Abstract
Treatment by All-Trans Retinoic Acid (ATRA) combined with an Anthracycline Chemotherapy has been shown to improve disease-free survival in patients with Acute Promyelocytic Leukemia (APL). Although ATRA is generally well tolerated, occurrence of Retinoic Acid Syndrome (RAS) during the induction chemotherapy in some patients is recognized as a distinct complication and a potential life-threatening side effect.
Isolated myocarditis as a result of RAS related to ATRA administration is uncommon and has been rarely reported in the literature.
We report a very rare case of ATRA-induced perimyocarditis accompanied by hemodynamic compromise. There was complete resolution of the signs and symptoms of peri-myocarditis when ATRA was temporary suspended. Finally, ATRA was safely resumed.
Keywords: ATRA syndrome, drug induced myocarditis, acute promyelocytic leukemia
Introduction
Myocarditis is an inflammatory disease of the myocardium characterized by leukocyte infiltration and cellular necrosis. It is most often due to a viral infection, but rarely associated with the use of a medicinal product.
Here, we report a case of fulminant peri-myocarditis following ATRA administration. Such a complication has been rarely documented.
Observation
A 27-year-old male patient, presented to the emergency department with a five-day history of fatigue, fever, and gingival bleeding. On physical examination, ecchymosis, petechiae on the lower limbs, and active gingival bleeding were observed. Results from a complete blood count revealed a pancytopenia. These abnormalities prompted a bone marrow biopsy. The patient was finally diagnosed with APL and subsequent Fluorescence In Situ Hybridisation (FISH) analysis documents at (15; 17) chromosomal translocation.
Screening echocardiography was performed before induction therapy, and it showed no wall motion abnormalities and a normal left ventricle ejection fraction (LVEF).
The patient was subsequently admitted to the hematology unit and received induction therapy with daunorubicin 60 mg/m2/day as 15–30 min IV infusion for 3 days plus oral ATRA 45 mg/m2/day in 2 equally divided doses.
No complication was observed until 10 day from initiation of remission induction therapy. However, the patient complained of orthopnea and left basal thoracic chest pain. The physical examination findings were hypotension, bilateral crackling in lungs, and pericardial friction on cardiac auscultation. The patient had no signs or symptoms of infection.
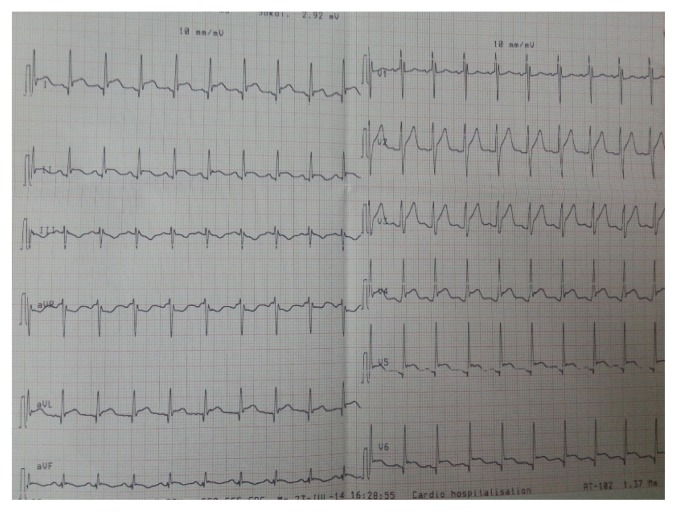
The electrocardiogram revealed ST segment elevation in most leads (Figure 1). Chest X-ray visualized a normally sized cardiac silhouette with bilateral alveolar syndrome.
Figure 1.
Electrocardiogram showing sinus tachycardia with diffuse ST segment elevation in relation to acute pericarditis.
Laboratory findings showed white blood cell (WBC) count of 1.03 × 109/L, creatine kinase (CK) of 524 IU/L (21–232), a brain natriuretic peptide (BNP) of 1133 pg/mL (0–100), and Troponin-I of 8.69 ng/mL (0.0–0.05).
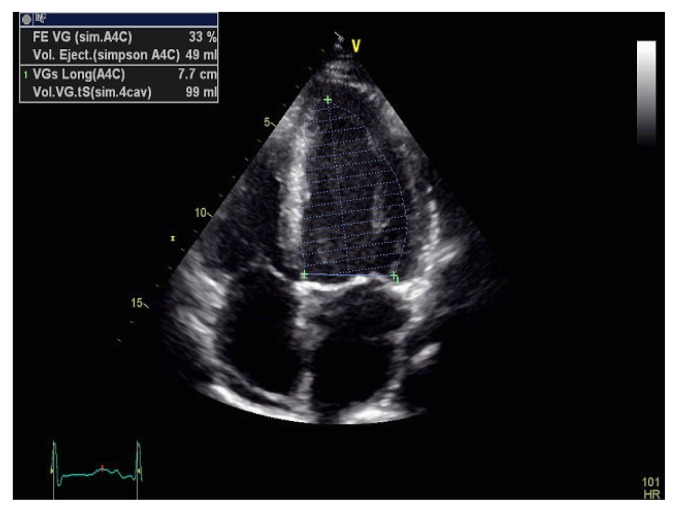
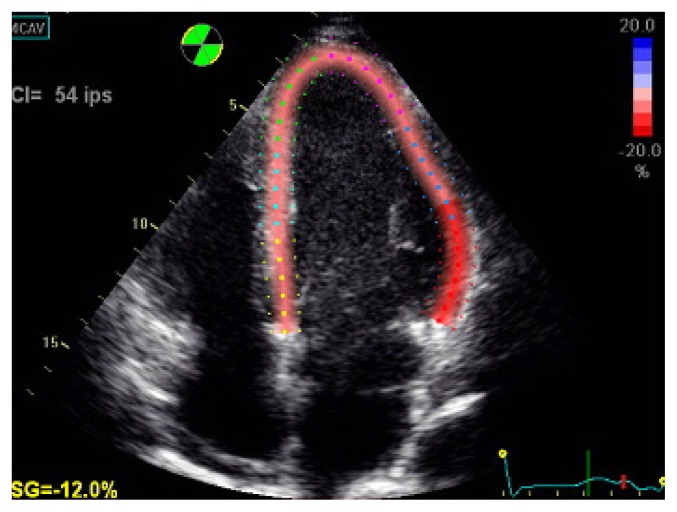
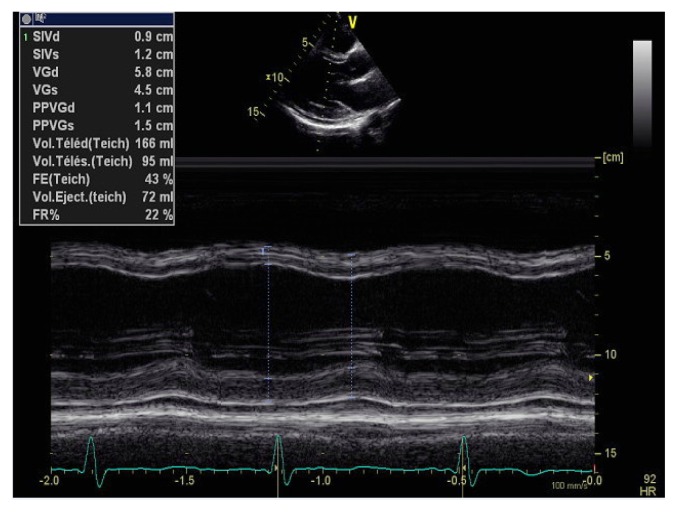
Echocardiography visualized left ventricle (LV) of normal size, diffuse LV wall motion abnormalities, and a reduced LVEF (lower than 33%) with a minimal pericardial effusion (Figure 2, 3 and 4). Serological tests for viral infections proved to be negative.
Figure 2.
Transthoracic echocardiography revealing left ventricular dysfunction with alteration of the ejection fraction.
Figure 3.
Transthoracic echocardiography revealing left ventricular dysfunction with longitudinal strain deterioration.
Figure 4.
Transthoracic echocardiography in TM mode showing a normal sized hypokinetic left ventricle with mild pericardial effusion.
A diagnosis of RAS as a result of ATRA intake was made. Consequently, ATRA was discontinued and vasopressor drugs were administrated in order to maintain hemodynamic parameters. The patient was then given diuretic, Converting Enzyme Inhibitor and beta-blockers.
A favorable outcome was obtained consisting of clinical improvement, regression of troponin serum level, disappearance of pericardial effusion and a complete recovery of LV systolic function.
The patient was on complete remission within 35 days post induction therapy. Two weeks later, he received his 2 consolidation cycles combining daunorubicin at a reduced dose: 45 mg/m2/day IV for 3 days and cytarabine 200 mg/m2/day IV for 7 days, followed by a maintenance therapy with ATRA 45 mg/m2/day for 15 days every 3 months, plus mercaptopurine 60 mg/m2/day per os and methotrexate 20 mg/m2 per os weekly for 2 years.
The patient remained in sustained remission for more than 3 years with no cardiac adverse effect.
Discussion
ATRA, a derivative of Vitamin A, is used as adjunctive therapy in combination with chemotherapy. It has transformed the management of APL by allowing a significant decrease in mortality rate [1].
First described by Frankel et al, RAS is a classic complication of treatment by ATRA occurring in up to 25% of patients with APL. This syndrome is characterized primarily by respiratory distress, pulmonary infiltrates, fever, body weight gain, pleural effusion, kidney failure, pericardial effusion, heart failure, and/or hypotension [2]. The morbidity and mortality of RAS is significant during the induction therapy. Reported mortality rate is as high as 9% [1].
De Botton et al. had analyzed a large series of RAS and found that 17% of ALP patients experienced cardiac failure during induction treatment in addition to other manifestations [3]. Isolated Cardiac manifestation of SRA, as in our case, was exceptionally reported in the literature [4,5].
The etiopathogenic mechanisms of RAS remain partially unknown. However, it may be due to cellular migration, endothelial activation, and release of interleukins and vascular factors responsible for tissue damage [6,7].
It is difficult to differentiate whether the myocarditis is part of RAS or toxicity-related, especially in the absence of a significant raise of WBC count. In our case, the diagnosis of isolated ATRA-induced myocarditis was mostly based on clinical manifestations supported by the striking response to ATRA discontinuation and the exclusion of other causes of myocarditis. Resuming ATRA far from the acute phase of myocarditis with no symptom recurrence is probably suggestive of myocarditis to be an isolated manifestation of RAS rather than a toxicity-related complication.
At the onset of the first symptoms of RAS, ATRA discontinuation and high-dose of dexamethasone administration seems likely to have dramatically reduced the mortality rate of RAS [7].
In our case, the complete restoration of myocardial function was obtained by discontinuation of ATRA, conventional therapy of heart failure, and without use of steroids. Similarly, Klein SK adopted the same approach with 2 cases of isolated symptomatic myocarditis induced by ATRA [5].
Conclusion
We present a rare case of ATRA-induced, isolated, and fulminant peri-myocarditis. The diagnosis was based on serologic and echocardiographic evidence with ad-integrum restitution of myocardial function after a temporarily withdrawal of ATRA. The patient recovered completely without use of steroids and was subsequently retreated with ATRA without recurrence of the peri-myocarditis.
Acknowledgements
The authors would like to thank the team of the department of Cardiology and department of Hematology of Mohammed V Military Hospital for providing support and helping in preparing this manuscript.
References
- 1.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 2.Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP., Jr The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292–296. doi: 10.7326/0003-4819-117-4-292. [DOI] [PubMed] [Google Scholar]
- 3.De Botton S, Dombret H, Sanz M, Miguel JS, Caillot D, Zittoun R, et al. Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1998;92:2712–2718. [PubMed] [Google Scholar]
- 4.Choi S, Kim HS, Jung CS, Jung SW, Lee YJ, Rheu JK, et al. Reversible Symptomatic Myocarditis Induced by All-Trans Retinoic Acid Administration during Induction Treatment of Acute Promyelocytic Leukemia: Rare Cardiac Manifestation as a Retinoic Acid Syndrome. J Cardiovasc Ultrasound. 2011;19:95–98. doi: 10.4250/jcu.2011.19.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein SK, Biemond BJ, van Oers MH. Two cases of isolated symptomatic myocarditis induced by all-trans retinoic acid (ATRA) Ann Hematol. 2007;86:917–918. doi: 10.1007/s00277-007-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenaux P, De Botton S. Retinoic acid syndrome. Recognition, prevention and management. Drug Saf. 1998;18:273–279. doi: 10.2165/00002018-199818040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3:e2011059. doi: 10.4084/MJHID.2011.059. [DOI] [PMC free article] [PubMed] [Google Scholar]