Abstract
Polychlorinated biphenyls (PCBs) consist of a range of toxic substances which are directly proportional to carcinogenesis and tumor-promoting factors as well as having neurotoxic properties. Reactive oxygen species, which are produced from PCBs, alter blood–brain barrier (BBB) integrity, which is paralleled by cytoskeletal rearrangements and redistribution and disappearance of tight junction proteins (TJPs) like claudin-5 and occludin. Brain-derived neurotrophic factor (BDNF), plays an important role in the maintenance, survival of neurons and synaptic plasticity. It is predominant in the hippocampal areas vital to learning, memory and higher thinking. Quercetin, a flavonoid, had drawn attention to its neurodefensive property. The study is to assess the role of quercetin on serum PCB, estradiol and testosterone levels and mRNA expressions of estrogen receptor α and β, TJPs and BDNF signaling molecules on the hippocampus of PCBs-exposed rats. Rats were divided into 4 groups of 6 each. Group I rats were intraperitoneally (i.p.) administered corn oil (vehicle). Group II received quercetin 50 mg/kg/bwt (gavage). Group III received PCBs (Aroclor 1254) at 2 mg/kg bwt (i.p). Group IV received quercetin 50 mg/kg bwt (gavage) simultaneously with PCBs 2 mg/kg bwt (i.p.). The treatment was given daily for 30 days. The rats were euthanized 24 h after the experimental period. Blood was collected for quantification of serum PCBs estradiol and testosterone. The hippocampus was dissected and processed for PCR and Western blot; serum PCB was observed in PCB treated animals, simultaneously quercetin treated animals showed PCB metabolites. Serum testosterone and estradiol were decreased after PCB exposure. Quercetin supplementation brought back normal levels. mRNA expressions of estrogen α and β were decreased in the hippocampus of PCB treated rats. TJPS and BDNF signalling molecules were decreased in hippocampus of PCB treated rats. Quercetin supplementation retrieved all the parameters. Quercetin alone treated animals showed no alteration. Thus in PCB caused neurotoxicity, quercetin protects and prevents neuronal damage in the hippocampus.
Keywords: TJPs, BDNF, PCBs, Quercetin, GC-MS
Introduction
Polychlorinated biphenyls (PCBs) are environmental toxicants widely used in electrical industry as coolants for transformers and capacitors (Safe, 1994). PCBs are lipophilic, resistant to biological decomposition and can accumulate in higher tropic levels through the food chain (Schneider et al., 2007). Gonadal hormones exert profound influence in the brain of developing and adult vertebrates, regulating the survival of neurons, the differentiation of neurons and glial cells, plasticity and function of synaptic contacts (McEwen et al., 2001). Estradiol is a pleiotropic hormone that enhances plasticity and survival of the brain in multiple models of injury (Garcia-Segura, 2009). It acts as a neurotrophic and neuroprotective factor. Epidemiological studies have reported a positive alliance between testosterone level and cognition with relevance to the incidence of Alzheimer’s disease. Testosterone is suggested to exert a protective effect on cognitive function (Muller et al., 1996).
PCBs induced toxic manifestations are associated with the production of free radicals (Allen and Tresini, 2000), which can damage the cellular elements in the developing nervous system (Venkataraman et al., 2007; Selvakumar et al., 2012a,b, c). ROS are reported to damage almost all macromolecules of the cell including membrane polyunsaturated fatty acids, causing impairment of cellular functions (Shimada & Sawabe, 1983). This phenomenon is termed lipid peroxidation (LPO), which is considered an index of oxidative stress. The brain regions are highly rich in polyunsaturated fatty acids and thus highly susceptible to oxidative stress.
The blood-brain barrier (BBB) is maintained by the homeostasis of the central nervous system (CNS) microenvironment. The BBB acts as a physical and metabolic barrier because a complex tight junction system between adjacent endothelial cells restricts most paracellular movement of ions and solutes across the brain endothelium (Pardridge, 2002). TJ proteins are significant in maintaining polarity of the cell barrier and are involved in cellular signaling. Disruption of the integrity of this BBB has been associated with several central nervous system pathologies (Weiss et al., 2009). TJPs form the most apical element of the junctional complex and are composed of an intricate complex of transmembrane, accessory, and cytoplasmic proteins that connect the TJPs to actin cytoskeleton and intracellular signaling systems (Abbott et al., 2006). The transmembrane proteins occludin and claudin-5 form the primary seal of the TJPs. which bind to zonula occludens (ZO-1, ZO-2) the intracellular proteins that couple the TJPs to the actin cytoskeleton of endothelial cells (Abbott et al., 2006). ALL-1 fusion partner at chromosome-6)/Afadin (AF6) is a multidomain actin-binding protein that serves as a scaffold protein between transmembrane proteins and the actin cytoskeleton (Boettner et al., 2000). However, the mechanisms by which PCBs cause these neurotoxic effects are not fully understood. Reports suggest that age associated decreases in circulating estrogen in females may adversely affect the structural composition of tight junctions and compromise the integrity of the barrier counterparts (Bake & Sohrabji, 2004).
BDNF neurotrophin family members play a significant role in cell proliferation, differentiation, neuronal protection, and help in the regulation of synaptic function in the central nervous system (CNS) via stimulating key intracellular signaling cascades (Huang & Reichardt 2003; Numakawa et al. 2004). BDNF assist in maintenance, plasticity and homeostasis of the central and peripheral nervous systems (Genzer et al., 2017). BDNF is known to be a strong survival-promoting factor against various insults. The molecular mechanisms of neurotrophin dependent survival when exposed to oxidative stress have been extensively studied. Furthermore, estrogens also regulate synaptic plasticity in addition to sex differentiation of the brain (Lee & Pfaff 2008; Brinton 2009; Tobet et al., 2009) and were found to exert protective actions against oxidative stress (Simpkins et al., 2010).
Activity-dependent changes in synaptic strength are considered mechanisms underlying learning and memory. One attractive candidate for modulating synaptic plasticity in learning and memory is BDNF (Tyler et al., 2002; Yamada et al., 2002), a member of the neurotrophin family, including nerve growth factor (NGF), neurotrophin-3 (NT-3), and NT-4/5. BDNF has been implicated in the modulation of synaptic function and plasticity (Schinder & Poo 2000). There is a good correlation between BDNF mRNA expression and behavioral performance in various learning and memory tests (Tyler et al., 2002; Yamada et al., 2002). Thus, hippocampus-dependent learning in the Morris water maze, contextual fear, and passive avoidance tests are associated with a rapid and transient increase in BDNF mRNA expression in the hippocampus (Hall et al., 2000; Tyler et al., 2002; Yamada et al., 2002). The role of BDNF in learning and memory has also been investigated with function-blocking anti-BDNF antibodies. Treatment with anti-BDNF antibodies causes impairment of memory in the water maze (Mu et al., 1999). The tropomyosin receptor kinase (trk) trkB-CRE mutant mice show severe deficits in the stressful water maze test and partial impairment in the 8-arm maze test, but no changes in simple passive avoidance learning, suggesting a role for BDNF/TrkB receptor signaling in complex learning. The findings also imply that procedural long-term memory is relatively spared, whereas short-term plasticity within the hippocampus is impaired in trkB-CRE mutant mice. The role of BDNF and the development of synaptic architecture have thoroughly been studied (Wang et al., 1995). Murray & Holmes (2011) found that BDNF activates Trk receptors which participate in the activation of many signaling cascades, including phospholipase c, phospho inositol-3-kinase (PI3K) and Ras which are involved in neuronal survival and neurite growth. TrkB receptors use both PI3K and MAPK cascades for cell survival, while TrkA receptors depend mainly on PI3K.
Quercetin and associated flavonoids present in fruit and vegetable elicit neuroprotection in different models of oxidative death (Echeverry et al., 2010). They have been reported to be strong oxygen radical scavengers and also good metal chelators. They were shown to scavenge superoxide in ischemia reperfusion injury. In addition, quercetin exerts its protective effect as chelator of divalent cations, free radical scavengers, as well as DNA damage protectors, and thus may be involved in preventing free radical–mediated cytotoxicity and lipid peroxidation (Zhang 2005). Pu et al. (2007) reported that quercetin increased brain GSH level, hydroxyl radical (.OH) scavenging capacity, and Na+/K+ ATPases activity but decreased brain NOS activity and mitochondrial malondialdehyde content, which consequently reversed in the improvement of spontaneous behavior and cognitive performance and enhancement of brain inherent antioxidant capacity. Quercetin preserved the tight junctional protein integrity in endothelial and epithelial cells (Chuenkitiyanon et al., 2010). Hence it is hypothesized that PCB exposure impairs BBB through TJP disruption and gonadal hormones via inducing ROS in rat brain. The present study was aimed at investigating the protective role of quercetin against adverse effects of PCBs on tight junctional proteins such as Ocln, Cldn5, JAM-3, ZO-1, ZO-2 , AF-6 and expression pattern of BDNF signaling molecules such as BDNF, TRKB, Ras, Raf, Mek-1, Mek-2, Erk-1, Erk-2 and CREB in the hippocampus. We analyzed serum testosterone, estradiol and also the level of PCBs. The mRNA expressions of estrogen α and β in the hippocampus were also studied.
Materials and methods
Reagents
Aroclor 1254 was purchased from Chem Service, West Chester, PA, (USA). Quercetin, total RNA isolation reagent (TRI) and primers were purchased from Sigma–Aldrich Private Limited (USA). Superscript-III Reverse Transcriptase was purchased from Invitrogen, (USA) and QPCR Ready Mix was purchased from KAPA-Biosystem (USA). C18 columns (Bond Elute C18) cartridges were purchased from Agilent Technologies, Chennai. All other chemicals were purchased from Sisco Research Laboratories (SRL) Pvt. Ltd., Mumbai, India and were of analytical grade.
Animals
Adult male albino rats of Wistar strain weighing about 180–200 g (age 90 days) were used in the present study which was approved by our ethical committee (Ref No.IAEC No 01/01/11- Experimental Set 3). Rats were maintained at room temperature (25 °C) and a normal light cycle (12 h light and 12 h dark) during the experiments. The animals were fed pellet diet (Amrut Laboratory Animal Feed, Maharashtra, India) and drinking water ad libitum.
Experimental protocol
Rats were divided into 4 groups of 6 animals each and treatment was given for 30 days. Body weights of the animals were monitored throughout the experimental period. Group I received corn oil (vehicle) intraperitoneally (i.p.). Group 2 rats received quercetin (50 mg/kg bwt/day) (gavage) alone. Group 3 was subjected to i.p. treatment with PCBs (Aroclor 1254) (2 mg/kg bwt/day). Group 4 received PCBs (2 mg/kg bw/day) (i.p.) simultaneously with quercetin (50 mg/kg bw/day) (gavage).
Blood and tissue collection
24 hrs after the 30-day treatment, the rats were euthanized. Blood samples were collected in the vaccutainer and allowed to clot and centrifuged at 5000 rpm for 20 min at 4 °C; the obtained serum was used for the analysis of testosterone, estradiol and estimation of PCB.
Radioimmunoassay of Serum Testosterone and Estradiol
Serum testosterone was assayed using ImmuChemTM Testosterone double antibody RIA kit obtained from ICN Biomedicals. Inc, CA. The sensitivity of the testosterone was 0.4 pg/dl, the intra- and interassay coefficient of variations were 4–11% and 7.3–11%, respectively. The cross-reactivity of the testosterone antiserum with estradiol was 0.02%.
Estradiol was also assayed by solid-phase RIA kit obtained from DPC, USA. The sensitivity of the estradiol assay was 0.08 pg/dl. The intra- and interassay coefficient of variations were 4–7% and 4–8%, respectively and the cross reactivity of the estradiol antiserum with testosterone was 0.001%
Assessment of PCBs in serum by gas chromatography and mass spectrometry (GC-MS)
GC/MS is a combination of two different analytical techniques, Gas Chromatography and Mass Spectrometry. GC/MS, with the use of internal standards, provides a multidimensional drug identification and quantitation procedure that is the leading confirmation method for forensic drug/toxicant testing (see Hajslova & Cajka, 2007).
Sample preparation
The serum sample was collected from the control and the treated rats. The sample was diluted with dis.H2O and pretreated with water-1-propanol (85:15) and mixed and centrifuged at 10,000 rpm for 10mins. The C18 columns (Bond Elute C18) cartridges were purchased from Agilent Technologies, Chennai. The column was conditioned using 2 column volumes of methanol and 2 column volumes of water-1-propanol (85:15). Then the clear supernatant of the pretreated serum samples was aspirated through the column. After elution, the column was washed twice with 500 µl of water-1-propanol (85:15) and dried in a steam of air. The final elution was done using n-hexane four times to obtain PCB.
The GC-MS analysis was performed on a combined GC-MS instrument (ITQ 900 Model of Thermo Fisher Scientific (USA) using a HP-5 fused silica gel capillary column. The method to perform the analysis was designed for both GC and MS using the XCaliber Software provided with the machine. A 1 µl-aliquot of sample was injected into the column using a Programmed Temperature Vaporization (PTV) injector whose temperature was set at 275 °C. The GC program was initiated by a column temperature set at 60 °C for 5 min, increased to 300 °C at a rate of 8 C/min, held for 10 min. Helium was used as the carrier gas (1.5 ml/min). The mass spectrometer was operated in Electron Impact ionisation (EI) mode with mass source set at 200 °C. The chromatogram and spectrum of the peaks were visualized using Qual Browser software. The particular compounds present in the samples were identified by matching their mass spectral fragmentation patterns of the respective peaks in the chromatogram with those stored in the National Institute of Standards and Technology Mass Spectral database (NIST-MS, 1998) library.
Total RNA isolation and RT-PCR
Total RNA was isolated from the hippocampus using TRI reagent following the method of Chomczynski & Sacchi (2006). 1µg of total RNA was subjected to two-step RT–PCR. First strand reaction: Complementary DNA (cDNA) is made from mRNA template using dNTPs & reverse transcriptase (Superscript-III Reverse Transcriptase, Invitrogen, USA). The components were combined with a DNA primer in a reverse transcriptase buffer for an hour at 37 °C. Second strand reaction: After the reverse transcriptase reaction was complete, cDNA was generated from the original single strand mRNA, standard PCR was initiated. PCR Ready Mix DNA polymerase was purchased from KAPA-Biosystem, (USA). PCR primers and conditions used in this study have been tabulated (see Table 1). The amplified products were separated by electrophoresis on 2% agarose gel and identified by ethidium bromide staining. Specificity was confirmed by the size of the amplified products with reference of 100 bp DNA ladder (Chromus Biotech, Chennai) and the band intensities were quantified by Quantity One Software, Bio-Rad, (USA).
Table 1.
Primer detail.
| Target Gene | Primer Sequence (5’->3’) | Amplicon size (bp) | Annealing Temp (°C) | GeneBank/Accession Number |
|---|---|---|---|---|
| Ocln | Sense : TGGCGGAGAGATGCACGTTCG Antisense : ACCGAAGCCGCTGCCGTAAG |
247 | 55.2 | NM_031329.2 |
| Cldn5 | Sense : CGTGACGGCGCAGACGACTT Antisense : TGCACTGAGCGCCGGTCAAG |
194 | 57.8 | NM_031701.2 |
| Jam3 | Sense : TCAAGGAGACCTGGCCGGTCG Antisense : GCCGAGGATAGCCCTCGCTCT |
254 | 62.9 | NM_001004269.1 |
| ZO-1 | Sense : GGCTACAGCCCGCGGCATTT Antisense : CCATGGGGCCCGCACATCAC |
213 | 56.7 | NM_001106266.1 |
| ZO-2 | Sense:TCCCGGGACTACAGCCGTGG Antisense : GCCGGGACCTAGACTCGGGG |
208 | 59.1 | NM_053773.1 |
| AF-6 | Sense : GCGATGGGCGAGGCTGAAACA Antisense : ATCAGGTGTCTCCCGCTCCACAG |
180 | 62.9 | NM_001007754.1 |
| β actin | Sense : TCCACCCGCGAGTACAACCTTC Antisense : GGGCCACACGCAGCTCATTGTA |
358 | 63.9 | NM_031144.2 |
| BDNF | Sense:TTGCCACAGCCCCAGGTGTGA Antisense : ACGCCTGTCACTGCGCCCTA |
134 | 62.0 | NM_012513.3 |
| TRKB | Sense:ACTCTGCCAGCCCTCTCCACC Antisense: CGGCTTGAGCTGGCTGTTGGT |
152 | 56.5 | NM_012731.2 |
| Ras | Sense:TGTGCTGTCCTGACACCAGGCT Antisense : TGGACTGGACTGGCTCCAGCA |
118 | 55.7 | NM_001105753.1 |
| Raf | Sense:TGGGATTGGCTCGGGCTCCT Antisense :TGCGCAAAACAGCCACCTCGT |
137 | 55.7 | NM_012639.2 |
| Mek 1 | Sense:GCTGGATGAGCAGCAGCGGA Antisense : CCACCATTGCCAGCCCCCAG |
117 | 56.5 | D14591.1 |
| Mek 2 | Sense:CATGGGGCTGTCGCTGGTGG Antisense : TGGGGCTCTCCATCTGCCCC |
120 | 56.5 | D14591.1 |
| Erk 1 | Sense:ACCGGGACCTGAAGCCCTCC Antisense:GGCTCGGTACCAGCGTGTGG |
146 | 62.9 | NM_017347.2 |
| Erk 2 | Sense:CTCCTCGTTCCCGATCGCCG Antisense:GGGCTCGACGCTTCGCGTTA |
100 | 58.6 | NM_053842.1 |
| CREB | Sense:TGATGCACCAGGGGTGCCAA Antisense:TGCTCCTCCCTGGGTAATGGC |
142 | 55.7 | NM_134443.1 |
Western blotting analysis
Tissue lysate was prepared with radio immuno precipitate assay buffer (RIPA) and protease inhibitor. Equal amounts of protein (60 µg) were electrophoresed on 10% SDS-PAGE. Following electrophoresis, separated proteins on SDS-PAGE gels were transferred on PVDF membrane (Millipore, USA). To block the nonspecific binding, the membranes were incubated with 5% skimmed milk for 2 h. Membranes were probed with primary antibodies Ras and Raf, Cell signaling, (USA). Blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:10000). The bands were developed using ECL kit (Millipore, USA) in Chemi Doc image scanner from Bio-Rad. The band intensity was quantified by Quantity One software, Bio Rad, (USA). The membranes were stripped and reprobed for ß-actin (1:5000) as an internal control.
Statistical analysis
Data were analyzed using One-way analysis of variance (ANOVA), followed by Post Hoc test Student’s Newman-Keul’s test (SNK) with Graphpad Prism5 software. In all cases, p<0.05 was considered as statistically significant.
Results
Effect of quercetin on serum PCBs levels in PCBs-exposed adult male rats
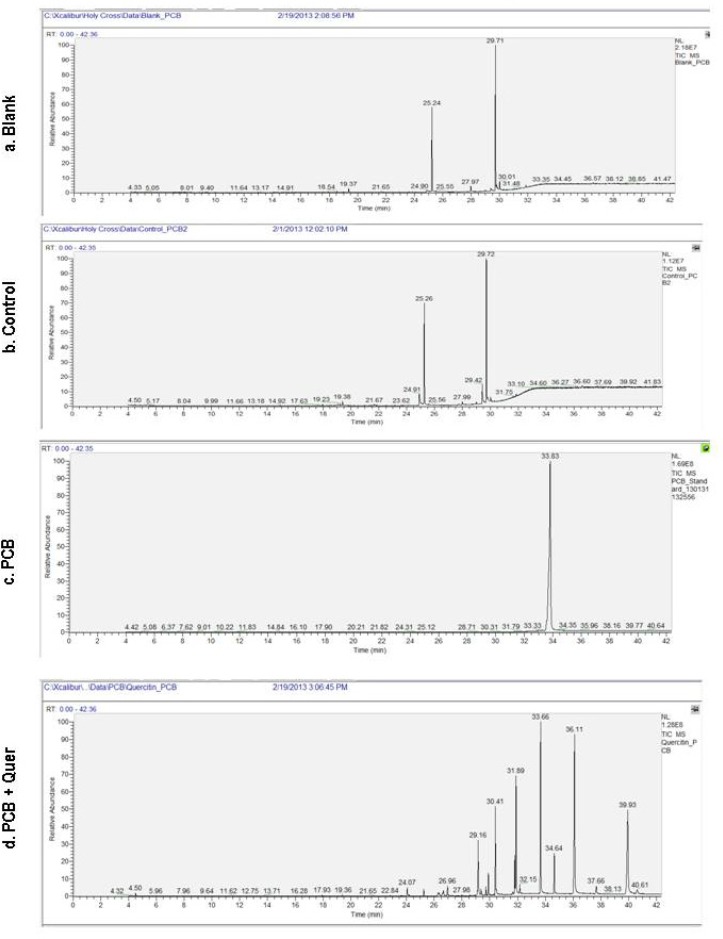
The use of the ion-trap GC-MS done to estimate PCB residues in the serum samples of control, PCB treated and simultaneous PCB+quer treated rats. Ion-trap GC-MS estimation of PCB was made according to these two criteria: (i) retention time (RT) and relative absorbance (RA). Figure 1 Blank and control shows a double peak at around RT 25 and 30 min and RA at 60 and 100, respectively, this indicates that there is no residue of PCBs in control treated rats which is similar to that blank and the peak is due to the solvent only. Figure1 PCB and PCB+quer, shows the serum levels of PCBs in PCB treated rats, there is a peak at RT 33.6 min and with RA of 100. This may be due to the accumulation of PCB. Figure 1 d, shows the serum levels of PCBs in simultaneous PCB+Quer treated rat, there are lots of peak at around RT 25 to 40 min and RA from 10 to 100 this indicates that various metabolites of PCBs are present at this RT of serum of simultaneous quercetin treated rats.
Figure 1.
Effect of quercetin on serum PCBs leves in PCBs-exposed adult rats.
Estimation of Serum testosterone and estradiol
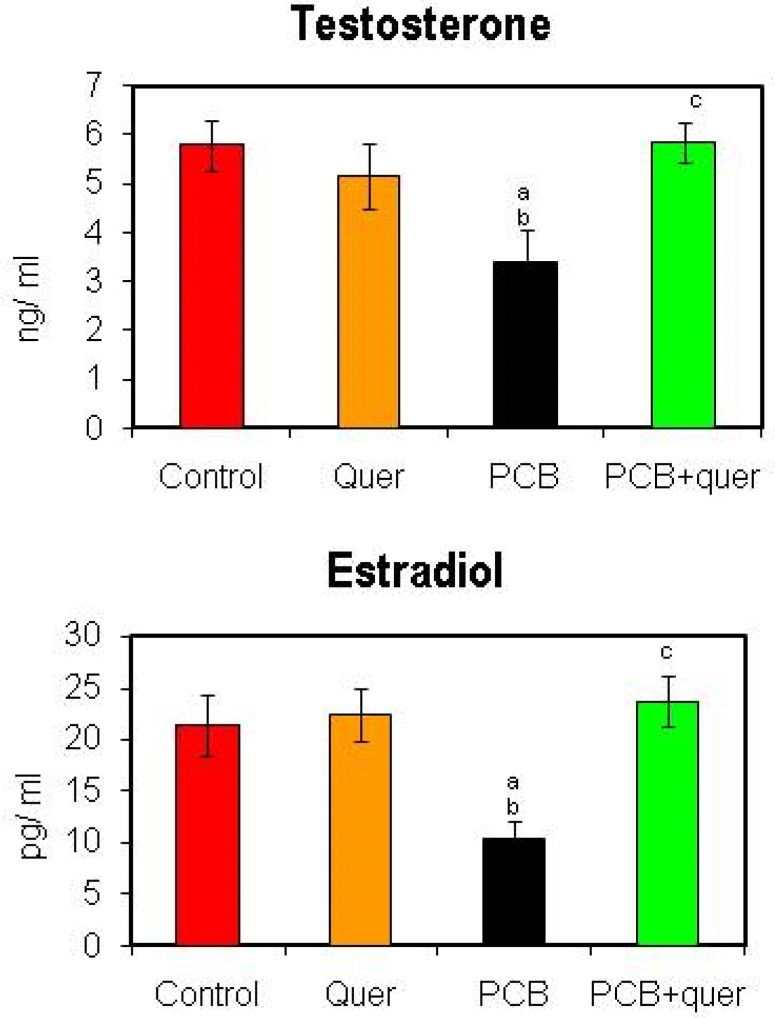
Figure 2 shows serum testosterone and estradiol levels in control and PCB treated adult rats. The serum testosterone and estradiol concentrations were decreased in PCB administrated rats when compared to vehicle treated control animals. It had been retrieved in the simultaneous quercetin group as that of control. No alteration was observed in quercetin alone treated group.
Figure 2.
Effect of quercetin on serum testosterone and estradiol of PCBs-exposed adult rats. Each bar represents mean ± SEM of 6 animals. Statistical significance at p<0.05. a- control vs. others; b- quercetin vs. others; c- PCB vs. PCB + quercetin.
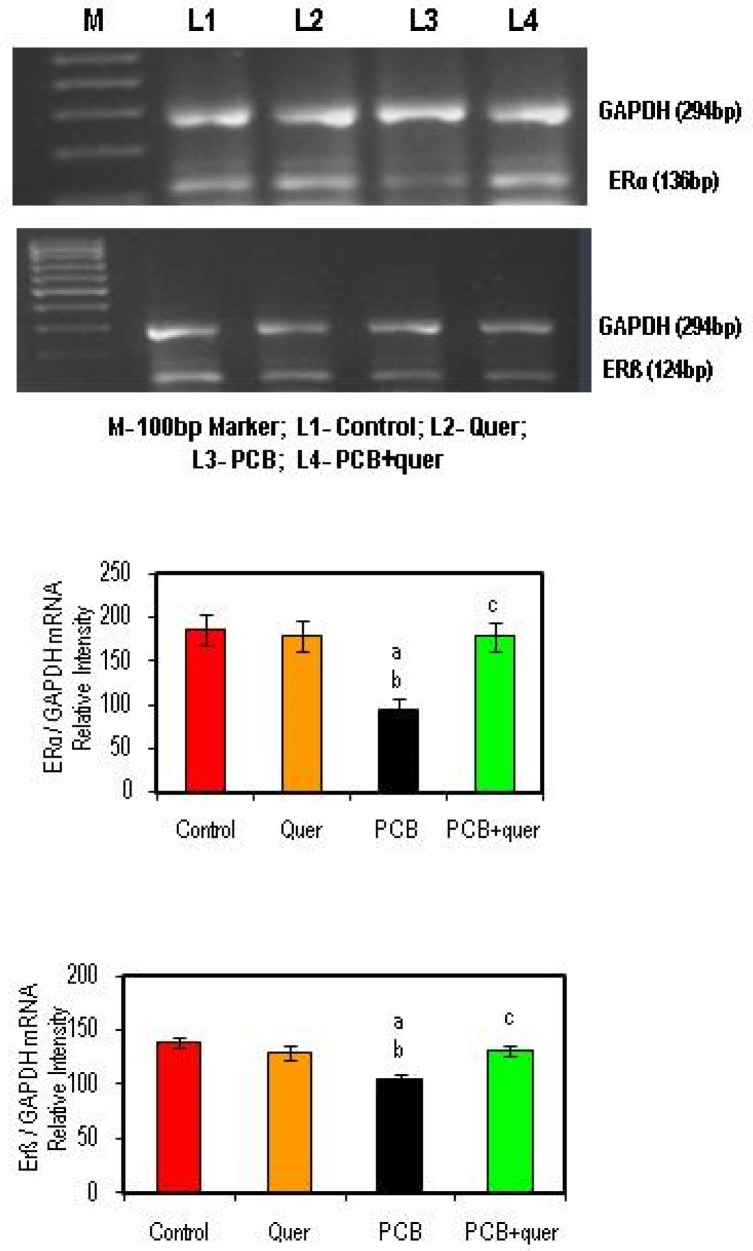
Effect of quercetin on mRNA expression of estrogen receptor α and ß in hippocampus of PCBs-exposed adult male rats
The mRNA expression of ERα and ERß were markedly reduced in the hippocampus of PCB treated rat while compared to control. The simultaneous supplementation of PCB+quercetin treatment retrieved the ERα and ERß gene expression as that of control (Figure 3). Quercetin alone treatment shows no change.
Figure 3.
Effect of quercetin on mRNA expression of estrogen receptor α and β in hippocampus of PCBs-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Statistical significance at p<0.05. a- control vs. others; b- quercetin vs. PCB, PCB + Quercetin; c- PCB vs. PCB + quercetin.
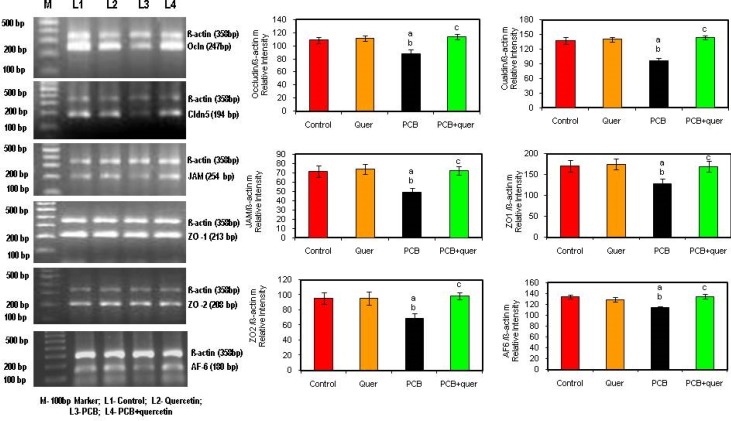
Effect of quercetin on mRNA expressions of (tight junctional proteins) Integral membrane and cytoplasmic accessory proteins in hippocampus of PCBs-exposed adult rats
Figure 4 depicts the effect of quercetin on mRNA expression of Integral membrane protein Occludin (Ocln), Claudin-5 (Cldn-5), cytoplasmic accessory proteins; Zona Occludens (ZO-1 and ZO-2) and AF-6 and Junction adhesion molecule (JAM-3) in hippocampus of PCBs-exposed adult male rats. Occludin, Claudin, Zona Occludens 1 and 2, AF 6 and Junction adhesion molecule mRNA expressions were decreased in PCBs-exposed rats while simultaneous supplementation of quercetin scavenges the ROS and decreases the degradation all the tight junctional proteins in hippocampus of PCBs-exposed adult male rats whereas simultaneous supplementation of quercetin brought levels back to normal. Quercetin alone didn’t show any significant change.
Figure 4.
Effect of quercetin on mRNA expression of (tight junctional proteins) Integral membrane and cytoplasmatic accessory proteins in hippocampus of PCBs-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Statistical significance at p<0.05. a- control vs. others; b- Quercetin vs. PCB, PCB + quercetin; c- PCB vs. PCB + quercetin.
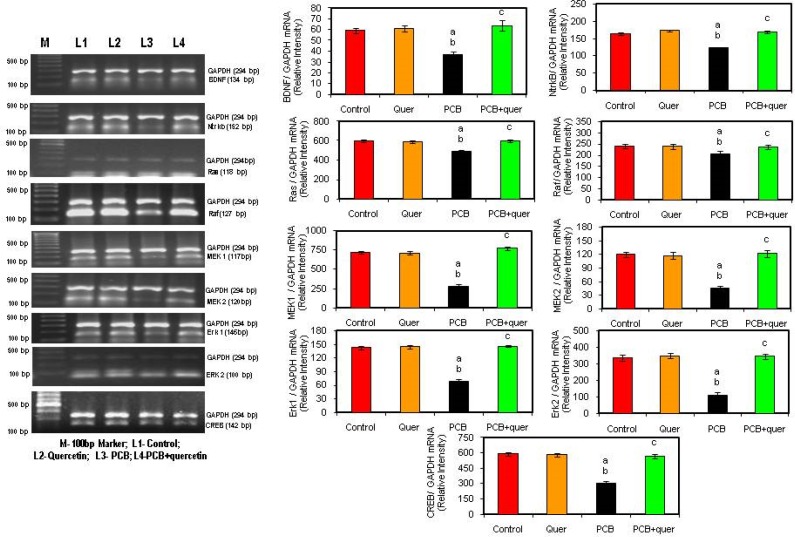
Effect of quercetin on mRNA expressions of BDNF signaling molecules in hippocampus of PCBs-exposed adult rats
BDNF, a member of the nerve growth factor family, has been shown to increase synaptic strength, survival, and growth of mature neurons through activation of a transmembrane receptor (NTrkB). The ligand BDNF, gene expression is decreased in hippocampus after PCB treatment (Figure 5). The simultaneous treatment of quercetin, a flavonoid brought back the level of BDNF expression to normal. The receptor NTrkB also get decreased in hippocampus of PCB treated rats, proclaiming the toxicity of PCB on neurotrophins and protective effect of quercetin against PCB induced toxicity.
Figure 5.
Effect of quercetin on mRNA expression of BDNF signaling molecules in hippocampus of PCBs-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Statistical significance at p<0.05. a- control vs. others; b- Quercetin vs. PCB, PCB + quercetin; c- PCB vs. PCB + quercetin.
Signal transduction cascades that underlie the actions of neurotrophic factors, including the phosphotidylinositol-3 kinase (PI3K)-Akt pathway and the Ras-mitogen-activated protein (MAPK) cascade. The MAPK cascade, which includes the extracellular signal regulated kinase (ERK), also supports cell survival and promotes synaptic plasticity by regulating several transcription factors, including the cyclic AMP response element binding protein (CREB). Figure 5 proclaims that the signaling molecules Ras, Raf, MAPK cascade were decreased during PCBs-treatment compared to control, whereas the quercetin treatment brought back the levels to normal. There is no difference in quercetin treated rats.
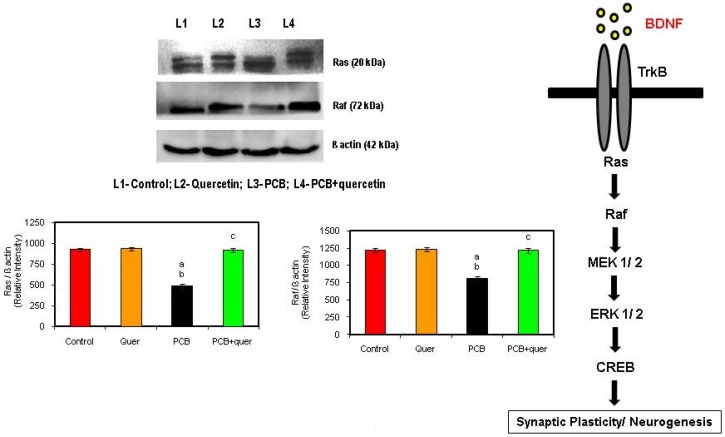
Effect of quercetin on protein expression of Ras and Raf in hippocampus of PCBs-exposed adult male rats
Ras and Raf proteins contribute to their aberrant regulation of growth stimulatory signaling pathways. Ras and Raf function as a relay switch that is positioned downstream of cell surface receptor tyrosine kinases and upstream of a cytoplasmic cascade of kinases that include the mitogen-activated protein kinases (MAPKs). During PCBs-treatment the Ras protein and its subsequent downstream signaling molecule Raf also get decreased compared to control stating the inhibition of growth stimulatory signal by PCBs (Figure 6). The simultaneous quercetin treatment restored the levels and proved as a neuroprotectant.
Figure 6.
Effect of quercetin on on protein expressions of BDNF signaling molecules in hippocampus of PCBs-exposed adult rats. Each bar represents mean ± SEM of 3 independent observations. Statistical significance at p<0.05. a- control vs. others; b- quercetin vs. PCB, PCB + Quercetin; c- PCB vs. PCB + quercetin.
Discussion
Previous studies in our laboratory also suggest that PCBs–quinones undergo redox cycling with the formation of ROS, thus becoming major source of oxidative stress in male Wistar rats. PCB-induced oxidative stress decreases the activities of antioxidant enzymes and disrupts the functional parameters in ventral prostate, testicular Leydig, Sertoli cells, and selected brain regions (Sridhar et al., 2004; Murugesan et al., 2005a; Venkataraman et al., 2007, 2008; Bavithra et al., 2012) and it can damage the cellular elements. Our recent study demonstrates that PCBs induced oxidative stress and apoptosis in the rat hippocampus (Selvakumar et al,. 2012a, b; 2013).
Quercetin is a strong oxygen radical scavenger and also a good metal chelator. It is believed to work via electron donation to directly detoxify free radicals, such as the highly toxic hydroxyl radicals. In vitro studies have suggested that quercetin has a potent inhibitory activity against production of nitric oxide and tumor necrosis factor in lipopolysaccharide stimulated Kupffer cells (Kawada et al., 1998) Quercetin was shown to scavenge superoxide in ischemia reperfusion injury (Huk et al., 1998). The 4-oxo group and 2, 3- double bond in the c ring of quercetin is thought to play an important role in its neuroprotective effects in repeated cerebral ischemic model (Pu et al., 2007). It was shown that the ROS produced by PCB had been scavenged by quercetin and protected the brain form neurodegeneration (Selvakumar et al., 2012a,b).
The BBB is primarily formed by specialized brain endothelial cells (ECs), which form a tight seal due to the presence of well-developed tight junctions (TJ) that impede the entrance of circulating molecules and immune cells into the CNS (Pachter et al., 2003). Loss of TJ proteins is commonly observed in neuroinflammatory and neurodegenerative disorders that are frequently associated with stroke (Brown & Davis, 2002), Alzheimer’s disease (Fiala et al., 2002), HIV-1 encephalitis (Persidsky et al., 2006), and traumatic brain injury (Morganti-Kossmann et al., 2002). The tight junctions (TJs) limit passive paracellular movement of solutes, ions, and water across the BBB. This barrier is variable and physiologically regulated, and its disruption contributes to human diseases (Powell, 1981). There is ample evidence that PCBs cause neurotoxicity in humans. The predominant mechanisms include alteration in TJ of human brain microvascular endothelial cells, intracellular signaling process and thyroid hormone metabolism (Kodavanti, 2005). PCB exposure induces TJ disruption of human brain microvascular endothelial cells, which may lead to perturbation of the BBB integrity (Eum et al., 2008).
The ROS alter BBB integrity, which is paralleled by cytoskeleton rearrangements and redistribution and disappearance of TJ proteins (Schreibelt et al., 2007). ROS, which are highly reactive molecules, are produced during monocyte migration and contribute to BBB injury and subsequent inflammation in the brain. In the present study, there is also decreased expression of tight junction protein in the microvascular endothelial cells in the hippocampus of PCB treated rats. Tight junctions (TJ) of the BBB are composed of an intricate combination of integral membrane proteins (Occludin Claudins and Junctional Adhesion Molecules [JAMs]) mediate cellular interaction between brain ECs and play a major role in TJ functioning and cytoplasmic accessory proteins [Zona Occludens (ZO-1, ZO-2) & AF6] provide a link between transmembrane TJ proteins and the actin cytoskeleton but also participate in intracellular signaling. This study prevails that the mRNA expression of Tight Junctional Proteins in the hippocampus significantly decreased at the PCB exposure. PCBs induced disruption of blood brain homeostasis, occurring predominantly by means of ROS induced tight junction disruption. The organization of TJ proteins occludin and ZO-1 is altered by exogenous ROS in brain ECs (Haorah et al., 2007). ROS selectively activate signaling cascades involving RhoA, PI3 kinase, and protein kinase B (PKB/Akt) leading to rearrangements of the actin cytoskeleton and spatial redistribution and disappearance of occludin and claudin-5, inducing altered BBB integrity (Schreibelt et al., 2007).
Previous studies in our laboratory showed neuronal damage, alteration of neuronal morphology of cerebral cortical layer, pyrimidal cells of hippocampal layers and the Purkinjee cellular layer in the cerebellum after PCB exposure (Venkataraman et al., 2010; Selvakumar et al., 2012a). It may be due to enhanced free radical generation in PCB exposed animals. Pyknotic nuclei with predominant perineuronal spaces were observed in coritical layer due to enhanced lipid peroxidation. In the present study, down regulation of both integral membrane proteins and cytoplasmic accessory proteins mRNA expression were observed in PCBs treated group. The possible mechanism behind this is the ROS induced disruption. In the present study also disruption of hippocampal layer in CA4 was seen in PCB treated rats. Quercetin supplemented rats showed restoration of pyramidal cells. Degeneration of pyramidal cells in hippocampal layer may be due to enhanced free radical generation in PCB treated rats. Degeneraton of cells was reduced in quercetin supplemented rats. Quercetin has been shown to possess genomic actions regulating the expression of several genes. Quercetin influences both antioxidant enzyme activity and cellular mRNA levels for these enzymes (Selvakumar et al., 2013a).
Kevil et al. (2000) studied oxidative stress induced hyperpermeability and suggested it to be related to phosphorylation of occludin and ZO1 at the tyrosine residues, down regulation of occludin and activation of MAPK signaling pathways. The action of quercetin has been linked to a number of enzymes involved in proliferation and signal transduction pathways including PKC, tyrosine kinase, PI3 kinase, NFkß, and MPK family. Chuenkitiyanon et al. (2012) studied the protective effects of quercetin suggesting that it might involve altered MAPK activities, in particular the decrease in p38 MAP signaling on epithelial and endothelial barriers.
Estrogens have broad and profound effects on the structure and function of the CNS. Estradiol enhances cognition, memory and learning in the hippocampus (Yildirim et al. 2008). In the present study, serum testosterone and estradiol and the expression of ERα and ERß were decreased after PCB exposure. The observed reduction in serum testosterone associated with decreased LH level was a result of diminished synthesis and secretion in rats subjected to PCB treatment (Murugesan et al. 2007). Our earlier studies also demonstrated that PCB treatment to adult male rats diminished Leydig cell LH receptor density (Murugesan et al. 2007). This was due to increased LPO and ROS in rats subjected to PCB. Andric et al. (2000) also reported that testosterone production was shown to be decreased by reduced activities of steroidogenic enzymes after PCBs exposure. The decreased serum estradiol may be due to impaired synthesis or enhanced metabolism, both of which are known to be influenced by PCB exposure. The synthesis of estradiol from testosterone depends on the activity of aromatase enzyme which was also shown to be decreased in PCB exposed male offspring (Hany et al. 1999). The study of aromatase enzyme is warranted. However simultaneous administration of quercetin maintained both testosterone and estradiol to near normal levels.
PCB is a neurotoxic compound which gets metabolized into hydroxylated PCB in the biological system (Tilson and Kodavanti 1998). Hence we planned to estimate the PCB levels in the serum of PCB-exposed animals. The results suggested in the serum of simultaneous PCB+quer treated rats a peak representing phenol, which may be due to the metabolization of PCB in vivo condition itself. Hence the results obtained show metabolites of PCB. There are reports suggesting that quercetin increased the cytochrome P450 metabolizing enzymes which would have metabolized the PCB and the ROS produced during PCB metabolism is scavenged by the antioxidant property of quercetin. Compared to the PCB results with the treated ones, both contain phenols but the intensity of its presence differs. In the treated samples the phenol levels are very minute but there is a high and unique peak in PCB treated samples showing its abundant presence. Thus the present study proved that the accumulation of PCB as such found in the PCB-exposed animals was due to continuous exposure. However the PCB+quercetin treated rats showed metabolites of PCBs, pointing to the influence of cytochrome P450 enhancement by quercetin.
Protein levels of BDNF and mRNA are decreased in patients and animal models of neurodegenerative diseases (Gines et al. 2006). The level of TrkB receptor has also been decreased in the hippocampus after PCB treatment because PCB decreased the synaptic strength, survival and growth of hippocampal neurons. The actions of neurotrophic factors including PI3/Akt pathway and Ras – mitogen activated protein (MAPK) cascade (MEK1 and MEK2), which includes the extracellular signal regulated kinase (ERK1 and ERK2), also support cell survival and promote synaptic plasticity by regulating cyclic AMP response element binding protein (CREB) and were decreased after PCBs-exposure in the hippocampus. BDNF/TrkB signaling pathway plays a major role in memory processes. The binding of BDNF to its receptor tyrosine kinase B (TrkB) leads to dimerization and autophosphorylation of tyrosine residues in the intracellular domain of the receptor and subsequent activation of cytoplasmic signaling pathways including MAPK, PLC, PI3K (Kaplan & Miller 2000). Murray & Holmes (2011) reported that BDNF increased protein synthesis by enhancing translation initiation via multiple signaling pathways including PI3k/Akt. Ying et al. (2002) demonstrated that BDNF triggers long term potentiation in the hippocampus in vivo through MAPK.
In the present study, BDNF signaling molecules were decreased in the hippocampus of PCB exposed rats. Ras is a small GTP binding protein, which is the common upstream molecule of several signaling pathways including Raf/MEK/ERK. EGF receptor mediated Ras activation was reported in ROS induced neurodegenerative disorder. During the learning process, cyclic AMP CREB is activated in the hippocampus (Mizuno et al. 2002). Ras and its effectors may be appropriate targets for therapeutic intervention. Quercetin supplementation inhibited the cell damage and prevented memory impairment and neuronal death. In addition to neurotrophic effects, regulation of synaptic plasticity is the primary function of BDNF. It induces several forms of synaptic plasticity in various brain areas including the hippocampus. Thus, BDNF is an attractive candidate molecule mediating learning and memory. Learning and memory behavior, anxiety and stress behavior evidence supports (Selvakumar et al., 2013) that BDNF is essential for at least certain forms of learning and memory.
PCBs-induced free radicals altered calcium channel expressions in the hippocampus. Quercetin is a potent free radical scavenger. The 4-oxo group and 2,3, double bond in the C ring of quercetin is thought to play an important role in the neuroprotective effect in repeated cerebral ischemic model (Pu et al. 2007). The alterations of excitatory and inhibitory dopamine receptors are seen in PCBs treated rats, whereas quercetin treated rats show the normal conditions. Several drug candidates, which were found to attenuate deleterious symptoms in various models of neurodegenerative diseases, are reported to upregulate the expression of neurotrophic factors including BDNF (Kaplan & Miller 2000). Considering this, it seems significant to further investigate the probable mechanisms behind such neurotrophic factor upregulation. On the other hand, estrogenic survival promotion hass also been well studied. Further investigation addressing how each ER contributes to neuronal protection against oxidative toxicity is required.
Conclusion
To conclude, PCBs disrupt the gene expression of transmembrane tight junctional proteins (Occludin, Claudin-5 and JAM) , cytoplasmic accessory tight junctional proteins (ZO-1, ZO-2 & AF-6), BDNF signaling molecules in the hippocampus and reduce the circulating gonadal hormone levels. Quercetin scavenges the PCBs induced ROS, thereby preventing transmembrane tight junctional proteins, cytoplasmic accessory tight junctional proteins in the hippocampus and maintaining the level of estradiol. thus protecting the BDNF signaling molecules in homeostasis. Thus quercetin might be clinically beneficial in reducing the potential threat to the brain system, particularly in the hippocampus.
Acknowledgement
The financial assistance to Mr. K. Selvakumar, Senior Research Fellow, Department of Endocrinology from University Grants Commission- Research Fellowship in Science for Meritorious Students (UGC- RFSMS-SRF) programme is gratefully acknowledged.
REFERENCES
- Anbalagan J, Kanagaraj P, Srinivasan N, Aruldhas MM, Arunakaran J. Effect of polychlorinated biphenyl, Aroclor 1254 on rat epididymis. Indian J Med Res. 2003;118:236–42. [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Andric SA, Kostic TS, Stojilkovic SS, Kovacevic RZ. Inhibition of rat testicular androgenesis by a polychlorinated biphenyl mixture aroclor 1248. Biol Reprod. 2000;62(6):1882–8. doi: 10.1095/biolreprod62.6.1882. [DOI] [PubMed] [Google Scholar]
- Awad HM, Boersma MG, Vervoort J, Rietjens IM. Peroxidase-catalyzed formation of quercetin quinone methide-glutathione adducts. Arch Biochem Biophys. 2000;378(2):224–33. doi: 10.1006/abbi.2000.1832. [DOI] [PubMed] [Google Scholar]
- Bancroft LW, Peterson JJ, Kransdorf MJ, Nomikos GC, Murphey MD. Soft tissue tumors of the lower extremities. Radiol Clin North Am. 2002;40(5):991–1011. doi: 10.1016/s0033-8389(02)00033-7. [DOI] [PubMed] [Google Scholar]
- Bavithra S, Selvakumar K, Pratheepa Kumari R, Krishnamoorthy G, Venkataraman P, Arunakaran J. Polychlorinated biphenyl (PCBs)-induced oxidative stress plays a critical role on cerebellar dopaminergic receptor expression: ameliorative role of quercetin. Neurotox Res. 2012;21(2):149–59. doi: 10.1007/s12640-011-9253-z. [DOI] [PubMed] [Google Scholar]
- Benjamins JA, Iwata R, Hazlett J. Kinetics of entry of proteins into the myelin membrane. J Neurochem. 1978;31(4):1077–85. doi: 10.1111/j.1471-4159.1978.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–35. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(Suppl):S2–9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: recent insights and remaining challenges. Learn Mem. 2001;8(3):121–33. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30(4):212–22. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, Ahlborg UG, van Leeuwen FX, Feeley MM. Report of the WHO working group on the assessment of health risks for human infants from exposure to PCDDs, PCDFs and PCBs. Chemosphere. 1998;37(9–12):1627–43. doi: 10.1016/s0045-6535(98)00230-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med. 2007;43(5):658–77. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Prot. 2006;1(2):581–5. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- Chuenkitiyanon S, Pengsuparp T, Jianmongkol S. Protective effect of quercetin on hydrogen peroxide-induced tight junction disruption. Int J Toxicol. 2010;29(4):418–24. doi: 10.1177/1091581810366487. [DOI] [PubMed] [Google Scholar]
- Devasagayam TP, Tarachand U. Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun. 1987;145(1):134–8. doi: 10.1016/0006-291x(87)91297-6. [DOI] [PubMed] [Google Scholar]
- Egan MF, Weinberger DR, Lu B. Schizophrenia, III: brain-derived neurotropic factor and genetic risk. Am J Psychiatry. 2003;160(7):1242. doi: 10.1176/appi.ajp.160.7.1242. [DOI] [PubMed] [Google Scholar]
- Faroon O, Jones D, de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol Ind Health. 2000;16(7–8):305–33. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- Genzer Y, Chapnik N, Froy O. Effect of brain-derived neurotrophic factor (BDNF) on hepatocyte metabolism. Int J Biochem Cell Biol. 2017;88:69–74. doi: 10.1016/j.biocel.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Gines S, Bosch M, Marco S, Gavaldà N, Díaz-Hernández M, Lucas JJ, Canals JM, Alberch J. Reduced expression of the TrkB receptor in Huntington’s disease mouse models and in human brain. Eur J Neurosci. 2006;23(3):649–58. doi: 10.1111/j.1460-9568.2006.04590.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Quinlan GJ. Malondialdehyde formation from lipid peroxides in the thiobarbituric acid test: the role of lipid radicals, iron salts, and metal chelators. J Appl Biochem. 1983;5(4–5):293–9. [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM, Jakoby WB. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974;71(10):3879–82. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature neuroscience. 2000;3(6):533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(Suppl 1):171–89. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158(3):231–43. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52(25):7514–7. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–91. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Venkataraman P, Arunkumar A, Vignesh RC, Aruldhas MM, Arunakaran J. Ameliorative effect of vitamins (alpha-tocopherol and ascorbic acid) on PCB (Aroclor 1254) induced oxidative stress in rat epididymal sperm. Reprod Toxicol. 2007;23(2):239–45. doi: 10.1016/j.reprotox.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Takebayashi M, Morinobu S, Yamawaki S. beta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J Pharmacol Exp Ther. 2004;311(1):237–45. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- Lee AW, Pfaff DW. Hormone effects on specific and global brain functions. J Physiol Sci. 2008;58(4):213–20. doi: 10.2170/physiolsci.RV007008. [DOI] [PubMed] [Google Scholar]
- Liu S, Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151(3):859–64. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E, Morch Andersen J, Fonnum F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol Appl Pharmacol. 1999;161(3):274–82. doi: 10.1006/taap.1999.8806. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behavioural brain research. 2002;133(2):135–41. doi: 10.1016/s0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835(2):259–65. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Murray PS, Holmes PV. An overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. Int J Pept. 2011;2011:654085. doi: 10.1155/2011/654085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan P, Kanagaraj P, Yuvaraj S, Balasubramanian K, Aruldhas MM, Arunakaran J. The inhibitory effects of polychlorinated biphenyl Aroclor 1254 on Leydig cell LH receptors, steroidogenic enzymes and antioxidant enzymes in adult rats. Reprod Toxicol. 2005a;20(1):117–26. doi: 10.1016/j.reprotox.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Effects of vitamins C and E on steroidogenic enzymes mRNA expression in polychlorinated biphenyl (Aroclor 1254) exposed adult rat Leydig cells. Toxicology. 2007;232(3):170–82. doi: 10.1016/j.tox.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Murugesan P, Senthilkumar J, Balasubramanian K, Aruldhas MM, Arunakaran J. Impact of polychlorinated biphenyl Aroclor 1254 on testicular antioxidant system in adult rats. Hum Exp Toxicol. 2005b;24(2):61–6. doi: 10.1191/0960327105ht500oa. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Kulkarni SK. Quercetin, a bioflavonoid, reverses haloperidol-induced catalepsy. Methods Find Exp Clin Pharmacol. 2004;26(5):323–6. doi: 10.1358/mf.2004.26.5.831321. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunugi H, Hashimoto R. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13(21):2699–708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- Olton DS. Mazes, maps, and memory. Am Psychol. 1979;34(7):583–96. doi: 10.1037//0003-066x.34.7.583. [DOI] [PubMed] [Google Scholar]
- Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1–2):161–70. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pratheepa Kumari R, Selvakumar K, Bavithra S, Zumaana R, Krishnamoorthy G, Arunakaran J. Role of quercetin on PCBs (Aroclor-1254) induced impairment of dopaminergic receptor mRNA expression in cerebral cortex of adult male rats. Neurochem Res. 2011;36(8):1344–52. doi: 10.1007/s11064-011-0449-7. [DOI] [PubMed] [Google Scholar]
- Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K, Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104(4):329–34. doi: 10.1254/jphs.fp0070247. [DOI] [PubMed] [Google Scholar]
- Puntarulo S, Cederbaum AI. Increased microsomal interaction with iron and oxygen radical generation after chronic acetone treatment. Biochim Biophys Acta. 1988;964(1):46–52. doi: 10.1016/0304-4165(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Seo BW, Crofton KM, Schantz SL. Gestational-lactational exposure to Aroclor 1254 impairs radial-arm maze performance in male rats. Toxicol Sci. 2000;57(1):121–30. doi: 10.1093/toxsci/57.1.121. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Moshtaghian J, Ness DK. Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam Appl Toxicol. 1995;26(1):117–26. doi: 10.1006/faat.1995.1081. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23(12):639–45. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Selvakumar K, Bavithra S, Krishnamoorthy G, Venkataraman P, Arunakaran J. Polychlorinated biphenyls-induced oxidative stress on rat hippocampus: a neuroprotective role of quercetin. The Scientific World Journal. 2012a;2012:980314. doi: 10.1100/2012/980314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar K, Bavithra S, Suganthi M, Benson CS, Elumalai P, Arunkumar R, Krishnamoorthy G, Venkataraman P, Arunakaran J. Protective role of quercetin on PCBs-induced oxidative stress and apoptosis in hippocampus of adult rats. Neurochem Res. 2012b;37(4):708–21. doi: 10.1007/s11064-011-0661-5. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev. 2008;57(2):421–30. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800(10):1113–20. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Staal GE, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta. 1969;185(1):39–48. doi: 10.1016/0005-2744(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, Searcy B, McClellan K. Brain sex differences and hormone influences: a moving experience? J Neuroendocrinol. 2009;21(4):387–92. doi: 10.1111/j.1365-2826.2009.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9(5):224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman P, Krishnamoorthy G, Vengatesh G, Srinivasan N, Aruldhas MM, Arunakaran J. Protective role of melatonin on PCB (Aroclor 1,254) induced oxidative stress and changes in acetylcholine esterase and membrane bound ATPases in cerebellum, cerebral cortex and hippocampus of adult rat brain. Int J Dev Neurosci. 2008;26(6):585–91. doi: 10.1016/j.ijdevneu.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Muthuvel R, Krishnamoorthy G, Arunkumar A, Sridhar M, Srinivasan N, Balasubramanian K, Aruldhas MM, Arunakaran J. PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: protective role of ascorbic acid. Neurotoxicology. 2007;28(3):490–8. doi: 10.1016/j.neuro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Sridhar M, Dhanammal S, Vijayababu MR, Arunkumar A, Srinivasan N, Arunakaran J. Effects of vitamin supplementation on PCB (Aroclor 1254)-induced changes in ventral prostatic androgen and estrogen receptors. Endocr Res. 2004;30(3):469–80. doi: 10.1081/erc-200035959. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15(7 Pt 1):4796–805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104(1):84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22(17):7580–5. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, Akama KT, McEwen BS, Milner TA, Morrison JH. Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience. 2008;152(2):360–70. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22(5):1532–40. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via estrogen receptor {beta} J Biol Chem. 2010;285(51):39575–9. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YS, Yen PM, Chin WW, Pfaff DW. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc Natl Acad Sci U S A. 1996;93(22):12587–92. doi: 10.1073/pnas.93.22.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]