Abstract
We present a generic immunoassay platform that uses enhanced total internal reflection fluorescence in the proximity of silver island films (SIFs), a surface coating consisting of metal (silver) particles. This platform is used with a model immunoassay where a protein antigen, rabbit immunoglobulin G, was immobilized on the SIF-coated glass surface. The signal from a fluorescent dye-labeled anti-rabbit antibody binding to the surface antigen was detected; different color dyes have been tested. Close placement of the fluorophore to surface-bound silver nanostructures results in dramatic signal enhancement (up to 40-fold) on the SIFs as compared with the glass slides. Use of the total internal reflection mode of excitation has significant advantages (over classic front-face excitation) for practical assay development. The limited evanescent wave excitation volume makes it possible to minimize the background signal and use the immunoassay with no need for any washing steps.
Keywords: Fluorescence immunoassay, Enhanced fluorescence, Immunoglobulin, Nano-size metallic particles, Silver island films
Fast and sensitive detection of physiological markers is a key element for preventive medicine and diagnostics. A successful sensing technology should offer high sensitivity and selectivity and ideally should not require any chemical or biological amplification steps. Fluorescence-based immunoassays have become the technology of choice for medical and clinical diagnostics [1–5]. Commonly used fluorescence assays are based on simple binding of labeled antigens or a sandwich format where second antibodies (Abs) have been labeled. During recent years, there has been a significant growth of interest in using surface-based immunoassays where the number of preparation steps can be significantly limited. Immobilization of the assay components on transparent surfaces presents great opportunities for technologies that use optical phenomena on the surface/interface to increase detection sensitivity. The practical uses of total internal reflection fluorescence (TIRF)1 [6–8], surface plasmon resonance [9–12], surface plasmon field-enhanced fluorescence [13–15] and surface plasmon-coupled directional emission [16–18] have already been demonstrated.
Recently, our laboratory [19–22] and others [23–25] have shown a significant fluorescence signal enhancement when fluorophores are placed in close proximity to a layer of metallic particles. Metal-enhanced florescence observed with silver island films (SIFs) can be used to improve the signal-to-noise level for quantitative bioanalyte detection. Experiments with SIFs have been made with front-face (FF) excitation where all volume above the sample layer has been excited. In this case, fluorescence from surface-associated molecules can be dwarfed by the fluorescence from unwanted nonassociated molecules in the adjunct detection volume. For this reason, all of our previous experiments with SIFs were performed using samples where volume above the SIFs was physically limited such as for thin spin-coated polyvinyl alcohol (PVA) films [26] and solution squeezed between two island layers [27,28].
TIRF is a technology that allows selective excitation of fluorescent molecules in close (200 nm) proximity to the surface [29–32]. The advantages of TIRF for surface-based bioassays have been recognized for many years; however, confinement and formation of the evanescent field above the highly scattering and absorbing layer of metallic particles have never been reported before.
In the current article, we describe a detection format that combines TIRF with SIF technology. Evanescent wave excitation confines the excitation volume to the assay surface, eliminating the need for washing the solution from above the assay surface. A thin layer has favorable properties that include surface-enhanced fluorescence, increased photostability, and rejection of unwanted background from the volume above the immunoassay surface. Such an assay format allows on-the-fly detection of binding with no need for washing steps. In the future, the use of a long wavelength near infrared fluorophores could allow direct marker detection in whole blood samples.
To test the applicability of the presented technology, we selected a model Ab-antigen interaction system that can be labeled with different color fluorophores. We present an immunoassay for a model antigen, rabbit immunoglobulin G (IgG), labeled with fluorescent dyes with emission from 550 to 750 nm.
Materials and methods
Materials
Rabbit and goat IgG (95% pure) were obtained from Sigma. Tetramethyl-rhodamine anti-rabbit IgG was obtained from Sigma and Molecular Probes. Rhodamine Red-X anti-rabbit IgG conjugate, Alexa Fluor-647 anti-rabbit IgG conjugate, Alexa Fluor-680 anti-rabbit IgG conjugate, and Alexa Fluor-750 anti-rabbit IgG conjugate were obtained from Molecular Probes. Buffer components and salts (e.g., bovine serum albumin [BSA], glucose, sucrose, AgNO3) were obtained from Sigma–Aldrich.
Silver island formation
SIF surface was formed similar to the procedures described elsewhere [19,20]. In particular, glass slides (3 × 1 inch, 1 mm thick, VWR) were cleaned by soaking in a 9:1 mixture (v/v) of H2SO4 (98%) and H2O2 (30%) for at least overnight and then were rinsed with Milli-Q water and dried on air before use. Slides were then coated with poly-L-lysine, with approximately 1.2 ml of the poly-L-lysine solution (freshly prepared solution: 8 ml water + 1.0 ml Na–phosphate buffer, 50 mM, pH7.4, + 1.0 ml poly-L-lysine solution [0.1%, Sigma]) being added to each slide and spin-coated. Spin-coating was performed using a P-6708D spin-coater (Specialty Coating Systems) at 2000 rpm for 3 s and at 3000 rpm for 1000 s (time ramps to reach 2000 rpm [from 0] and 3000 rpm [from 2000] were 3 s each, and time ramp to slow down from 3000 rpm to 0 was 10 s). Silver deposition was performed in a glass 100-ml beaker as follows. At intensive stirring, 20 drops of NaOH solution (5 M) were added to AgNO3 solution (500 mg) in Milli-Q water (60 ml), and dark brown precipitate formed immediately. Approximately 2 ml of 30% NH4OH solution was added to dissolve the precipitate (at continuous intense stirring), and the clear solution was cooled with ice to 10–15 °C (~10 min). A fresh glucose solution (720 mg D(+)glucose in 15 ml Milli-Q water) was added to the mixture, and glass slides were immediately half-inserted (half-soaked) into the mixture. Soaking of slides was performed for pairs of slides, so only one surface of each slide was exposed to the reaction mixture. Stirring of the mixture was continued in an ice bath for 2 min, and then ice was removed and the solution was stirred at warming(i.e., at medium heating) until 30 °C for approximately 2 min. Then the heating was turned off, and the solution continued to be mixed intensively for an additional 3–4 min or so (the temperature increased to 35 °C). After the color of the slides became greenish-brown and the solution became opaque, the slides were removed from the beaker and washed with water two times with sonication for approximately 25 s. After rinsing several times with water, the slides were stored in water at room temperature (several hours to months) until use. Several typical absorbance spectra of the SIF-coated glass slide are given in Fig. 1.
Fig. 1.
Some examples of absorption spectra of SIF-coated glass slides. a.u., arbitrary units.
Coating slides with IgG
Slides were noncovalently coated with rabbit IgG as a sample slide or with goat IgG as a control slide. First, slides were dried with air and covered with tape containing punched holes (regular-size hole puncher) to form wells on the surface of the slides. The size of the wells corresponds to the well size routinely used in high-throughput screening 96-well plates. A coating solution of IgG (10–30 μg/ml of IgG dissolved in Na–phosphate buffer, 50 mM, pH 7.4) was added to each well (25 μl/well), and slides were incubated for 2 to 4 h at room temperature in a humid chamber. Slides were then rinsed with water, washing solution (0.05% Tween 20 in water), and water. Blocking was performed by adding blocking solution (1% BSA, 1% sucrose, 0.05% NaN3, and 0.05% Tween-20 in 50 mM Tris–HCl buffer, pH 7.4, 35 μl per well) and incubating at room temperature for 2–4 h (or overnight at 4 °C) in a humid chamber. Slides were rinsed with water, washing solution (0.05% Tween-20 in water), and water, covered with Na–phosphate buffer (50 mM, pH7.4) or blocking solution, and stored at 4 °C until use.
End-point binding experiment
Dye-labeled conjugate dye anti-rabbit IgG (diluted to 10 μg/ml with Na–phosphate buffer, 50 mM, pH 7.4) was added to the sample slide (coated with rabbit IgG) or the control slide (coated with goat IgG) (25 μl/well), and slides were incubated at room temperature in a humid chamber for 1–2 h. Slides were then rinsed with water, washing solution (0.05% Tween-20 in water), and water, coated with blocking buffer, and stored at 4 °C before measurement. A scheme of the model immunoassay is presented in Fig. 2.
Fig. 2.
Scheme of the model immunoassay.
Spectroscopic measurements
Absorption spectra of SIF surfaces and dye-labeled Abs (in solution and bound to the slide surfaces) were measured using a Hewlett–Packard model 8543 spectrophotometer. Emission spectra in solution were measured using a Varian Cary Eclipse fluorometer (Varian Analytical Instruments). Fluorescence measurements of the samples on glass and glass SIF slides were performed by placing the slides horizontally on the total internal reflection (TIR) stage, as shown in Fig. 3. The TIR stage consisted of a coupling prism mounted in the custommade holder. The holder was mounted on a translational stage that may move in x and y directions on the horizontal plane. For excitation, we used small solid-state laser diode (651 nm, used for commercial laser pointers), and the second harmonic (532 nm) of the diode pumped Nd:YVO4 laser (compact laser pointer design). The excitation beam was directed to the coupling prism by the metallic mirror mounted on the holder movable in the y direction (Fig. 3), with the polarization plane forming a 45° angle with the vertical. Such a configuration allowed for easy changes of the incident angle and the evanescent spot position. Emission spectra were collected by fiber-optics mounted on x and y positions on the top using a Fiber-Optics Spectrometer (SD2000, Ocean Optics). The fiber-optic detection end was mounted in the protecting head containing a custom-cut filter and a collimating lens (Fig. 3). For observation, we used 550- or 650-nm cutoff plastic filters to attenuate the excitation line. The protecting head for the fiber-optic end significantly limited the scattering and ambient external light.
Fig. 3.
TIR measurement platform.
The enhancement ratio was calculated as a ratio of the average SIF signal to the average glass signal. The signal for a single spot was taken as the average of the fluorescence signal at the emission maximum ±5 nm. For all reported intensities, we averaged the signal from four or more different locations on the slide.
Results and discussion
Model immunoassay
To demonstrate the principles and effectiveness of bioassays based on metal-enhanced fluorescence with TIR excitation, we used the model immunoassay format shown in Fig. 2. A model antigen, rabbit IgG, was immobilized on the surfaces (SIF-coated surface and noncoated glass surface as a control), and the specific or nonspecific labeled anti-rabbit Ab was allowed to bind to the antigen. For end-point binding measurements, we incubated plates with Ab for approximately 1–2 h to complete the binding reaction. Then the plates were rinsed with water, a buffer was added, and the fluorescence signal was measured. To monitor binding kinetics, the solution of labeled Ab was added to the well and the fluorescence signal was immediately monitored in TIR mode.
TIR excitation
Excitation of fluorophores or macromolecules at a glass–liquid interface can be accomplished using the principle of TIR. It is well known that for TIR excitation, the optical field propagates into the liquid phase (which has a lower refractive index) for distance comparable to the wavelength of the light, and the depth of penetration depends on the angle of incidence, the wavelength, and the values of the refractive indexes for glass and liquid [29,30,33,34]. Depending on the angle of incidence (θ) relative to an axis normal to the interface (Fig. 3), the beam can either penetrate into the liquid phase with the refractive angle or undergo TIR. The latter occurs for angles greater than the critical angle θc. The critical angle is given by
| (1) |
where n1 is the refractive index of the liquid phase and n2 is the refractive index of the glass. For TIR to occur, n2 must be greater then n1. An example is our BK7 glass–water interface where n1 = 1.34 and n2 = 1.52, θc is 66.2, and TIR occurs for θ > 66.2°. If the angle of incidence (θ) is smaller than the critical angle (θc), the beam passes into the aqueous phase. For angles θ > θc, the beam is totally reflected back into the glass, but some of the energy propagates into the aqueous phase in the form of an evanescent wave. The intensity of this wave decreases exponentially with the distance z from the surface
where
| (2) |
where d describes the characteristic distance (depth of penetration) and is comparable to or smaller than the incident wavelength. I(0) is the initial intensity on the interface. Fig. 4 shows simulated examples of spatial distributions of the evanescent waves for excitation wavelength 532 nm as a function of incident angle θ. The evanescent field decreases exponentially with the distance from a glass–water interface. For angles significantly greater than θc, the effective penetration depth is smaller than 300 nm. Currently, we do not know whether the depth of the evanescent field is modified by silver particle on the glass surface.
Fig. 4.
Simulated spatial distribution (according to Eq. (2)) of the normalized evanescent wave intensity along the z axis for excitation 532 nm for different incident angles in the glass (BK7, n = 1.52) and water (n = 1.34) interface.
First, we examined the TIR phenomena for glass–air, glass–water, glass/SIF–air, and glass/SIF–water interfaces. Using the stage shown in Fig. 3, the TIR angle on glass and glass coated with SIFs can be measured by observing the increased reflection correlated with the disappearance of the transmitted beam on top of the stage. The measured critical angle (θc) for bare glass and glass coated with SIFs were the same within our experimental uncertainty of approximately 0.5°. For the glass–air (glass/SIF-air) interface, we measured an angle of 41.5°, and for the glass–water (glass/SIF-water) interface, we measured an angle of 62°. The calculated angles are 41.3 and 61.8° for glass–air and glass–water, respectively, and they are in very good agreement with experimental values.
Next, for one selected system (Rhodamine Red-X labeled anti-rabbit Ab bound to the surface-immobilized antigen), we examined dependence of fluorescence intensity as a function of incident angle. Fig. 5 shows the fluorescence intensities for glass (dashed) and SIFs (solid). Total fluorescence intensity on both glass and SIFs decreases slightly as incident angle increases. This is consistent with the simulation in Fig. 4 where effective penetration depth decreases slightly with the angle θ.
Fig. 5.
Fluorescence spectra of the labeled anti-rabbit Ab bound to the surface-immobilized antigen in TIR mode at different angles θ. For a water-glass interface, the critical angle is approximately 62°. a.u., arbitrary units.
FF vs. TIR excitation
To test the feasibility of TIR excitation with SIFs we have used our model system, anti-rabbit Ab labeled with Rhodamine Red-X binding to the immobilized rabbit IgG antigen. A microscope slide, half covered with SIFs, was coated with antigen as described earlier. The measurements were done in FF excitation mode, where incident excitation light was coming from the top (Fig. 3), and in the TIR mode as described earlier. The fluorescence signal was observed from the top independently of the excitation mode. Both measurements were performed for two kinds of sample preparations. First, the binding reaction was done and the labeled Ab was carefully washed out and wells were filled with pure buffer solution. Second, after the binding reaction, the wells were not emptied from the supernatant of the labeled Ab and no washing steps were applied. Fig. 6 shows the fluorescence signal observed with FF excitation and TIR excitation on glass and SIFs, respectively. In both cases, a significant fluorescence signal enhancement (~fivefold) for SIFs was observed. The fluorescence signal and enhancement observed in TIR mode were slightly higher than in FF excitation. This small difference may be due to the different geometry of the excited spot. A dramatic difference in the performance of the assay was found when the sample was directly excited, not using TIR, and when no washing steps were performed. In this case, the supernatant solution contained labeled Ab (at an IgG concentration of 20 μg/ml), forming a thin layer (<1 mm) in the reaction well over the immunoassay surface. The measurement in FF configuration on glass is dominated by bulk solution, and binding cannot be observed. Because of fluorescence enhancement in close proximity to the silver particles, an increase of fluorescence signal can be observed for SIFs, indicating binding to the surface. However, this change depends on layer thickness and is quickly diminished for a thicker layer or a higher concentration of Abs.
Fig. 6.
Fluorescence signal of Rhodamine Red-X-labeled Ab bound to the antigen immobilized on the slide surface observed with TIR excitation and FF excitation on glass and SIF. a.u., arbitrary units; Rh, rhodamine.
Clearly, TIR excitation is well suited for detecting enhanced fluorescence in close proximity to the surface and has significant advantages for surface assay development.
Immunoassay specificity
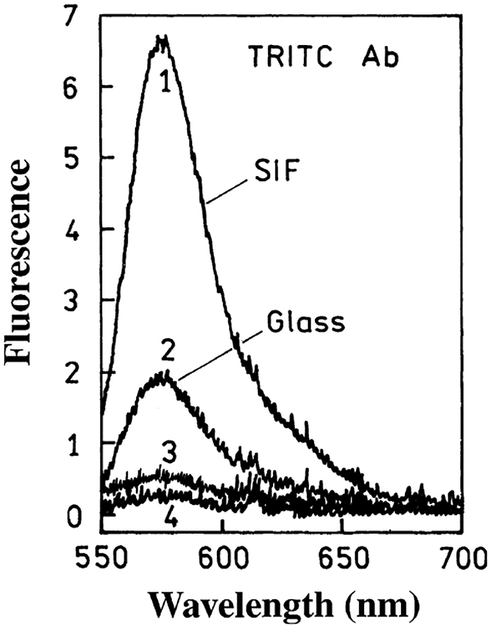
We tested the specificity of enhanced TIRF assay configuration by comparing the binding of the labeled (tetramethyl-rhodamine isothiocyanate modified, TRITC) anti-rabbit Ab with the “right” (rabbit IgG) and “wrong” (goat IgG) antigens. Control slides were coated with a wrong antigen (goat IgG). Thus, the signal from control slides shows the nonspecific binding of the TRITC anti-rabbit IgG Ab. The respective emission spectra on SIF-coated and noncoated (glass) slides are shown in Fig. 7. The background was visible for both SIF and glass (spectra 3 and 4 in Fig. 7); however, its level was much lower than that of the respective specific signal (spectra 1 and 2 in Fig. 7). Similar results were observed for Abs labeled with other fluorophores.
Fig. 7.
Fluorescence signal of labeled Ab (TRITC anti-rabbit IgG, dye/Ab = 1.4) bound to the antigen immobilized on the slide surface:(1) rabbit IgG on SIF; (2) rabbit IgG on glass; (3) goat IgG on SIF (control SIF); and (4) goat IgG on glass (control glass).
Enhancement of the signal from SIF vs. glass
We tested several fluorescent labels to check enhancement of the signal for the model immunoassay detected from SIFs vs. glass slides. Fig. 8 shows examples of the emission spectra of labeled anti-rabbit IgG bound to the antigen (rabbit IgG) on SIFs and We normally observed more than a fivefold increase intensity from the silver-coated part of the slide as pared with the unsilvered part of the slide. Depending on the particular silver-coated slide, the intensity increase may be slightly lower or higher. Appropriate enhancements obtained for other labeling dyes are presented in Table 1. These results indicate that the observed enhancement is larger for red color dyes.
Fig. 8.
Examples of the fluorescence spectra of the labeled anti-rabbit Ab bound to the surface-immobilized antigen. Rh, rhodamine; Alexa, Alexa Fluor-647.
Table 1.
Fluorescence standard deviations (n = 6) and enhancements of anti-rabbit IgG (labeled with different dyes) bound to the antigen (rabbit IgG) immobilized on the SIF substrate or glass substrate
| Antigen IgG | Standard deviation SIF (%) | Standard deviation glass (%) | Dye/IgG, mol/mol | Emission wavelength (nm) | Enhancement ratio | |
|---|---|---|---|---|---|---|
| Excited at 632 nm | ||||||
| Alexa Fluor-555 | Rabbit | 22 | 40 | 4.5 | 568 | 7.9 |
| TRITC | Rabbit | 25 | 34 | 1.4 | 575 | 6.6 |
| TRITC | Rabbit | 12 | 36 | 3.9 | 579 | 9.6 |
| Rhodamine Red-X | Rabbit | 18 | 22 | 3.8 | 592 | 4.6 |
| Alexa Fluor-594 | Sheep | 3 | 26 | 4.0 | 617 | 4.9 |
| Alexa Fluor-647 | Human | 16 | 25 | 4.9 | 670 | 5.2 |
| Alexa Fluor-647 | Rabbit | 21 | 38 | 4.5 | 674 | 7.6 |
| Alexa Fluor-660 | Goat | 11 | 71 | 4.5 | 692 | 18 |
| Alexa Fluor-680 | Rabbit | 23 | 126 | 3.2 | 705 | 7.0 |
| Alexa Fluor-750 | Rabbit | 15 | 21 | 3.0 | 582 | 4.0 |
| Excited at 651 nm | ||||||
| Alexa Fluor-647 | Rabbit | 31 | 51 | 4.5 | 677 | 16 |
| Alexa Fluor-680 | Rabbit | 27 | 24 | 3.2 | 708 | 42 |
| Alexa Fluor-750 | Rabbit | 40 | 26 | 3.0 | 773 | 33 |
| Excited at 663 nm (fluorometer) | ||||||
| Alexa Fluor-680 | Rabbit | 3.2 | 707 | 8.5 | ||
Because of the nonhomogeneous slide surfaces variations in SIF thickness, we observed variations the enhancement ratio as well. Selected examples of signal variation for TRITC-labeled and Rhodamine Red-X-labeled anti-rabbit Abs are shown on Fig. 9 We would expect the enhancement to be more consistent with careful control of the silver deposition.
Fig. 9.

Variations in the fluorescence signal of the TRITC anti-rabbit antibody (A, dye/IgG = 1.4) and the Rhodamine (Rh) Red-X anti-rabbit antibody (B) bound to the antigen (rabbit IgG) immobilized on SIF, vs. SIF extinction at 450 nm. a.u., arbitrary units.
We varied SIF thickness by changing the SIF-forming times and tried to estimate optimal SIF slide bance. We discovered that absorbance should be than 0.3 and that the optimal absorbance interval SIFs is approximately 0.5 < OD < 0.8 (Fig. 9).
The enhancement ratio may depend on the type of fluorescent label used, on the labeling degree (dye/protein tio), and on the excitation wavelength. Table 1 presents summary of the enhancement data for the model immunoassay using various fluorescent labels and excitation wavelengths. We would expect larger enhancement for high dye/protein ratio, which is consistent with the higher enhancement observed for a 3–4 dye/protein ratio rather than a 1–2 ratio. This larger enhancement for heavily labeled samples was expected due to self-quenching and the lower quantum yields of such samples. The enhancement appears higher when exciting at longer wavelengths, but this conclusion should be considered only as preliminary (due to large deviations in this case) and requires further investigation.
An important question to test is how the fluorescence signal is enhanced by the real fluorophore–metallic particle interactions. There are few effects that may lead to the increased fluorescence signals. The most important one is possible differences in protein binding to glass and SIFs. We carefully tested whether the calculated enhancement can be explained by more effective binding to the SIF-coated surface than to the glass surface. For this, we performed the following indirect test to estimate relative binding on both surfaces (SIF-coated glass and non-coated glass). In the same experiment, we measured both SIF and glass fluorescence signals from surfaces, and also the decrease of fluorescence of the supernatant labeled Ab after its incubation on the SIF and glass surfaces, as compared with the fluorescence level of the supernatant before incubation. Assuming that the decrease in the supernatant signal is proportional to the binding, the binding to the SIF surface is approximately 20–30% better, which itself cannot explain the four- to fivefold enhancement of the SIF surface signal vs. glass (Fig. 10).
Fig. 10.
Relative fluorescence signal on surface and decrease of signal in supernatant aspirated from surface (SIF vs. glass). Label: TRITC (dye/IgG = 1.4 mol/mol). a.u., arbitrary units.
Kinetics of binding
The important demonstration of quality and applicability of any immunoassay is time-dependent binding. The binding kinetics is an important characteristic parameter for practical immunoassay that clearly demonstrates the physical binding process. It is necessary to continuously monitor the fluorescence signal immediately after the addition of labeled Ab solution. Our experimental stage allowed us to continuously monitor the fluorescence signal (fluorescence spectra) with subsecond resolution.
Fig. 11 shows an example of binding kinetics for Rhodamine Red-X-labeled anti-rabbit Ab to the rabbit IgG immobilized on the SIF or glass surface. The kinetics was monitored starting immediately after the addition of the labeled Ab solution to the well. For the first 5 min, the signal increase was very fast; within approximately 10 min, the signal approached the plateau; and later, within the next 20–30 min, only a slight increase could be monitored. When a wrong antigen was used for the model immunoassay (nonspecific binding of labeled anti-rabbit Ab to the goat IgG antigen), no significant increase of the signal was observed within the first 30 min. The kinetic approach can be very useful to decrease unwanted fluorescence background for biological sample matrices.
Fig. 11.
Increase of the fluorescence signal of Rhodamine (Rh) Red-X-labeled anti-rabbit Ab binding to the antigen, rabbit IgG, immobilized on SIF or glass slide surface. a.u., arbitrary units.
Fluorescence lifetime in the proximity of SIF
As discussed in our earlier articles [19,20], there are two simultaneous phenomena that result in an increased fluorescence signal for fluorophores on SIFs [21]: a local field enhancement that increases efficiency of excitation and an increase in the radiative decay rate. An increase in the radiative decay rate or a decrease in the nonradiative decay rate each results in increased intensities; however, the former is accompanied by a decrease in the fluorescence lifetime, whereas the latter is accompanied by an increase in the fluorescence lifetime [20]. The fluorescence lifetime is the only parameter that allows unambiguous distinguishing of the metallic particle-induced fluorescence enhancement.
We measured the frequency domain intensity decays of Rhodamine Red-X anti-rabbit IgG unbound and bound to the antigen (rabbit IgG) at different experimental conditions (in solution or bound to the SIF or glass surface) (Table 2). Average lifetime and amplitude-weighted lifetime 〈τ〉 are approximately the same for the labeled Ab in solution with or without bound nonlabeled antigen (rabbit IgG). The time-resolved intensity decays of Rhodamine Red-X anti-rabbit IgG bound to the antigen on the glass (–●–) or SIFs (–○–) are shown on Fig. 12. As seen from the decays and Table 2, the lifetime on SIFs is three- to fourfold shorter than that on glass surfaces. The increased fluorophore brightness with simultaneous decrease of the fluorescence lifetime prove that the observed enhancements are due to an increased radiative decay rate. Shortening the fluorescence lifetime will also result in increased photostability [19,20] of dyes on the SIF, and this is another advantage of surface-enhanced immunoassay technology.
Table 2.
Multiexponential analysis of Rhodamine Red-X anti-rabbit IgG bound to the antigen (rabbit IgG) at different experimental conditions (in solution or bound to the SIF or glass surface)
| Condition | 〈τ〉 (ns) | (ns) | αi | fi | τi (ns) | |
|---|---|---|---|---|---|---|
| Reference (solution, not bound to the antigen) | 0.83 | 2.41 | 0.620 | 0.063 | 0.08 | 1.0 |
| 0.187 | 0.223 | 0.99 | ||||
| 0.193 | 0.714 | 3.06 | ||||
| In solution | 0.82 | 2.42 | 0.629 | 0.072 | 0.09 | 0.9 |
| 0.228 | 0.340 | 1.23 | ||||
| 0.143 | 0.588 | 3.39 | ||||
| On glass surface | 0.54 | 1.32 | 0.606 | 0.105 | 0.09 | 0.9 |
| 0.308 | 0.534 | 0.93 | ||||
| 0.086 | 0.361 | 2.26 | ||||
| On SIF-coated surface | 0.15 | 0.40 | 0.585 | 0.162 | 0.04 | 1.8 |
| 0.358 | 0.511 | 0.21 | ||||
| 0.057 | 0.327 | 0.86 |
Note. , average lifetime; 〈τ〉, amplitude-weighted lifetime.
Fig. 12.
Frequency domain intensity decays on the glass or SIFs. The studied system was Rhodamine Red-X-labeled anti-rabbit Ab bound to the antigen, rabbit IgG. The excitation was 514 nm, and observation was 590 nm.
Conclusions
In this article, we have presented our initial results demonstrating the possibilities of using a TIR mode of excitation for surfaces coated with SIFs. The results demonstrate high fluorescence enhancements for surface-bound dye-labeled Abs. The enhancement is largest for the TIRF mode and is practically independent of sample layer thickness above the assay surface. Application of the TIRF mode allows performing the immunoassay in the presence of a highly fluorescent background without washing steps. This indicates the high potential of this technology as a generic platform for highly sensitive immunoassay development as well as for any general bioaffinity reactions on surfaces.
Acknowledgments
This work was supported by the National Center for Research Resource (RR-08119), the National Institute of Biomedical Imaging and Bioengineering (EB-1690), and Philip Morris USA Inc. and Philip Morris International. The authors are grateful to Mikhail Matveev for writing the software for enhancement calculations.
Footnotes
Abbreviations used: Ab, antibody; TIRF, total internal reflection fluorescence; SIF, silver island film; FF, front-face excitation; PVA, polyvinyl alcohol; IgG, immunoglobulin G; BSA, bovine serum albumin; TIR, total internal reflection; TRITC, tetramethyl-rhoda-mine isothiocyanate modified.
References
- [1].Gosling JP, A decade of development in immunoassay methodology, Clin. Chem 36 (1980) 1408–1427. [PubMed] [Google Scholar]
- [2].Hemmila IA, Applications of Fluorescence in Immunoassays, John Wiley, New York, 1991. [Google Scholar]
- [3].Vo-Dinh T, Sepaniak MJ, Griffn GD, Alarie JP, Immuno-sensors: principles and applications, Immunol. Methods 3 (1993) 85–92. [Google Scholar]
- [4].Dickson EF, Pollak A, Diamandis EP, Ultrasensitive bioanalytical assays using time-resolved fluorescence detection, Pharmacol. Ther 66 (1995) 207–235. [DOI] [PubMed] [Google Scholar]
- [5].Pearson JE, Gill A, Vadgama P, Analytical aspects of biosensors, Ann. Clin. Biochem 37 (2000) 119–145. [DOI] [PubMed] [Google Scholar]
- [6].Lehr HP, Reimann M, Brandenburg A, Sulz G, Klapproth H, Real-time detection of nucleic acid interactions by total internal reflection fluorescence, Anal. Chem 75 (2003) 2414–2420. [DOI] [PubMed] [Google Scholar]
- [7].Schuderer J, Akkoyun A, Brandenburg A, Bilitewski U, Wagner E, Development of a multichannel fluorescence affnity sensor system, Anal. Chem 72 (2000) 3942–3948. [DOI] [PubMed] [Google Scholar]
- [8].Asanov AN, Wilson WW, Oldham PB, Regenerable biosensor platform: a total internal reflection fluorescence cell with electro-chemical control, Anal. Chem 70 (1998) 1156–1163. [DOI] [PubMed] [Google Scholar]
- [9].Kooyman RPH, Kolkman H, Van Gent J, Greve J, Surface plasmon resonance immunosensors: sensitivity considerations, Anal. Chim. Acta 213 (1988) 35–45. [Google Scholar]
- [10].Melendez J, Carr R, Bartholomew DU, Kukanskis K, Elkind J, Yee S, Furlong C, Woodbury R, A commercial solution for surface plasmon sensing, Sensor. Actuator. B 35/36 (1996) 212–216. [Google Scholar]
- [11].Suzuki M, Ozawa F, Sugimoto W, Aso S, Miniature surface-plasmon resonance immunosensors: rapid and repetitive procedure, Anal. Bioanal. Chem 372 (2002) 301–304. [DOI] [PubMed] [Google Scholar]
- [12].Attridge JW, Daniels PB, Deacon JK, Robinson GA, Davidson GP, Sensitivity enhancement of optical immunosensors by the use of a surface plasmon resonance fluoroimmunoassay, Biosens. Bioelectron 6 (1991) 201–214. [DOI] [PubMed] [Google Scholar]
- [13].Liebermann T, Knoll W, Surface plasmon field-enhanced fluorescence spectroscopy, Coll. Surf. A 171 (2000) 115–130. [Google Scholar]
- [14].Yu F, Yao D, Knoll W, Surface plasmon field-enhanced fluorescence spectroscopy studies of the interaction between an antibody and its surface-coupled antigen, Anal. Chem 75 (2003) 2610–2617. [DOI] [PubMed] [Google Scholar]
- [15].Neumann T, Johansson M-L, Kambhampati D, Knoll W, Surface-plasmon fluorescence spectroscopy, Adv. Funct. Mater 12 (2002) 575–584. [Google Scholar]
- [16].Lakowicz JR, Radiative decay engineering: III. Surface plasmon-coupled directional emission, Anal. Biochem 324 (2004) 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR, Radiative decay engineering: IV. Experimental studies of surface plasmon coupled directional emission, Anal. Biochem 324 (2004) 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matveeva E, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Multi-wavelength immunoassays using surface plasmon-coupled emission, Biochem. Biophys. Res. Commun 313 (2004) 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lakowicz JR, Radiative decay engineering: biophysical and biomedical applications, Anal. Biochem 298 (2001) 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lakowicz JR, Shen Y, DÕAuria S, Malicka J, Fang J, Gryczynski Z, Gryczynski I, Radiative decay engineering: II. Effects of silver island films on fluorescence intensity lifetimes and resonance energy transfer, Anal. Biochem 301 (2002) 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Effects of fluorophore-to-silver distance on the emission of cyanine-dye-labeled oligonucleotides, Anal. Biochem 315 (2003) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Malicka J, Gryczynski I, Lakowicz JR, DNA hybridization assays using metal-enhanced fluorescence, Biochem. Biophys. Res. Commun 306 (2003) 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stich N, Gandhum A, Matushin V, Mayer C, Bauer G, Schalkhammer T, Nanofilms and nanoclusters: energy sources driving fluorophores of biochip bound labels, J. Nanosci. Nanotechnol 1 (2001) 397–405. [DOI] [PubMed] [Google Scholar]
- [24].Lochner N, Lobmaier C, Wirth M, Leitner A, Pittner F, Gabor F, Silver nanoparticle enhanced immunoassays: one step real time kinetic assay for insulin in serum, Eur. J. Pharmacol. Biopharmacol 56 (2003) 469–477. [DOI] [PubMed] [Google Scholar]
- [25].Lochner N, Pittner F, Wirth M, Gabor F, Preparation, characterization, and application of artificial Caco-2 cell surfaces in the silver nanoparticle enhanced fluorescence technique, J. Control Release 89 (2003) 249–259. [DOI] [PubMed] [Google Scholar]
- [26].Gryczynski I, Malicka J, Holder E, DiCesare N, Lakowicz JR, Effects of metallic silver particles on the emission properties of [Ru(bpy)3]2+, Chem. Phys. Lett 372 (2003) 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Malicka J, Gryczynski I, Fang J, Kusba J, Lakowicz JR, Increased resonance energy transfer between fluorophores bound to DNA in proximity to metallic silver particles, Anal. Biochem 315 (2003) 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Malicka J, Gryczynski I, Kusba J, Lakowicz JR, Effects of metallic silver island films on resonance energy transfer between N,N’-(dipropyl)-tetramethyl-indocarbocyanine (Cy3)- and N,N’-(dipropyl)-tetramethyl-indodicarbocyanine (Cy5)-labeled DNA, Biopolymers 70 (2003) 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Axelrod D, Hellen EH, Fulbright RM, Total internal reflection fluorescence, in: Lakowicz JR (Ed.), Topics in Fluorescence Spectroscopy, Biochemical Applications, vol. 3, Plenum, New York, 1992, pp. 289–343. [Google Scholar]
- [30].Axelrod D, Total internal reflection fluorescence microscopy, Methods Cell Biol. 30 (1989) 245–270. [DOI] [PubMed] [Google Scholar]
- [31].Stock K, Sailer R, Strauss WS, Lyttek M, Steiner R, Schneckenburger H, Variable-angle total internal reflection fluorescence microscopy (VA-TIRFM): realization and application of a compact illumination device, J. Microsc 211 (2003) 19–29. [DOI] [PubMed] [Google Scholar]
- [32].Ajo-Franklin CM, Kam L, Boxer SG, High refractive index substrates for fluorescence microscopy of biological interfaces with high z contrast, Proc. Natl. Acad. Sci. USA 98 (2001) 13643–13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lakowicz JR, Gryczynski Z, Gryczynski I, On the possibility of evanescent wave excitation distal from a solid–liquid interface using light quenching, Photochem. Photobiol 64 (1996) 636–641. [DOI] [PubMed] [Google Scholar]
- [34].Gryczynski I, Gryczynski Z, Lakowicz JR, Two-photon excitation by the evanescent wave from total internal reflection, Anal. Biochem 247 (1997) 69–76. [DOI] [PubMed] [Google Scholar]