Abstract
Objectives
To assess safety and efficacy of 90Y resin microspheres administration using undiluted non-ionic contrast material (UDCM) {100% 0mnipaque-300 (Io-hexol)} in both the “B” and “D” lines.
Materials and methods
We reviewed all colorectal cancer liver metastases patients treated with 90Y resin micro-spheres radioembolization (RAE) from 2009 to 2017. As of April 2013, two experienced operators started using UDCM (study group) instead of standard sandwich infusion (control group). Occurrence of myelosuppression (leukopenia, neutropenia, erythrocytopenia or/and thrombocytopenia), stasis, nontarget delivery (NTD), median fluoroscopy radiation dose (FRD), median infusion time (IT), liver progression-free (LPFS) and overall survivals (OS) was evaluated. Complications within 6 months post-RAE were reported according to CTCAE v3.0 criteria.
Results
Study and control groups comprised 23(28%) and 58(72%) patients, respectively. Median follow-up was 9.1 months. There was no statistically significant difference in myelosuppression incidence within 6 months post-RAE between groups. Median FRD and IT for study and control groups were 44.6 vs. 97.35 Gy/cm2 (p = 0.048) and 31 vs. 39 min (p = 0.006), respectively. A 38% lower stasis incidence in study group was not significant (p = 0.34). NTD occurred in 1/27(4%) study vs. 5/73(7%) control group procedures (p =1). Grade 1–2 and grade 3–4 toxicities between study and control group patients were 36%(8/22) vs. 45%(26/58), p = 0.61 and 9%(2/22) vs. 16%(9/58), p = 0.72, respectively. There was no difference in LPFS and OS between groups.
Conclusion
Administration of 90Y resin microspheres using UDCM in both lines is safe and effective, resulting in lower fluoroscopy radiation dose and shorter infusion time, without evidence of myelosuppression or increased stasis incidence.
Keywords: Radioembolization, Myelosuppression, Yttrium-90, Hepatic malignancy, Colorectal cancer liver metastases, SIR-spheres, Arterially directed therapies, Selective internal radiation therapy, SIRT
Introduction
90Y resin microspheres (SIR-Spheres—Sirtex Medical, Sydney, Australia) radioembolization (RAE) is an FDA approved brachytherapy device, recommended through the National Comprehensive Cancer Network and European Society for Medical oncology guidelines for the treatment of chemo-refractory colorectal cancer liver metastases (CLM) in selected patients with liver-only or liver-dominant disease [1–13].
The manufacturer recommends using a sandwich technique during 90Y resin microspheres administration [14, 15]. This technique includes a vehicle {sterile water or 5% dextrose solution (D5W)} to preclude direct contact of iodinated contrast material with the resin microspheres. With the sandwich technique, the contrast agent is never used in the “D” line (authorized user line). Since neither the 90Y microspheres nor the vehicle is radiopaque, the sandwich technique does not allow continuous monitoring of the microsphere flow toward the target tumor. This limits the ability to detect microspheres’ stasis or reflux, which can result in nontarget delivery with unnecessary toxicity, as well as diminished radioactivity reaching the target tumor. This is particularly important when treating relatively hypovascular tumors such as CLM, where the incidence of stasis may be higher than 30% [16–18].
Although the administration of 90Y resin microspheres with contrast agent in the “D” line is not recommended [15], there are only anecdotal and controversial data regarding potential disadvantages of this technique. The main concern of using a contrast agent as the delivery medium during 90Y resin microspheres administration includes a theoretical possibility of 90Y leaching from the resin microspheres. This could cause systemic nontarget radiation delivery [19]. Resin-based yttrium microspheres normally have 0.01–0.4% unbound or unprecipitated 90Y [20], with recommended amount of 90Y leaching of less than 5% [21]. Another concern is the possibility of microspheres clumping, resulting in microcatheter occlusion. This was observed in vitro in the 1990’s, when resin microspheres were exposed to ionic contrast medium, although the details of that experiment remain unknown. Resin microspheres aggregation did not occur with isotonic solutions of non-ionic contrast agent and 5% dextrose [22]. The safety of a modified 90Y microspheres infusion techniques using diluted non-ionic contrast agent in the “B” line-only [14, 22] or in both “B” and “D” lines has been demonstrated [23]. The administration of 90Y resin microspheres using 50% non-ionic contrast agent in both lines resulted in lower incidence of stasis, shorter resin microspheres infusion and fluoroscopy times without complications [23].
Fluoroscopic image conspicuity is improved as the opacity degree increases. This is directly proportional to the concentration of iodinated contrast agent used [24]. Since no adverse event was noted when using diluted contrast material, we started using undiluted contrast medium for 90Y resin microspheres administration in 2013.
This work was undertaken to specifically address the safety and efficacy of 90Y resin microspheres RAE for CLM using undiluted non-ionic contrast material (UDCM) in both “B” and “D” lines.
Materials and methods
IRB waiver of approval was obtained for this retrospective review of our prospectively created and maintained HIPAA registered and compliant CLM RAE database. All patients with CLM treated with 90Y resin microspheres RAE from 9/15/2009 to 1/19/2017 were included. Beginning in April 2013, two experienced operators started using 100% non-ionic contrast agent {100% Omnipaque-300 (Iohexol 300 mgl/ml, GE Healthcare Inc.)} in both “B” and “D” lines (study group) instead of the standard sandwich infusion technique (control group) for 90Y resin microspheres administration. In the study group, intermittent clearance with D5W or sterile water solution was performed from both “B” and “D” lines as needed not unlike any other embolization technique.
Standard sandwich technique for microspheres administration was used in the control group per manufacturer’s instructions, which included administration of sterile water or D5W in both “B” and “D” lines with intermittent checking for the progress of radioembolization by using non-ionic contrast agent in “B” line.
Primary study objectives were as follows:
-
(1)
Comparison of incidence of myelosuppression and lymphopenia within 6 months post-RAE between the groups;
-
(2)
Comparison of incidence of stasis and/or reflux during RAE between the groups;
-
(3)
Comparison of nontarget microspheres delivery between the groups;
-
(4)
Comparison of incidence of RAE-related hyperbilirubinemia, side effects and complications within 6 months post-RAE;
-
(5)
Comparison of 90Y infusion time between the groups;
-
(6)
Comparison of fluoroscopic radiation dose between the groups;
-
(7)
Comparison of UDCM amount administered between the groups.
Secondary study objectives included:
-
(1)
Analysis of mean laboratory values changes post-RAE between the groups;
-
(2)
Liver progression-free survival (LPFS);
-
(3)
Overall survival (OS).
Definitions of study objectives were presented in Supplement 1.
All patients were followed at 1–4, 5–8, 9–16 and 17–26 weeks after RAE with clinic visits and laboratory tests to evaluate for possible radiation-induced hepatotoxicity, systemic and any other toxic effect. All laboratory values were recorded by the blinded reader at above-mentioned follow-up time points and compared to baseline. In cases of multiple laboratory values within a specific follow-up period available, the value closest to the middle of the specific period was chosen.
Imaging follow-up was obtained at 5–8 weeks and 9–16 weeks post-procedure to assess treatment response and every 3 months thereafter to detect disease progression.
Statistical analysis methodology was described in Supplement 2.
Results
Eighty-one patients underwent 146 90Y resin microspheres infusions. In 23 (28%) patients, 90Y delivery was performed using UDCM (study group), whereas in 58 (72%) patients standard sandwich infusion technique was used (control group). Median follow-up time was 9.1 months. One patient in study group was lost to follow-up. Study and control group demographics and post-RAE therapies were described in Supplements 3–4.
Complete Blood Count (CBC) Values Change After Radioembolization: Evidence of Myelosuppression and Lymphopenia
Laboratory values on at least one follow-up time point were available for 20/23 (87%) patients in the study and 56/58 (97%) patients in the control group. On the last follow-up, laboratory values were available for 13/23 (57%) patients in the study and 40/58 (69%) in the control group.
Analysis of CBC values at baseline and follow-ups between the study and control groups showed no evidence to support myelosuppression (Tables 1, 2, Figs. 1, 2). When comparing study and control groups, the incidence of lymphopenia was statistically significantly lower in the study group at 5–8 weeks post-RAE (p = 0.05).
Table 1.
Incidence of myelosuppression within 6 months after 90Y radioembolization
| Laboratory value | Control group | Study group | P value (between two groups) |
|---|---|---|---|
| Leucopenia (< 4 K/mcl) | |||
| Before treatment | 10/57 (17%) | 0/22 (0%) | 0.06 |
| After 1–4 weeks | 11/53 (21%) | 1/15 (7%) | 0.28 |
| After 5–8 weeks | 15/54 (28%) | 3/17 (18%) | 0.53 |
| After 9–16 weeks | 16/49 (33%) | 3/14 (21%) | 0.52 |
| After 17–26 weeks | 10/40 (25%) | 3/13 (23%) | 1 |
| Neutropenia (< 1.5 K/mcl) | |||
| Before treatment | 2/57 (4%) | 0/22 (0%) | 1 |
| After 1–4 weeks | 1/53 (2%) | 0/15 (0%) | 1 |
| After 5–8 weeks | 2/54 (4%) | 0/17 (0%) | 1 |
| After 9–16 weeks | 5/49 (10%) | 0/14 (0%) | 0.17 |
| After 17–26 weeks | 2/40 (5%) | 3/13 (23%) | 0.09 |
| Erythrocytopenia (< 4.2 M/mcl) | |||
| Before treatment | 31/57 (54%) | 15/22 (68%) | 0.32 |
| After 1–4 weeks | 30/53 (57%) | 11/15 (73%) | 0.37 |
| After 5–8 weeks | 29/54 (54%) | 10/17 (59%) | 0.78 |
| After 9–16 weeks | 23/49 (47%) | 10/14 (71%) | 0.14 |
| After 17–26 weeks | 24/40 (60%) | 8/13 (62%) | 1 |
| Thrombocytopenia (< 160 K/mcl) | |||
| Before treatment | 24/57 (42%) | 8/22 (36%) | 0.08 |
| After 1–4 weeks | 20/53 (38%) | 8/15 (53%) | 0.37 |
| After 5–8 weeks | 28/54 (52%) | 5/17 (29%) | 0.16 |
| After 9–16 weeks | 25/49 (51%) | 9/14 (64%) | 0.54 |
| After 17–26 weeks | 21/40 (53%) | 9/13 (69%) | 0.35 |
| Lymphocytopenia (< 0.5 K/mcl) | |||
| Before treatment | 3/55 (5%) | 3/20 (15%) | 0.33 |
| After 1–4 weeks | 15/50 (30%) | 4/16 (25%) | 1 |
| After 5–8 weeks | 16/50 (32%) | 1/16 (6%) | 0.05 |
| After 9–16 weeks | 10/47 (21%) | 3/13 (23%) | 1 |
| After 17–26 weeks | 8/38 (21%) | 0/13 (0%) | 0.17 |
Table 2.
Complete blood count value changes after RAE
| Laboratory value | Coefficienta | p value | 95% CI | |
|---|---|---|---|---|
| 1. | WBC (K/mcl) Diff between infusion groups | 0.02 | 0.98 | [− 1,59; 1,63] |
| 2. | ANC (K/mcl) Diff between infusion groups | − 0.4 | 0.95 | [− 1,46; 1,37] |
| 3. | RBC (M/mcl) Diff between infusion groups | 0.11 | 0.17 | [− 1,46; 1,37] |
| 4. | PLT (K/mcl) Diff between infusion groups | − 10.21 | 0.45 | [− 36.41; 16.0] |
| 5. | ALC (K/mcl) Diff between infusion groups | 0.14 | 0.11 | [− 0.03; 30] |
Analysis was made using multilevel mixed-effects linear regression model
Diff—difference; WBC—white blood cell count, ANC—absolute neutrophil count, RBC—red blood cell count, PLT—platelet count, ALC—absolute lymphocyte count. There was no statistically significant difference between study and control groups complete blood count changes over 6 months period
Negative coefficients reflect lower mean values in study group during follow-up period, positive coefficients—higher mean values in study group
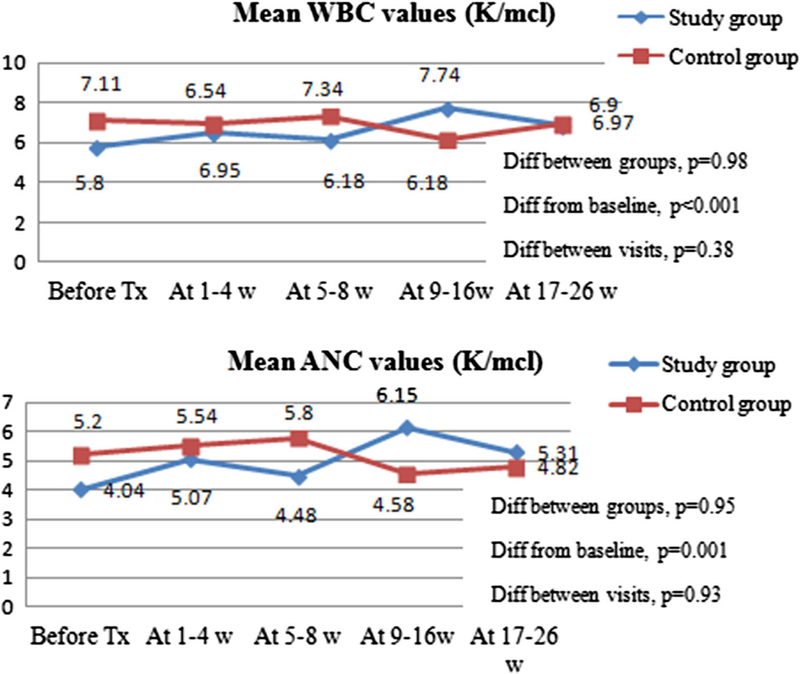
Fig. 1.
Mean blood count values within 6 months post-RAE (both RAE sessions included). WBC—white blood cell count; ANC—absolute neutrophil count; Diff between Tx groups—difference between standard and control groups. Diff from baseline—difference between baseline and follow-up values regardless of infusion group. Diff between visits— difference in values among the follow-up visits irrespective of infusion group. P values accounted for the patients with two RAE sessions, using multilevel mixed-effects linear regression model. The figures demonstrated that there was no statistically significant difference in mean white blood cell (p = 0.98) and absolute neutrophil count values (p = 0.95) between study and control groups during 6-months follow-up
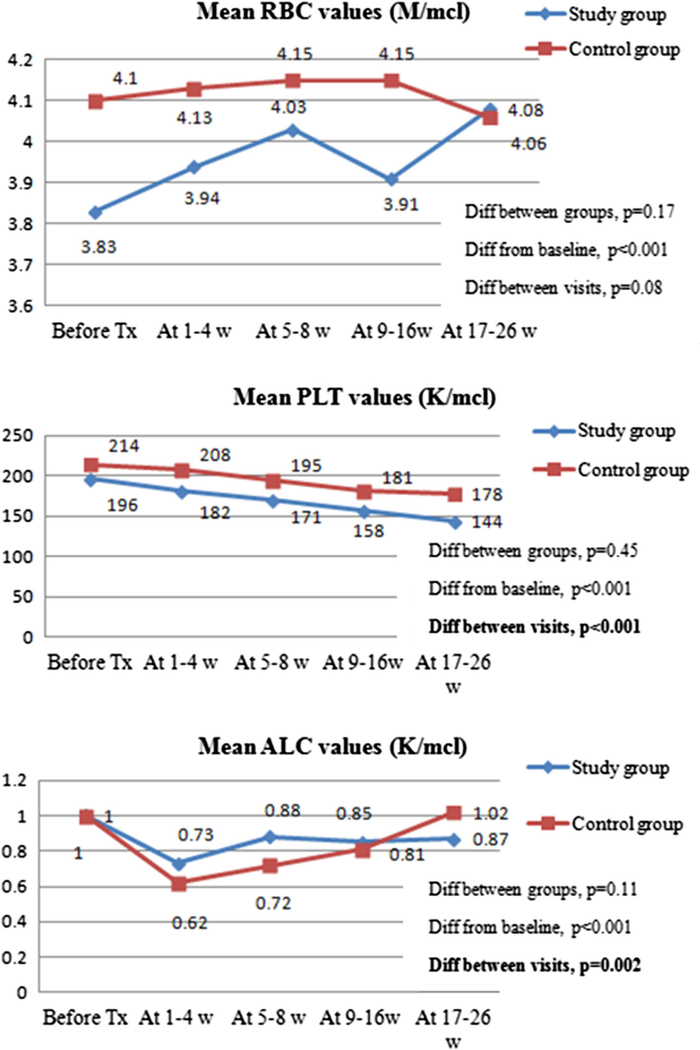
Fig. 2.
Mean blood count values within 6 months post-RAE (both RAE sessions included). RBC—red blood cell count; PLT—platelet count; ALC—absolute lymphocyte count; Diff between Tx groups—difference between study and control groups. Diff from baseline—difference between baseline and follow-up values regardless of infusion group. Diff between visits-difference in values among the follow-up visits irrespective of infusion group. P values accounted for the patients with two RAE sessions, using multilevel mixed-effects linear regression model. The figures demonstrated that there was no statistically significant difference in mean red blood cell (p = 0.17), platelet count (p = 0.45) and absolute lymphocyte count values (p = 0.11) between study and control groups during 6-months follow-up
Detailed description of low CBC values within 6 months post-RAE with related clinical symptoms and treatment in the study group patients was described in Supplement 5.
Incidence of Stasis, Catheter Occlusion and Amount of Prescribed Radiation Dose Delivered
Incidence of stasis in study and control groups was 23% (95% CI 10–36%) vs. 28% (95% CI 19–37%). When accounting for multiple infusions per patients using generalized estimated equations test, the study group patients had 38% lower incidence of stasis (HR 0.62, p = 0.34). The mean percentage of prescribed 90Y dose administered was 90% in study group (95% CI 85–95%) versus 85% in control group (95% CI 81–89%), p = 0.21. No catheter occlusion was noted.
Median Fluoroscopy Radiation Dose, Microspheres Infusion Time and Amount of Used UDCM
Median microsphere infusion time was 31 vs. 39 min for the study vs. control groups (p = 0.006).
A total of 71 single-infusion procedures were identified and used for the median fluoroscopy radiation dose and median amount of administered UDCM analyses; 8/71 (11%) infusions were excluded from the analysis as they required additional vascular embolization prior to RAE. After exclusion, 12 procedures in the study and 51 in the control group were analyzed.
Median single-infusion RAE fluoroscopy radiation dose for the study and control groups was 44.6 Gy/cm2 (range, 19.4–221.8 Gy/cm2) and 97.35 Gy/cm2(range, 23.2–398.2 Gy/cm2), respectively, p = 0.048. There was no statistically significant difference in mean patients BMI values between study (26.6) and control group (28.4), p = 0.3. Median amount of UDCM administered in the study and control groups was exactly the same: 150 ml (range, 101–200 ml) and 150 ml (range, 60–275 ml), respectively, p = 0.72 (Table 3).
Table 3.
Comparison of time from resin microspheres infusion start to post-implantation vial radioactivity measurement, fluoroscopy radiation doses and amount on contrast medium (Omnipaque-300) administered between study and control groups
| Study group | Control groupa | |
|---|---|---|
| 1. Resin microspheres infusion time {from infusion start to post-implantation vial radioactivity measurement (for single-infusion procedures)} | ||
| Number of patients | 12 | 37 |
| Median | 31 min | 39 min |
| Range | 22–50 min | 20–110 min |
| P value | 0.0058 | |
| 2. Fluoroscopy radiation dose (Gy/cm2, for single-infusion procedures) | ||
| Number of patients | 12 | 46 |
| Median | 44.6 | 97.35 |
| Range | 19.4–221.8 Gy/cm2 | 23.23–398.16 Gy/cm2 |
| P value | 0.048 | |
| 3. Amount of Omnipaque administered (ml, for single-infusion procedures) | ||
| Number of patients | 12 | 47 |
| Median | 150 ml | 150 ml |
| Range | 101–200 ml | 60–275 ml |
| P value | 0.72 | |
Bold values are statistically significant
Analyzed parameters data were not available per reports for all control group patients. The table demonstrated statistically significantly shorter resin microspheres infusion time (p = 0.0058) and lower fluoroscopy radiation dose in study group (p = 0.048), compared to control group. There was no statistically significant difference between groups regarding amount of contrast medium (Omnipaque) administered
Incidence of Reflux and Evidence of Nontarget Delivery on Bremsstrahlung SPECT/CT Imaging
Reflux was not observed in the study group. It was recorded in 3/58 (5%) of infusions in the control group. The incidence of nontarget 90Y delivery on Bremsstrahlung SPECT/CT between study and control groups was 1/27 (4%, 95% CI 0–11%) vs. 5/73 (7%, 95% CI 1–13%), p = 1. In the study group, tracer uptake along the distribution of the falciform artery was recorded. The vessel was identified in pretreatment mapping but could not be embolized. Ice packs were placed on the skin during 90Y microspheres delivery; however, activity in the distribution of this vessel was detected in the Bremsstrahlung SPECT/CT scan [25]. The patient remained asymptomatic and without skin changes during follow-up.
Side Effects and Complications after 90Y Radioembolization
Side effects during 90Y microspheres infusion were observed only in the control group (in 6/58 (9%) of patients), which included pain, hypersensitivity reaction (flushing and swelling) and arterial hypertension. There were no side effects in the study group. There was no difference in incidence of hyperbilirubinemia, grade 1−2 and grade 3−4 toxicities (Tables 4, 5).
Table 4.
Grade 1–2 toxicities
| Complication (multiple possible per patient) | Study group | Control group |
|---|---|---|
| Fatigue/weakness | 5/22 (23%) | 26/58 (45%) |
| Abdominal pain/distension | 3/22 (14%) | 17/58 (29%) |
| Shortness of breath | 1/22 (5%) | 9/58 (16%) |
| Fever | 1/22 (5%) | 9/58 (16%) |
| Change in appetite/weight changes | 0 | 8/58 (14%) |
| Nausea | 1/22 (5%) | 7/58 (12%) |
| Vomiting | 0 | 4/58 (7%) |
| Ascites | 0 | 5/58 (9%) |
| Constipation/diarrhea | 0 | 13/58 (45%) |
| Light colored stool/dark urine | 0 | 9/58 (16%) |
| Hiccups | 0 | 1/58 (2%) |
| Portal hypertension | 1/22 (5%) | 0 |
| Total patients number | 8/22 (36%) | 26/58 (45%) |
Table 5.
Grade 3–4 toxicities
| Complication (multiple possible per patient) | Days post-RE | Study group | Control group |
|---|---|---|---|
| Post-SIRT radioembolization syndrome | 1–90 days | 1/22 (5%) | 4/58 (7%) |
| Grade 3 abdominal pain/distension | 10–12 days | 1/22 (5%) | 2/58 (3%) |
| Grade 3 fevers | 3 days | 0 | 1/58 (2%) |
| Acute liver failure/failure to thrive/grade 3 hyperbilirubinemia | 14 days | 0 | 1/58 (2%) |
| Grade 3 dyspnea | 30 days | 0 | 1/58(2%) |
| Bile duct obstruction + Budd-Chiari syndrome + stop chemotherapy for 6 weeks | 1 day/60 days | 1/22 (5%) | 0 |
| Total patients number | 2/22 (9%) | 9/58 (16%) | |
Median Liver PFS and OS
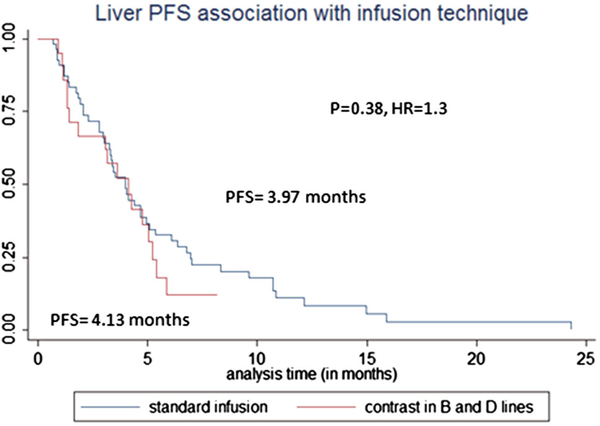
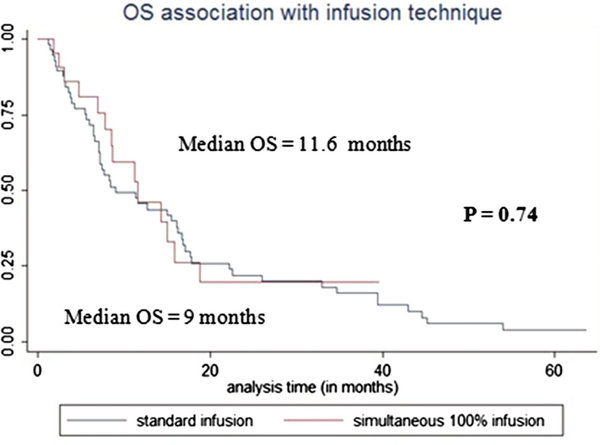
Median liver PFS for study and control groups was 4.13 vs. 3.97 months (HR = 1.29, p = 0.38) (Fig. 3). OS for study and control groups was 11.6 months vs. 9 months (p = 0.74, HR = 0.9) (Fig. 4).
Fig. 3.
Liver progression-free survival (PFS) association by infusion technique. The figure demonstrated that there was no statistically significant difference in liver PFS between study and control groups (p = 0.38)
Fig. 4.
Overall survival (OS) association with infusion technique. The figure demonstrated that there was no statistically significant difference in OS between study group (simultaneous 100% contrast medium infusion) and control group (standard sandwich technique infusion), p = 0.74
Discussion
This study demonstrated no indirect evidence of 90Y leaching when using UDCM for 90Y resin microspheres administration in both “B” and “D” lines. This was indicated by no difference in complete blood count values at baseline and follow-ups between the study and control groups. The comparison with the control group was used as reference to anticipated complete blood count changes post-RAE, such as thrombocytopenia and lymphopenia [26−28]. No abnormal β-emission was detected on Bremsstrahlung SPECT/CT in the study group, which further supported the lack of 90Y leaching in the study. There was no difference in peri- or post-procedural toxicity, hepatic progression-free and overall survival between the groups. These findings indicated that no additive toxicity occurred during direct infusion of resin microspheres using UDCM. Chao et al., similarly, reported no complications when infusing 90Y resin microspheres with 50% non-ionic contrast medium in saline [23].
Statistically significantly lower median fluoroscopy radiation dose was recorded in the study group, compared to control (44.6 vs. 97.4 Gy/cm2, p = 0.048), accounting for patients’ BMI. The amount of contrast agent used was similar between the groups. A significantly shorter median resin microspheres infusion time in the study group may have attributed to the lower fluoroscopy radiation dose and the same amount of contrast agent used between groups. This is concordant with reported findings by Chao et al. (7 min infusion time for the 50% of Isovue vs. 22 min for the standard sandwich technique) [23]. A 38% reduction in the incidence of stasis in the study group did not reach statistical significance in our study. However, when using 50% of concentration non-ionic contrast medium, a significant decrease in the incidence of stasis was documented [23]. Reflux never occurred during UDCM infusion in our study, compared to three cases in the control group. Non-target delivery occurred in 5/73 (7%) of infusions in the control group compared to 1/27 (4%) in the study group (all asymptomatic).
No gastrointestinal ulceration was noted, comparing favorably to historical data. Gastrointestinal ulceration is the most frequent major complication, reported in 0–20% of RAEs with a median incidence of 8% when using advanced antireflux protective techniques and in 6% of cases requiring surgical ulcer management [29].
90Y administration using UDCM was as effective as sandwich technique in terms of liver progression-free and overall survival.
Limitations of the study include its single-center, retrospective nature and the relatively small number of patients. The lack of pathologic assessment such as bone marrow biopsy at different time points after RAE to detect early microscopic bone marrow morphologic changes was another study limitation. Direct 90Y leaching detection should be performed in blood or urine samples in certified radioactive material laboratories, according to radiation safety standards [21]. The lack of these assessments was limitations of this study. Leaching and radiotoxicity were assessed only indirectly, through post-RAE serial complete blood count, Bremsstrahlung SPECT/CT as well as laboratory and clinical toxicity evaluations. The fact, that 90Y Bremsstrahlung SPECT/CT has low quantitative accuracy and that it was only done at one-time point (within hours of RAE, according to routine practice), limits the potential for leaching detection [30, 31]. Considering that the half-life of 90Y is 2.7 days, ideally such study should be performed in several time points within 11 days after RAE to detect all emitted β-radiation in both target and nontarget organs. However, such information would have little if any impact on clinical patient management due to the absence of symptoms and laboratory values, indicating myelosuppression and radiotoxicity. Further prospective studies could address these limitations and optimize the direct administration of 90Y resin microspheres with non-ionic contrast medium.
Conclusion
Despite the limitations, the study met its goal and demonstrated that direct 90Y resin microspheres infusion with undiluted non-ionic contrast medium was safe and effective. Significant reduction in 90Y resin microspheres infusion time and fluoroscopic radiation exposure were documented.
Supplementary Material
Acknowledgments
Funding The research funded by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Compliance with Ethical Standards
Ethical Approval IRB waiver of approval was obtained for this retrospective cohort study. The database was HIPAA registered and compliant.
Informed Consent The patients signed informed consent for the procedure.
Clinical Relevance Statement 90Y resin microsphere administration using undiluted non-ionic contrast material was safe and effective, providing continuous real-time infusion monitoring as well as decreasing infusion time and fluoroscopy radiation dose.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00270-018-1985-1) contains supplementary material, which is available to authorized users.
Conflict of interest C. T. Sofocleous has received research support in the past and was a consultant for Sirtex Medical. Other authors have no conflicts of interest.
References
- 1.Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Nat Comprehen Cancer Netw JNCCN. 2017;15(3):370–98. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(8):1386–422. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. [DOI] [PubMed] [Google Scholar]
- 4.Sotirchos VS, Petre EN, Brown KT, Brody LA, D’Angelica MI, DeMatteo RP, et al. Safe and successful yttrium-90 resin microsphere radioembolization in a heavily pretreated patient with chemorefractory colorectal liver metastases after biliary stent placement above the Papilla. Case Rep Hepatol. 2014;2014:921406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofocleous CT, Garcia AR, Pandit-Taskar N, Do KG, Brody LA, Petre EN, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13(1):27–36. [DOI] [PubMed] [Google Scholar]
- 6.Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(23):3687–94. [DOI] [PubMed] [Google Scholar]
- 7.Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88(2):78–85. [DOI] [PubMed] [Google Scholar]
- 8.Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol Off J Eur Soc Med Oncol. 2001;12(12):1711–20. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RA, Wasan HS, Hazel GAV, Heinemann V, Sharma NK, Taieb J, et al. Overall survival analysis of the FOXFIRE prospective randomized studies of first-line selective internal radiotherapy (SIRT) in patients with liver metastases from colorectal cancer. J Clin Oncol 2017;35(15 suppl):3507 [Google Scholar]
- 10.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C . Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60(5):1552–63. [DOI] [PubMed] [Google Scholar]
- 11.Sharma RA, Van Hazel GA, Morgan B, Berry DP, Blanshard K, Price D, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(9):1099–106. [DOI] [PubMed] [Google Scholar]
- 12.Vente MA, Wondergem M, van der Tweel I, van den Bosch MA, Zonnenberg BA, Lam MG, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19(4):951–9. [DOI] [PubMed] [Google Scholar]
- 13.Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103(3):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paprottka KJ, Todica A, Ilhan H, Rubenthaler J, Schoeppe F, Michl M, et al. Evaluation of Visualization using a 50/50 (contrast media/glucose 5% solution) technique for radioembolization as an alternative to a standard sandwich technique. Cardiovasc Interv Radiol. 2017;40(11):1740–7. [DOI] [PubMed] [Google Scholar]
- 15.SIR-Spheres® Y-90 resin microspheres Package Insert. [.
- 16.Sofocleous CT, Violari EG, Sotirchos VS, Shady W, Gonen M, Pandit-Taskar N, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin Colorectal Cancer. 2015;14(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piana PM, Bar V, Doyle L, Anne R, Sato T, Eschelman DJ, et al. Early arterial stasis during resin-based yttrium-90 radioembolization: incidence and preliminary outcomes. HPB Off J Int Hepato Pancreato Biliary Assoc. 2014;16(4):336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy R, Xiong H, Nunez R, Cohen AC, Barron B, Szklaruk J, et al. Yttrium 90 resin microspheres for the treatment of unresectable colorectal hepatic metastases after failure of multiple chemotherapy regimens: preliminary results. J Vascul Interv Radiol JVIR. 2005;16(7):937–45. [DOI] [PubMed] [Google Scholar]
- 19.Mantravadi RV, Spigos DG, Tan WS, Felix EL. Intraarterial yttrium 90 in the treatment of hepatic malignancy. Radiology. 1982;142(3):783–6. [DOI] [PubMed] [Google Scholar]
- 20.Lambert B, Sturm E, Mertens J, Oltenfreiter R, Smeets P, Troisi R, et al. Intra-arterial treatment with (9)(0)Y microspheres for hepatocellular carcinoma: 4 years experience at the Ghent University Hospital. Eur J Nucl Med Mol Imaging. 2011;38(12):2117–24. [DOI] [PubMed] [Google Scholar]
- 21.Patents.google.com. (2017). W02002034300A1—Polymer based radionuclide containing particulate material—Google Patents. https://patents.google.com/patent/W02002034300A1/en?assignee= Sirtex+Medical+Ltd [Accessed 26 Jul. 2017].
- 22.Sirtex Medical Ltd. Technical Bulletin, August 7, 2013. Available from Sirtex Medical [Google Scholar]
- 23.Chao C, Stavropoulos SW, Mondschein JI, Dagli M, Sudheendra D, Nadolski G, et al. Effect of Substituting 50% isovue for sterile water as the delivery medium for SIR-Spheres: improved dose delivery and decreased incidence of stasis. Clin Nucl Med. 2017;42(3):176–9. [DOI] [PubMed] [Google Scholar]
- 24.https://www.drugbank.ca/drugs/DB01362 [3.26.2018].
- 25.Wang DS, Louie JD, Kothary N, Shah RP, Sze DY. Prophylactic topically applied ice to prevent cutaneous complications of nontarget chemoembolization and radioembolization. J Vascul Interv Radiol JVIR. 2013;24(4):596–600. [DOI] [PubMed] [Google Scholar]
- 26.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123(2):224–7. [PubMed] [Google Scholar]
- 28.Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy R, Brown DB, Salem R, Meranze SG, Coldwell DM, Krishnan S, et al. Gastrointestinal complications associated with hepatic arterial Yttrium-90 microsphere therapy. Journal of vascular and interventional radiology: JVIR. 2007;18(4):553–61; quiz 62. [DOI] [PubMed] [Google Scholar]
- 30.Dezarn WA, Cessna JT, DeWerd LA, Feng W, Gates VL, Halama J, et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys. 2011;38(8):4824–45. [DOI] [PubMed] [Google Scholar]
- 31.Rong X, Du Y, Ljungberg M, Rault E, Vandenberghe S, Frey EC. Development and evaluation of an improved quantitative (90)Y bremsstrahlung SPECT method. Med Phys. 2012;39(5):2346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.