Abstract
In recent decades, biomedical research has focused on understanding the functionality of the human translated genome, which represents a minor part of all genetic information transcribed from the human genome. However, researchers have become aware of the importance of non-coding RNA species that constitute the vast majority of the transcriptome. In addition to their crucial role in tissue development and homeostasis, mounting evidence shows non-coding RNA to be deregulated and functionally contributing to the development and progression of different types of human disease including cancer both in adults and children. Small non-coding RNAs (i.e., microRNA) are in the vanguard of clinical research which revealed that RNA could be used as disease biomarkers or new therapeutic targets. Furthermore, many more expectations have been raised for long non-coding RNAs, by far the largest fraction of non-coding transcripts, and still fewer findings have been translated into clinical applications. In this review, we center on PVT1, a large and complex long non-coding RNA that usually confers oncogenic properties on different tumor types. We focus on the compilation of early advances in the field of pediatric tumors which often lags behind clinical improvements in adult tumors, and provide a rationale to continue studying PVT1 as a possible functional contributor to pediatric malignancies and as a potential prognostic marker or therapeutic target.
Keywords: PVT1, lncRNA, epigenetic, 8q32, pediatric cancer
Pediatric Cancer
Pediatric cancers are rare diseases which differ from adult malignancies owing to their etiology, biology, response to treatment, and outcome. The most frequent adult cancers are of epithelial origin and, in some cases, are caused by environmental factors. By contrast, pediatric tumors tend to be of hematologic, mesenchymal, or nervous system origin and their etiology is often little known (1). Approximately 160,000 cases of cancer in children and adolescents are diagnosed every year worldwide, accounting for up to 2% of all cancers (2). In recent decades, with the introduction of multimodal treatments, the outcome of children and adolescents has improved, reaching ~80% of overall survival. Despite this significant improvement, cancer remains the leading cause of death in children, and adolescents worldwide (3). Furthermore, two thirds of patients may suffer severe side effects associated with these intensive treatments (4). Thus, the development of safe and more effective therapies is a must.
LncRNAs and Cancer
The Emerging Opportunity of Long Non-coding RNAs (LncRNAs)
Current therapies are directed at targeting the functionality of the human translated genome (i.e., proteins), barely 2% of all transcribed genetic information (5). Therefore, use of the largest part of the transcribed genome for therapeutic, diagnostic, and prognostic purposes remains unexplored (6). Recent advances in high-throughput sequencing technologies have been crucial for improving understanding of non-coding RNA (ncRNA), which represents a significant fraction of all transcribed RNAs, and in the past, were considered evolutionary junk or transcriptional noise (7, 8). However, ncRNA are now known to be important regulators of biological processes such as gene expression (9). Furthermore, interest in ncRNAs has grown owing to their implication in different diseases, such as cancer (6, 10).
In general, ncRNAs are classified according to their length as small ncRNAs (sncRNAs) or long ncRNAs (lncRNAs), smaller than 200 nt and larger than 200 nt, respectively (11).
Functions of LncRNAs
LncRNAs functionality depends on their subcellular distribution (12). LncRNAs located in the nucleus can regulate gene expression at different levels: (a) LncRNAs can interact with transcription factors or chromatin-remodeling complexes to regulate gene expression in cis, i.e., when the lncRNAs locus is proximal to the regulated gene, or in trans, when the gene affected and lncRNAs are at distant locations of the genome (13). For example ANRIL is a lncRNA that interacts with the polycomb repressive complex-1 (PRC1) and−2 (PRC2) and mediates transcriptional silencing of INK4b-ARF-INK4a locus. Specifically, ANRIL binds to Suz12 to recruit PRC2 complex which initiates H3K27me3, a post-translational histone mark indicative of transcriptional repression (14); (b) LncRNAs may bind directly to DNA, causing chromatin remodeling or looping, where the lncRNAs can recruit chromatin modifiers (e.g., histone methyltransferases, DNA methyltransferases) to enhance or repress gene transcription (15). In fact, there are evidence of sequence-specific interactions of lncRNAs with DNA via triple-helix (triplex) formation, a structure that allows lncRNAs to recruit protein complexes to specific genomic regions and regulate gene expression. For example, the GATA6-AS lncRNA regulates the expression of several genes related to cardiac development using this mechanism (16); (c) finally, other functions of lncRNAs such as their participation in splicing and export or translation of mRNA have also been described (17). One of these examples is the lncRNA Fas-antisense or Saf, which interacts with Fas receptor pre-mRNA and with the human splicing factor 45 (SPF45) in the nucleus of the cell. As a consequence, the exclusion of exon 6 from the Fas mRNA is produced. The resulting protein product is a soluble Fas that protects cells against FasL-induced apoptosis (18).
When lncRNAs are in cytoplasm, they can interact with proteins, thereby either activating or inhibiting its function (19). For example, lincRNA-21 is able to interact with the Heterogeneous Nuclear Ribonucleoprotein K, and modulates the P53 transcriptional response (20). Furthermore, lncRNAs may also regulate mRNA stability and translation processes. For instance, the TMPO-AS1 lncRNA regulates the expression of Thymopoietin, a protein involved in the maintenance of the nuclear envelope structure. Qin et al. demonstrated that the knockdown of TMPO-AS1 also resulted in a decrease of TMPO mRNA and protein levels (21).
However, one of the most described roles of lncRNAs in cytoplasm is microRNA (miRNA) sequestration. LncRNAs may contain multiple miRNA binding sites and can sequester them, thereby reducing their availability for their target genes (22). In particular, lncRNAs may regulate the expression of a certain mRNA by having a miRNA recognition element (MRE) in its sequence. This competition for a miRNA gives name to the competing endogenous RNA (ceRNA) hypothesis formulated by Salmena et al. (23). One of such examples is the lncRNA FER1L4, which modulates PTEN expression by sponging miR-106a-5p in gastric (24) or in colon cancer (25).
LncRNAs present evolutionarily-conserved secondary structure and function, although their exon sequence is much less conserved than protein-coding genes (8, 26). LncRNAs have been reported to present tissue-specific expression and their deregulation has been associated with cancer development, metastasis, and patient outcome (13) often by regulating oncogenes or tumor suppressors.
For example, one of the most studied lncRNAs is the Hox transcript antisense intergenic RNA (HOTAIR). HOTAIR, has been shown to be overexpressed in multiple human tumors such as breast, pancreatic, liver, lung, and hepatocellular cancer among others, and has been involved in different hallmarks of cancer (6, 27). Mechanistically, HOTAIR acts as a scaffold for the chromatin repressors polycomb repressor complex 2 (PRC2) and histone lysine-specific demethylase 1 (LSD1) and may perform a cis and trans-regulation of HOX genes and HOXD cluster, respectively (28). While the bibliography is abundant for tumors in adult patients, examples are scant in the field of pediatric cancer. One such example is the lncRNA neuroblastoma-associated transcript 1 (NBAT-1) which was associated with good prognosis in high-risk neuroblastoma patients (29). Mechanistically, NBAT-1 epigenetically controls the expression of target genes involved in cell proliferation, differentiation and cell invasion by interacting with EZH2, a subunit of PRC2 (29).
Plasmacytoma Variant Translocation 1 (PVT1)
One of the lncRNAs that is attracting attention in both pediatric and adult tumors is the plasmacytoma variant translocation 1 (PVT1). Initially described in the middle 80's, this gene was identified as a breakpoint site in chromosome 6;15 translocations, which are associated with murine plasmacytomas (30). In humans, this kind of translocation occurs in rare variants of Burkitt's lymphoma (31). Herein, we focus on this complex and multi-functional lncRNA to raise awareness and pave the way toward better characterization of its use as a potential prognostic marker or therapeutic target in pediatric cancer.
PVT1 Gene Structure
“Plasmacytoma variant translocation 1” or PVT1 is a long intergenic ncRNA encoded in the human PVT1 gene (Figure 1). PVT1 gene is located at the chromosomal locus 8q24.21 which contains the MYC oncogene. This region is frequently altered in many types of cancer owing to chromosomal translocations, amplifications or deletions, single nucleotide polymorphisms (SNP) and/or viral integrations (32, 33). The PVT1 gene contains nine exons, which produce multiple transcripts between 2.7 and 3.3 kb in length by alternative splicing (32). Thus, far, none of these transcripts have been shown to be translated into a protein product (32).
Figure 1.

Graphical representation of the 8q24 genomic locus.
In addition to these splicing variants, PVT1 contains a cluster of five different microRNA (i.e., miR-1204, miR-1205, miR-1206, miR-1207, and miR-1208) which are encoded in the intronic regions of PVT1, except miR-1204, which is located in exon 1b (32, 34). Furthermore, PVT1 encodes a circular RNA, which is presented as a covalent closed loop structure without polyadenylated tail (35, 36). This structure, indeed, also has the capacity to sequester miRNAs such as miR-497 (37, 38) or miR-125 (39, 40).
PVT1 Participates in Embryonic Development
Pediatric cancers are frequently originated in developing organs where the integration of multiple signaling pathways related to proliferation, growth factor signaling, developmental angiogenesis and programmed cell death, take place (41). It is in this scenario where lncRNA such as PVT1 become critical for proper physiologic development (42). One of such examples is a recent report showing that PVT1 is involved in the early stages of development, such as pre-implantation and oocyte maturation (43). Moreover, the loss of PVT1 function may also be implicated in human disease. For example, PVT1 expression was found to be significantly lower in pregnant women with preeclampsia than that in women with normal pregnancies. Loss of function experiments in trophoblast cell lines showed that PVT1 silencing resulted in cell proliferation inhibition and apoptosis induction. Conversely, ectopic expression of PVT1 increased trophoblastic cell proliferation (44).
In following steps of embryonic development, PVT1 has also been shown to play a significant role in neuronal differentiation. Apparently, PVT1 expression is high in pluripotent stem cells, whereas it decreases during neuronal differentiation (45).
Functional Role of PVT1 in Cancer
Several studies reported that PVT1 is overexpressed in a wide variety of cancers compared to non-tumoral tissues, including breast and ovarian cancer (46), pancreatic ductal adenocarcinoma (47), cholangiocarcinoma (47), thyroid carcinoma (47), malignant pleural mesothelioma (47), pediatric malignant astrocytoma (46), acute myeloid leukemia (46), and Hodgkin's lymphoma (46). Less evidence, however, also supports a potential role of PVT1 as a tumor suppressor (48).
PVT1 may impact on tumor biology through different mechanisms:
Role of the miRNA Cluster
PVT1 encodes a cluster of five miRNA (miR-1204, miR-1205, miR-1206, miR-1207, and miR-1208) (34) which have been shown to play both oncogenic and tumor-suppressive roles. For example, miR-1204 has been shown to be overexpressed and associated with poor prognosis in breast cancer (BC). The ectopic expression of miR-1204 promoted proliferation, epithelial to mesenchymal transition and invasion of BC cells by targeting the vitamin D receptor (49). MiR-1204 was also shown to promote cell proliferation in different types of cancer such as ovarian squamous cell carcinoma (50), non-small cell lung cancer (51), or in hepatocellular carcinoma (52). In line with these results, miR-1205 expression was found to be increased in primary prostate tumors and castration-resistant prostate cancer cell lines. Functional analyses revealed that miR-1205 induced cell proliferation and cell cycle progression (53). Concurring with these results, miR-1205 inhibition impaired osteosarcoma proliferation in vitro and in vivo by suppressing Wnt/β-catenin activation (54). Overexpression of miR-1207-5p has been shown to promote cancer stem cells traits in ovarian cancer cells by targeting inhibitors of the Wnt/β-catenin pathways (55) and induce breast cancer cell proliferation by targeting STAT6 (56).
Conversely, tumor suppressive roles for some of these miRNAs have also been reported. For example, ectopic expression of miR-1205 significantly inhibited tumor growth in NSCLC mouse xenograft by targeting MDM4 and E2F1. In addition, multiple circRNA have been suggested to sponge miR-1205 in glioma (57–59) and papillary thyroid cancer (60) to increase oncogenicity of these tumors. MiR-1207-5p inhibition induced stem phenotype and expression of stem cell markers, such as OCT4, C-MYC, and SOX2, in nasopharyngeal cancer (61). MiR-1207-5p also has shown to reduce cell invasion in vitro and metastasis in a lung cancer mouse xenograft model (62).
PVT1-MYC Interaction
The proximal location between the well-established oncogene MYC and PVT1 in the 8q24 region prompted researchers to analyze whether this lncRNA may somehow regulate or be regulated by MYC, a master regulator of cell growth, proliferation, and differentiation, widely implicated in cancer (47). In fact, genomic co-amplification of MYC and PVT1 has been reported in different types of tumor (63). The first evidence found for the interaction between these two molecules was reported by Carramusa et al. who found that PVT1 can be transcriptionally regulated by MYC proteins (64). Further in depth studies revealed that PVT1 interacts with MYC in the nucleus and inhibits its degradation by blocking MYC phosphorylation at threonine 58 (65). Moreover, stabilized MYC protein can activate a positive feedback loop, thereby activating PVT1 expression. Hence, MYC and PVT1 enhance each other's oncogenic effects (65). Concurring with these observations, siRNA-mediated silencing of PVT1 resulted in a reduction in MYC protein levels in different tumor types such as colorectal carcinoma, breast, and ovarian cancer (33). Another level of regulation is also possible, since MYC can be targeted by miR-1205, one of the miRNA encoded in the PVT1 RNA (66). Other authors, however, suggested that the oncogenic activities of MYC and PTV1 are independent of each other (67).
PVT1 as a Mediator of the Tumor Suppressor Role of p53
Since p53 modulates the expression of several coding and non-coding genes, Barsotti et al. analyzed a potential relationship between p53 and PVT1. Experimental evidence demonstrated that PVT1 contains several p53-binding elements and that certain stress-inducing agents such as DNA-damaging drugs, induced p53-dependent expression of PVT1. The authors went further and found mature levels of miR-1204 to be raised after p53 activation and that it was one of the mediators of the tumor-suppressive functions of p53 in colon cancer cell lines (46).
PVT1 as a Competing Endogenous RNA (ceRNA)
ceRNA are transcripts that may contain multiple miRNA binding sites (alternatively known as mRNA response elements) that regulate gene expression by sequestering miRNA which otherwise would be bound to their target mRNA. Several studies reported that ceRNA are key regulators of cancer progression (68, 69). Several miRNA have been reported to interact directly with PVT1 in different cancers (Table 1) and, importantly, restoration of miRNA levels partially rescues the oncogenic effect of PVT1 overexpression (40, 102).
Table 1.
Examples of PVT1-interacting microRNAs.
| Type of cancer | Effects of PVT1 silencing | MiRNA | Targets | References |
|---|---|---|---|---|
| Bladder cancer | ↓ Tumor growth | miR-31-5p | CDK1 | (70) |
| ↓ Tumor growth | miR-128-3p | VEGFC | (71) | |
| Cervical cancer | ↑ Cell apoptosis | miR-195-5p | SMAD3 | (72) |
| Colorectal cancer | ↓ Tumor growth | miR-455-5p | RUNX2 | (73) |
| ↓ Cell proliferation, invasion, and migration | miR-26b | - | (74) | |
| ESCC | ↓ Tumor growth | miR-203a-3p | LASP1 | (75) |
| Hepatocellular carcinoma | ↓ Tumor growth | miR-150-5p | HIG2 | (76) |
| - | miR-365-3p | ATG3 | (77) | |
| ↓ Cell proliferation and invasion | miR-186-5p | YAP1 | (78) | |
| HUVEC* | ↓ Cell migration | miR-26b-5p | ANGPT2 and CTGF | (79) |
| Gallbladder cancer | ↓ Cell proliferation and invasion | miR-143-3p | HK2 | (80) |
| Gastric cancer | - | miR-152-3p | CD151, FGF2 | (81) |
| - | miR-216a-5p | YBX1 | (82) | |
| ↓ Cell proliferation and migration | miR-186-5p | - | (83) | |
| Glioma | ↓ Tumor growth | miR-190a-5p | MEF2C | (84) |
| ↓ Tumor growth | miR-488-3p | |||
| ↓ Invasion and migration | miR-200a-3p | - | (85) | |
| ↓ Tumor growth | miR-128-3p | GREM1 | (86) | |
| ↓ Cell proliferation | miR-186-5p | - | (87) | |
| Lung cancer | ↓ Cell proliferation | miR-126-3p | SLC7A5 | (88) |
| LSCC | ↓ Cell proliferation and migration | miR-519d-3p | - | (89) |
| Melanoma | ↓ Cell proliferation | miR-26b-5p | - | (90) |
| NSCLC | ↓ Tumor growth | miR-195-5p | - | (91) |
| ↓ Tumor growth | miR-497-5p | - | (92) | |
| ↓ Cell proliferation | miR-200a-5p | MMP9 | (93) | |
| miR-200b-5p | ||||
| - | miR-216b | Beclin 1 | (94) | |
| ↓ Cell proliferation and invasion | miR-125b-5p | E2F2 | (40) | |
| Osteosarcoma | ↓ Tumor growth | miR-195-5p | - | (95) |
| ↑ Chemoresistance to gemcitabine | miR-152-3p | c-MET | (96) | |
| ↓ Cell proliferation | miR-497-5p | HK2 | (97) | |
| Ovarian cancer | ↓ Cell proliferation, invasion, and migration | miR-133a-3p | - | (98) |
| ↓ Cell proliferation | miR-140-5p | - | (99) | |
| Pancreatic cancer | - | miR-488-3p | - | (100) |
| ↓ Cell proliferation, invasion and migration | miR-448 | SERBP1 | (100) | |
| ↓ Tumor growth | miR-20a-5p | ULK1 | (101) | |
| PTC | ↓ Cell proliferation and invasion | miR-30a-5p | IGF1R | (102) |
| Prostate cancer | ↓ Cell proliferation and migration | miR-186-5p | Twist1 | (103) |
| Renal cell carcinoma | ↓ Cell proliferation and invasion | miR-16-5p | - | (104) |
| Retinoblastoma | ↓ Tumor growth | miR-488-3p | Notch2 | (105) |
ESCC, Esophageal squamous cell carcinoma; LSCC, Laryngeal squamous cell carcinoma; NSCLC, Non-small cell lung cancer; PTC, Papillary thyroid carcinoma; HUVEC, Human umbilical vein endothelial cells.
Indicates that experiments were performed in non-tumoral human cell lines.
PVT1 and Pediatric Cancer
Very little is known on the molecular mechanisms that could alter PVT1 expression in pediatric tumors. While genetic aberrations at the 8q24 locus could be responsible for deregulated PVT1 levels in adult malignancies, few cases have been described in pediatric tumors. For example, recurrent translocations of PVT1-MYC or PVT1-NDRG1 were identified in a large study on medulloblastoma, the most malignant brain tumor in children (106), or genomic amplifications of MYC/PVT1 in pediatric gliomas (107). Additional genetic alterations have been reported in the St. Jude PeCAn Data Portal. The analysis of the PVT1 gene in a cohort of 3,769 pediatric cancer samples from the projects PCGP (St. Jude-WashU Pediatric Cancer Genome Project) (108) and TARGET (Therapeutically Applicable Research to Generate Effective Treatments) (109) reveals 62 different intronic single nucleotide variants and small insertions and deletions, 8 copy neutral loss of heterozygosity, 50 copy number variants, 43 DNA structural variants, and 6 RNA-seq fusions. However, the clinical relevance of these alterations remains to be determined.
Regardless of these examples, transcriptomic analysis of PVT1 showed it to be expressed in different pediatric tumors, with higher levels corresponding to hematologic malignancies and sarcoma, and more moderate expression in nervous system tumors (Figure 2), thereby suggesting that PVT1 has the potential to be used as a prognostic marker or as a therapeutic target in some pediatric tumors. Further studies, however, are needed to reveal the mechanism(s) controlling PVT1 expression in childhood tumors.
Figure 2.
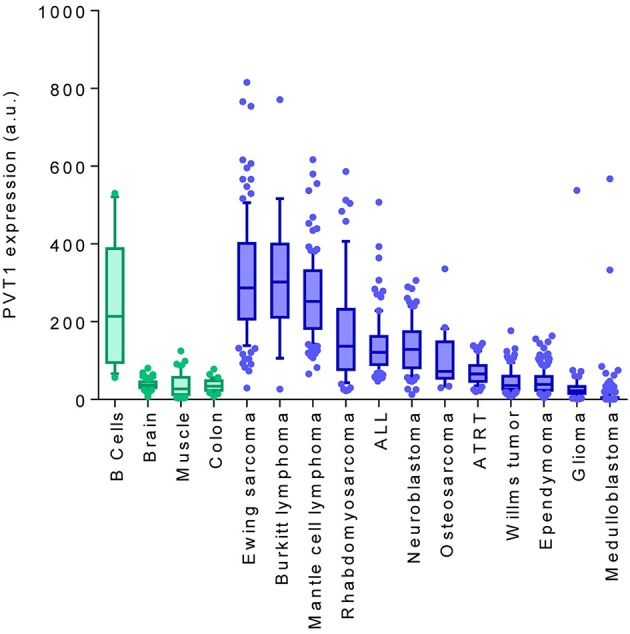
PVT1 expression comparing healthy tissues (green) with pediatric tumors (blue). PVT1 RNA expression levels were obtained from publicly-available Affymetrix expression array (u133p2) datasets using the “R2: Genomics Analysis and Visualization Platform” software. B cells (GSE12366), brain (GSE11882), muscle (GSE9103), colon (GSE8671), Ewing sarcoma (GSE34620), burkitt lymphoma (GSE26673), mantle cell lymphoma (GSE93291), rhabdomyosarcoma (GSE66533), ALL (GSE68720), neuroblastoma (GSE16476), osteosarcoma (GSE14827), ATRT (GSE70678), wilm's tumor (R2 ID: ps_avgpres_wilmsocg125_u133p2), ependymoma (GSE64415), glioma (GSE19578), medulloblastoma (R2 ID: ps_avgpres_mb500affym223_u133p2). a.u, Arbitraty units.
Prognostic Value of PVT1 in Pediatric Cancer
While mounting evidence suggests that PVT1 could be a prognostic marker in several adult malignancies (110), very few analyses have been carried out in pediatric tumors. Indeed, Song et al. analyzed PVT1 expression in osteosarcoma (OS), the most common malignant bone tumor in children, adolescents and young adults. The authors found PVT1 to be overexpressed in OS cell lines and in tumor samples compared with healthy tissue. Furthermore, high PVT1 RNA levels correlated with poor overall survival (97).
In line with these observations, we sought to determine whether PVT1 could also be a prognostic marker in other pediatric cancers. Data mining of publicly-available databases which contain PVT1 expression along with annotated clinical parameters was performed for wilm's tumor (n = 148, R2 ID:ps_avgpres_wilmsocga148_u133a), neuroblastoma (n = 476, GSE45547), mantle cell lymphoma (n = 122, GSE93291), ewing's sarcoma (n = 52, GSE17679), osteosarcoma (n = 88, GSE42352), and pediatric glioma (n = 47, SE19578), and revealed that, in neuroblastoma, the expression of PVT1 could have prognostic value (Figure 3). In addition to PVT1 full RNA, other authors used circular RNA (circRNA) of PTV1 as a potential biomarker. CircRNA are uncapped RNA molecules characterized by a covalently closed loop structure without a 3′ polyadenylated tail (111). Kun-Peng et al. found circPVT1 to be upregulated in OS tumors compared with normal tissues. Moreover, higher circPVT1 levels were found in chemoresistant tumors and, furthermore, high levels of circPVT1 were associated with poor overall survival. Finally, those authors demonstrated that the expression of circPVT1 in serum had better sensitivity and specificity than currently-used OS markers such as alkaline phosphatase (112). Concurring with these observations, high levels of circPVT1 (but not total PVT1) were found to be overexpressed in acute lymphoblastic leukemia (ALL) compared to normal bone marrow samples (113); however, their potential to predict patient outcome remains to be determined.
Figure 3.
Kaplan-Meier survival curves based on PVT1 expression in different pediatric tumors. Kaplan-Meier plots were generated using the “R2: Genomics Analysis and Visualization Platform” software. Patient samples were split according to high (above median) or low (below median) PVT1 expression levels from the following datasets: Wilm's tumor (n = 148, R2 ID:ps_avgpres_wilmsocga148_u133a), neuroblastoma (n = 476, GSE45547), mantle cell lymphoma (n = 122, GSE93291), Ewing's sarcoma (n = 52, GSE17679), osteosarcoma (n = 88, GSE42352), and pediatric glioma (n = 47, SE19578).
PVT1 as a ceRNA in Pediatric Tumors
The most common oncogenic attribute of lncRNA is their capacity to sequester tumor-suppressive miRNA. Some examples of PVT1 have already been described in adult and pediatric malignancies (Table 1). For example, Song et al. showed that PVT1 acts as a sponge to repress miR-497-5p in osteosarcoma cells. In fact, silencing PVT1 resulted in upregulation of miR-497-5p levels which, in turn, target hexokinase 2 (HK2), a key metabolic enzyme that regulates glucose metabolism. Conversely, overexpression of PVT1 reduced miR-497-5p levels and HK2 was upregulated, thereby contributing to enhanced glycolysis, proliferation and motility (97). Similar observations were reported for the interaction of miR-195-5p and PVT1 also in osteosarcoma. In that case, the authors demonstrated that PVT1 silencing resulted in increased migration, invasion potential and cell survival, effects that were partially mediated by miR-195-5p (95). Interestingly, miR-195-5p and miR-497-5p are in the same cluster and belong to the same family, i.e., are potentially regulators of the same biological processes.
Finally, a recent study identified PVT1 as an important contributor to resistance to gemcitabine, a nucleoside analog currently used for the treatment of osteosarcoma. Sun et al. showed that PVT1 interacts and inhibits the function of miR-152-3p, thereby promoting increased resistance to gemcitabine by enhancing the activation of C-MET/PI3K/AKT pathway (96).
These first examples are just the tip of the iceberg, since PVT1 can potentially bind to many more miRNA and should therefore be considered in future studies. Indeed, mining the data available at the experimentally-validated miRNA-LnRNA interactions of the LncBASE (114), revealed that more than 30 different miRNAs are able to interact with PVT1.
Potential Use of PVT1 as a Therapeutic Target in Pediatric Cancer
RNA-based therapies are an emerging alternative to conventional treatments owing, in part, to their potential to target all the transcriptome, thereby expanding the number of potential targets. Indeed, proof of concept experiments which silence PVT1, such as those mentioned previously [i.e., osteosarcoma (97) and ALL (113)] have already shown that targeting PVT1 yielded an anti-tumoral response. RNA interference (RNAi), antisense oligonucleotides (ASO) and genome editing (i.e., CRISPR/Cas9 system) are the only currently-available tools to silence lncRNAs [reviewed in (115, 116)]. Furthermore, the function of PVT1 that needs to be targeted will depend on its subcellular distribution. For example, cytosolic PVT1 could be sensitive to siRNA or shRNA-based strategies, whereas nuclear PVT1 would be more sensitive to ASO. One of the examples in which targeting PVT1 could be a new therapeutic strategy was reported recently. Wu et al. showed that siRNA-mediated silencing of PVT1 caused a marked reduction in cell viability in retinoblastoma cell lines. Furthermore, shRNA-mediated loss of PVT1 function also resulted in a reduced tumor growth in vivo (105).
In addition to, or instead of, targeting the whole PVT1 molecule, targeting single components of PVT1 may be necessary. For example, in a tumor context where the miRNA encoded within PVT1 participate in disease progression, the use of anti-miR would be recommended. Alternatively, if the oncogenic function of PVT1 is related to its capacity to bind and sequester miRNA, miRNA restoration therapies using miRNA mimetics would be the best approach (117).
Conclusions and Future Perspectives
Several indicators suggest that PVT1 could be a future prognostic and therapeutic target for some pediatric tumors. First of all, PVT1 is a necessary element for the correct embryonic development and differentiation of certain cellular lineages. Aberrant PVT1 expression may account for the “undifferentiated” state observed in almost all pediatric malignancies. Second, the mutational burden of pediatric tumors is ~14 times lower than adult tumors (118), which suggests that epigenetic alterations are more likely to participate in tumorigenesis or tumor progression than in adult tumors. Third, the fact that PVT1 has the capacity to bind and modulate the function of multiple tumor-suppressive miRNA, places PVT1 as a master regulator of several biological processes regulated by these miRNA. Further safety studies analyzing the impact of a systemic PVT1 loss of function and finding an appropriate clinical formulation to administer small RNA molecules targeting PVT1, are the next steps in implementing the use of PVT1-based therapies for the treatment of tumors of childhood and adolescence.
Author Contributions
AB, MM, and RA revised bibliography. CJ designed and created the figures. AS, JR, JS, and SG provided intellectual support and expertise in the field of pediatric cancer. AB, MM, and MS wrote the original manuscript. All authors contributed to the edition and the critical review of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ms. Christine O'Hara for English language correction and the members of the Group of Translational Research in Child and Adolescent Cancer for helpful suggestions and discussion. The authors apologize to their colleagues whose work could not be cited in this paper due to space limitations.
Footnotes
Funding. The funding was received by Ministerio de Educación, Cultura y Deporte (Grant no. FPU16/01099), Instituto de Salud Carlos III (Grant no. CP16/00006), Instituto de Salud Carlos III (Grant no. PI17/00564), and Asociación Española Contra el Cáncer (Grant no. LABAE18009SEGU).
References
- 1.Roma J, Almazán-Moga A, Sánchez de Toledo J, Gallego S. SMF. miRNAQ16 targeted therapies in the most prevalent pediatric solid tumors. In: International S, editor. MicroRNA Targeted Cancer Therapy. Springer International Publishing; (2014). p. 239–63. 10.1007/978-3-319-05134-5_14 [DOI] [Google Scholar]
- 2.Rodriguez-Galindo C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. (2015) 33:3065–73. 10.1200/JCO.2014.60.6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-2010: a population-based registry study. Lancet Oncol. (2017) 18:719–31. 10.1016/S1470-2045(17)30186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saletta F, Seng MS, Lau LM. Advances in paediatric cancer treatment. Transl Pediatr. (2014) 3:156–82. 10.3978/j.issn.2224-4336.2014.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J, Kapranov P, Drenkow J, Dike S, Brubaker S, Patel S, et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science. (2005) 308:1149–54. 10.1126/science.1108625 [DOI] [PubMed] [Google Scholar]
- 6.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. (2015) 21:1253–61. 10.1038/nm.3981 [DOI] [PubMed] [Google Scholar]
- 7.Ponjavic J, Ponting CP, Lunter G. Functionality or transcriptional noise? Evidence for selection within long non-coding RNAs. Genome Res. (2007) 17:556–65. 10.1101/gr.6036807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long non-coding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. (2012) 22:1775–89. 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. (2012) 482:339–46. 10.1038/nature10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JJ, Tay Y. Non-coding RNA:RNA regulatory networks in cancer. Int J Mol Sci. (2018) 19:1310 10.3390/ijms19051310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. (2007) 316:1484–8. 10.1126/science.1138341 [DOI] [PubMed] [Google Scholar]
- 12.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. (2009) 10:155–9. 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 13.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. (2011) 1:391–407. 10.1158/2159-8290.CD-11-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilo F, Zhou MM, Walsh MJ. Long non-coding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res. (2011) 71:5365–9. 10.1158/0008-5472.CAN-10-4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. (2018) 19:143–57. 10.1038/nrm.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo CC, Hanzelmann S, Senturk Cetin N, Frank S, Zajzon B, Derks JP, et al. Detection of RNA-DNA binding sites in long non-coding RNAs. Nucleic Acids Res. (2019) 47:e32 10.1093/nar/gkz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanly DJ, Esteller M, Berdasco M. Interplay between long non-coding RNAs and epigenetic machinery: emerging targets in cancer? Philos Trans R Soc Lond B Biol Sci. (2018) 373:20170074. 10.1098/rstb.2017.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villamizar O, Chambers CB, Riberdy JM, Persons DA, Wilber A. Long non-coding RNA Saf and splicing factor 45 increase soluble Fas and resistance to apoptosis. Oncotarget. (2016) 7:13810–26. 10.18632/oncotarget.7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt AM, Chang HY. Long non-coding RNAs in cancer pathways. Cancer Cell. (2016) 29:452–63. 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. A large intergenic non-coding RNA induced by p53 mediates global gene repression in the p53 response. Cell. (2010) 142:409–19. 10.1016/j.cell.2010.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Z, Zheng X, Fang Y. Long non-coding RNA TMPO-AS1 promotes progression of non-small cell lung cancer through regulating its natural antisense transcript TMPO. Biochem Biophys Res Commun. (2019) 516:486–93. 10.1016/j.bbrc.2019.06.088 [DOI] [PubMed] [Google Scholar]
- 22.Kondo Y, Shinjo K, Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. (2017) 108:1927–33. 10.1111/cas.13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. (2011) 146:353–8. 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia T, Chen S, Jiang Z, Shao Y, Jiang X, Li P, et al. Long non-coding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci Rep. (2015) 5:13445 10.1038/srep13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue B, Sun B, Liu C, Zhao S, Zhang D, Yu F, et al. Long non-coding RNA Fer-1-like protein 4 suppresses oncogenesis and exhibits prognostic value by associating with miR-106a-5p in colon cancer. Cancer Sci. (2015) 106:1323–32. 10.1111/cas.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balas MM, Johnson AM. Exploring the mechanisms behind long non-coding RNAs and cancer. Non-coding RNA Res. (2018) 3:108–17. 10.1016/j.ncrna.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q, Hann SS. HOTAIR: an oncogenic long non-coding RNA in human cancer. Cell Physiol Biochem. (2018) 47:893–913. 10.1159/000490131 [DOI] [PubMed] [Google Scholar]
- 28.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by non-coding RNAs. Cell. (2007) 129:1311–23. 10.1016/j.cell.2007.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey GK, Mitra S, Subhash S, Hertwig F, Kanduri M, Mishra K, et al. The risk-associated long non-coding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell. (2014) 26:722–37. 10.1016/j.ccell.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 30.Cory S, Graham M, Webb E, Corcoran L, Adams JM. Variant (6;15) translocations in murine plasmacytomas involve a chromosome 15 locus at least 72 kb from the c-myc oncogene. EMBO J. (1985) 4:675–81. 10.1002/j.1460-2075.1985.tb03682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham M, Adams JM. Chromosome 8 breakpoint far 3' of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. (1986) 5:2845–51. 10.1002/j.1460-2075.1986.tb04578.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huppi K, Pitt JJ, Wahlberg BM, Caplen NJ. The 8q24 gene desert: an oasis of non-coding transcriptional activity. Front Genet. (2012) 3:69. 10.3389/fgene.2012.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo T, Farina L, Macino G, Paci P. PVT1: a rising star among oncogenic long non-coding RNAs. Biomed Res Int. (2015) 2015:304208 10.1155/2015/304208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huppi K, Volfovsky N, Runfola T, Jones TL, Mackiewicz M, Martin SE, et al. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res. (2008) 6:212–21. 10.1158/1541-7786.MCR-07-0105 [DOI] [PubMed] [Google Scholar]
- 35.Xiang JF, Yang L, Chen LL. The long non-coding RNA regulation at the MYC locus. Curr Opin Genet Dev. (2015) 33:41–8. 10.1016/j.gde.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 36.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. (2013) 9:e1003777. 10.1371/journal.pgen.1003777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verduci L, Ferraiuolo M, Sacconi A, Ganci F, Vitale J, Colombo T, et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. (2017) 18:237. 10.1186/s13059-017-1368-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin S, Zhao Y, Lim G, Lin H, Zhang X, Zhang X. Circular RNA PVT1 acts as a competing endogenous RNA for miR-497 in promoting non-small cell lung cancer progression. Biomed Pharmacother. (2018) 111:244–50. 10.1016/j.biopha.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Li Y, Zheng Q, Bao C, He J, Chen B, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. (2017) 388:208–19. 10.1016/j.canlet.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 40.Li X, Zhang Z, Jiang H, Li Q, Wang R, Pan H, et al. Circular RNA circPVT1 promotes proliferation and invasion through sponging miR-125b and activating E2F2 signaling in non-small cell lung cancer. Cell Physiol Biochem. (2018) 51:2324–40. 10.1159/000495876 [DOI] [PubMed] [Google Scholar]
- 41.Federico S, Brennan R, Dyer MA. Childhood cancer and developmental biology a crucial partnership. Curr Top Dev Biol. (2011) 94:1–13. 10.1016/B978-0-12-380916-2.00001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcu KB, Bossone SA, Patel AJ. myc function and regulation. Annu Rev Biochem. (1992) 61:809–60. 10.1146/annurev.biochem.61.1.809 [DOI] [PubMed] [Google Scholar]
- 43.Wu F, Liu Y, Wu Q, Li D, Zhang L, Wu X, et al. Long non-coding RNAs potentially function synergistically in the cellular reprogramming of SCNT embryos. BMC Genomics. (2018) 19:631. 10.1186/s12864-018-5021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Lian Y, Zhang Y, Huang S, Zuo Q, Yang N, et al. The long non-coding RNA PVT1 represses ANGPTL4 transcription through binding with EZH2 in trophoblast cell. J Cell Mol Med. (2018) 22:1272–82. 10.1111/jcmm.13405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin M, Pedrosa E, Shah A, Hrabovsky A, Maqbool S, Zheng D, et al. RNA-Seq of human neurons derived from iPS cells reveals candidate long non-coding RNAs involved in neurogenesis and neuropsychiatric disorders. PLoS ONE. (2011) 6:e23356. 10.1371/journal.pone.0023356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-dependent induction of PVT1 and miR-1204. J Biol Chem. (2012) 287:2509–19. 10.1074/jbc.M111.322875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui M, You L, Ren X, Zhao W, Liao Q, Zhao Y. Long non-coding RNA PVT1 and cancer. Biochem Biophys Res Commun. (2016) 471:10–4. 10.1016/j.bbrc.2015.12.101 [DOI] [PubMed] [Google Scholar]
- 48.Cho SW, Xu J, Sun R, Mumbach MR, Carter AC, Chen YG, et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell. (2018) 173:1398–412 e22. 10.1016/j.cell.2018.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Bi L, Wang Q, Wen M, Li C, Ren Y, et al. miR-1204 targets VDR to promotes epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene. (2018) 37:3426–39. 10.1038/s41388-018-0215-2 [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Gu X, Yang X, Meng Y. MiR-1204 promotes ovarian squamous cell carcinoma growth by increasing glucose uptake. Biosci Biotechnol Biochem. (2018) 83:1–6. 10.1080/09168451.2018.1527208 [DOI] [PubMed] [Google Scholar]
- 51.Jiang W, He Y, Shi Y, Guo Z, Yang S, Wei K, et al. MicroRNA-1204 promotes cell proliferation by regulating PITX1 in non-small-cell lung cancer. Cell Biol Int. (2018) 43:253–64. 10.1002/cbin.11083 [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Sun L, Wang Y, Yao B, Liu R, Chen T, et al. miR-1204 promotes hepatocellular carcinoma progression through activating MAPK and c-Jun/AP1 signaling by targeting ZNF418. Int J Biol Sci. (2019) 15:1514–22. 10.7150/ijbs.33658 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Wang Y, Li X, Liu W, Li B, Chen D, Hu F, et al. MicroRNA-1205, encoded on chromosome 8q24, targets EGLN3 to induce cell growth and contributes to risk of castration-resistant prostate cancer. Oncogene. (2019) 38:4820–34. 10.1038/s41388-019-0760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z, Shi W, Jiang C. Overexpressing circular RNA hsa_circ_0002052 impairs osteosarcoma progression via inhibiting Wnt/beta-catenin pathway by regulating miR-1205/APC2 axis. Biochem Biophys Res Commun. (2018) 502:465–71. 10.1016/j.bbrc.2018.05.184 [DOI] [PubMed] [Google Scholar]
- 55.Wu G, Liu A, Zhu J, Lei F, Wu S, Zhang X, et al. MiR-1207 overexpression promotes cancer stem cell-like traits in ovarian cancer by activating the Wnt/beta-catenin signaling pathway. Oncotarget. (2015) 6:28882–94. 10.18632/oncotarget.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan C, Chen Y, Kong W, Fu L, Liu Y, Yao Q, et al. PVT1-derived miR-1207-5p promotes breast cancer cell growth by targeting STAT6. Cancer Sci. (2017) 108:868–76. 10.1111/cas.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang M, Li G, Fan L, Zhang G, Xu J, Zhang J. Circular RNA circ_0034642 elevates BATF3 expression and promotes cell proliferation and invasion through miR-1205 in glioma. Biochem Biophys Res Commun. (2019) 508:980–5. 10.1016/j.bbrc.2018.12.052 [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Zhang Y, Chen B, Ding L, Mu Z, Li Y. Elevation of circular RNA circ-POSTN facilitates cell growth and invasion by sponging miR-1205 in glioma. J Cell Biochem. (2019) 120:16567–74. 10.1002/jcb.28916 [DOI] [PubMed] [Google Scholar]
- 59.Xiong J, Wang T, Tang H, Lv Z, Liang P. Circular RNA circMAN2B2 facilitates glioma progression by regulating the miR-1205/S100A8 axis. J Cell Physiol. (2019) 234:22996–3004. 10.1002/jcp.28860 [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Ding L, Li Y, Xuan C. Hsa_circ_0039411 promotes tumorigenesis and progression of papillary thyroid cancer by miR-1179/ABCA9 and miR-1205/MTA1 signaling pathways. J Cell Physiol. (2019). 10.1002/jcp.29048. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 61.Cui M, Chang Y, Fang QG, Du W, Wu JF, Wang JH, et al. Non-coding RNA Pvt1 promotes cancer stem cell-like traits in nasopharyngeal cancer via inhibiting miR-1207. Pathol Oncol Res. (2018) 25:1411–22. 10.1007/s12253-018-0453-1 [DOI] [PubMed] [Google Scholar]
- 62.Dang W, Qin Z, Fan S, Wen Q, Lu Y, Wang J, et al. miR-1207-5p suppresses lung cancer growth and metastasis by targeting CSF1. Oncotarget. (2016) 7:32421–32. 10.18632/oncotarget.8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. (2014) 512:82–6. 10.1038/nature13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carramusa L, Contino F, Ferro A, Minafra L, Perconti G, Giallongo A, et al. The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J Cell Physiol. (2007) 213:511–8. 10.1002/jcp.21133 [DOI] [PubMed] [Google Scholar]
- 65.Tseng YY, Bagchi A. The PVT1-MYC duet in cancer. Mol Cell Oncol. (2015) 2:e974467. 10.4161/23723556.2014.974467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michelle K, Naidoo DKD, Adeodat Ilboudo, Akintunde Orunmuyi, Gabriel O, Ogun SA. editors. MicroRNA-1205 as a tumor suppressor in castration resistant prostate cancer [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; Chicago, PA: AACR; Cancer Res; (2018). [Google Scholar]
- 67.Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. (2007) 13:5745–55. 10.1158/1078-0432.CCR-06-2882 [DOI] [PubMed] [Google Scholar]
- 68.Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. (2015) 52:710–8. 10.1136/jmedgenet-2015-103334 [DOI] [PubMed] [Google Scholar]
- 69.Qu J, Li M, Zhong W, Hu C. Competing endogenous RNA in cancer: a new pattern of gene expression regulation. Int J Clin Exp Med. (2015) 8:17110–6. [PMC free article] [PubMed] [Google Scholar]
- 70.Tian Z, Cao S, Li C, Xu M, Wei H, Yang H, et al. LncRNA PVT1 regulates growth, migration, and invasion of bladder cancer by miR-31/ CDK1. J Cell Physiol. (2019) 234:4799–811. 10.1002/jcp.27279 [DOI] [PubMed] [Google Scholar]
- 71.Yu C, Longfei L, Long W, Feng Z, Chen J, Chao L, et al. LncRNA PVT1 regulates VEGFC through inhibiting miR-128 in bladder cancer cells. J Cell Physiol. (2019) 234:1346–53. 10.1002/jcp.26929 [DOI] [PubMed] [Google Scholar]
- 72.Shen CJ, Cheng YM, Wang CL. LncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J Drug Target. (2017) 25:637–44. 10.1080/1061186X.2017.1307379 [DOI] [PubMed] [Google Scholar]
- 73.Chai J, Guo D, Ma W, Han D, Dong W, Guo H, et al. A feedback loop consisting of RUNX2/LncRNA-PVT1/miR-455 is involved in the progression of colorectal cancer. Am J Cancer Res. (2018) 8:538–50. [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang R, Li J, Yan X, Jin K, Li W, Liu X, et al. Long non-coding RNA plasmacytoma variant translocation 1 (PVT1) promotes colon cancer progression via endogenous sponging miR-26b. Med Sci Monit. (2018) 24:8685–92. 10.12659/MSM.910955 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Li PD, Hu JL, Ma C, Ma H, Yao J, Chen LL, et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget. (2017) 8:34164–76. 10.18632/oncotarget.15878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, Luo X, He W, Chen G, Li Y, Li W, et al. Long non-coding RNA PVT1/miR-150/ HIG2 axis regulates the proliferation, invasion and the balance of iron metabolism of hepatocellular carcinoma. Cell Physiol Biochem. (2018) 49:1403–19. 10.1159/000493445 [DOI] [PubMed] [Google Scholar]
- 77.Yang L, Peng X, Jin H, Liu J. Long non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by sponging microRNA-365 in hepatocellular carcinoma. Gene. (2019) 697:94–102. 10.1016/j.gene.2019.02.036 [DOI] [PubMed] [Google Scholar]
- 78.Lan T, Yan X, Li Z, Xu X, Mao Q, Ma W, et al. Long non-coding RNA PVT1 serves as a competing endogenous RNA for miR-186-5p to promote the tumorigenesis and metastasis of hepatocellular carcinoma. Tumour Biol. (2017) 39:1010428317705338. 10.1177/1010428317705338 [DOI] [PubMed] [Google Scholar]
- 79.Zheng J, Hu L, Cheng J, Xu J, Zhong Z, Yang Y, et al. lncRNA PVT1 promotes the angiogenesis of vascular endothelial cell by targeting miR26b to activate CTGF/ANGPT2. Int J Mol Med. (2018) 42:489–96. 10.3892/ijmm.2018.3595 [DOI] [PubMed] [Google Scholar]
- 80.Chen J, Yu Y, Li H, Hu Q, Chen X, He Y, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. (2019) 18:33. 10.1186/s12943-019-0947-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li T, Meng XL, Yang WQ. Long non-coding RNA PVT1 acts as a “sponge” to inhibit microRNA-152 in gastric cancer cells. Dig Dis Sci. (2017) 62:3021–8. 10.1007/s10620-017-4508-z [DOI] [PubMed] [Google Scholar]
- 82.Zeng X, Liu Y, Zhu H, Chen D, Hu W. Downregulation of miR-216a-5p by long non-coding RNA PVT1 suppresses colorectal cancer progression via modulation of YBX1 expression. Cancer Manag Res. (2019) 11:6981–93. 10.2147/CMAR.S208983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang T, Liu HW, Chen JQ, Wang SH, Hao LQ, Liu M, et al. The long non-coding RNA PVT1 functions as a competing endogenous RNA by sponging miR-186 in gastric cancer. Biomed Pharmacother. (2017) 88:302–8. 10.1016/j.biopha.2017.01.049 [DOI] [PubMed] [Google Scholar]
- 84.Xue W, Chen J, Liu X, Gong W, Zheng J, Guo X, et al. PVT1 regulates the malignant behaviors of human glioma cells by targeting miR-190a-5p and miR-488-3p. Biochim Biophys Acta Mol Basis Dis. (2018) 1864(5 Pt A):1783–94. 10.1016/j.bbadis.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Yang G, Luo Y. Long non-coding RNA PVT1 promotes glioma cell proliferation and invasion by targeting miR-200a. Exp Ther Med. (2019) 17:1337–45. 10.3892/etm.2018.7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu C, Li D, Zhang X, Liu N, Chi G, Jin X. LncRNA PVT1 facilitates tumorigenesis and progression of glioma via regulation of MiR-128-3p/GREM1 axis and BMP signaling pathway. Neurotherapeutics. (2018) 15:1139–57. 10.1007/s13311-018-0649-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Ma Y, Wang P, Xue Y, Qu C, Zheng J, Liu X, et al. PVT1 affects growth of glioma microvascular endothelial cells by negatively regulating miR-186. Tumour Biol. (2017) 39:1010428317694326. 10.1177/1010428317694326 [DOI] [PubMed] [Google Scholar]
- 88.Li H, Chen S, Liu J, Guo X, Xiang X, Dong T, et al. Long non-coding RNA PVT1-5 promotes cell proliferation by regulating miR-126/SLC7A5 axis in lung cancer. Biochem Biophys Res Commun. (2018) 495:2350–5. 10.1016/j.bbrc.2017.12.114 [DOI] [PubMed] [Google Scholar]
- 89.Zheng X, Zhao K, Liu T, Liu L, Zhou C, Xu M. Long non-coding RNA PVT1 promotes laryngeal squamous cell carcinoma development by acting as a molecular sponge to regulate miR-519d-3p. J Cell Biochem. (2019) 120:3911–21. 10.1002/jcb.27673 [DOI] [PubMed] [Google Scholar]
- 90.Wang BJ, Ding HW, Ma GA. Long non-coding RNA PVT1 promotes melanoma progression via endogenous sponging miR-26b. Oncol Res. (2018) 26:675–81. 10.3727/096504017X14920318811730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu D, Li Y, Zhang H, Hu X. Knockdown of Lncrna PVT1 enhances radiosensitivity in non-small cell lung cancer by sponging Mir-195. Cell Physiol Biochem. (2017) 42:2453–66. 10.1159/000480209 [DOI] [PubMed] [Google Scholar]
- 92.Guo D, Wang Y, Ren K, Han X. Knockdown of LncRNA PVT1 inhibits tumorigenesis in non-small-cell lung cancer by regulating miR-497 expression. Exp Cell Res. (2018) 362:172–9. 10.1016/j.yexcr.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 93.Chen W, Zhu H, Yin L, Wang T, Wu J, Xu J, et al. lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell Biol. (2017) 36:787–93. 10.1089/dna.2017.3725 [DOI] [PubMed] [Google Scholar]
- 94.Chen L, Han X, Hu Z, Chen L. The PVT1/miR-216b/Beclin-1 regulates cisplatin sensitivity of NSCLC cells via modulating autophagy and apoptosis. Cancer Chemother Pharmacol. (2019) 83:921–31. 10.1007/s00280-019-03808-3 [DOI] [PubMed] [Google Scholar]
- 95.Zhou Q, Chen F, Zhao J, Li B, Liang Y, Pan W, et al. Long non-coding RNA PVT1 promotes osteosarcoma development by acting as a molecular sponge to regulate miR-195. Oncotarget. (2016) 7:82620–33. 10.18632/oncotarget.13012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun ZY, Jian YK, Zhu HY, Li B. lncRNAPVT1 targets miR-152 to enhance chemoresistance of osteosarcoma to gemcitabine through activating c-MET/PI3K/AKT pathway. Pathol Res Pract. (2019) 215:555–63. 10.1016/j.prp.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 97.Song J, Wu X, Liu F, Li M, Sun Y, Wang Y, et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun. (2017) 490:217–24. 10.1016/j.bbrc.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 98.Yang Q, Yu Y, Sun Z, Pan Y. Long non-coding RNA PVT1 promotes cell proliferation and invasion through regulating miR-133a in ovarian cancer. Biomed Pharmacother. (2018) 106:61–7. 10.1016/j.biopha.2018.06.112 [DOI] [PubMed] [Google Scholar]
- 99.Ding Y, Fang Q, Li Y, Wang Y. Amplification of lncRNA PVT1 promotes ovarian cancer proliferation by binding to miR-140. Mamm Genome. (2019) 30:217–25. 10.1007/s00335-019-09808-1 [DOI] [PubMed] [Google Scholar]
- 100.Zhao L, Kong H, Sun H, Chen Z, Chen B, Zhou M. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol. (2018) 233:4044–55. 10.1002/jcp.26072 [DOI] [PubMed] [Google Scholar]
- 101.Huang F, Chen W, Peng J, Li Y, Zhuang Y, Zhu Z, et al. LncRNA PVT1 triggers Cyto-protective autophagy and promotes pancreatic ductal adenocarcinoma development via the miR-20a-5p/ULK1 Axis. Mol Cancer. (2018) 17:98. 10.1186/s12943-018-0845-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Feng K, Liu Y, Xu LJ, Zhao LF, Jia CW, Xu MY. Long non-coding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA-30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother. (2018) 104:686–98. 10.1016/j.biopha.2018.05.078 [DOI] [PubMed] [Google Scholar]
- 103.Chang Z, Cui J, Song Y. Long non-coding RNA PVT1 promotes EMT via mediating microRNA-186 targeting of Twist1 in prostate cancer. Gene. (2018) 654:36–42. 10.1016/j.gene.2018.02.036 [DOI] [PubMed] [Google Scholar]
- 104.Ren Y, Huang W, Weng G, Cui P, Liang H, Li Y. LncRNA PVT1 promotes proliferation, invasion and epithelial-mesenchymal transition of renal cell carcinoma cells through downregulation of miR-16-5p. Onco Targets Ther. (2019) 12:2563–75. 10.2147/OTT.S190239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu XZ, Cui HP, Lv HJ, Feng L. Knockdown of lncRNA PVT1 inhibits retinoblastoma progression by sponging miR-488-3p. Biomed Pharmacother. (2019) 112:108627. 10.1016/j.biopha.2019.108627 [DOI] [PubMed] [Google Scholar]
- 106.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. (2012) 488:49–56. 10.1038/nature11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. (2012) 124:439–47. 10.1007/s00401-012-0998-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Downing JR, Wilson RK, Zhang J, Mardis ER, Pui CH, Ding L, et al. The pediatric cancer genome project. Nat Genet. (2012) 44:619–22. 10.1038/ng.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma X, Liu Y, Liu Y, Alexandrov LB, Edmonson MN, Gawad C, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. (2018) 555:371–6. 10.1038/nature25795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeng Y, Wang T, Liu Y, Su Z, Lu P, Chen X, et al. LncRNA PVT1 as an effective biomarker for cancer diagnosis and detection based on transcriptome data and meta-analysis. Oncotarget. (2017) 8:75455–66. 10.18632/oncotarget.20634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. (2015) 12:381–8. 10.1080/15476286.2015.1020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kun-Peng Z, Xiao-Long M, Chun-Lin Z. Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int J Biol Sci. (2018) 14:321–30. 10.7150/ijbs.24360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu J, Han Q, Gu Y, Ma J, McGrath M, Qiao F, et al. Circular RNA PVT1 expression and its roles in acute lymphoblastic leukemia. Epigenomics. (2018) 10:723–32. 10.2217/epi-2017-0142 [DOI] [PubMed] [Google Scholar]
- 114.Paraskevopoulou MD, Georgakilas G, Kostoulas N, Reczko M, Maragkakis M, Dalamagas TM, et al. DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. (2013) 41:D239–45. 10.1093/nar/gks1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. (2016) 44:863–77. 10.1093/nar/gkv1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. (2018) 24:257–77. 10.1016/j.molmed.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soriano A, Jubierre L, Almazan-Moga A, Molist C, Roma J, de Toledo JS, et al. microRNAs as pharmacological targets in cancer. Pharmacol Res. (2013) 75:3–14. 10.1016/j.phrs.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 118.Grobner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, et al. The landscape of genomic alterations across childhood cancers. Nature. (2018) 555:321–7. 10.1038/nature25480 [DOI] [PubMed] [Google Scholar]



