Abstract
The objective of the present study was to evaluate the safety of long term consumption of ethanolic fraction of Neurocalyx calycinus leaves (NCEF) in rodents. The NCEF was subjected to detect the presence of various phytoconstituents. In acute oral toxicity study, graded doses of NCEF was administered in mice and were observed up to 14 days. In sub-chronic oral toxicity study, NCEF was administered to Wistar rats at doses of 50, 500 and 1000 mg/kg b.w. per day for 90 days and after that, observed up to 28 days. NCEF showed the presence of alkaloids, steroids, phenolics and glycosides. In acute toxicity study, there was no mortality and no behavioural signs of toxicity at the highest dose level (6400 mg/kg b.w.). In sub-chronic oral toxicity study, there were no significant difference observed in the consumption of food and water, body weight and relative organ weights. Haematological, serum biochemical, hepatic oxidative stress marker analysis and urine analysis revealed the non-adverse effects of prolonged oral consumption of NCEF. The histopathologic examination did not show any differences in vital organs. Based on our findings, NCEF, at dosage levels up to 1000 mg/kg b.w., is non-toxic and safe for long term oral consumption.
Keywords: Neurocalyx calycinus, sub-chronic toxicity, histopathology
ABBREVIATIONS
- ASL
above sea level
- ABR
Agasthyamalai Biosphere Reserve
- NCEF
ethanolic fraction of leaves of Neurocalyx calycinus
- b.w.
body weight
- v/v
volume/volume
- w/v
weight/volume
- EDTA
ethylenediaminetetraacetic acid
- WBC
white blood cell count
- RBC
red blood cell count
- HGB
hemoglobin
- HCT
hematocrit
- MCV
mean corpuscular volume
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- RDW
red blood cell distribution width
- PLT
platelet count
- MPV
mean platelet volume
- PDW
platelet distribution width
- PCT
plateletcrit
- LYM
lymphocyte count
- MONO
monocyte count
- GRAN
granulocyte count
- ALT
alanine transaminase
- AST
aspartate transaminase
- ALP
alkaline phosphatase
- GGT
γ-glutamyltransferase
- LDH
L-lactate dehydrogenase
- TSP
total serum protein
- ALB
albumin
- TBIL
total bilirubin
- GLU
glucose
- CK
creatine kinase
- CRE
creatinine
- BUN
urea nitrogen
- UA
uric acid
- TG
serum triglyceride
- TC
total cholesterol
- Ca2+
calcium
- Na+
sodium
- K+
potassium
- Cl–
chlorine
- PBS
phosphate buffer saline
- THP
total hepatic protein
- BSA
bovine serum albumin
- CAT
catalase
- GSH
reduced glutathione
- SOD
superoxide dismutase
- MDA
malondialdehyde
- GPx
glutathione peroxidase
- GLU
glucose
- BIL
bilirubin
- KET
ketone
- SG
specific gravity
- BLO
blood
- PRO
protein
- URO
urobilinogen
- NIT
nitrite
- LEU
leukocytes
- DTNB
(5,5’-dithio-bis-[2-nitrobenzoic acid])
- NBT
nitro blue tetrazolium
- NADH
nicotinamide adenine dinucleotide
- TBA
thiobarbituric acid
- H2O2
hydrogen peroxide
Introduction
The diverse ecological conditions in India make it a treasure house of biodiversity, covering about 8% of global biodiversity. The forests of India are estimated to harbour 90% of India’s medicinal plant diversity in a wide range of forest types (Aneesh et al., 2009). With its rich ethnic diversity and strong traditional knowledge in herbal medicine, India has been using herbal drugs for thousands of years. Due to high prices and harmful side effects of synthetic drugs, people rely more on herbal drugs as evidenced by its rapidly growing trends in international and national markets through exploration of ethnopharmacology and traditional medicine (Fabricant & Farnsworth, 2001). The modern drug discovery screening techniques, mainly based on traditional knowledge, have given clues to the development of valuable drugs (Hashmi & Singh, 2003). Most of the traditional knowledge about medicinal plants was in the form of oral knowledge that has been eroded or distorted due to the persistent invasions and cultural adaptations. Locally available medicinal plants are primary raw material for the development of traditional drug formulations.
Neurocalyx Hook is an endemic taxon in the family Rubiaceae, mainly distributed in South Western Ghats of India and Sri Lanka (Bremer, 1979). Neurocalyx calycinus (R. Br. ex Benn.) Rob. is a wild ornamental herbaceous plant classified under the tribe Ophiorrhizeae (Bremer, 1987; Viswanathan et al., 2005; Takhtajan, 2009). It grows up to 20 inches and is dispersed on rocky crevices at the banks of streams in the tropical wet evergreen forests at higher altitudes (1200–1600 m ASL). Cholanaickans, the last remaining hunter-gatherer tribes of South India, are the most primitive and vanishing native tribal communities, living in caves called Alas spread in the Karulai and Chungathara forest ranges in Nilambur, Malappuram district of Kerala, India (Menon, 1996; Mathur, 2013). Cholanaickans use the fresh leaves of N. calycinus, locally known as ‘Pachachedi’, prepared in the form of paste and applied externally to arrest bleeding due to bear bites and to heal fresh wounds, inflammation and pain. Decoction of the leaves (30 mL) is administered orally thrice a day for one week to one month, depending upon the conditions of the symptoms (Saradamma et al., 1994).
From the review of literature, it was evident that a sparse record is available about the usage, safety parameters and medicinal properties of N. calycinus. To develop a novel drug with least side effects and multi therapeutic effect, preclinical studies along with toxicity analysis should be conducted in vivo with pertinent animal models. The present work was done to evaluate the acute and sub-chronic (90 days) toxicity effect of the ethanolic fraction of leaves of Neurocalyx calycinus (NCEF) in Wistar rats.
Material and methods
Chemicals
Bovine serum albumin (BSA), Catalase, 5,5'-Dithiobis (2-nitrobenzoic acid) (DTNB), L-Glutathione, Superoxide Dismutase (SOD), Nitro Blue Tetrazolium (NBT) and Nicotinamide Adenine Dinucleotide (NADH) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Biochemical kits were purchased from Coral Clinical System, Goa, India. All other reagents, chemicals, and solvents were of analytical grade and were purchased from HiMedia Laboratories (India). Phosphate Buffered Saline (PBS) and other reagents were prepared according to protocol.
Plant material
The fresh leaves of Neurocalyx calycinus (R. Br. ex Benn.) Rob. (vernacular name: Pachachedi) were collected from the evergreen forest streams of the upper hill of Ezhumadakka (Latitude 8°37’28”N, Longitude 77°12’53”E) in Athirumala at the Agasthyamalai Biosphere Reserve (ABR) region, Thiruvananthapuram, Kerala, India during the month of April 2014. The plant material was taxonomically identified by Dr. A.G. Pandurangan, Plant Taxonomist, Plant Systematics and Evolutionary Science Division, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Thiruvananthapuram, Kerala, India. A voucher specimen (TBGT 86801 dated 14/12/2015) was certified (JNTBGRI/PS/213/2015) and deposited at the institutional herbarium.
Preparation of the plant extract
The fresh leaves of Neurocalyx calycinus were thoroughly washed, segmented, shade dried and powdered (Usha Shriram (India), Noida, UMA 29103448). The powder (100 g) was sequentially extracted with petroleum ether, chloroform and ethanol (96% v/v) in a Soxhlet apparatus for 24 h each. Each time before extracting with the next solvent, marc was air dried at below 35±5 °C. The ethanolic extract was concentrated under reduced pressure at 30±10 mbar in a rotary evaporator at 30–60 °C (Rotavapor R-215, Buchi, Switzerland) to a syrupy consistence (6.28±0.47% w/w) named NCEF, finally dried in a desiccator (Auto Secador 401110, USA) and stored in airtight containers in a refrigerator at 4 °C.
Qualitative phytochemical screening
The NCEF was subjected to qualitative phytochemical tests to detect the presence of various phytoconstituents (Sofowora, 1982; Harborne, 1998) such as carbohydrates (Molisch test, Fehling test, Barfoed test and Benedict test), proteins (Millon test, Biuret test and Ninhydrin test), alkaloids (Mayer test, Wagner test, Hager test and Dragendorff test), glycosides (Borntrager test, Legal test, Keller kiliani test and Kedde test), phenolic compounds (ferric chloride test, gelatin test, lead acetate test, alkaline reagent test and Shinoda test), phytosterols (Libermann-Burchard test and Salkowski test), fixed oils and fats (spot test and saponification test), saponins (foam test), gum and mucilage (alcohol 95% test), volatile oils (steam distillation), anthraquinones (chloroform – 10% ammonia test) and iridoids (Trim-Hill reagent test).
Experimental animals
Adult female and male Swiss Albino mouse (Mus musculus), 25±2 g, and Wistar Albino rat (Rattus norvegicus), 155±3 g, were obtained from the animal house of JNTBGRI, India. All animals were housed in standard polypropylene cages at controlled temperature (25±2 °C), with light conditions (12 h light and dark cycle), room air changes 15±3 times/h and relative humidity (65±5%). The animals were provided with pellet diet (Lipton India Ltd. Mumbai) and water ad libitum. The animals were allowed to acclimatize to the new environment for 7 days before starting the experiment. All experimental protocols described in this study were approved by the Institutional Animal Ethics Committee, JNTBGRI and were in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA), Government of India (B-01/03/2015/EM-EP/05).
Acute oral toxicity study
The acute oral toxic effect of NCEF was studied in Swiss albino mice (Lorke, 1983). Animals (54) of both sexes were randomized into 9 groups of 6 each (Group A to Group I). Before starting the experiment, all groups were fasted for 12 h and weighed. The NCEF was freshly prepared by suspending with 1.5% v/v Tween-80 in distilled water. 0.5 mL of the vehicle and test materials were administered orally via gavage No. 16 to Group B to I (NCEF at 50, 100, 200, 400, 800, 1600, 3200, 6400 mg/kg b.w. respectively) and Group A, vehicle control, was given 0.5 mL 1.5% v/v Tween-80 in distilled water. Food and drinking water were provided to the mice approximately an hour after treatment. The animals were observed 30 min after dosing, followed by hourly observation for 8 h and once a day for the next 14 days. Cage side observations were systematically recorded for each animal on day 1, 7, 14 and visual observations for mortality, behavioral pattern, body weight, changes in physical appearance, injury, pain and signs of illness were examined daily.
Sub-chronic oral toxicity study
The sub-chronic oral toxicity studies were conducted according to OECD Guidelines No. 408 (OECD, 1998). Wistar rats (48) were randomized into 4 groups and received 1 mL of the test materials orally once a day consecutively for 90 days. Group A (8 rats/sex) served as vehicle-control and they were given 1.5% v/v Tween-80 in distilled water. Group B and C (4 rats/group/sex) received NCEF at 50 and 500 mg/kg b. w. respectively. Group D (8 rats/sex) received NCEF at 1000 mg/kg b.w. After 90 days of treatment, 4 animals of each sex from group A and D were assigned as satellite groups and kept for further observation after the treatment for a period of 28 days, for any reversibility or persistence of toxic effects.
Mortality checks and behavioral observations
All the animals were observed every day in the morning for mortality and signs of morbidity. Any changes in the skin, fur, subcutaneous swellings, eyes, mucous membranes, excretions, autonomic activity, changes in gait, posture and response to handling as well as bizarre behavior were noted during the entire period of the study.
Food intake and water consumption
The quantities of supplied and remaining food and water were measured daily and average weekly consumption was calculated.
Body weight and relative organ weight
The body weight of all animals was recorded before starting oral administration and continued once a week up to the day of sacrifice. Based on the body weight, the quantities of NCEF were calculated again to ensure administration of fixed dose. The overnight fasted animals were sacrificed on the 91st day and 119th day (satellite group) for dissecting internal organs such as liver, kidneys, lungs, spleen, heart, brain, ovaries and testes. The relative organ weight of each animal was then calculated by the following formula:
Relative organ weight = [absolute organ weight (g)/body weight of rat on sacrifice day (g)] × 100
Hematological analysis
The blood samples were collected from overnight fasted animals through cardiac puncture on day 91 and day 119. One part was collected into ethylenediaminetetraacetic acid (EDTA)-coated vials and analysis such as white blood cell count (WBC), 2.9 to 15.3×103/µL; red blood cell count (RBC), 5.60 to 7.89×106/µL; hemoglobin (HGB) concentration, 12 to 15 g/dL; hematocrit (HCT), 36 to 46%; mean corpuscular volume (MCV), 53.0 to 68.8 fL; mean corpuscular hemoglobin (MCH), 16.0 to 23.1 pg; mean corpuscular hemoglobin concentration (MCHC), 30.0 to 34.1 g/dL; red blood cell distribution width (RDW), 11.0 to 15.5%; platelet count (PLT), 100 to 1610×103/µL; mean platelet volume (MPV), 3.8 to 6.2 fL; platelet distribution width (PDW); plateletcrit (PCT); lymphocyte count (LYM), 2.6 to 13.5×103/µL; monocyte count (MONO), 0.0 to 0.5×103/µL; granulocyte count (GRAN), 0.4 to 3.2×103/µL; LYM%, 63.7 to 90.1%; MONO%, 1.5 to 4.5% and GRAN%, 7.3 to 30.1% were performed by auto hematology analyzer (BC-2800Vet, Mindray, Shenzhen, China).
Biochemical analysis
The second part of blood was collected into plain vials and allowed to coagulate for 1 h at room temperature followed by centrifugation at 3 000×g for 15 min at 37 °C (R8C Remi, Laboratory centrifuge, Remi Industries Pvt. Ltd, India). The serum was separated, transferred into small test tubes for biochemical analysis. Liver function enzymes such as alanine transaminase (ALT; EC 2.6.1.2), aspartate transaminase (AST; EC 2.6.1.1), alkaline phosphatase (ALP; EC 3.1.3.1), γ-glutamyltransferase (GGT; EC 2.3.2.2) and L-lactate dehydrogenase (LDH; EC 1.1.1.27) were determined. Total serum protein (TSP), albumin (ALB), total bilirubin (TBIL) and glucose (GLU) were also assessed. Creatine kinase (CK, EC 2.7.3.2), creatinine (CRE), urea nitrogen (BUN), uric acid (UA) concentrations were determined to evaluate kidney function. The concentrations of serum triglyceride (TG) and total cholesterol (TC) were also determined to give an indication of the influence of any adverse effect of NCEF on the lipid profile. Serum electrolyte parameters such as calcium (Ca2+), sodium (Na+); potassium (K+) and chlorine (Cl–) were determined. All the above parameters were evaluated by using the commercial kits.
Hepatic oxidative stress analysis
Fresh liver samples of all groups were parted into two. Each portion was weighted and homogenized separately using a Potter-Elvehjem tissue homogenizer (Universal motor, RQ 127A, Remi Motors Ltd, India). One portion (10% w/v) was homogenized in 50 mM, pH 7.4 (ION 2700, pH meter 2113190, Eutech Instruments, Singapore) phosphate buffer saline (PBS). The tissue suspensions were centrifuged at 6 000×g for 15 min at 3 °C (LAG 412, Remi cooling centrifuge, Remi Industries Pvt. Ltd, India) to remove the cell debris, unbroken cells, nuclei and erythrocytes (Gonzalez-Flecha et al., 1993). The pellet was discarded and the supernatant was used to assess the following oxidative stress markers. Determination of total hepatic protein (THP; mg/g wet tissue) was done by the modified Lowry method using bovine serum albumin (BSA) as standard (Hartree, 1972). Catalase determination (CAT; EC 1.11.1.6; U/mg protein) was performeded by spectrophotometric method (Sinha, 1972). One unit of enzyme activity is defined as the amount of catalase which catalyzed the oxidation of 1 μmol H2O2 per min per mg protein under assay conditions. Determination of reduced glutathione (GSH; µmol/g wet tissue) was performed by the reduction of 5, 5’dithio-bis (2-nitrobenzoic acid) (DTNB) method (Tietze, 1969). Superoxide dismutase (SOD; EC 1.15.1.1; U/mg protein) was determined by the inhibition of reduced nicotinamide adenine dinucleotide (NADH)-dependent-nitroblue tetrazolium (NBT) reduction method (Kakkar et al., 1984) using a spectrophotometer at 560 nm (G9821A, Cary 100 UV-Vis Spectrophotometer, Agilent Technologies, United States). The second portion (10% w/v) was homogenized with icecold 150 mM KCl-Tris-HCl buffer, (pH 7.2) for the determination of malondialdehyde (MDA; nmol/g wet tissue) during lipid peroxidation using the thiobarbituric acid method (Ohkawa et al., 1979) and glutathione peroxidase (GPx; EC 1.11.1.9; U/mg protein) was determined by the method of Pinto & Bartley (1969) in which GPx was found as a result of reaction between glutathione and H2O2.
Urine analysis
At the 91st and 119th day, 24 h prior to euthanasia, the animals were housed separately in metabolic cages for urine collection. The samples were observed for color, transparency, odor and turbidity. The volume of collected urine from all animals was recorded, centrifuged at 3 000×g for 10 min at 4°C; supernatant was collected for the estimation of glucose (GLU); bilirubin (BIL); ketone (KET); specific gravity (SG); blood (BLO); pH; protein (PRO); urobilinogen (URO); nitrite (NIT); leukocytes (LEU) by test strips (DIRUI A10) and the sediments were analyzed microscopically for blood cells, casts, crystals, microorganisms and squamous cells.
Histopathological examination
Organs (liver, kidney, lung, spleen, heart, brain, ovary and testis) from different groups were fixed in neutral buffered 10% v/v formalin solution for histopathological assessment. After fixation, the organs were dehydrated in graded alcohol (TP1020, semi-enclosed benchtop tissue processor, Leica Biosystems, Germany), embedded in paraffin (EG1130 and EG1150, cold plate for cooling embedding molds and paraffin blocks and modular tissue embedding center, Leica Biosystems, Germany), sectioned into 4 µm thick (RM2255, fully automated rotary microtome, Leica Biosystems, Germany) and stained with Weigert’s Hematoxylin and Eosin (Suvarna et al., 2012). Visualization and microphotographs were captured under a light microscope (Axiostar plus 1169-149, Carl Zeiss, Germany) at magnification 10×.
Statistical analysis
All the data were expressed as mean ± standard error of the mean. The comparisons between normal control and different dose levels of drug samples treated groups were performed by two-way analysis of variance (ANOVA) followed by Dunnett’s test using GraphPAD Prism 7.01 program (Trial-version) for Windows 10 (GraphPAD, San Diego, California, USA). The p<0.05 was considered statistically significant.
Results
Qualitative phytochemical screening
The qualitative study for the phytochemical analysis of the leaf extract of N. calycinus (NCEF) showed the presence of carbohydrates, proteins, alkaloids, steroids, phenolic compounds and glycosides, while saponins, anthraquinones, gums, mucilages and volatile oils were not found (Table 1).
Table 1.
Qualitative phytochemical analysis of Neurocalyx calycinus leaves ethanolic fraction.
| Phytoconstituents | NCEF | |
|---|---|---|
| Carbohydrates | Molish’s test | + + + |
| Fehling’s test | + + | |
| Barfoed’s test | + + | |
| Benedict’s test | + | |
| Proteins and amino acids | Millon’s test | + |
| Biuret test | + | |
| Ninhydrin test | + | |
| Alkaloids | Mayer’s reagent | + |
| Wagner’s reagent | + + + | |
| Hager’s reagent | + + + | |
| Dragendorff’s reagent | + | |
| Glycosides | Borntrager’s test | – |
| Legal’s test | – | |
| Keller – Kiliani test | + + + | |
| Kedde test | + + | |
| Phenolic compounds | Ferric chloride test | + + + |
| Gelatin test | + | |
| Lead acetate test | + + | |
| Alkaline reagent test | + + + | |
| Shinoda’s test | + | |
| Phytosterols | Libermann Burchard | – |
| Salkowski reaction | + | |
| Fixed oils and fats | Spot test | – |
| Saponification test | – | |
| Saponins | Foam test | – |
| Gum and mucilage | Alcohol 95% test | – |
| Volatile oils | Steam distillation | – |
| Iridoids | Trim- Hill reagent test | + + + |
| Anthraquinones | Chloroform - 10% ammonia test | – |
+ = slightly present, + + = moderately present, + + + = highly present, – = absent. All the tests were carried out three times. Observations were based on the color intensity and precipitation with appropriate reagents.
Acute oral toxicity study
The control and NCEF treated groups did not show any behavioral changes and mortality during the 14-day post-treatment period after single oral administration. The body weight of the NCEF treated animal group of both sexes exhibited statistically no significant (p<0.05) differences when compared with the control group (Table 2). Hence the median lethal dose (LD50) of NCEF was greater than 6 400 mg/kg b.w.
Table 2.
Effect of Neurocalyx calycinus leaves ethanolic fraction on body weight and behavioral symptoms in acute oral toxicity study in Swiss albino mice.
| Female | Male | ||||||
|---|---|---|---|---|---|---|---|
| 1st day | 7th day | 14th day | 1st day | 7th day | 14th day | ||
| Control | 21.14±0.11 | 21.71±0.17 | 22.47±0.24 | 24.51±0.21 | 25.26±0.36 | 26.10±0.34 | None |
| 50 mg/kg | 20.88±0.29ns | 21.42±0.24ns | 22.14±0.23ns | 25.01±0.34ns | 25.76±0.41ns | 26.60±0.23ns | None |
| 100 mg/kg | 22.17±0.19ns | 22.76±0.27ns | 23.51±0.23ns | 26.56±0.39ns | 27.29±0.42ns | 28.12±0.27ns | None |
| 200 mg/kg | 20.84±0.25ns | 21.51±0.18ns | 22.35±0.23ns | 25.40±0.24ns | 26.08±0.22ns | 26.91±0.28ns | None |
| 400 mg/kg | 21.92±0.31ns | 22.57±0.24ns | 23.45±0.29ns | 25.42±0.23ns | 26.07±0.29ns | 26.95±0.32ns | None |
| 800 mg/kg | 22.94±0.27ns | 23.61±0.32ns | 24.42±0.29ns | 26.56±0.32ns | 27.29±0.37ns | 28.12±0.35ns | None |
| 1600 mg/kg | 21.42±0.39ns | 22.03±0.27ns | 22.81±0.33ns | 23.90±0.13ns | 24.56±0.19ns | 25.36±0.21ns | None |
| 3200 mg/kg | 22.96±0.19ns | 23.64±0.24ns | 24.42±0.25ns | 25.40±0.35ns | 26.08±0.31ns | 26.91±0.27ns | None |
| 6400 mg/kg | 20.17±0.11ns | 20.72±0.15ns | 21.43±0.20ns | 23.90±0.15ns | 24.56±0.24ns | 25.37±0.17ns | None |
Each value in the table is expressed as Mean ± SEM, n=3 ns = no significant differences (p<0.05) were observed using two-way ANOVA, followed by Dunnett’s multiple comparison test.
Sub-chronic oral toxicity
Behavioral observation and mortality
The control and NCEF treated animal groups showed neither mortality and nor clinical signs of toxicity in their skin, fur, eyes, mucous membranes, gait, secretions, excretions, autonomic activity, posture handling, clonic or tonic movements, stereotypes and bizarre behavior during the 90 days of repeated oral administration (dosage: 50, 500, 1000 mg/kg/b.w.) followed by 28 days of recovery period.
Food and water consumption
No changes were noted in the consumption of food and water for the animal groups treated with NCEF orally for 90 consecutive days. Hence the water intake and food consumption (Figure 1) of NCEF treated animal groups of both sexes showed statistically no significant (p<0.05) differences when compared with the control group.
Figure 1.
Effect of ethanolic fraction of Neurocalyx calycinus leaves on (A) water intake and (B) food intake in sub-chronic oral toxicity study in Wistar rats. The values are expressed as Mean ± SEM, n=4.
Body weight and relative organ weight
During the 90 days of oral administration of NCEF, both male and female rats in either group did not show statistically significant differences when compared with the control group (Figure 2). The NCEF treated animal groups showed no significant variations (p<0.05) in relative organ weights as compared to control animals in both sexes (Table 3).
Figure 2.
Effect of ethanolic fraction of Neurocalyx calycinus leaves on body weight in sub-chronic oral toxicity study in Wistar rats. The values are expressed as Mean ± SEM, n=4. Male and female rats in all the groups did not show any statistically significant differences when compared with the control group by using two-way ANOVA followed by Dunnett’s multiple comparison test.
Table 3.
Effect of Neurocalyx calycinus leaves ethanolic fraction on relative organ weights in sub-chronic oral toxicity study in Wistar rats.
| #Organs | Treatment group | Satellite group | ||||
|---|---|---|---|---|---|---|
| Control | NCEF 50 mg/kg | NCEF 500 mg/kg | NCEF 1000 mg/kg | Control | NCEF 1000 mg/kg | |
| Female | ||||||
| Liver | 3.14 ± 0.13 | 3.05 ± 0.08ns | 2.96 ± 0.12* | 2.93 ± 0.15** | 3.02 ± 0.09 | 3.03 ± 0.14ns |
| Kidney (L) | 0.33 ± 0.01 | 0.32 ± 0.04ns | 0.32 ± 0.02ns | 0.34 ± 0.02ns | 0.32 ± 0.02 | 0.32 ± 0.02ns |
| Kidney (R) | 0.34 ± 0.02 | 0.29 ± 0.02ns | 0.32 ± 0.03ns | 0.35 ± 0.02ns | 0.31 ± 0.01 | 0.34 ± 0.01ns |
| Lungs | 0.55 ± 0.01 | 0.59 ± 0.02ns | 0.63 ± 0.06ns | 0.55 ± 0.04ns | 0.55 ± 0.02 | 0.54 ± 0.01ns |
| Spleen | 0.29 ± 0.04 | 0.32 ± 0.05ns | 0.37 ± 0.01ns | 0.31 ± 0.01ns | 0.35 ± 0.04 | 0.32 ± 0.04ns |
| Heart | 0.36 ± 0.01 | 0.34 ± 0.01ns | 0.36 ± 0.02ns | 0.34 ± 0.02ns | 0.33 ± 0.02 | 0.33 ± 0.01ns |
| Brain | 0.49 ± 0.01 | 0.55 ± 0.03ns | 0.53 ± 0.03ns | 0.52 ± 0.03ns | 0.4 ± 0.01 | 0.51 ± 0.03ns |
| Ovary (L) | 0.02 ± 0.01 | 0.02 ± 0.01ns | 0.02 ± 0.01ns | 0.02 ± 0.01ns | 0.02 ± 0.01 | 0.02 ± 0.01ns |
| Ovary (R) | 0.02 ± 0.01 | 0.02 ± 0.01ns | 0.02 ± 0.01ns | 0.02 ± 0.01ns | 0.02 ± 0.01 | 0.02 ± 0.01ns |
| Male | ||||||
| Liver | 2.86 ± 0.05 | 2.91 ± 0.16ns | 2.89 ± 0.11ns | 2.67 ± 0.08** | 2.82 ± 0.03 | 2.84 ± 0.14ns |
| Kidney (L) | 0.31 ± 0.02 | 0.30 ± 0.01ns | 0.32 ± 0.01ns | 0.32 ± 0.02ns | 0.30 ± 0.02 | 0.30 ± 0.01ns |
| Kidney (R) | 0.31 ± 0.02 | 0.32 ± 0.01ns | 0.33 ± 0.01ns | 0.35 ± 0.02ns | 0.32 ± 0.01 | 0.31 ± 0.02ns |
| Lungs | 0.50 ± 0.01 | 0.51 ± 0.02ns | 0.52 ± 0.02ns | 0.48 ± 0.01ns | 0.54 ± 0.05 | 0.47 ± 0.01ns |
| Spleen | 0.28 ± 0.02 | 0.29 ± 0.03ns | 0.23 ± 0.03ns | 0.29 ± 0.04ns | 0.27 ± 0.04 | 0.26 ± 0.03ns |
| Heart | 0.35 ± 0.02 | 0.33 ± 0.02ns | 0.34 ± 0.01ns | 0.36 ± 0.01ns | 0.33 ± 0.01 | 0.34 ± 0.01ns |
| Brain | 0.49 ± 0.03 | 0.49 ± 0.04ns | 0.46 ± 0.01ns | 0.46 ± 0.01ns | 0.44 ± 0.03 | 0.42 ± 0.02ns |
| Testis (L) | 0.35 ± 0.01 | 0.34 ± 0.01ns | 0.35 ± 0.02ns | 0.36 ± 0.01ns | 0.33 ± 0.01 | 0.34 ± 0.01ns |
| Testis (R) | 0.35 ± 0.01 | 0.35 ± 0.01ns | 0.34 ± 0.01ns | 0.35 ± 0.01ns | 0.32 ± 0.01 | 0.34 ± 0.01ns |
The values are expressed as Mean ± SEM, n=4. #g/100 g body weight. ns = non-significant, *p<0.05, **p<0.01 significantly different from the control group by using two-way ANOVA followed by Dunnett’s multiple comparison test. L, left; R, right.
Hematological analysis
There were no significant (p<0.05) variations in hematological parameters in the groups treated with NCEF compared to control animals of each sex (Table 4). However, the platelet count of NCEF treated animals at doses 50, 500, 1000 mg/kg/b.w showed in both sexes statistically significant (p<0.0001) increases in dose-dependent manner when compared with the control animals during the 90 days of repeated oral administration followed by 28 days of recovery period.
Table 4.
Effect of Neurocalyx calycinus ethanolic fraction of leaves on hematological parameters in sub-chronic oral toxicity study in Wistar rats.
| Parameters | Treatment group | Satellite group | ||||
|---|---|---|---|---|---|---|
| Control | NCEF 50 mg/kg | NCEF 500 mg/kg | NCEF 1000 mg/kg | Control | NCEF 1000 mg/kg | |
| Female | ||||||
| WBC (×103/µL) | 11.88±0.96 | 10.98±0.50ns | 11.23±0.73ns | 11.45±1.08ns | 10.58±0.85 | 10.48±0.77ns |
| RBC (×106/µL) | 7.60±0.42 | 6.71±0.56ns | 5.96±0.78ns | 6.71±0.89ns | 6.16±0.53 | 7.10±0.50ns |
| HGB (g/dL) | 14.11±0.23 | 13.78±0.35ns | 12.35±0.40ns | 13.88±0.34ns | 13.76±0.36 | 13.20±0.43ns |
| HCT (%) | 40.10±2.00 | 36.48±3.15ns | 31.52±4.11ns | 35.77±4.90ns | 31.96±2.68 | 37.16±2.89ns |
| MCV (fL) | 52.82±0.31 | 54.35±0.39ns | 52.91±0.32ns | 53.22±0.24ns | 51.91±0.25 | 52.25±0.43ns |
| MCH (pg) | 18.74±1.13 | 20.86±1.28ns | 21.71±2.51ns | 21.97±3.22ns | 22.71±1.49 | 19.01±2.02ns |
| MCHC (g/dL) | 35.92±1.69 | 42.46±5.15ns | 37.04±6.78ns | 47.62±6.28ns | 38.99±1.98 | 39.37±4.55ns |
| RDW (%) | 13.55±1.05 | 13.15±1.64ns | 15.24±1.42ns | 14.28±1.23ns | 15.60±0.93 | 13.80±0.96ns |
| PLT (×103/µL) | 1088.00±24.62 | 1125.50±32.95ns | 1194.25±37.12**** | 1227.00±34.07**** | 1069.75±42.29 | 1209.75±39.22†††† |
| MPV (fL) | 5.05±0.18 | 4.78±0.13ns | 4.49±0.14ns | 4.20±0.04ns | 5.55±0.12 | 4.65±0.12ns |
| PDW | 16.45±0.80 | 17.05±0.50ns | 15.90±0.65ns | 16.75±0.35ns | 16.73±0.27 | 16.25±0.36ns |
| PCT (%) | 0.55±0.01 | 0.64±0.01ns | 0.68±0.02ns | 0.65±0.02ns | 0.59±0.02 | 0.68±0.01ns |
| LYM (×103/µL) | 7.63±0.85 | 7.10±0.58ns | 6.88±0.60ns | 7.68±0.67ns | 6.83±0.63 | 6.63±0.73ns |
| MONO (×103/µL) | 0.45±0.06 | 0.40±0.04ns | 0.35±0.03ns | 0.28±0.05ns | 0.30±0.04 | 0.30±0.04ns |
| GRAN (×103/µL) | 3.80±0.24 | 3.48±0.36ns | 4.00±0.38ns | 3.50±0.53ns | 3.45±0.29 | 3.55±0.36ns |
| LYM% | 63.78±2.48 | 64.58±3.82ns | 61.13±3.25ns | 67.23±2.31ns | 64.37±1.22 | 62.93±3.91ns |
| MONO% | 3.88±0.61 | 3.65±0.38ns | 3.11±0.14ns | 2.48±0.49ns | 2.99±0.64 | 2.89±0.41ns |
| GRAN% | 32.34±2.03 | 31.77±3.47ns | 35.75±3.20ns | 30.29±2.70ns | 32.64±1.12 | 34.17±3.51ns |
| Male | ||||||
| WBC (×103/µL) | 12.20 ± 0.79 | 12.18 ± 0.95ns | 11.48±0.40ns | 10.78±0.98ns | 10.93±0.99 | 11.05±0.37ns |
| RBC (×106/µL) | 7.85 ± 0.21 | 7.88 ± 0.49ns | 7.21±0.47ns | 6.96±0.56ns | 7.16±0.66 | 7.10±0.50ns |
| HGB (g/dL) | 13.86 ± 0.38 | 13.53 ± 0.23ns | 12.60±0.23ns | 13.63±0.19ns | 13.26±0.26 | 12.95±0.56ns |
| HCT (%) | 44.38 ± 0.76 | 46.08 ± 3.34ns | 41.22±2.74ns | 39.87±2.71ns | 40.19±3.67 | 41.14±2.72ns |
| MCV (fL) | 56.57 ± 0.52 | 58.35 ± 0.59ns | 57.16±0.76ns | 57.47±0.83ns | 56.16±0.43 | 58.00±0.45ns |
| MCH (pg) | 17.68 ± 0.58 | 17.39 ± 1.22ns | 17.76±1.42ns | 19.96±1.56ns | 19.04±1.96 | 18.70±2.20ns |
| MCHC (g/dL) | 31.24 ± 0.90 | 29.87 ± 2.36ns | 31.09±2.59ns | 34.64±2.28ns | 33.92±3.51 | 32.20±3.67ns |
| RDW (%) | 12.55 ± 0.58 | 11.65 ± 0.42ns | 12.99±0.55ns | 13.28±1.10ns | 13.10±0.86 | 13.05±0.67ns |
| PLT (×103/µL) | 1088.50 ± 24.03 | 1110.50 ± 23.26ns | 1206.25±28.14**** | 1244.00±24.78**** | 1069.50±43.49 | 1214.25±18.20†††† |
| MPV (fL) | 4.83 ± 0.18 | 4.65 ± 0.15ns | 4.35±0.12ns | 4.10±0.04ns | 4.95±0.18 | 4.45±0.13ns |
| PDW | 16.20 ± 0.60 | 16.80 ± 0.23ns | 15.65±0.70ns | 16.00±0.75ns | 16.73±0.24 | 16.55±0.55ns |
| PCT (%) | 0.52 ± 0.03 | 0.61 ± 0.01ns | 0.65±0.01ns | 0.62±0.01ns | 0.53±0.04 | 0.63±0.01ns |
| LYM (×103/µL) | 7.83 ± 0.76 | 8.10 ± 0.58ns | 7.78±0.47ns | 7.18±0.79ns | 7.08±0.65 | 7.38±0.48ns |
| MONO (×103/µL) | 0.40 ± 0.07 | 0.38 ± 0.06ns | 0.33±0.05ns | 0.35±0.06ns | 0.35±0.03 | 0.28±0.05ns |
| GRAN (×103/µL) | 3.98 ± 0.14 | 3.70 ± 0.36ns | 3.38±0.29ns | 3.25±0.36ns | 3.50±0.33 | 3.40±0.47ns |
| LYM% | 63.72 ± 2.27 | 66.64 ± 1.14ns | 67.69±2.72ns | 66.36±2.12ns | 64.74±0.79 | 66.86±4.32ns |
| MONO% | 3.35 ± 0.70 | 3.03 ± 0.37ns | 2.84±0.40ns | 3.41±0.86ns | 3.24±0.22 | 2.48±0.40ns |
| GRAN% | 32.93 ± 0.10 | 30.33 ± 1.35ns | 29.47±2.54ns | 30.23±2.55ns | 32.02±0.62 | 30.66±3.92ns |
The values are expressed as Mean ± SEM, n=4. ns = no significant, ****p<0.0001 significantly different from the control group, ††††p<0.0001 significantly different from the satellite control group by using two-way ANOVA followed by Dunnett’s multiple comparison test. WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width; PCT, plateletcrit; LYM, lymphocyte count; MONO, monocyte count; GRAN, granulocyte count. µL, microliter; dL, deciliter; fL, femtoliter; pg, picogram.
Biochemical analysis
Analysis of serum biochemical parameters showed no significant (p<0.05) variations among the groups treated with NCEF and the control of the both sexes (Table 5). However, the LDH and CK levels were found significantly decreased, gradually reaching normal level during the recovery period in both sexes as compared with the normal control group.
Table 5.
Effect of Neurocalyx calycinus leaves ethanolic fraction on serum biochemical parameters in sub-chronic oral toxicity study in Wistar rats.
| Parameters | Treatment group | Satellite group | ||||
|---|---|---|---|---|---|---|
| Control | NCEF 50 mg/kg | NCEF 500 mg/kg | NCEF 1000 mg/kg | Control | NCEF 1000 mg/kg | |
| Female | ||||||
| ALT (U/L) | 35.25±1.65 | 33.25±2.21ns | 30.75±1.75ns | 32.50±1.32ns | 32.25±1.65 | 31.00±2.58ns |
| AST (U/L) | 64.25±2.32 | 65.25±1.89ns | 61.00±2.65ns | 65.00±2.38ns | 65.50±2.75 | 67.25±2.78ns |
| ALP (U/L) | 101.50±3.10 | 105.50±3.30ns | 103.50±2.40ns | 104.75±2.81ns | 106.25±4.09 | 102.00±2.38ns |
| GGT (U/L) | 8.25±1.31 | 6.25±1.11ns | 8.00±1.29ns | 7.75±1.11ns | 9.00±1.47 | 7.00±1.08ns |
| LDH (U/L) | 533.75±21.54 | 504.75±12.07ns | 523.00±41.94ns | 492.75±16.13** | 549.25±30.40 | 522.25±39.64ns |
| TSP (g/L) | 60.25±1.76 | 61.00±1.58ns | 58.50±1.73ns | 62.75±1.99ns | 59.75±1.99 | 61.75±1.70ns |
| ALB (g/L) | 49.75±1.49 | 48.25±1.25ns | 47.25±1.93ns | 46.25±1.55ns | 45.25±1.25 | 47.75±1.84ns |
| TBIL (µmol/L) | 4.90±0.14 | 5.03±0.09ns | 4.80±0.13ns | 4.85±0.16ns | 5.00±0.16 | 4.95±0.14ns |
| CK (U/L) | 360.50±17.49 | 348.75±26.22ns | 380.75±32.02ns | 320.75±24.99** | 387.00±21.30 | 344.05±17.08ns |
| CRE (µmol/L) | 44.90±2.27 | 46.74±3.53ns | 45.93±2.42ns | 47.08±3.58ns | 42.75±3.47 | 44.25±3.33ns |
| BUN (mmol/L) | 14.85±0.59 | 14.28±0.84ns | 13.68±0.39ns | 15.45±0.72ns | 13.53±0.49 | 14.95±0.43ns |
| UA (µmol/L) | 38.50±4.03 | 34.50±2.47ns | 36.00±5.40ns | 42.25±3.35ns | 36.50±4.21 | 39.00±4.55ns |
| GLU (mmol/L) | 6.43±0.13 | 6.28±0.11ns | 6.30±0.18ns | 6.23±0.13ns | 6.18±0.19 | 6.06±0.12ns |
| TC (mmol/L) | 2.06±0.23 | 2.19±0.19ns | 2.34±0.28ns | 1.96±0.12ns | 2.16±0.12 | 2.05±0.12ns |
| TG (mmol/L) | 0.95±0.08 | 0.96±0.10ns | 0.84±0.06ns | 0.94±0.08ns | 0.91±0.06 | 0.95±0.09ns |
| Ca (mmol/L) | 0.89±0.11 | 0.81±0.07ns | 0.88±0.05ns | 0.83±0.07ns | 0.86±0.05 | 0.86±0.11ns |
| Na (mmol/L) | 133.00±3.76 | 138.00±3.34ns | 133.25±5.62ns | 133.75±3.17ns | 137.25±4.52 | 135.75±4.42ns |
| K (mmol/L) | 3.36±0.10 | 3.56±0.21ns | 3.72±0.28ns | 3.61±0.25ns | 3.66±0.19 | 3.77±0.15ns |
| Cl (mmol/L) | 97.30±4.27 | 96.75±4.33ns | 99.00±3.03ns | 98.75±3.57ns | 97.50±3.30 | 102.50±2.02ns |
| Male | ||||||
| ALT (U/L) | 39.75±1.11 | 34.75±0.85ns | 33.50±1.85ns | 36.75±2.02ns | 37.50±1.71 | 35.50±2.72ns |
| AST (U/L) | 61.50±1.71 | 63.25±1.11ns | 62.50±2.02ns | 59.75±3.01ns | 62.25±1.65 | 63.25±3.15ns |
| ALP (U/L) | 96.50±2.50 | 100.50±2.63ns | 95.75±4.37ns | 103.00±3.76ns | 99.75±2.32 | 102.75±2.95ns |
| GGT (U/L) | 11.50±1.44 | 10.25±1.31ns | 10.75±1.25ns | 13.50±1.71ns | 11.75±1.38 | 9.75±1.55ns |
| LDH (U/L) | 708.75±31.87 | 729.50±24.84ns | 730.50±41.56ns | 678.25±49.44* | 723.25±31.30 | 724.50±27.32ns |
| TSP (g/L) | 55.25±1.66 | 54.80±1.87ns | 56.00±1.98ns | 55.50±1.46ns | 55.12±1.21 | 54.50±1.54ns |
| ALB (g/L) | 42.00±1.29 | 41.00±1.22ns | 42.50±1.85ns | 40.25±1.75ns | 41.75±1.38 | 42.25±1.55ns |
| TBIL (µmol/L) | 4.60±0.13 | 4.43±0.23ns | 4.38±0.17ns | 4.40±0.18ns | 4.62±0.13 | 4.48±0.20ns |
| CK (U/L) | 437.25±21.58 | 429.50±12.69ns | 435.25±15.70ns | 399.75±14.10* | 436.50±16.29 | 430.25±24.50ns |
| CRE (µmol/L) | 39.55±2.27 | 42.05±2.80ns | 43.13±3.45ns | 38.43±3.17ns | 40.30±2.49 | 37.55±2.68ns |
| BUN (mmol/L) | 14.03±0.34 | 13.90±0.42ns | 14.38±0.40ns | 14.53±0.39ns | 13.63±0.37 | 14.73±0.89ns |
| UA (µmol/L) | 32.65±2.83 | 35.00±3.11ns | 33.75±3.84ns | 36.50±3.62ns | 36.50±2.99 | 38.50±3.88ns |
| GLU (mmol/L) | 6.40±0.12 | 6.53±0.11ns | 6.60±0.13ns | 6.45±0.10ns | 6.56±0.13 | 6.43±0.15ns |
| TC (mmol/L) | 1.86±0.20 | 1.63±0.18ns | 1.71±0.25ns | 1.83±0.17ns | 1.77±0.19 | 1.80±0.16ns |
| TG (mmol/L) | 1.35±0.18 | 1.28±0.11ns | 1.24±0.10ns | 1.35±0.12ns | 1.33±0.08 | 1.39±0.10ns |
| Ca (mmol/L) | 0.77±0.08 | 0.71±0.06ns | 0.78±0.09ns | 0.77±0.06ns | 0.78±0.04 | 0.72±0.10ns |
| Na (mmol/L) | 131.00±2.80 | 132.75±3.77ns | 135.25±4.96ns | 139.00±3.24ns | 135.50±3.80 | 128.25±4.35ns |
| K (mmol/L) | 3.37±0.11 | 3.59±0.18ns | 3.69±0.27ns | 3.50±0.23ns | 3.90±0.19 | 3.44±0.23ns |
| Cl (mmol/L) | 95.35±3.65 | 89.25±4.33ns | 91.00±2.12ns | 100.00±4.42ns | 97.00±3.42 | 96.00±3.92ns |
The values are expressed as Mean ± SEM, n=4. ns = no significant differences (p<0.05) were observed using two-way ANOVA, followed by Dunnett’s multiple comparison test. *p<0.05, **p<0.01 significantly different from the control group by using two-way ANOVA followed by Dunnett’s multiple comparison test. ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; GGT, γ-glutamyltransferase; LDH, lactate dehydrogenase; TSP, total serum protein; ALB, albumin; TBIL, total bilirubin; GLU, glucose; CK, creatine kinase; CRE, creatinine; BUN, urea nitrogen; UA, uric acid; TG, triglyceride; TC, total cholesterol; Ca, calcium; Na, sodium; K, potassium; Cl, chlorine. U/L, units per litre; g/L, gram per litre; µmol/L, micromoles/litre; mmol/L, millimole per litre.
Hepatic oxidative stress analysis
The hepatic oxidative stress marker index (CAT, GSH, SOD, MDA and GPx) of orally administered NCEF treated groups of female and male Wistar rats is shown in Figure 3. A dose-dependent non-significant increase (p<0.05) in total protein content (Figure 3A) and a dose-dependent decrease in CAT activity were observed in both female and male rats (Figure 3B). However, a significant reduction (p<0.01) was observed in female rats at NCEF 1000 mg/kg when compared to the control group, which was normalized after the recovery period. A dose-dependent increase in GSH activity was found in both female and male rat (Figure 3C). Further, a significant increase was observed in female rats at NCEF 500 mg/kg (p<0.05), 1000 mg/kg (p<0.01) and male rats at NCEF 1000 mg/kg (p<0.05) as compared to the control group, which was seen normalized during the recovery period. A dose-dependent increase in SOD activity was observed in both female and male rats (Figure 3D). However, a significant increase (p<0.05) was observed in female rats at NCEF 1000 mg/kg, as compared to the control group, which became regularized after the recovery period. A dose-dependent non-significant increase (p<0.05) in MDA level was observed in both female and male rats (Figure 3E). A dose-dependent increase in GPx activity was observed in both female and male rats (Figure 3F). A significant increase in GPx was observed in female rats at NCEF 500 mg/kg (p<0.05), 1000 mg/kg (p<0.01) and in male rats at NCEF 1000 mg/kg (p<0.01) as compared to the control group, which was normalized after the recovery period.
Figure 3.
Effect of ethanolic fraction of Neurocalyx calycinus leaves on hepatic biochemical markers for oxidative stress in sub-chronic oral toxicity study of both female and male Wistar rats. (A) TP (total protein) expressed as mg/g (milligram/gram) wet tissue; (B) CAT (catalase activity expressed as U/mg (units/milligram) protein; (C) GSH (reduced glutathione) activity expressed as µmol/g (micromole per gram) wet tissue; (D) SOD (superoxide dismutase) activity expressed as U/mg protein; (E) MDA (malondialdehyde) activity expressed as nmol/g (nanomole/gram) wet tissue; (F) GPx (glutathione peroxidase) activity expressed as U/mg protein). The results are expressed as Mean ± SEM, n=4. ns, no significant differences (p<0.05); *p<0.05, **p<0.01 significantly different from the control group by using two-way ANOVA followed by Dunnett’s multiple comparison test.
Urine analysis
There were no significant changes in the urinary parameters observed in the group treated with NCEF compared to controls of both sexes (Table 6). Urine samples showed yellow color and a characteristic odor. Blood cells, casts, crystals, microorganisms and epithelial cells were not seen in the microscopic examination of urine samples collected from the NCEF treated rats.
Table 6.
Effect of Neurocalyx calycinus ethanolic fraction of leaves on urine analysis in sub-chronic oral toxicity study in Wistar rats.
| Parameters | Treatment group | Satellite group | ||||
|---|---|---|---|---|---|---|
| Control | NCEF 50 mg/kg | NCEF 500 mg/kg | NCEF 1000 mg/kg | Control | NCEF 1000 mg/kg | |
| Female | ||||||
| *Volume (mL/24 h) | 6.84±1.78 | 6.45±2.00ns | 6.01±2.14ns | 5.37±1.04ns | 6.92±1.57 | 6.17±2.64ns |
| GLU | – | – | – | – | – | – |
| BIL | – | – | – | + | – | – |
| KET | – | – | + | + | – | – |
| SG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| BLO | – | – | – | – | – | – |
| PH | 1# | 1# | 1# | 1# | 1# | 1# |
| PRO | ++ | ++ | ++ | +++ | ++ | ++ |
| LEU | t | t | t | t | t | t |
| NIT | – | – | – | – | – | – |
| URO | – | – | – | – | – | – |
| *CRE (µmol/L) | 124.47±9.35 | 105.11±14.58ns | 112.58±17.43ns | 139.84±12.45ns | 127.65±11.26 | 131.72±10.87ns |
| Male | ||||||
| *Volume (mL/24 h) | 5.29±0.98 | 5.86±1.76ns | 6.25±1.07ns | 5.99±2.40ns | 5.07±1.88 | 5.59±2.01ns |
| GLU | – | – | – | – | – | – |
| BIL | – | – | + | + | – | – |
| KET | – | – | – | + | – | – |
| SG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| BLO | – | – | – | – | – | – |
| pH | 1# | 1# | 1# | 1# | 1# | 1# |
| PRO | + | ++ | ++ | +++ | ++ | ++ |
| LEU | t | t | t | t | t | t |
| NIT | – | – | – | – | – | – |
| URO | – | – | – | – | – | – |
| *CRE (µmol/L) | 104.36±8.24 | 111.27±12.35ns | 98.75±16.25ns | 109.77±11.21ns | 96.41±15.64 | 101.89±13.78ns |
*The values are expressed as Mean ± SEM, n=4. ns = no significant differences (p<0.05) were observed using two-way ANOVA, followed by Dunnett’s multiple comparison test. #pH = between 6 and 9. µmol/L, micromoles/litre; GLU, Glucose; BIL, bilirubin; KET, ketone; SG, specific gravity; BLO, blood; PRO, protein; LEU, leukocytes; NIT, nitrite; URO, urobilinogen (URO); CRE, creatinine. + = slightly present, + + = moderately present, + + + = highly present, – = absent, t = trace.
Histopathological examination
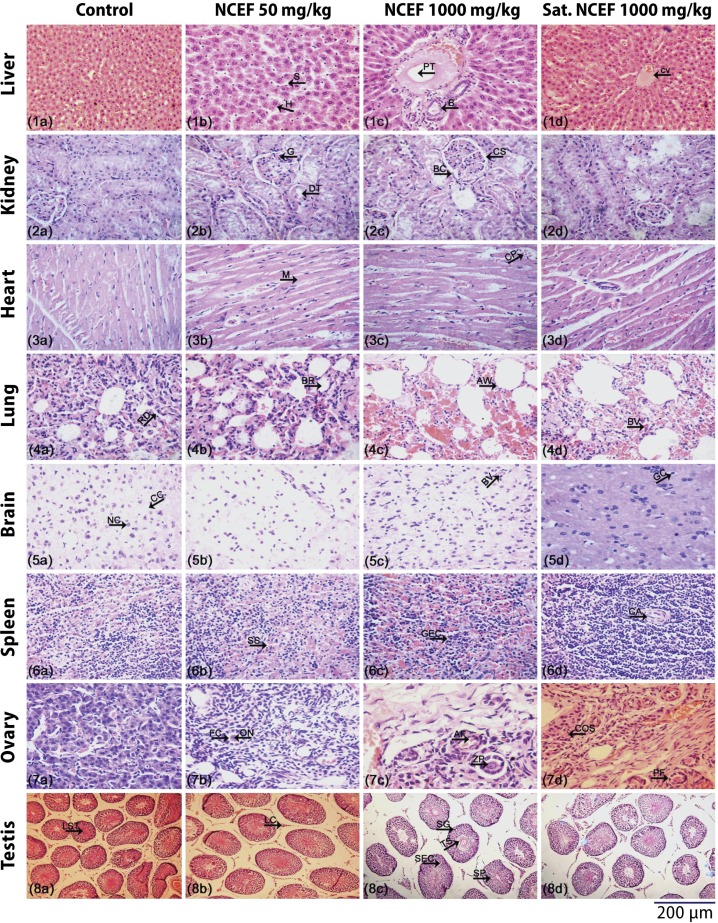
There were no irregularities in the structural integrity of organs observed in the histopathological examination after 90 days of oral administration of NCEF 50, 500 and 1000 mg/kg dose groups. Microphotographs of the liver showed normal sinusoids, portal vein and hepatocytes, which are radially arranged from the central vein. Absence of fatty cells, cytoplasmic vacuolation, necrosis and minimum of Kupffer cell infiltration indicates that NCEF is safe for oral administration (Figures 4.1, a–d). Cross section of the kidney showed normal architecture. Tubular necrosis and lymphocyctic infiltration were absent in the entire NCEF treated groups (Figures 4.2, a–d). The myocardium layer of the heart showed normal cardiac muscle fibers and capillaries. Infiltration of inflammatory cells, fibrosis and ganglionic abnormalities were not detected (Figures 4.3, a–d). Lung tissues showed normal delicate alveolar septa, uniform distribution of alveoli, bronchioles lined by single ciliated epithelium. No septal breakage, alveoli expansion and bronchiolar inflammation were observed (Figures 4.4, a–d). Brain histologic examination showed normal architecture in the cerebral cortex. No necrosis and vacuolar changes were found (Figures 4.5, a–d). Spleen histology showed no differences in the splenic cords, sinusoids and central arteriole (Figures 4.6, a–d). Histology of the ovary showed normal architecture in cortex and medullary region. Primordial follicles, thick-walled blood vessels and rete cells were normally functioning (Figures 4.7, a–d). Normal interstitial tissues, Leydig cells, Sertoli cells, seminiferous tubules, primary spermatocytes are seen in the testis (Figures 4.8, a–d).
Figure 4.
Microphotographs of histopathological evaluation of organs in Wistar rats treated with leaves ethanolic fraction of Neurocalyx calycinus in sub-chronic oral toxicity study. Histological sections of selected groups stained with hematoxylin and eosin a. Control, b. NCEF 50 mg/kg, c. NCEF 1000 mg/kg, d. Satellite 1000 mg/kg. (1a-1d) Liver, (2a-2d) Kidney (right), (3a-3d) Heart, (4a-4d) Lung, (5a-5d) Brain, (6a-6d) Spleen, (7a-7d) Ovary (right) and (8a-8d) Testis (right). S = Sinusoid; H = Hepatocyte; PT = Portal vein; B = Bile duct; CV = Central Vein; G = Glomerulus; DT = Distal tubules; BC = Bowman’s capsule; CS = Capsular space; M = Myofibrils; CP = Capillaries; RD = Respiratory ducts; BR = Bronchioles; AW = Alveolar walls; BV = Blood vessel; CC = Cerebral cortex; NC = Neuronal cells; GC= Glial cell; SS = Splenic sinuses; GEC = Germinal center; CA = Central arteriole; FC = Follicular cavity; ON = Oocyte nuclei; AF = Atretic follicle; ZP = Zona pellucida; COS = Cortical stroma, PF = Primary follicle; LST = Lumen of seminiferous tubule; LC = Leydig cell; SG = Spermatogonium; TS = Tails of spermatozoa; SEC = Sertoli cell; SP = Spermatozoon.
Discussion
The leaves of N. calycinus belonging to the family Rubiaceae were extensively used by the Cholanaickan tribe of Kerala for treating wounds and inflammations. The worldwide acceptance of plant-based medicines is possible only if they fulfil the same efficacy, quality and safety parameters as synthetic products (Rates, 2001). Based on these assumptions, our present study focuses on acute and 90-day sub-chronic toxicity studies for understanding the impacts of long-term consumption of N. calycinus leaves as medicine.
In the present study, different solvent extracts of leaves of N. calycinus were checked for various in vivo and in vitro experiments. The ethanolic fraction showed the most promising activity. Preliminary phytochemical screening of NCEF of leaves showed the presence of major phytocompounds like alkaloids, glycosides, iridoids and phenolic compounds like coumarins, flavonoids and tannins. The presence of these compounds depends upon the environmental conditions, the plant part and the extraction solvents used for the study. The presence of phenols is considered to be potentially toxic to the growth and development of pathogens. Although anthraquinones, iridoids and alkaloids are the secondary metabolites in Rubioideae (Martins & Nunez, 2015), anthraquinones were absent in NCEF.
Toxicity studies are the essential criteria for pharmacological evaluation and standardization of unknown substances. Acute oral toxicity study of NCEF showed no mortality even at its highest dose level (6400 mg/kg b.w.). Acute toxicity studies provide information about the therapeutic index and fix the absolute dose for pharmacological tests in rodents (Chinedu et al., 2013). The chemical labeling and classification of acute toxicity based on oral LD50 values recommended by the OECD guideline 423 (OECD, 2002) also showed LD50 value for ethanolic fraction of N. calycinus leaves to be greater than 5000 mg/kg body weight, thus coming under category 5. Body weight is one of the most sensitive indicators of the condition of an animal if it is monitored frequently and carefully during the study. In the toxicity studies no difference in body weight was found at any of the doses throughout the experiment. Body weight is measured at least once a week during toxicity studies. A treatment dose which causes more than 10% reduction in body weight is considered to be toxic (Kushwaha et al., 2013). Our results confirmed that the extract did not possess any acute oral toxicity in mice.
Based on the acute toxicity data, a lower (50 mg/kg bw), middle (500 mg/kg bw) and higher (1000 mg/kg bw) dose of the NCEF were selected for sub-chronic oral toxicity study in both sexes of Wistar rats. In the sub-chronic oral toxicity study, NCEF did not induce any signs of morbidity and mortality, and that not even in the group treated with the highest dose (1000 mg/kg bw) during the 90 days of treatment and 28 days of recovery period.
Water intake, food consumption, body weight and relative organ weight are interrelated and are the primary indicators of adverse effects of a toxic substance in rodents (Speakman & McQueenie, 1996; Shokryazdan et al., 2016). Any variation in food consumption and water intake will directly affect the normal metabolism of animals (Mukinda & Syce, 2007). Any changes in body weight can be used as a rapid assessment for side effects of a drug (Teo et al., 2002). In the present study, female and male rats of all the NCEF treated groups showed a regular intake of water and food similar to the control rats. Increase in body weight of NCEF treated animals was not significantly different from the control group. This general increase may be due to the quality of food and water. These results confirmed that NCEF did not affect growth retardation and appetite of rats. The mean relative organ weight of kidney, lungs, spleen, heart, brain, testis and ovary did not show any significant differences when compared to the control group. However, the liver of both female and male rats showed a significant decrease in weight at the highest dose of NCEF (1000 mg/kg b.w.). Understanding the organ to body weight ratio on studying herbal products is mandated to identify the target organs, mechanism of action and toxicokinetics (Bailey et al., 2004; Sellers et al., 2007; Adewale et al., 2016). Based on these findings, NCEF is safe and non-toxic to animals for long-term oral administration.
Blood cells, the most sensitive connective tissue in mammals, responsible for the transport of nutrients and foreign bodies, are the target areas of toxic substances. Any damage in these cells will directly indicate the physiological condition, inflammations and infections of the animals (Petterino & Argentino-Storino, 2006; Ezeja et al., 2014). No significant variations were observed in any of the blood parameters of NCEF treated groups and control groups in both sexes, except in the platelet count. A significant dose-dependent increase in platelet count of both female and male rats was observed during the 90 days of treatment , and even after the recovery period. The increases in platelet count might be an indication of the platelet augmentation property of N. calycinus. Further studies are required to scientifically validate the platelet augmentation effect of the plant. Increased platelet count plays a significant role in hemostasis (Anitua et al., 2004) and also the release of potent growth factors for repairing various wounds (Knighton et al., 1986).
The liver is the vital organ for drug biotransformation. Abnormalities present in the hepatic cells will affect the normal detoxification mechanism of animals. A wide variety of toxic substances is extensively biotransformed into metabolites with markedly different toxicological properties. Such biotransformation takes variable times in different tissues and different species, convolving the cellular toxicological effects (Nicholson et al., 2002). Serum biochemical studies will provide a significant inference about the nature of toxic effects on the liver (Wolf, 1999). Oral administration of NCEF did not cause any significant changes in the serum enzyme levels of ALT, AST, ALP and GGT as compared to control rats of both sexes. However, LDH level was significantly reduced in both female and male rats at the highest dose (NCEF 1000 mg/kg bw). The standard level of the LDH was regained after 28 days of the recovery period. An increase in the level of serum markers will give information about the nature and type of chronic liver damage (Sheweita et al., 2001; Ramaiah, 2007). Among these parameters, ALT enzymatic activity is highly sensitive (Ozer et al., 2008). However, NCEF oral consumption did not elevate the enzyme level in all dose groups. Other serum parameters such as TSP, ALB and TBIL showed no significant changes as compared to normal rats of both sexes, indicating that NCEF did not cause any adverse effects in animals. Hepatotoxic substances change the standard level of serum enzymes (ALT, AST, ALP, GGT and LDH), which are vital diagnostic parameters in liver disease and cell injury (Galle et al., 1990; Sutcu et al., 2006). In the present study, no variations were observed in the CRE, BUN, UA and electrolyte levels such as Na, K, Cl and Ca. The CRE levels were also found to be normal during the recovery period of 28 days. These serum biochemical markers are prominent indicators of kidney function and any fluctuations in the normal level of these parameters directly reflect the metabolism (Vasan, 2006). Our study suggests that NCEF has no adverse effect on hepatic and renal cells, indicating its non-toxicity. There were no significant changes observed in the GLU, TC and TG levels of NCEF treated rats compared to control rats. The non-significant difference of hepatic and renal serum marker levels (Hilaly et al., 2004) between the control and treated groups suggests that repeated daily oral consumption of NCEF did not alter the normal biologic process of animals. Furthermore, detailed molecular profiling like genomics, proteomics, and metabolomics analysis (Hellmold et al., 2002) is desirable to understand the effect of oral consumption of NCEF on whole-organism functional integrity overtime after drug exposure.
The liver plays a critical role in the maintenance of body homoeostasis. The imbalance between the excess formation of pro-oxidants and the antioxidant defence mechanism of a cell leads to oxidative stress, which causes tissue damage and cell death (Sanders, 2001; Mates et al., 2008; Weydert & Cullen, 2009). No significant difference was observed in the activity of antioxidant enzymes (CAT, GPx and SOD) together with GSH and the level of oxidation product MDA in any of the groups studied. SOD helps the endogenous antioxidant defence system to scavenge radicals and maintain cellular redox balance. GPx catalyzes the reduction of H2O2 and other peroxides. GSH protects cells against lipid peroxidation (Yao et al., 2007). In the present study, we confirmed that repeated 90-day oral consumption of NCEF did not induce any oxidative stress and liver toxicity and normal hepatic non-enzymatic and enzymatic antioxidant status was maintained in rats.
Kidney is one of the primary targets of toxic substances. Urinary parameters, especially BUN and CRE, assist in the detection of renal failure (Hoffmann et al., 2010). Physicochemical and microscopical urine test will give vital information about the abnormalities in renal and urinary tract diseases. But, there was no significant difference in the urinary parameters between the NCEF treated and control animals. However, in the animals of either sex treated with highest dose, the urine color changed to pale yellowish. The microscopic examination of urine sediments did not find blood cells, casts, crystals, squamous cells and microorganisms. A negative nitrite test confirmed the absence of microorganisms.
The vital organs are extremely sensitive to toxic elements, and the resulting hypertrophy of these organs provides a direct indication of toxicity which may be useful in predicting toxicity level, enzyme induction, physiologic perturbations and acute injury (Michael et al., 2007). Histopathological examination of the liver, kidneys, lungs, spleen, heart, brain, ovaries and testes after repeated 90-day oral consumption of NCEF did not show any anatomical disorder and detrimental changes at any dose level compared with normal rats. No signs of inflammations, lesions, color changes, texture and hypertrophy confirmed the non-toxic nature of NCEF. In our biochemical observations slight variations of normal parameters had no effect on the routine function of vital organs, which was corroborated by histological findings. These findings will help to fix dose-dependent safety margins for using NCEF as a drug and its role in targeted visceral organs (Kramer et al., 2007).
Conclusion
The sub-chronic toxicity effect of ethanolic fraction of leaves of Neurocalyx calycinus will be a stepping stone for various preclinical and clinical pharmacological studies in future. The acute toxicity study at NCEF 6400 mg/kg/b.w. did not cause any mortality and adverse effects. Repeated oral consumption of NCEF for 90 days among male and female Wistar rats did not show any deleterious changes in food and water consumption, body weight, organ weight, hematological, biochemical, oxidative stress and histopathological parameters. Therefore, based on these findings, it is confirmed for the first time that prolonged oral consumption of NCEF is safe, non-toxic and also not adversely affecting the normal physiological functions of the animal. Our present findings also facilitate the future step for the development of a potent drug candidate in nearby future from the leaves of N. calycinus.
Acknowledgements
The authors are thankful to the Director, JNTBGRI for providing required facilities and Kerala State Council for Science, Technology and Environment (KSCSTE) for financial assistance.
REFERENCES
- Adewale OB, Onasanya A, Anadozie SO, Abu MF, Akintan IA, Ogbole CJ, Olayide II, Afolabi OB, Jaiyesimi KF, Ajiboye BO, Fadaka AO. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J Ethnopharmacol. 2016;188:153–158. doi: 10.1016/j.jep.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Aneesh TP, Hisham M, Sekhar S, Madhu M, Deepa TV. International market scenario of traditional Indian herbal drugs – India declining. Int J green pharm. 2009;3:184. [Google Scholar]
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: What is the best analytical Endpoint? Toxicol Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- Bremer B. The genus Neurocalyx Rubiaceae– Argostemmateae) in Ceylon. Bot Not. 1979;132:399–407. [Google Scholar]
- Bremer B. Genus Neurocalyx. In: Dassanayake MD, Fosberg FR, editors. A revised handbook to the flora of Ceylon. New Delhi: Oxford and IBH publishing Co. Pvt. Ltd.; 1987. pp. 322–326. [Google Scholar]
- Chinedu E, Arome D, Ameh FS. A new method for determining acute toxicity in animal models. Toxicol Int. 2013;20:224–226. doi: 10.4103/0971-6580.121674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeja MI, Anaga AO, Asuzu IU. Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J Ethnopharmacol. 2014;151:1155–1164. doi: 10.1016/j.jep.2013.12.034. [DOI] [PubMed] [Google Scholar]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12:486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flecha B, Evelson P, Sterin-Speziale N, Boveris A. Hydrogen peroxide metabolism and oxidative stress in cortical, medullary and papillary zones of rat kidney. Biochim Biophys Acta Gen Subj. 1993;1157:155–161. doi: 10.1016/0304-4165(93)90059-h. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. 3rd ed. New York: Springer-Verlag; 1998. [Google Scholar]
- Hartree EF. Determination of proteIn: A modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hashmi S, Singh VK. Importance of Pharmacognosy as an aid to drug standardisation programme: a review. In: Singh VK, Govil JN, Hashmi S, Singh G, editors. Recent progress in medicinal plants: Ethnomedicine and Pharmacognosy-II. Vol. 7. United States: Studium press LLC.; 2003. pp. 339–346. [Google Scholar]
- Hellmold H, Nilsson CB, Schuppe-Koistinen I, Kenne K, Warngard L. Identification of end points relevant to detection of potentially adverse drug reactions. Toxicol Lett. 2002;127:239–243. doi: 10.1016/s0378-4274(01)00505-7. [DOI] [PubMed] [Google Scholar]
- Hilaly JE, Israili ZH, Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol. 2004;91:43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Adler M, Vaidya VS, Rached E, Mulrane L, Gallagher WM, Callanan JJ, Gautier JC, Matheis K, Staedtler F, Dieterle F, Brandenburg A, Sposny A, Hewitt P, Ellinger-Ziegelbauer H, Bonventre JV, Dekant W, Mally A. Performance of novel kidney Biomarkers in preclinical toxicity studies. Toxicol Sci. 2010;116:8–22. doi: 10.1093/toxsci/kfq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic non-healing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF) Ann Surg. 1986;204:322–330. doi: 10.1097/00000658-198609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JA, Sagartz JE, Morris DL. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat Rev Drug Discov. 2007;6:636–649. doi: 10.1038/nrd2378. [DOI] [PubMed] [Google Scholar]
- Kushwaha SK, Dashora A, Dashora N, Patel JR, Kori ML. Acute oral toxicity studies of the standardized methanolic extract of Phyllanthus amarus Schum & Thonn. J Pharm Res. 2013;6:720–724. [Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Martins D, Nunez CV. Secondary metabolites from Rubiaceae species. Molecules. 2015;20:13422–13495. doi: 10.3390/molecules200713422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates JM, Segura JA, Alonso FJ, Marquez J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273–299. doi: 10.1007/s00204-008-0304-z. [DOI] [PubMed] [Google Scholar]
- Mathur PRG. Traditional Knowledge of the Cholanaickan and Kurumba: The hunter gatherers of Kerala. J Traditional Folk practices. 2013;1:19–30. [Google Scholar]
- Menon M. The Encyclopaedia of Dravidian Tribes, second vol. Thiruvananthapuram, India: The International school of Dravidian Linguistics; 1996. [Google Scholar]
- Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007;35:742–750. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- Mukinda JT, Syce JA. Acute and chronic toxicity of the aqueous extract of artemisia afra in rodents. J Ethnopharmacol. 2007;112:138–144. doi: 10.1016/j.jep.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: A platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- OECD (The Organisation of Economic Co-operation Development) Test guidelines No. 408: Repeated dose 90-day oral toxicity study in Rodents. Paris: OECD Publishing; 1998. [Google Scholar]
- OECD (The Organisation of Economic Co-operation Development) Test guidelines No. 423: Acute Oral toxicity – Acute Toxic Class Method. Paris: OECD Publishing; 2002. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol. 2006;57:213–219. doi: 10.1016/j.etp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food and Chemical Toxicology. 2007;45:1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Rates SM. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Sanders RA, Rauscher FM, Watkins JB. Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2001;15:143–149. doi: 10.1002/jbt.11. [DOI] [PubMed] [Google Scholar]
- Saradamma L, Nair CPR, Bhat AV, Rajasekharan S. Final technical Report: All India Co-ordinate Research Project on Ethnobiology-Phase II. 1994. [Google Scholar]
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K. Society of Toxicologic pathology position paper: Organ weight recommendations for toxicology studies. Toxicol Pathol. 2007;35:751–755. doi: 10.1080/01926230701595300. [DOI] [PubMed] [Google Scholar]
- Sheweita SA, El-Gabar MA, Bastawy M. Carbon tetrachloride-induced changes in the activity of phase II drug-metabolizing enzyme in the liver of male rats: Role of antioxidants. Toxicology. 2001;165:217–224. doi: 10.1016/s0300-483x(01)00429-2. [DOI] [PubMed] [Google Scholar]
- Shokryazdan P, Jahromi MF, Liang JB, Kalavathy R, Sieo CC, Ho YW. Safety assessment of Two new Lactobacillus strains as Probiotic for human using a rat model. PLOS ONE. 2016;11:e0159851. doi: 10.1371/journal.pone.0159851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Sofowora A. Medicinal plants and traditional medicine in Africa. United Kingdom: John Wiley and Sons; 1982. [Google Scholar]
- Speakman JR, McQueenie J. Limits to sustained metabolic rate: The link between food intake, Basal metabolic rate, and morphology in reproducing mice, mus musculus. Physiol Zool. 1996;69:746–769. [Google Scholar]
- Sutcu R, Altuntas I, Yildirim B, Karahan N, Demirin H, Delibas N. The effects of subchronic methidathion toxicity on rat liver: Role of antioxidant vitamins C and E. Cell Biol Toxicol. 2006;22:221–227. doi: 10.1007/s10565-006-0039-7. [DOI] [PubMed] [Google Scholar]
- Suvarna KS, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. 7th ed. Oxford: Elsevier Science Health Science div; 2012. [Google Scholar]
- Takhtajan A. Flowering plants. 2nd ed. New York: Springer Science; 2009. [Google Scholar]
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. A 90-day oral gavage toxicity study of d-methylphenidate and d, l-methylphenidate in Sprague–Dawley rats. Toxicology. 2002;179:183–196. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- Viswanathan MB, Manikandan U, Tangavelou AC. A new species of Neurocalyx (Rubiaceae) from Peninsular India. Nord J Bot. 2005;23:389–394. [Google Scholar]
- Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2009;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PL. Biochemical diagnosis of liver disease. Indian J Clin Biochem. 1999;14:59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P, Li K, Song F, Zhou S, Sun X, Zhang X, Nussler AK, Liu L. Heme oxygenase-1 upregulated by ginkgo biloba extract: Potential protection against ethanol-induced oxidative liver damage. Food Chem Toxicol. 2007;45:1333–1342. doi: 10.1016/j.fct.2007.01.016. [DOI] [PubMed] [Google Scholar]