Abstract
We report the first findings of Metal-Enhanced Fluorescence (MEF) from modified plastic substrates. In the past several years our laboratories have reported the favorable effects of fluorophores in close proximity to silver nanoparticles. These effects include, enhanced fluorescence intensities, (increased detectability), and reduced lifetimes, (enhanced fluorophore photostability). All of these reports have featured silver nanostructures and fluorophores which have been immobilized onto clean glass or quartz surfaces. In this report we show how plastic surfaces can be modified to obtain surface functionality, which in turn allows for silver deposition and therefore metal-enhanced fluorescence of fluorophores positioned above the silver using a protein spacer. Our findings show that plastic substrates are ideal surfaces for metal-enhanced phenomena, producing similar enhancements as compared to clean glass surfaces. Subsequently, we speculate that plastic substrates for MEF will find common place, as compared to the more expensive and less versatile traditional silica based supports.
Keywords: Metal-enhanced fluorescence, radiative decay engineering, plastics, modified plastics, polycarbonate
INTRODUCTION
Fluorescence detection is the central technology in biological research and biotechnology. Many fluorescent probes have become widely available over the last 10 years with diverse and appealing properties. These properties were engineered into fluorophores by modification of their structures, driven by the requirements from biological research and biotechnology. However, until a couple of years ago, all these modifications did not alter the intrinsic emissive decay rate of fluorophores, that is, the spontaneous rate at which a fluorophore emits photons.
In the past couple of years our laboratories have developed many metal-fluorophore combinations and geometries [1–5], which ultimately yielded significantly brighter and more photostable fluorophores. These advances are not due to chemical structure modifications, but are due to the control of the intrinsic fluorophore radiative decay rate. We have subsequently named this new technology both metal-enhanced fluorescence (MEF) [1,5] and radiative decay Engineering (RDE) [2,3]. In all of these examples of MEF, we have for the most part primarily used silver nanostructures deposited onto clean glass microscope slides or quartz plates. This has been because the chemistries of the surface of glass are well established and therefore the covalent immobilization of silver nanostructures onto glass less arduous and indeed reproducibly reliable.
However, polymer substrates are known to be very promising substrates for a variety of applications. Industrial interest in utilizing plastics is primarily driven by the fact that these materials are less expensive and easier to mass produce than silica based substrates [6]. Techniques like plasma etching [7], reactive ion etching [7], laser ablation [8], imprinting [9,10], and injection molding [11] are typically applicable to the fabrication of both devices and substrates in plastic materials [6]. There is also a wide variety of materials to choose from with an even greater array of chemical and physical properties [6].
Subsequently we have attempted to ascertain whether polymeric substrates can be used as substrates for metal-enhanced fluorescence, which, given their cost, are likely to be much better received by industry. While hydrophilic polymers are readily available with a high surface density of either hydroxyl or amine groups, which are readily used for silver deposition, we questioned whether plastics with a low density of surface functionality could be surface modified. This modification would potentially allow for MEF to be introduced into already existing plastic based technologies, such as with plastic high throughput screening well plates and fluorescence based clinical assays.
The practical approaches to polymer surface modification are corona discharge treatment, plasma, surface graft, light, and chemical modification [12]. In this paper we have chosen to surface modify a polycarbonate film (PC) for silver deposition and therefore metal-enhanced fluorescence, using chemical modification of the surface. Base catalyzed hydrolysis of the PC film readily creates additional surface functionality for silver island film deposition. Silver island films (SIFs) [3,5] have been widely used on glass slides in our laboratories for a variety of applications typically producing >5 fold enhancements in fluorescein emission intensity, as compared to a control sample containing no silver. Polycarbonate was chosen as the polymer of interest due to its widespread use in biotechnology [6,12,13]. While not discussed much in this paper, we have also tested a polypropylene film for MEF. However, the lack of any available functionalities, even after treatment, meant that it was not suitable for chemical modification.
Metal-enhanced fluorescence is known to be a through space phenomenon, where the close proximity of fluorophores to silver nanostructures results in quenching of the emission [1,2,5]. Subsequently, we have positioned the fluorophore a mean distance of 4 nm from the surface using a labeled protein, namely FITC-HSA. Our results clearly show that plastics can indeed be modified for silver deposition and notable enhancements in fluorescence emission can be achieved from the plastic substrates, similar to that observed from glass [5].
MATERIALS AND METHODS
Polycarbonate (PC) films with ≈50 μm thickness were cut into 75 × 25 mm pieces and placed onto Fisher brand glass microscope slides in order to provide support for the films. PC films were hydrolyzed in 2 M aqueous NaOH solution for 1 min and rinsed with deionized water. PC films were then transferred onto new glass slides and finally dried under a stream of cooled air. The hydrolyzed PC films were silanized with a 2% v/v solution of 3- (aminopropyl) triethoxysilane (APS) in denatured ethanol for 2 h. The APS coated PC films were removed from the solution and rinsed several times with ethanol and deionized water to remove the unbound APS. Silver island films (SIFs) were formed on half of the silanized PC films (the non-silvered half is used as a control) similar to our previous procedure [3,5]. SIFs were also formed on virgin PC films, i.e. unmodified films.
In previous reports of metal-enhanced fluorescence (MEF), our laboratories have coated silvered surfaces with fluorophore labeled protein [5]. This experimental format has been adopted for two main reasons, the first, being that the protein coverage with human serum albumin (HSA) is known to bind to silvered surfaces and indeed forms a monolayer [4,5] and secondly, the dimensions of the protein being such that the protein allows for a mean ≈4 nm separation of the silver and the fluorophore, MEF being a through-space phenomenon, as demonstrated by late T. Cotton and indeed our laboratories [1,2,5]. In contrast, surface enhanced raman scattering (SERS) is known to be a consequence of contact between the species of interest and the silvered surface [2].
Binding the FITC-HSA to the PC films was accomplished by soaking in a 10 μM FITC-HSA solution for 2 h, followed by rinsing with water to remove the unbound material. PC films were then transferred onto new glass slides. Both the unsilvered and silvered PC films were coated with labeled HSA, which is known to passively absorb to noble metal surfaces and form a ≈4 nm thick protein monolayer, allowing us to study the fluorescence spectral properties of noncovalent FITC-HSA complexes in the absence and presence of SIFs. By equally coating a PC film with FITC-HSA we were also able to determine the enhancement factor (benefit) obtained from using the silver, i.e.
given that both surfaces are known to have an approximate equal monolayer coverage [5].
All absorption measurements were performed using a HP 8453 UV-Vis spectrophotometer. Fluorescence measurements on PC films were performed by placing the films on a stationary stage equipped with a fiber-optic mount on a 15 cm long arm (normal to sample). The output of the fiber was connected to an Ocean Optics HD2000 spectrofluorometer to measure the fluorophore emission spectra. The excitation was from the second harmonic (470 nm) of the diode-pumped Nd:YVO4 laser (compact laser pointer design, output power ≈30 mW) at angle of45◦. The emission was observed through a 500 nm longpass filter (Edmund Scientific).
The real-color photographs of FITC-HSA on nonsilvered PC films and PC films with SiFs, were taken with a Olympus digital camera (C-740, 3.2 Mega Pixel, 10× optical zoom) using the same long-pass filter that was used for the emission spectra.
Time-resolved intensity decays were measured using reverse start-stop time-correlated single-photon counting (TCSPC) [14] with a Becker and Hickl gmbh 630 SPC PC card and an un-amplified MCP-PMT. Vertically polarized excitation at ≈440 nm was obtained using a pulsed laser diode, 1 MHz repetition rate. The intensity decays were analyzed in terms of the multi-exponential model:
| (1) |
whereαI are the amplitudes and τI the decay times, αI = 1.0. The fractional contribution of each component to the steady-state intensity is given by:
| (2) |
The mean lifetime of the excited state is given by:
| (3) |
and the amplitude-weighted lifetime is given by:
| (4) |
The values of αI and τI were determined by non-linear least squares impulse reconvolution with a goodness-of-fit criterion [14].
RESULTS AND DISCUSSION
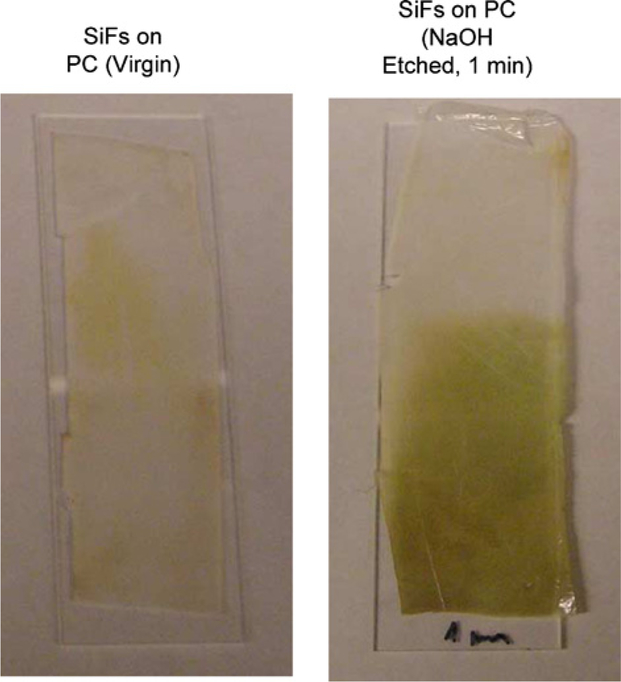
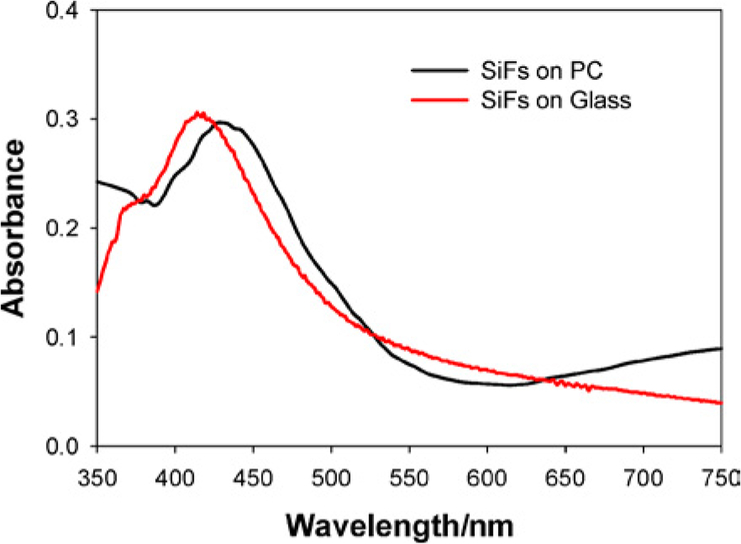
Our initial attempts at directly depositing silver island films (SIFs) onto plastic substrates resulted in relatively poor silver attachment to the virgin polycarbonate (PC) surface (Fig. 1, Left). However, after etching the PC film in 2 M NaoH for 1 min, followed by amino coating the surface using APS, 3-(aminopropyl) triethoxysilane, silver island films were readily formed (Fig. 1, right), and could not be washed from the surface. We reasoned that the strong base was providing additional surface hydroxyl groups for APS attachment by hydrolyzing the PC film, a procedure previously reported by Dauginet et al. [12]. For the thin films used in this report, immersion in 2 M hydroxide for 1 min was found to be sufficient for SIF preparation on the plastic surface, where the SIFs have a maximum optical density around 0.3, consistent with our previous reports [3,5]. The plasmon absorption band for the SIFs was also found to be slightly red-shifted on the PC film as compared to that typically observed on glass substrates, Fig. 2 [3,5].
Fig. 1.
Photograph of two plastic films mounted on glass slides. Left SIFs deposited on unmodified PC, Right SIFs deposited on NaOH etched PC.
Fig. 2.
Absorption spectra of silver island films, SIFs, grown on both modified PC and glass.
To test the silver coated plastic surfaces for metal-enhanced fluorescence we equally coated unmodified and modified films with fluorescein labeled HSA (Fig. 3) where the FITC-HSA has been shown to be an ideal labeled protein [3–5], with regard to positioning the fluorophore a couple of nanometers from the silver nanoparticles to facilitate metal-enhanced fluorescence [1,2].
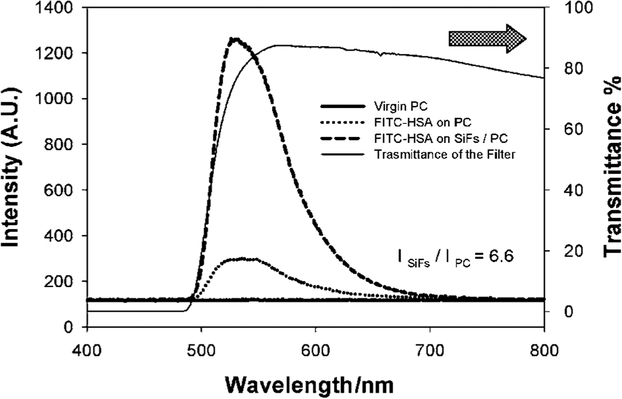
Fig. 3.
Emission spectra of FITC-HSA monolayers on modified PC with and without SiFs and on virgin PC (unmodified). The transmission spectra of the 500 nm cut-off filter is also shown.
Figure 3 shows that the emission of fluorescein is substantially greater on the silver island film coated modified PC films as compared to an equal coating on PC, but without any SIFs. In addition, no emission could be observed from the FITC-HSA coated virgin PC, demonstrating the need to modify the surface for metal-enhanced fluorescence. Interestingly, even without silver, etching the plastic with hydroxide provided for a greater protein coverage than the virgin PC film ( Fig. 3). The transmission of the cut-off filter used is also shown in Fig. 3 and accounts for the sharp rising edge of the emission spectra.
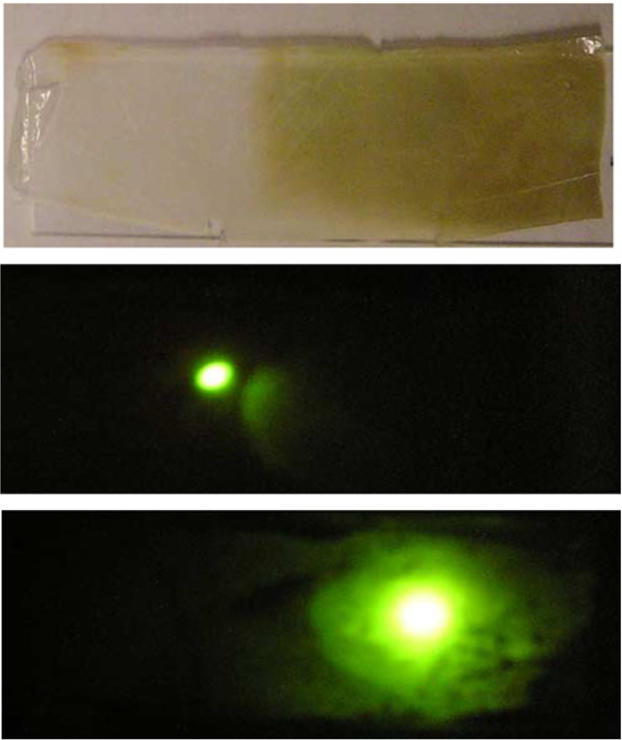
The metal-enhanced fluorescence from the silver coated modified plastic film was found to be approximately 7 times greater than the modified PC film but with no SIFs (i.e. the control sample). This relatively large increase in emission intensity could also be seen visually (Fig. 4), through the same long pass filter as used in Fig. 3. As the 470 nm laser excitation is moved from the unsilvered plastic (Fig. 4, middle) to the silvered plastic side, (Fig. 4, bottom), we see a dramatic increase in fluorescein emission.
Fig. 4.
Photographs: SiF coated modified plastic, Top, the emission of FITC-HSA on the unsilvered modified PC, Middle, and on the silvered and modified PC, Bottom.
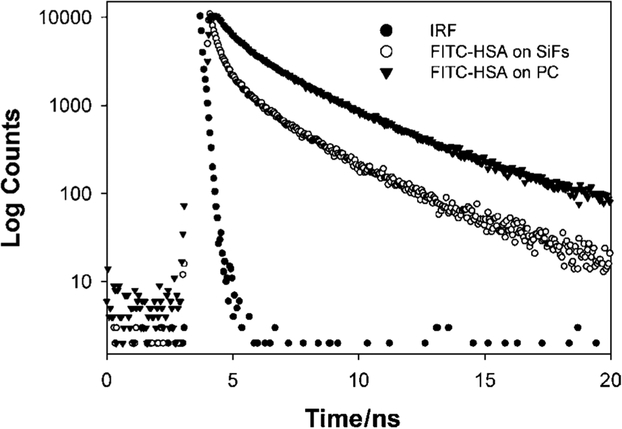
Metal-enhanced fluorescence results in both an increased emission intensity, accompanied by a reduction in fluorophore lifetime, i.e. a radiative rate modification [1–5]. Figure 5 shows the reduction in lifetime on the SiFs as compared to the modified plastic. The amplitude weighted lifetime was found to decrease from 2.58 ns on the unsilvered plastic to 1.68 ns on the silvered plastic, (Fig. 5 and Table I).
Fig. 5.
Intensity decays of FITC-HSA on silvered and unsilvered modified PC. The instrumental response function, IRF, is also shown.
Table I.
Analysis of the Intensity Decay of FITC-HSA on Silvered and Unsilvered Modified PC, Measured Using the Reverse Start-Stop Time-Correlated Single Photon Counting Technique and the Multi-Exponential Model
| Sample | αi | τI (ns) | α2 | τ2 (ns) | α3 | τ3 (ns) | 〈τ〉 (ns) | ||
|---|---|---|---|---|---|---|---|---|---|
| FITC-HSA on SiFs | 0.290 | 0.090 | 0.330 | 0.946 | 0.380 | 3.54 | 3.00 | 1.68 | 1.14 |
| FITC-HSA on PC | 0.079 | 0.289 | 0.289 | 1.182 | 0.632 | 3.50 | 3.16 | 2.58 | 0.89 |
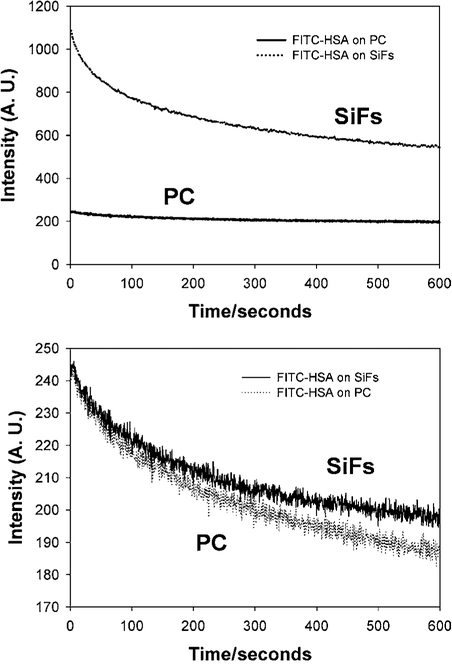
Finally we measured the photostability of the FITC-HSA on both the unsilvered and silvered modified PC film (Fig. 6). Using the same laser power we observe significantly more fluorescence from the silvered plastic, by simply considering the integrated area under the respective curves (Fig. 6, Top). However, when we attenuate the laser power on the silver surface to give the same initial emission intensity as observed on the unsilvered but modified plastic, we see similar photostability characteristics (Fig. 6, bottom).
Fig. 6.
Emission intensity vs. time of FITC-HSA on both silvered and unsilvered modified PC with constant 470 nm excitation, Top, and with the laser power adjusted to give the same initial steady state fluorescence intensity, Bottom.
CONCLUSIONS
To the best of our knowledge this is the first report of MEF from plastic substrates.
By base hydrolysis of thin polycarbonate films we have been able to provide more surface functionality for silver deposition. Subsequently, by coating these silvered surfaces with a labeled protein we have been able to observe metal-enhanced fluorescence, an approximate 7- fold increase in fluorescein emission intensity observed from modified and silvered plastic as compared to a modified but unsilvered PC film. Further, by comparing the emission intensity from the virgin PC film to the modified and silvered film, a substantial increase in fluorophore emission intensity can be realized. Given the widespread use of plastic substrates in fluorescence based clinical assays and in drug discovery (e.g. HTS well plates), simple surface modifications of plastics could facilitate silver depositions for metal-enhanced fluorescence. Alternatively, unmodified hydrophilic plastics with an abundance of surface hydroxyl or even amino groups [15] could be ideal for silver deposition and alleviate the need for surface plastic modification. In addition, there have been recent reports of surface plastic modification using light [13], specific wavelengths used to break covalent bonds and therefore provide for additional polymer surface functionality. This reagentless approach could readily be used to prepare plastics for silver deposition. Work is currently underway in our laboratories in this regard and will be reported in due course.
ACKNOWLEDGMENTS
This work was supported by the NIH, GM070929–01.
ABBREVIATIONS:
- MEF
metal-enhanced fluorescence
- RDE
radiative decay engineering
- PC
polycarbonate plastic films
- APS
3- (aminopropyl) triethoxysilane
- SIFs
silver island films
- FITC-HSA
fluorescein labeled human serum albumin
REFERENCES
- 1.Geddes CD and Lakowicz JR (2002). Metal-enhanced fluorescence. J. Fluoresc 12(2), 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakowicz JR (2001). Radiative decay engineering: Biophysical and biomedical applications. Appl. Biochem 298, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Grcyzynski Z, and Gryczynski I (2002). Radiative decay engineering 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer, Anal. Biochem 301, 261– 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geddes CD, Cao H, Gryczynski I, Gryczynski Z, Fang J, and Lakowicz JR (2003). Metal-enhanced fluorescence due to silver colloids on a planar surface: Potential applications of Indocyanine green to in vivo imaging. J. Phys. Chem. A 107, 3443– 3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geddes CD, Aslan K, Gryczynski I, Malicka J, and Lakowicz JR (2004) in Geddes CD (Ed.) Noble Metal Nanostructure for Metal-Enhanced Fluorescence, Reviews in Fluorescence 2004, Springer, New York, pp. 365–401. [Google Scholar]
- 6.Liu Y, Ganser D, Schneider A, Grodzinski P, and Kroutchinina N (2001). Microfabricated polycarbonate CE devices for DNA analysis. Anal. Chem 73, 4196–4201. [DOI] [PubMed] [Google Scholar]
- 7.Boerner M, Kohl M, Pantenburg F, Bacher W, Hein H, and Schomburg W (1996). Microsyst. Technol 2, 149–152. [Google Scholar]
- 8.Roberts MA, Rossier JS, Bercier P, and Girault H (1997). UV laser machined polymer substrates for the development of microdiagnostic systems. Anal. Chem 69, 2035– 2042. [DOI] [PubMed] [Google Scholar]
- 9.Martynova L, Locascio LE, Gaitan M, Kramer GW, Christensen RG, and MacCrehan W (1997). Fabrication of plastic microfluid channels by imprinting methods. Anal. Chem 69, 4783– 4789. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Locascio L, Gaitan M, and Lee CS (2000). Room-temperature imprinting method for plastic microchannel fabrication. Anal. Chem 72, 1930–1933. [DOI] [PubMed] [Google Scholar]
- 11.McCormick RM, Nelson RJ, Alonso-Amigo MG, Benvegnu DJ, and Hooper HH (1997). Microchannel electrophoretic separations of DNA in injection-molded plastic substrates. Anal. Chem 69, 2626–2630. [DOI] [PubMed] [Google Scholar]
- 12.Dauginet L, Duwez A-S, Legras R, and Demoustier- Champagne S. Surface modification of polycarbonate and poly(ethyleneterephthalate) films and membranes by polyelectrolyte deposition. Langmuir 17, 3952–3957. [Google Scholar]
- 13.Xu Y, Vaidya B, Patel AB, Ford SM, McCarley RL, and Soper SA (2003). Solid-phase reversible immobilization in microfluidic chips for the purification of dye-labeled DNA sequencing fragments. Anal. Chem 75, 2975–2984. [DOI] [PubMed] [Google Scholar]
- 14.Lakowicz JR (1999). Principles of Fluorescence Spectroscopy, Kluwer, New York. [Google Scholar]
- 15.Ward W and McCarthy TJ (1989) in Mark HF, Bikales NM, Overberger CG, Menges G, and Kroschwitz JI (Eds.), Encyclopedia of Polymer Science and Engineering, 2nd ed. John Wiley and Sons, New York, 1989, supplvol. pp. 674–689. [Google Scholar]