPA causes toxicity in epididymis of mice by generating free radicals, which may be a possible reason for reduction in sperm parameters.
Keywords: bisphenol A, cauda epididymis, biochemical study, in vivotoxicity
ABBREVIATIONS
- BPA
bisphenol A
- LPO
lipid peroxidation
- MD
medium dose
- LD
low dose
- HD
high dose
- ROS
reactive oxygen species
- ACP
acid phosphatase
- CAT
catalase
- SDH
succinate dehydrogenase
- T.S
transverse section
Introduction
The use of plastics has become one of the defining characteristics of modern life. But many of the plastic products people use on a daily basis contain components that can prove harmful to human health and the environment. One such component is a chemical called bisphenol A [2,2-bis(4-hydroxyphenyl)propane], It is one of the highest volume chemicals produced worldwide (Vandenberg et al., 2010). It is a key monomer in the production of epoxy resins and polycarbonate plastic used in manufacture of many household products (Staples et al., 1998; Ranjit et al., 2010). As epoxy resins are used as coatings inside of almost all food and beverage cans, it leaches into food and thus food is considered the main source of its exposure (Vandenberg et al.,2007; Carwile et al., 2009).
Bisphenol A mimics estrogen activity that interferes with the hormonal system in animals and human beings and contributes to adverse health effects (Rochester et al., 2013). It induces hepatic damage and mitochondrial dysfunction by increasing oxidative stress in the liver and other vital organs (Sangai & Verma, 2012; Hassan et al., 2012; Chen et al., 2012; Xia et al., 2014, Elswefy et al., 2016). A study carried out by Verma and Sangai (2009) showed that treatment with bisphenol A causes cytotoxicity in human erythrocytes, which may be due to oxidative stress. However, its effects on the epididymis, highly specialized tissue responsible for maturation and storage of spermatozoa, is not understood.
According to our previous study (Samova et al., 2016), BPA treatment for 45 days causes significant (p<0.05), dose-dependent decrease in sperm count (r=0.992: BPA-LD 80.86%; BPA-MD 47.89%; BPA-HD 25.60%), sperm motility (r=0.995: BPA-LD 73.48%; BPA-MD 51.78%; BPA-HD 24.78%) and sperm viability (r= 0.996: BPA-LD 76.07%; BPA MD 55.85%; BPA HD 25.05%) as compared to vehicle treated control.
As there was reduction in sperm functional parameters, our aim was to investigate what changes affect sperm function. We investigated the sub-chronic toxic effect of bisphenol A in the cauda epididymis of mice by analyzing the oxidative stress, enzymatic antioxidants, energy metabolism, and total contents.
Materials and methods
Chemicals
Bisphenol A was procured from Hi Media Laboratories Pvt. Ltd., Mumbai, India. Olive oil was obtained from Figaro, Madrid, Spain. All the other chemicals used in the present study were of analyzer grade reagent.
Experimental animals
In this study, inbred healthy adult Swiss strain male albino mice weighing 30–35 g were obtained from Cadila pharmaceutical Center, Ahmedabad, India. Animals were kept in the Animal House of Zoology Department of Gujarat University, Ahmedabad, India. They were housed in an air-conditioned room at a temperature of 22±3 °C and 45–55% relative humidity with a 12 h light/dark cycle throughout the experiment. The animals were fed certified pelleted rodent food supplied by Amrut Feeds, Pranav Agro Industries Ltd., Pune, India and potable water ad libitum. All the experimental protocols were approved by the Committee for the Purpose of Control and Supervision of Experiment on Animals (Reg.-167/1999/CPCSEA), New Delhi, India. Animals were handled according to the guidelines published by the Indian National Science Academy, New Delhi, India (1991).
Experimental design
Dose selection
Different doses of BPA were selected on the basis of LD50 value (Kimura et al., 2007). Animals of BPA-treated groups received three different doses of bisphenol A, i.e. 1/10th, 1/20th and 1/30th of LD50 (240,120 and 80 mg/kg bw/day respectively) for 45 days.
Experimental protocol
The experimental protocol is shown in Table 1.The 50 mice were randomly divided into five groups each containing 10 animals. Animals of Group I (untreated control) were kept without any treatment and given free access to food and water. Group II (vehicle control) animals were treated with olive oil (0.2 ml/animal/day), as olive oil was used to dissolve bisphenol A. Animals of group III, IV and V received three respectively different doses of BPA (80, 120 and 240 mg/kg bw/day) for 45 days.
Table 1.
Experimental protocol.
| Sr. No. | Experimental Groups | Number of animals | Duration of treatment (Days) | Day of necropsy |
|---|---|---|---|---|
| Control groups | ||||
| I | Untreated control | 10 | 45 | 46th |
| II | Vehicle control (0.2 ml olive oil/animal/day) | 10 | 45 | 46th |
| BPA-treated groups | ||||
| III | BPA-Low dose (80 mg/kg body weight/day) | 10 | 45 | 46th |
| IV | BPA-Medium dose (120 mg/kg body weight/day) | 10 | 45 | 46th |
| V | BPA-High dose (240 mg/kg body weight/day) | 10 | 45 | 46th |
All treatments were given orally using a feeding tube attached to a hypodermic syringe.
Necropsy
After treatment, the animals were sacrificed using anesthetic ether. The epididymis was dissected out quickly, blotted free from blood and used for the histopathological study and biochemical parameters such as lipid peroxidation, protein and sialic acid contents, as well as the activities of enzymatic antioxidants, acid phosphatase and mitochondrial enzymes (ATPase and succinate dehydrogenase).
Biochemical parameters
Lipid Peroxidation
The levels of lipid peroxidation were measured as malondialdehyde (MDA) using a colorimetric method, as previously described by Ohkawa et al. (1979) with modifications. The results were expressed as nano moles of MDA per gram of protein.
Enzymatic antioxidants
Catalase (E.C.1.11.1.6) activity was analyzed using the method described by Luck (1963), utilizing hydrogen peroxide as substrate. Decrease in absorption was noted at 240 nm. The enzyme activity was expressed as μmoles H2O2 consumed/mg protein/min. Superoxide dismutase (E.C.1.15.1.1) activity (SOD) was measured by the method of Kakkar et al. (1984). The enzyme activity was expressed as units/mg protein. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of O2 •.The glutathione peroxidase (E.C.1.11.1.9) activity (GSH-Px) in the testis was assayed by the modified method of Paglia and Valentine (1967). The decrease in absorbance was recorded for 3 min at 340 nm. The enzyme activity was expressed as units/mg protein/min, where 1 unit of GSH-Px equals to nmoles of NADPH consumed/mg protein/min.
Energy metabolism and phosphatase activity
The adenosine triphosphatase (ATPase) activity in the cauda epididymis was assayed by the method of Quinn and White (1968). The enzyme activity was expressed as μmoles inorganic phosphate released/mg protein/30 min. Succinic dehydrogenase (SDH) activity was assayed by the method of Beatty et al. (1966). The enzyme activity was expressed as μg formazon formed/mg protein/15 min. The acid phosphatase (ACP) activity was assayed by the method as described in Sigma Technical Bulletin (Sigma Technical Bulletin, MO, USA. 2001). The enzyme activity was expressed as μmoles p-nitrophenol released/mg protein/30 min.
Total content estimation
Protein content in the cauda epididymis was estimated by the method of Lowry et al. (1951). The protein content was expressed as mg/100 mg tissue weight. The concentration of sialic acid was assessed by the method of Jourdian et al. (1971). The sialic acid content was expressed as μg/mg tissue weight.
Statistical analysis
Statistical analysis was performed by analysis of variance (ANOVA) followed by Tukey’s test using Graph Pad Instant software version 5.03. Data are expressed as the means ± S.E.M. The level of significance was accepted at p<0.05. Pearson’s correlation analysis was used to determine the correlation between controls and treated animals.
Histopathological studies
Histopathological studies were carried out using the standard technique of hematoxylin and eosin staining. The cauda epididymis of all control and treated groups of animals were dissected out, blotted free of blood and fixed in Bouin’s solution immediately after autopsy. The preserved tissues were dehydrated by passing through ascending grades of alcohol, cleared in xylene and embedded in paraffin wax (58 to 60 °C). Sections of 5 μm were cut on a rotary microtome and stained with H & E, dehydrated in alcohol, cleared in xylene, mounted in DPX and examined under a light microscope.
Results
Absolute and relative weights
Table 2 shows results of BPA treatment on absolute and relative weights of cauda epididymis. No significant changes were observed in absolute and relative weights of cauda epididymis between different control groups of animals (Groups I–II). The treatment of BPA (Groups III–V) for 45 days caused significant (p<0.05) reduction in absolute and relative weights of cauda epididymis as compared to vehicle control group of animals (Group II). These effects were dose-dependent (r=0.940, 0.891 respectively). The maximum reductions in absolute and relative weights were up to 33.84% and 30.16% respectively.
Table 2.
Bisphenol A induced changes in absolute and relative weight of cauda epididymis of mice.
| Sr. No. | Experimental groups | Absolute weight | Relative weight |
|---|---|---|---|
| Control groups | |||
| I | Untreated control | 24.12 0.26 | 61.53±0.39 |
| II | Vehicle control (0.2 ml olive oil/animal/day) | 24.08±0.16 | 61.48±0.71 |
| BPA-treated groups | |||
| III | BPA-Low dose (80 mg/kg bodyweight/day) | 21.32±0.34* (11.44) | 54.99±0.87* (10.54) |
| IV | BPA-Medium dose (120 mg/kg bodyweight/day) | 18.54±0.27* (22.98) | 49.54±1.06* (19.56) |
| V | BPA-High dose(240 mg/kg bodyweight/day) | 15.98±0.28* (33.84) | 42.94±1.00* (30.16) |
Values are mean±S.E.M., n=10. Values shown in parenthesis indicate: Brackets – Percent change in BPA-treated from vehicle treated control group. Significance at the level of *p<0.05, as compared with vehicle control group. No significant difference was noted between untreated and vehicle control group. Units: Absolute weight – mg; Relative weight – mg/100 gm body weight.
Lipid peroxidation and activity of enzymatic antioxidants
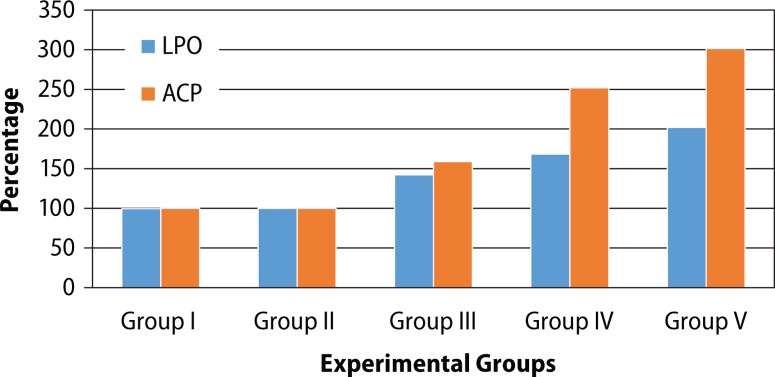
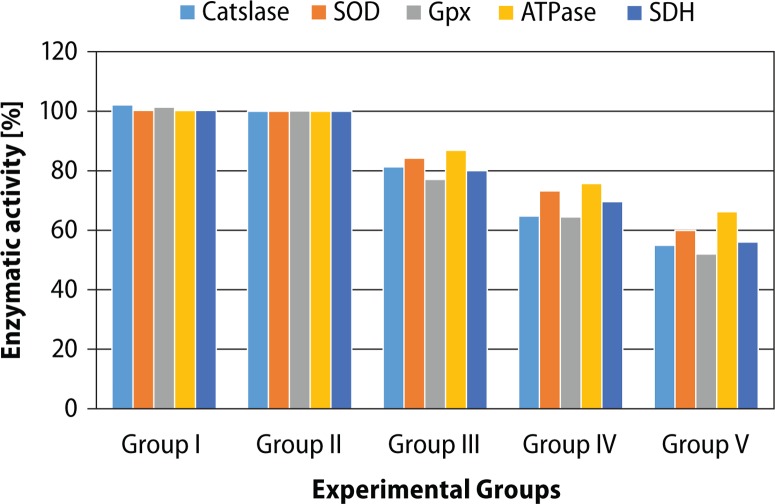
The effect of BPA treatment on lipid peroxidation as well as enzymatic antioxidants in cauda epididymis is shown in Table 3. No significant difference was noted in LPO and enzymatic antioxidants between different control groups of animals (Groups I–II). Oral administration of BPA (Groups III–V) for 45 days caused significant (p<0.05), dose-dependent (r=0.876) increase in LPO (LD: 42.34%, MD: 68.43% and HD: 102.14%) as compared to vehicle control (Group II). The activities of enzymatic antioxidants such as CAT, SOD and GSH-Px were significantly lowered in BPA-treated mice as compared to vehicle control. These effects were in dose-dependent manner (r=0.870, 0.864, 0.770, respectively). The maximum reduction was observed with BPA-HD (Figure 1).
Table 3.
Bisphenol A-induced changes on lipid peroxidation and enzymatic antioxidants in cauda epididymis of mice
| Sr. No. | Experimental group | LPO | Enzymatic Antioxidants | ||
|---|---|---|---|---|---|
| Catalase | SOD | GSH-Px | |||
| Control groups | |||||
| I | Untreated control | 1.462±0.11 | 2.066±0.05 | 6.725±0.31 | 0.407±0.03 |
| II | Vehicle control (0.2 ml olive oil /animal/day) | 1.470±0.09 | 2.022±0.05 | 6.707±0.28 | 0.402±0.02 |
| BPA-treated groups | |||||
| III | BPA-Low dose (80mg/kg bodyweight/day) | 2.092±0.12* (42.33) | 1.643±0.07* (18.75) | 5.648±0.18* (15.79) | 0.310±0.02* (22.90) |
| IV | BPA-Medium dose (120 mg/kg bodyweight/day) | 2.476±0.10* (68.43) | 1.309±0.05* (35.24) | 4.911±0.12* (26.78) | 0.259±0.01* (35.53) |
| V | BPA-High dose (240 mg/kg bodyweight/day) | 2.971±0.06* (102.14) | 1.111±0.12* (45.04) | 4.021±0.11* (40.05) | 0.209±0.02* (47.99) |
Values are mean±S.E.M., n=10, Values shown in parenthesis indicate: Brackets – Percent change in BPA-treated from vehicle control. Level of significance; ap <0.05 as compared to vehicle control, bp<0.05 as compared to BPA-HD -treated. No significant difference was noted between different control groups (Groups I-III). Units: LPO – nmoles MDA formed/mg protein/60 min; Catalase – μmoles H2O2 consumed/mg protein/min; SOD – units/mg protein; GSH-Px- nmoles of NADPH consumed/mg protein/min.
Figure 1.
Percentages of lipid peroxidation and acid phosphatase activity in experimental groups.
Mitochondrial enzymes and phosphatase activities
BPA-induced changes in biochemical parameters in cauda epididymis are shown in Table 4. No significant difference was noted in activities of ACP, ATPase and SDH between different control groups of animals (Groups I–II). Similarly, as compared to vehicle control, BPA treatment caused significant (p<0.05), dose-dependent (r=0.929) increase in ACP activity (LD: 58.98%, MD: 151.862% and LD: 201.71%), as compared to vehicle control (Figure 2).
Table 4.
Bisphenol A induced changes in phosphatase activity and energy metabolism in cauda epididymis of mice.
| Sr. No. | Experimental groups | Phosphatase activity (ACP) | Energy Metabolism | |
|---|---|---|---|---|
| ATPase | SDH | |||
| Control groups | ||||
| I | Untreated control | 0.46±0.06 | 1.62±0.02 | 19.21±0.26 |
| II | Vehicle control (0.2 ml olive oil/animal/day) | 0.45±0.01 | 1.61±0.01 | 19.17±0.36 |
| BPA-treated groups | ||||
| III | BPA-Low dose (80 mg/kg bodyweight/day) | 0.61±0.01* (58.98) | 1.40±0.02* (13.16) | 15.33±0.26* (19.96) |
| IV | BPA-Medium dose (120 mg/kg bodyweight/day) | 1.00±0.02* (151.862) | 1.22±0.02* (24.31) | 13.34±0.48* (30.38) |
| V | BPA-High dose (240 mg/kg bodyweight/day) | 1.16±0.05* (201.71) | 1.07±0.02* (33.80) | 1073±0.43* (43.99) |
Values are mean±S.E.M., n=10. Values shown in parentheses indicate: Brackets – Percent change in BPA treated from vehicle treated control group. Significance at the level of *p<0.05, as compared with vehicle control,No significant difference was noted between untreated and vehicle control groups. Units: ACP – μmoles p-nitrophenol released/mg protein/30 min; ATPase – μmoles inorganic phosphate released/mg protein/30 min; SDH – μg formazon formed/mg protein/15 min
Figure 2.
Percentages of enzymatic activities in experimental groups.
BPA treatment also caused significantly (p<0.05) decreased activities of ATPase and SDH. The effect was dose-dependent (r=0.944, 0.919, respectively).
Total protein and ascorbic acid
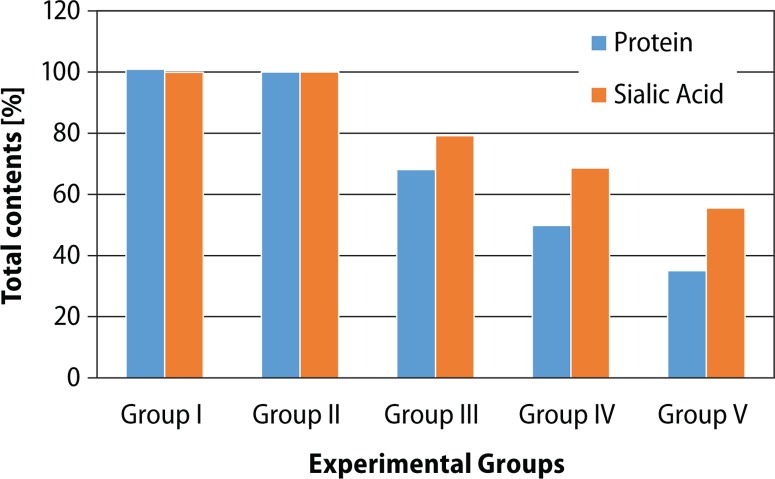
Reduction in total content is shown in Table 5. Oral administration of BPA (Groups III–V) caused significant (p<0.05) decrease in protein (BPA-LD: 31.84%, BPA-MD: 50.14%, BPA-HD: 64.91%,) and sialic acid (BPA-LD: 20.79%, BPA-MD: 31.33%, BPA-HD: 44.43%) contents as compared to vehicle treated control group (Group II). These effects were dose-dependent (r=0.942, 0.948) (Figure 3).
Table 5.
Bisphenol A induced changes in total contents in cauda epididymis of mice.
| Sr. No. | Experimental groups | Protein | Sialic acid |
|---|---|---|---|
| Control groups | |||
| I | Untreated control | 12.28±0.58 | 10.03±0.16 |
| II | Vehicle control (0.2 ml olive oil/animal/day) | 12.17±0.39 | 10.04±0.13 |
| BPA-treated groups | |||
| III | BPA-Low dose (80 mg/kg bodyweight/day) | 8.295±0.16* (31.84) | 7.95±0.15* (20.79) |
| IV | BPA-Medium dose (120 mg/kg bodyweight/day) | 6.07±0.81* (50.14) | 6.89±0.16* (31.33) |
| V | BPA-High dose (240 mg/kg bodyweight/day) | 4.27±0.17* (64.91) | 5.58±0.14* (44.43) |
Values are mean±S.E.M., n=10. Values shown in parentheses indicate:Bracket – Percent change in BPA-treated from vehicle treated control group. Significance at the level of *p<0.05, as compared with vehicle control, No significant difference was noted between untreated and vehicle control groups. Units: Protein – mg/100 mg tissue weight; Sialic acid – μg/mg tissue weight.
Figure 3.
Percentages of total contents in experimental groups.
Histopathological analysis
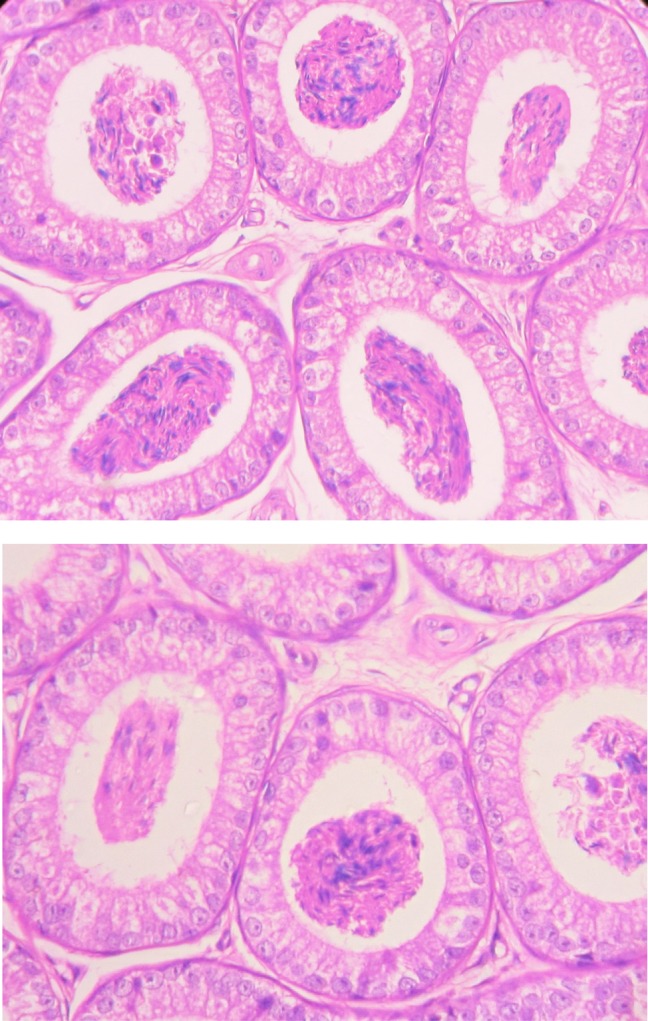
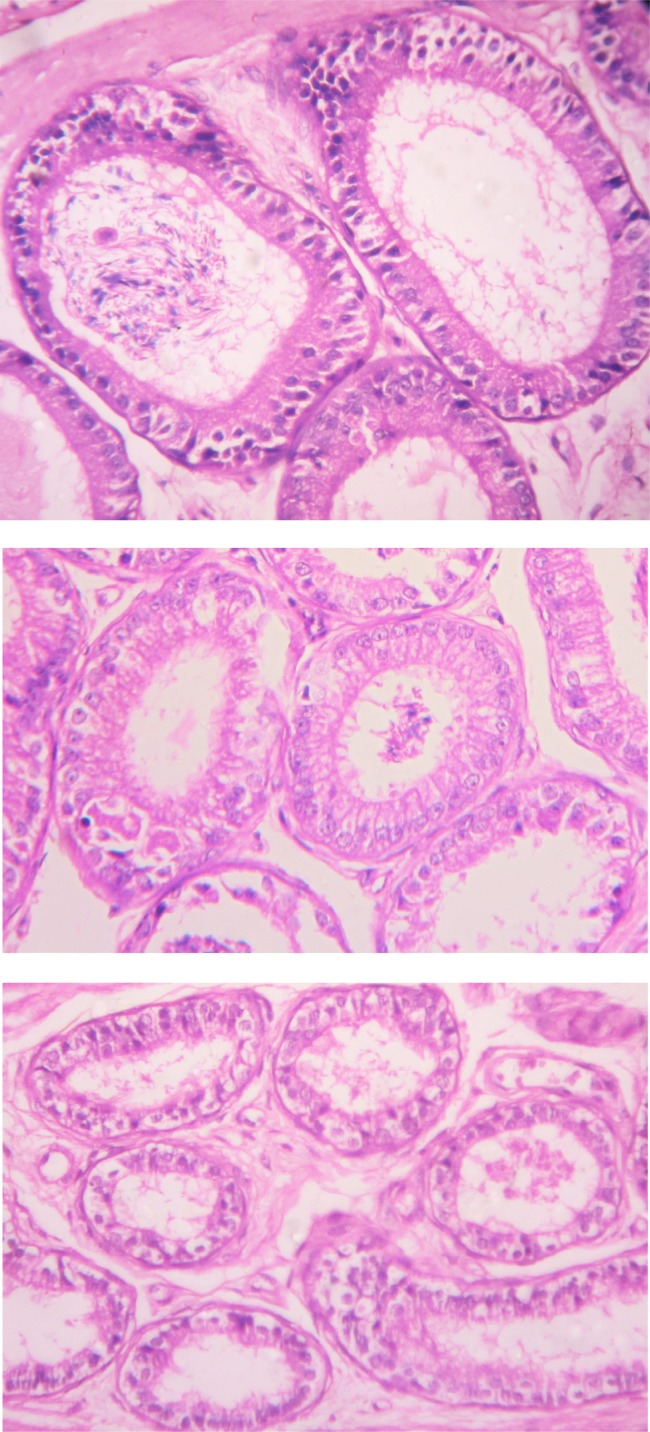
The cauda epididymis of all control groups (Groups I–II) of animals showed normal tubules with pseudostratified epithelium lined by stereocilia and containing dense sperm bundles in the lumen of the tubules (Figure 4) However, BPA treatment (Groups III–V) for 45 days resulted in alterations in the cauda epididymis. Bisphenol A treatment caused degeneration in epithelium, decrease in stereo cilia, and reduction in sperm density and wider space between tubules (Figure 5). The effect was more pronounced in BPA-HD-treated group (Group V).
Figure 4.
T.S. of cauda epididymis of untreated control mice (group I-II) showing normal tubules with pseudostratified epithelium lined by stereocilia and containing dense sperm bundles in the lumen of the tubules (H & E staining, 400×).
Figure 5.
T.S. of cauda epididymis of BPA-HD-treated mice (group III-V) showing degenerated epithelium with decrease in stereocilia, reduction in sperm density and wider space between tubules as per increases the exposure of BPA (H & E staining, 400×).
Discussion
The epididymis is an important organ in the male reproductive system in which the testicular spermatozoa undergo maturation (Cornwall 2009). Sperm maturation depends on the secretary product of the epididymis. The epididymis provides a luminal microenvironment for sperm maturation and storage under androgen control (Robaire et al., 1988; Serre & Robaire 1998). Previous studies showed BPA to cause generation of free radicals (Chitra et al., 2003; Kabuto et al., 2003).
In the present study, BPA was administered in order to evaluate its effect through a complete spermatogenic cycle, which takes approximately 45 days in mice (Clermont, 1972; Hess et al., 2009) and the length of spermatogenic cycle is considered as biological constant controlled by germ cell, thus we exposed mice to BPA for 45 days. Gravimetric analysis revealed that BPA treatment for 45 days caused dose-dependent significant decrease in absolute and relative weights of cauda epididymis (Chitra et al., 2003). Vom Saal et al. (1998) and Delclos et al. (2014) also reported that decreases in epididymis weights were associated with degenerative changes with hypospermia. Histopathology showed reduction in sperm bundles (Chitra et al., 2003; 2001). Reduction in weights of cauda epididymis could be due to degenerative changes in epithelium and lower sperm concentration in lumen. Moreover, it may be due to the inhibition of spermatogenesis, decreased elongated spermatids and steroidogenic enzyme activity (Takahashi & Oishi, 2001; Lanning et al., 2002).
Overproduction of reactive oxygen species (ROS) and free radicals constitutes oxidative stress that can be detrimental to sperm as associated with male fertility (Agarwal et al., 2014). If spermatozoa are exposed to excessive levels of ROS, their fertilizing capacity and genetic integrity could be compromised (Aitken et al., 2014; 2016). Oral administration of BPA generates oxidative stress (ROS) which damages the lipid membrane and moreover reduces the activities of enzymatic antioxidants in cauda epididymis of mice. BPA exerts some of its effects by binding to the nuclear steroid receptors for estrogen to subsequently impact expression of estrogen-responsive gene products (Wetherill et al., 2007). Moreover, the study by Qiu et al. indicates that a low BPA concentration can induce spermatogenesis disorders mainly through decreasing androgen receptor expression (Qiu et al., 2013). Unfavorable condition for sperm leads to deterioration of the fertility rate (Gabrielsen et al.,2016). El-Missiry et al. (2014) explained that bisphenol A elicits depletion of antioxidant defense system and induces oxidative stress. Another study done by Hassan et al. (2012) reported that bisphenol A induces hepatotoxicity through oxidative stress and ultimately decreases the antioxidant enzymes. Catalase and glutathione peroxidase are important enzymes of antioxidant defense systems, which protect tissue against oxidative stress induced by reactive oxygen species (Lei et al., 2016). Both these enzymes catalyze the hydrolysis of H2O2 into water and oxygen molecules to prevent tissue injury (Prescott et al., 2016).
The cell has numerous defense mechanisms to fight against oxidative stress. Our result shows the decrease in activity of CAT. This indicates that H2O2 was most probably present in high levels, moreover CAT is involved predominantly in the detoxification of high H2O2 levels. Reduction in the activity of catalase may reflect incapability of mitochondria and eliminate hydrogen peroxide produced after exposure to BPA (Bindhumol et al., 2003).
The present study shows that exposure to BPA causes significant, dose-dependent decrease in energy metabolism. ATPase is required for enzymatic hydrolysis of ATP, which is important for intracellular transfer of energy (Zeisel, 2012). Activity of SDH, also significantly decreased in cauda epididymis by BPA administration. SDH is a key enzyme of mitochondrial Krebs cycle and it is mainly concerned with aerobic oxidation of acetylCoA and generation of ATP (Iacobazzi et al., 2014). Of the Krebs cycle dehydrogenases, SDH is more active than any other enzyme (Putilina et al., 1969). Thus reduction in ATPase and SDH activity could be due to alteration in mitochondria and this could lead to reduction in sperm viability and motility (Ramalho-Santos et al., 2009; Piomboni et al., 2012). Oral administration of bisphenol A for 45 days caused significant dose dependent reduction in activities of SDH and ATPase in epididymis of mice. The effect was comparatively more pronounced in high-dose bisphenol A-treated group than with a low dose. These results indicate that the onset of cytotoxicity caused by BPA may depend on the intracellular energy status and that mitochondria are important targets of the compound (Amaral et al., 2016). The toxicity caused by inhibition of ATP synthesis may be related to the concentration of unmetabolized free BPA remaining in the cell suspensions. Oral administration of BPA for 45 days caused dose-dependent significant increase in ACP activity in cauda epididymis of mice (Graph.1). Acid phosphatase is a marker enzyme for the lysosomal integrity and important for tissue reorganization and tissue repair (Collins and Lewis et al., 1971, Gómez-Sintes et al., 2016). Increased levels of ACP show the tissue damage or nercosis, which is seen in histopathology (Fridovich, 1978).
Bisphenol A treatment caused significant decrease in protein content in cauda epididymis of mice (Figure 3). Proteins are major targets of free radicals (Barnes et al., 2008). Oxidative modifications such as breakdown of peptide bonds can alter protein structures which are related to reduction of protein by elevated oxidative free radicals (Stadman, 2001). Oral exposure of BPA for 45 days caused significant, dose-dependent decrease in sialic acid content in the cauda epididymis of mice. The altered sialic acid content might affect structural integrity of the acrosomal membrane of sperm (Agarwal et al., 2016). This could also affect sperm morphology. It is also concerned with changing the membrane surface of maturing spermatozoa including stabilization of the acrosome and its membrane during sperm maturation and the coating of spermatozoa with certain antigens, which play an important role in the development of fertilizing capacity of spermatozoa (Levinsky et al., 1983; La Spina et al., 2017). According to Samova et al. (2016), BPA exposure causes the morphological abnormality in mice spermatozoa. El-Missiry reported that ascorbic acid had a protective effect against oxidative damage by free radicals. Reduction in the content of ascorbic acid should relate to the reduction in fertility (Wright et al., 2014). Histopathological alteration was seen in different doses of BPA administration. Similar degenerative changes in histopathology were also observed by Chitra et al. (2003).
Conclusion
The present investigation revealed that oral administration of BPA is toxic in a way that induces oxidative stress by generating free radicals as well as reducing the activities of enzymatic antioxidants. Moreover, reduction in metabolism and the contents like protein and sialic acid are also seen. This can be a possible reason for reduction in sperm count and increase in sperm morphological abnormalities and may be the reason for reduction in fertility.
REFERENCES
- Agarwal A, Bertolla RP, Samanta L. Sperm proteomics: potential impact on male infertility treatment. Expert Rev Proteomics. 2016;13(3):285–296. doi: 10.1586/14789450.2016.1151357. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reproduction Fertil Dev. 2016;28(2):1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16(1):31. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral SS Tavares R, Baptista MI, Sousa M, Silva A, Escada-Rebelo S, Paiva PC, Ramalho-Santos J. Mitochondrial Functionality and Chemical Compound Action on Sperm Function. Curr Med Chem. 2016;23(31):3575–3606. doi: 10.2174/0929867323666160425113518. [DOI] [PubMed] [Google Scholar]
- Barnes S, Shonsey EM, Eliuk SM, Stella D, Barrett K, Srivastava OP, Kim H, Renfrow MB. High-resolution mass spectrometry analysis of protein oxidations and resultant loss of function. Biochem Soc Trans. 2008;36(5):1037–1044. doi: 10.1042/BST0361037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty CH, Basinger GM, Dully CC, Bocek RM. Comparison of red and white voluntary skeletal muscles of several species of primates. Journal of Histochemistry & Cytochemistry. 1966;14(8):590–600. [Google Scholar]
- Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188(2):117–124. doi: 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117(9):1368. doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Xu B, Ji W, Qiao S, Hu N, Hu Y, Wu W, Qiu L, Zhang R, Wang Y, Wang S. Bisphenol A alters n-6 fatty acid composition and decreases antioxidant enzyme levels in rat testes: a LC-QTOF-based metabolomics study. PloS one. 2012;7(9):e44754. doi: 10.1371/journal.pone.0044754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra KC, Rao KR, Mathur PP. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: a histological and biochemical study. Asian J Androl. 2003;5(3):203–208. [PubMed] [Google Scholar]
- Chitra KC, Sujatha R, Latchoumycandane C, Mathur PP. Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian J Androl. 2001;3(3):205–8. [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52(1):198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Collins AJ, Lewis DA. Lysosomal enzyme levels in the blood of arthritic rats. Biochem Pharmacol. 1971;20(1):251–253. doi: 10.1016/0006-2952(71)90496-5. [DOI] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15(2):213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, da Costa GG, Woodling KA, Bryant MS. Toxicity evaluation of bisphenol A administered by gavage to Sprague-Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139(1):174–97. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Missiry MA, Othman AI, Al-Abdan MA, El-Sayed AA. Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. J Neurol Sci. 2014;347(1):251–256. doi: 10.1016/j.jns.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Elswefy SES, Abdallah FR, Atteia HH, Wahba AS, Hasan RA. Inflammation, oxidative stress and apoptosis cascade implications in bisphenol A‐induced liver fibrosis in male rats. Int J Exp Pathol. 2016;97(5):369–379. doi: 10.1111/iep.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: the impacts of environment, diet, and drug use on spermatogenesis. Andrology. 2016;4(4):648–661. doi: 10.1111/andr.12198. [DOI] [PubMed] [Google Scholar]
- Gómez-Sintes R, Ledesma MD, Boya P. Lysosomal cell death mechanisms in aging. Ageing Res Rev. 2016;32:150–168. doi: 10.1016/j.arr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, AlOlayan EM. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev. 2012;2012:194829. doi: 10.1155/2012/194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Molecular Mechanisms in Spermatogenesis. New York: Springer; 2009. Spermatogenesis and cycle of the seminiferous epithelium; pp. 1–15. [Google Scholar]
- Iacobazzi V, Infantino V. Citrate–new functions for an old metabolite. Biol Chem. 2014;395(4):387–399. doi: 10.1515/hsz-2013-0271. [DOI] [PubMed] [Google Scholar]
- Jourdian GW, Dean L, Roseman S. The sialic acids XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971;246(2):430–435. [PubMed] [Google Scholar]
- Kabuto H, Hasuike S, Minagawa N, Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003;93(1):31–35. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kimura T, Kimura N, Totsukawa K. Effect of Compound Exposure to Bisphenol A and Nonylphenol on the Development and Fertility of Fetal Mice. J Mamm Ova Res. 2007;24(1):35–41. [Google Scholar]
- La Spina FA, Stival C, Krapf D, Buffone MG. Molecular and cellular aspects of mammalian sperm acrosomal exocytosis. In: Schatten H, Constantinescu GM, editors. Animal Models and Human Reproduction. Wiley-Blackwell; 2017. pp. 409–426. [Google Scholar]
- Lanning LL, Creasy DM, Chapin RE, Mann PC, Barlow NJ, Regan KS, Goodman DG. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Pathol. 2002;30(4):507–520. doi: 10.1080/01926230290105695. [DOI] [PubMed] [Google Scholar]
- Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR, Holmgren A, Arnér ES. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev. 2016;96(1):307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsky H, Singer R, Barnet M, Sagiv M, Allalouf D. Sialic acid content of human spermatozoa and seminal plasma in relation to sperm counts. Arch Androl. 1983;10(1):45–46. doi: 10.3109/01485018308990169. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Luck H. A spectrophotometric method for the estimation of catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York, NY, USA: Academic Press; 1963. pp. 886–887. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Transl Res. 1967;70(1):158–169. [PubMed] [Google Scholar]
- Parker RM. Testing for reproductive toxicity. In: Hood RD, editor. Developmental and Reproductive Toxicology. Boca Raton: Taylor & Francis; 2006. pp. 425–488. [Google Scholar]
- Piomboni P, Focarelli R, Stendardi A, Ferramosca A, Zara V. The role of mitochondria in energy production for human sperm motility. Int J Androl. 2012;35(2):109–124. doi: 10.1111/j.1365-2605.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- Prescott C, Bottle SE. Biological Relevance of Free Radicals and Nitroxides. Cell Biochem Biophys. 2016;75(2):227–240. doi: 10.1007/s12013-016-0759-0. [DOI] [PubMed] [Google Scholar]
- Putilina PE, Eschanko ND. Activity of some Kreb’s cycle dehydrogenases in the brain, liver and kidneys. Yestln. Leningrad. Univ Ser Biol. 1969;24:112–116. [PubMed] [Google Scholar]
- Qiu LL, Wang X, Zhang XH, Zhang Z, Gu J, Liu L, Wang Y, Wang X, Wang SL. Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A. Toxicol Lett. 2013;219(2):116–124. doi: 10.1016/j.toxlet.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Quinn PJ, White IG. Distribution of adenosinetriphosphatase activity in ram and bull spermatozoa. J Reprod Fertil. 1968;15(3):449–452. doi: 10.1530/jrf.0.0150449. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Human Reprod Update. 2009;15(5):553–572. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30(1):2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. In: Knobil E, et al., editors. The Physiology of Reproduction. New York, NY: Raven Press; 1988. pp. 999–1080. [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Samova S, Doctor H, Verma RJ. Spermatotoxic effect of bisphenol a and its amelioration using quercetin. World J Pharm Pharm Sci. 2016;5(5):1161–1175. [Google Scholar]
- Sangai NP, Verma RJ. Quercetin ameliorates bisphenol A-induced toxicity in mice. Acta Pol Pharm Drug Res. 2012;69(3):557–563. [PubMed] [Google Scholar]
- Serre V, Robaire B. Segment-specific morphological changes in aging brown Norway rat epididymis. Biol Reprod. 1998;58(2):497–513. doi: 10.1095/biolreprod58.2.497. [DOI] [PubMed] [Google Scholar]
- Sigma Technical Bulletin No. 104, Sigma Chemical Co., 3500. MO, USA: Dekoib St. Louis 18; 2001. [Google Scholar]
- Stadman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928(1):22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36(10):2149–2173. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Oishi S. Testicular toxicity of dietary 2, 2-bis (4-hydroxyphenyl) propane (bisphenol A) in F344 rats. Arch Toxicol. 2001;75(1):42–51. doi: 10.1007/s002040000204. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Verma RJ, Sangai NP. The ameliorative effect of black tea extract and quercetin on bisphenol A-induced cytotoxicity. Acta Pol Pharm. 2009;66(1):41–44. [PubMed] [Google Scholar]
- Vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14(1–2):239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24(2):178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28(6):684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Xia W, Jiang Y, Li Y, Wan Y, Liu J, Ma Y, Mao Z, Chang H, Li G, Xu B, Chen X. Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PloS one. 2014;9(2):e90443. doi: 10.1371/journal.pone.0090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res. 2012;733(1):34–38. doi: 10.1016/j.mrfmmm.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]