Abstract
Background:
The long-term effects of sigmoidoscopy screening on colorectal cancer (CRC) incidence and mortality in men and women is unclear.
Objective:
To determine the effectiveness of flexible sigmoidoscopy screening after 15 years follow-up in men and women.
Design:
Randomized controlled trial ()
Setting:
Oslo city and Telemark County, Norway.
Participants:
Individuals, aged 50–64 years at baseline, without prior CRC.
Intervention:
Screening (between 1999 and 2001) with flexible sigmoidoscopy with and without additional fecal blood testing, or no screening. Screen-positives were offered colonoscopy.
Measurements:
Age-adjusted colorectal cancer incidence and mortality stratified by sex
Results:
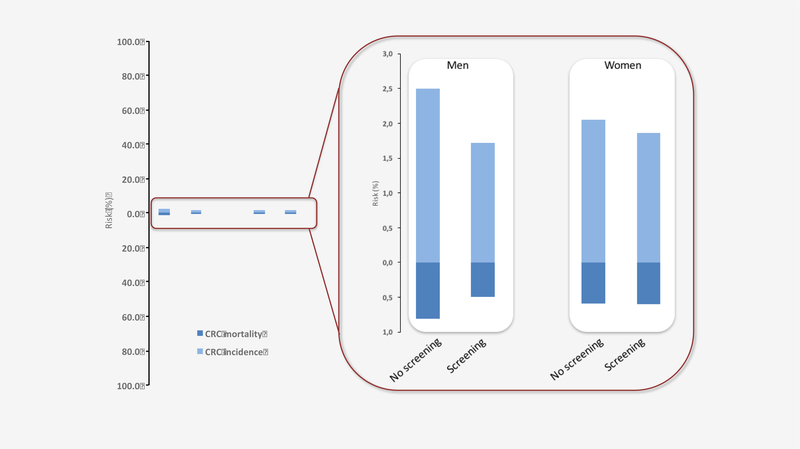
Of 98,678 individuals, 20,552 were randomized to screening and 78,126 to no screening. Attendance was 61.4% in men and 64.7% in women. Median follow-up was 14.8 years. The absolute risk of CRC in men was 1.72% in the screening-group and 2.50% in the control-group; risk-difference −0.78% (95% Confidence-Interval [CI] −1.08% to-0.48%), hazard-ratio[HR] 0.66(95%CI 0.57–0.78). In women, the corresponding risks were 1.86% and 2.05%; risk-difference −0.19%(95%CI −0.49% to 0.11%), HR 0.92(95%CI 0.79–1.07) (P-value for heterogeneity: 0.004). The absolute risk of CRC-mortality in men was 0.49% in the screening-group and 0.81% in the control-group; risk-difference −0.33%(95%CI −0.49 to −0.16), HR 0.63(95% CI 0.47–0.83). The corresponding CRC-mortality risk in women was 0.60% and 0.59%, respectively; risk-difference 0.01% (95%CI −0.16% to 0.18%), HR 1.01(95%CI 0.77–1.33) (P-value for heterogeneity: 0.014).
Limitation:
Follow-up through national registries.
Conclusion:
Offering sigmoidoscopy screening in Norway reduced colorectal cancer incidence and mortality in men, but had little or no effect in women.
Primary funding source:
Norwegian government, Norwegian Cancer Society, NIH grant P01 CA134294.
Recent recommendations from the United States Preventive Services Task Force include several screening tests, amongst them sigmoidoscopy or the combination of sigmoidoscopy and immunochemical fecal occult blood testing (FOBT) for colorectal cancer (CRC) screening in men and women aged 50 to 75 years.(1) Colorectal cancer screening with sigmoidoscopy has been introduced in the United Kingdom and other countries, including Norway.
Previous analyses of four randomized trials have indicated that sigmoidoscopy screening reduces CRC incidence by 18–26% and CRC mortality by 22–31% after 10 to 17 years of follow-up.(2–5) It is important to evaluate the duration of the effect of sigmoidoscopy screening because this may enable guideline makers to recommend evidenced-based screening intervals, thus to reduce health-care costs, patient inconvenience and adverse events related to screening.
There is still uncertainty with regard to the effectiveness of sigmoidoscopy screening in women. In three of the sigmoidoscopy screening trials, there is a larger absolute reduction in CRC incidence among men (0.40% to 1.05%) compared with women (0.13% to 0.42%), while one trial (with shorter follow-up) found a larger reduction in women compared with men (0.38% versus 0.26%, respectively). Sex-specific CRC mortality reduction was consistently larger in men (0.15% to 0.36%) than women (0.04% to 0.17%).(2–6) (and Carle Senore, personal communication). Longer follow-up times are needed to confirm data on the effectiveness of sigmoidoscopy screening in women and men.
We here report data on colorectal cancer incidence and mortality after up to 17 years follow-up in 98,678 men and women based on the Norwegian Colorectal Cancer Prevention Trial (NORCCAP), a population-based, randomized trial of sigmoidoscopy screening with and without additional immunochemical FOBT versus no screening.
Methods
Patients and design
The study design has been described elsewhere (7) and in the appendix. In brief, between 1999 and 2000, all men and women aged 55–64 years in the City of Oslo and Telemark County, Norway, were identified through the Population Registry and randomly selected to once-only sigmoidoscopy screening or no screening (appendix section 1). Individuals in the screening group were further randomized (1:1) to receive sigmoidoscopy, or sigmoidoscopy and a single round of fecal immunochemical occult blood testing (FOBT) (FlexSure OBT, Beckman-Coulter, Palo Alto, CA, USA) (7). Both randomization procedures were performed by an independent body (IBM Norway) using computerized algorithms. At the end of 2000, it was decided to also include all individuals 50–54 years of age living in the trial areas, as previously described (6) and in the appendix section 1. Screening interventions took place during 1999 and 2000 for individuals 55–64 year of age and in 2001 for individuals 50–54 years of age. The only trial exclusion criterion was personal history of CRC. During the study period, there was no organized CRC screening in the trial areas, and very little opportunistic screening (3). All participants who attended the screening examination provided written informed consent. The study was approved by the Ethics Committee of South-East Norway and the Norwegian Data Protection Authority. The trial is registered at ().
Screening examinations were performed at three dedicated centers (7, 8). During sigmoidoscopy, detected polyps were removed or biopsied and subjected to histopathology. Participants assigned to sigmoidoscopy and FOBT received the FOBT kit by mail and returned it to the screening center on attendance for sigmoidoscopy. A positive screening test was defined as any polyp ≥10 millimeters (irrespective of histology), any adenoma, CRC, or positive FOBT. All screen-positive individuals were referred for colonoscopy for polyp removal of all detected polyps, and diagnosis of cancer. Post-polypectomy surveillance was carried out according to Norwegian guidelines (5 or 10-year colonoscopy intervals for high-risk adenomas; no surveillance for low-risk adenomas)(9).
Endpoint ascertainment
Primary study endpoints were CRC incidence and mortality. Predefined secondary endpoints included CRC incidence and mortality in men and women, and in the distal (defined as rectum and the sigmoid colon) and proximal colon. All endpoints were retrieved by linkage of the individuals’ national identity number to Norwegian registries (Cancer Registry, Cause of Death Registry, Population Registry) (7). Colorectal cancer was defined as adenocarcinoma of the colon or rectum.
Statistics
All primary analyses followed the intention-to-treat principle, that is, all individuals were classified into their allocated group (screening or control), regardless of compliance with the screening intervention. All individuals were followed from study entry date until diagnosis of colorectal cancer, death, emigration, or December 31st 2015, whichever occurred first. The number of individuals eligible for analyses is updated from previous publications for current registry data status for individuals who had emigrated or were dead prior to study start. Details of the sample size calculations have been published previously and are also available in the appendix section 2.1. (3, 7).
We computed both rates and 15-year cumulative probabilities (risks) of CRC incidence and mortality, as well as rate differences and 15-year risk differences. All estimates were age-adjusted as explained in the appendix section 2.2 and elsewhere (3); 95% confidence intervals for the differences were calculated via a nonparametric bootstrap with 10,000 samples. We estimated hazard ratios (HR) from Cox models (appendix section 2.2).
Sex-stratified analysis is a predefined subgroup analysis in the NORCCAP trial. We tested for effect heterogeneity by sex on the additive scale using bootstrap (7). Because there was substantial heterogeneity between men and women for both CRC incidence (P=0.004) and mortality (P=0.014), the NORCCAP trial steering committee decided to present results for men and women separately. Results for men and women combined are available in the appendix section 3. As secondary analyses, we estimated the per-protocol effect among screening compliers via instrumental variable estimation (see Appendix section 2.3 for details).
All analyses were conducted with Stata 14.1 software (StataCorp, College Station, Texas, USA).
Role of the funding source
The study was funded by grants from Norwegian government and the Norwegian Cancer Society, and by the U.S. National Institutes of Health. The funders had no role in design, conduct or reporting of the trial.
Results
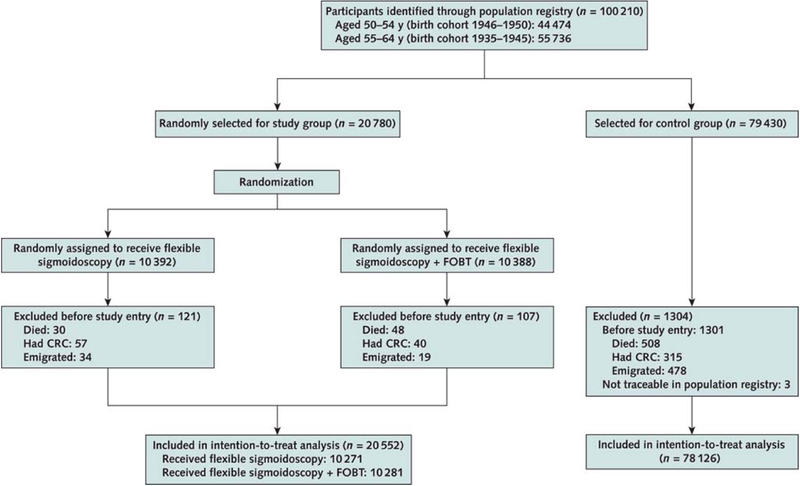
Of 100,210 randomized individuals, 1,532 (1.5%) were excluded (due to CRC, death, or emigration before study entry, or because they were not traceable through the Population Registry), leaving 98,678 individuals eligible for analyses (20,552 in the screening group, 78,126 in the control group (Appendix Figure 1).
In the screening group, 10,271 individuals were randomized to sigmoidoscopy screening and 10,281 individuals were randomized to the combination of sigmoidoscopy and FOBT. Men accounted for 49.8% of the study population.
The screening attendance rate was 61.4% in men, and 64.7% in women. Colonoscopy for positive screening was performed in 2,520 (19.5%) of screened individuals, 16.2% of women and 22.9% of men. Surveillance was recommended to 493 (7.4%) women and 775 (12.3%) men. Adherence to colonoscopy after positive sigmoidoscopy or FOBT was high (96%) in both men and women. The median follow up was 14.8 years for both CRC incidence and mortality.
Colorectal cancer incidence in women
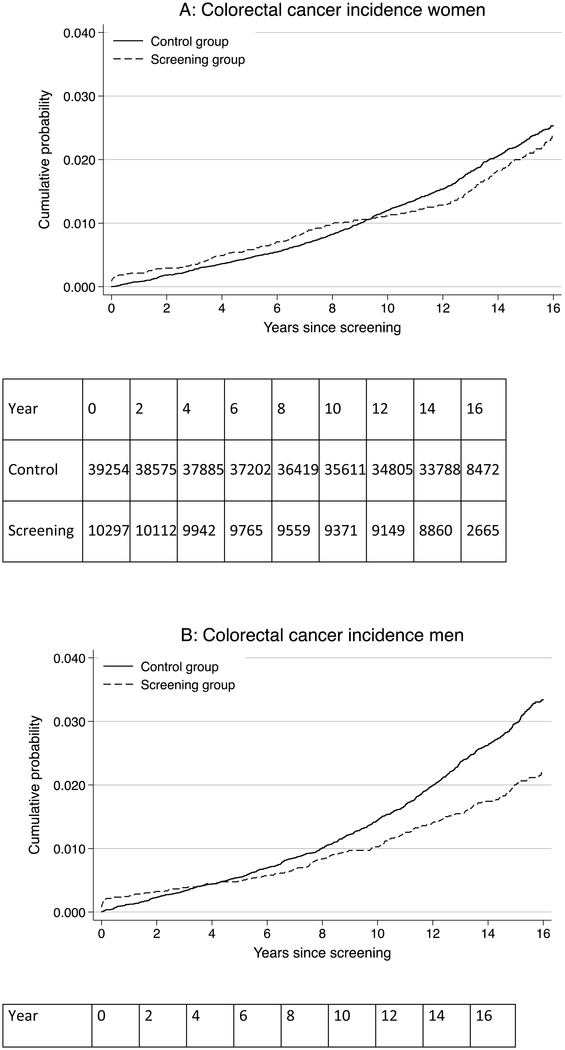
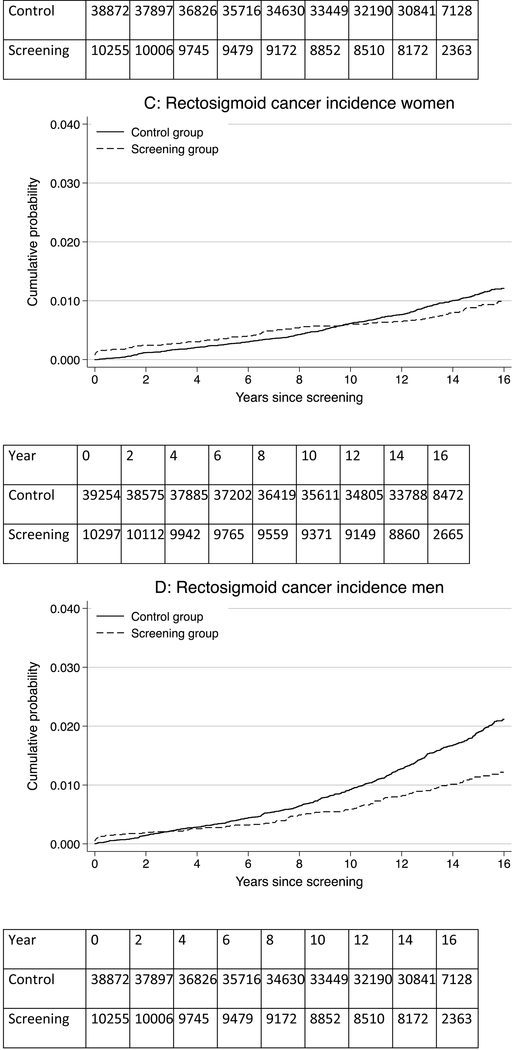
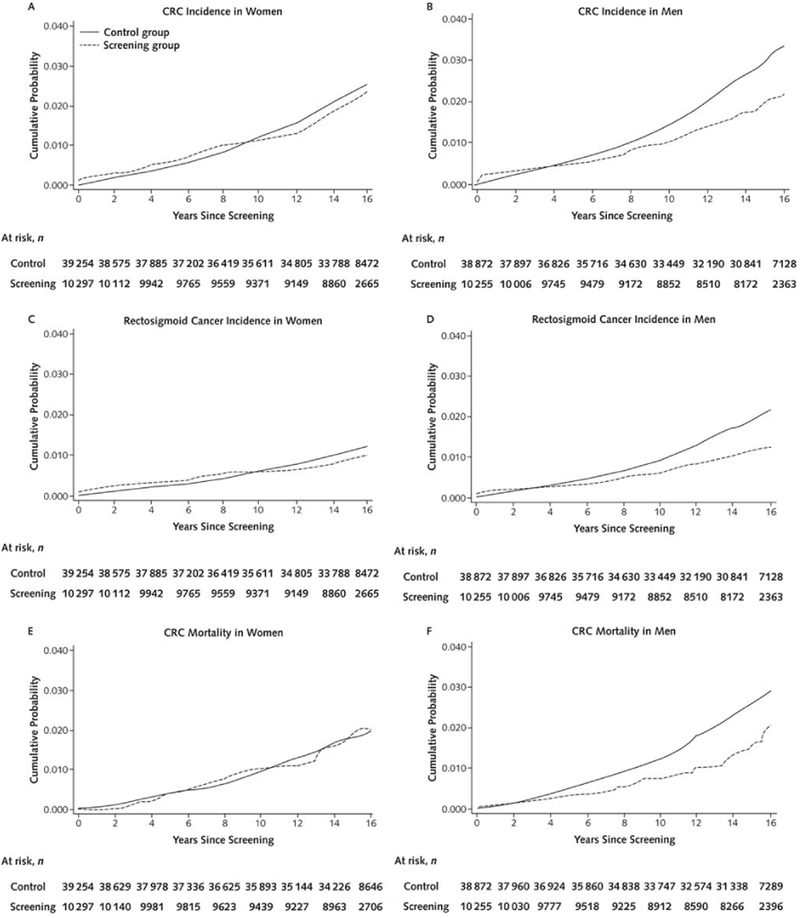
During 704,219 person-years of observation in women, 207 colorectal cancers were diagnosed in the screening group and 789 in the control group (Table 1). The CRC incidence rate per 100,000 person-years was 140.1 in the screening group and 153.6 in the control group, corresponding to 13.5 fewer cases per 100,000 person-years in the screening group (95% CI −35.4 to 8.5) and a HR of 0.92 (95% CI 0.79–1.07) (Table 1; Figure 1). The 15-year CRC risk was 1.86% in the screening group and 2.05% in the control group; risk difference-0.19% (95% CI −0.49% to 0.11%) (Figure 2). There was little difference in CRC incidence rate between the screening and control group regardless of tumor location (distal versus proximal colon) or screening modality (sigmoidoscopy screening alone or additional FOBT).
Table 1:
Age-adjusted hazard ratios and rate differences for colorectal cancer incidence and mortality in the screening versus the control group in women and men
| Women | Men | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening group (N=10,297) | Control group (N=39,254) | Screening group (N=10,255) | Control group (N=38,872) | ||||||||||
| n | Cases/100,000 py |
n | Cases/100,000 py* |
Hazard ratio (95% CI) | Rate difference/100,000 py (95%CI) | n | Cases/100,000 py |
n | Cases/100,000 py* |
Hazard ratio (95% CI) | Rate difference/100,000 py (95%CI) | P-value for heterogeneity | |
| Colorectal cancer incidence | 207 | 140.1 | 789 | 153.6 | 0.92 (0.79–1.07) | −13.5 (−35.4 to 8.5) | 186 | 131.4 | 962 | 196.9 | 0.66 (0.57–0.78) | −65.5 (−80.8 to −36.9) | 0.004 |
| Person-years of observation | 147,762 | 556,457 | 141,510 | 528,317 | |||||||||
| Location** | |||||||||||||
| Distal | 89 | 60.2 | 389 | 74.3 | 0.81 (0.64–1.02) | −14.1 (−28.9 to 1.10) | 105 | 74.2 | 611 | 124.3 | 0.59 (0.48–0.73) | −50.1 (−62.0 to 28.6) | 0.006 |
| Proximal | 113 | 76.5 | 383 | 76.1 | 1.01 (0.82–1.25) | 0.35 (−15.7 to 16.4) | 78 | 55.1 | 326 | 67.6 | 0.81 (0.63–1.04) | −12.5 (−24.3 to 3.5) | 0.312 |
| Age group | |||||||||||||
| 50–54 years | 39 | 81.0 | 237 | 94.2 | 0.86 (0.61–1.21) | −13.2 (−41.3 to 14.9) | 38 | 81.9 | 317 | 126 | 0.65 (0.46–0.91) | −44.1(−73.6 to −14.6) | 0.25 |
| 55–64 years | 168 | 168.7 | 552 | 181.1 | 0.93 (0.78–1.11) | −12.4 (−42.1 to 17.2) | 148 | 155.6 | 645 | 233.1 | 0.67 (0.56–0.80) | −77.5 (−108.4 to −46.7) | 0.009 |
| Screening modality | |||||||||||||
| Sigmoidoscopy | 104 | 140.6 | 789 | 153.6 | 0.92 (0.75–1.13) | −12.9 (−41.2 to 17.0) | 85 | 119.9 | 962 | 196.9 | 0.60 (0.48–0.75) | −77.0 (−91.9 to −35.7) | 0.002 |
| Sigmoidoscopy + FOBT | 103 | 139.5 | 789 | 153.6 | 0.91 (0.74–1.11) | −14.0 (−42.7 to 15.8) | 101 | 142.9 | 962 | 196.9 | 0.72 (0.59–0.89) | −54.0 (−72.0 to 10.7) | 0.274 |
| Colorectal cancer mortality | 65 | 43.7 | 225 | 43.3 | 1.01 (0.77–1.33) | 0.43 (−11.7 to 12.6) | 57 | 40 | 305 | 63.3 | 0.63 (0.47–0.83) | −23.2 (−32.8 to −8.7) | 0.014 |
| Person-years of observation | 148,705 | 575,166 | 142,370 | 539,415 | |||||||||
| Location** | |||||||||||||
| Distal | 33 | 22.2 | 99 | 18.8 | 1.17 (0.79–1.73) | 3.36 (−4.9 to 12.0) | 35 | 24.6 | 180 | 37.3 | 0.65 (0.45–0.93) | −12.8 (−20.7 to −1.9) | 0.023 |
| Proximal | 27 | 18.2 | 114 | 22.2 | 0.83 (0.54–1.26) | −4.0 (−12.0 to 4.0) | 20 | 14.1 | 112 | 23.3 | 0.60 (0.37–0.96) | −9.2 (−15.5 to −1.1) | 0.43 |
| Age group | |||||||||||||
| 50–54 years | 12 | 24.8 | 70 | 27.7 | 0.90 (0.49–1.65) | −2.86 (−18.3 to 12.6) | 8 | 17.2 | 88 | 34.8 | 0.49 (0.24–1.02) | −17.6 (−31.6 to −3.7) | 0.21 |
| 55–64 years | 53 | 52.8 | 155 | 50.5 | 1.05 (0.77–1.42) | 2.3 (−14.0 to 18.6) | 49 | 51.1 | 217 | 77.7 | 0.66 (0.48–0.90) | −26.6 (−44.2 to −8.9) | 0.038 |
| Screening modality | |||||||||||||
| Sigmoidoscopy | 35 | 47.1 | 225 | 43.3 | 1.09 (0.76–1.56) | 3.78 (−12.9 to 20.5) | 29 | 40.7 | 305 | 63.3 | 0.64 (0.43–0.93) | −22.6 (−34.6 to −1.9) | 0.010 |
| Sigmoidoscopy + FOBT | 30 | 40.4 | 225 | 43.3 | 0.94 (0.64–1.37) | −2.93 (−17.9 to 13.2) | 28 | 39.3 | 305 | 63.3 | 0.62 (0.42–0.91) | −23.9 (−35.3 to −3.3) | 0.084 |
| All-cause mortality | 1571 | 1056.4 | 5427 | 1047.5 | 1.02 (0.96–1.07) | 8.92 (−49.3 to 66.6) | 2238 | 1572.0 | 8006 | 1638.1 | 0.96 (0.91–1.00) | −66.1 (−137.5 to 5.77) | 0.11 |
| Age group | |||||||||||||
| 50–54 years | 315 | 652.4 | 1651 | 654.0 | 1.00 (0.88–1.13) | −1.5 (−80.2 to 77.1) | 463 | 995.3 | 2528 | 1001.8 | 0.99 (0.90–1.10) | −6.5 (−105.2 to 92.2) | 0.96 |
| 55–64 years | 1256 | 1251.4 | 3776 | 1230.0 | 1.02 (0.95–1.08) | 21.4 (−58.2 to 101.0) | 1775 | 1853.0 | 5478 | 1964.0 | 0.94 (0.89–1.00) | −111.0 (−211.7 to −10.3) | 0.08 |
Rates are age-standardized.
The sum of distal and proximal cancers are lower than the total due to unknown location for some cancers.
Men versus women. py: person-years. CI: confidence interval. FOBT: fecal occult blood test. n denotes the number of cases (colorectal cancer diagnosis and deaths)
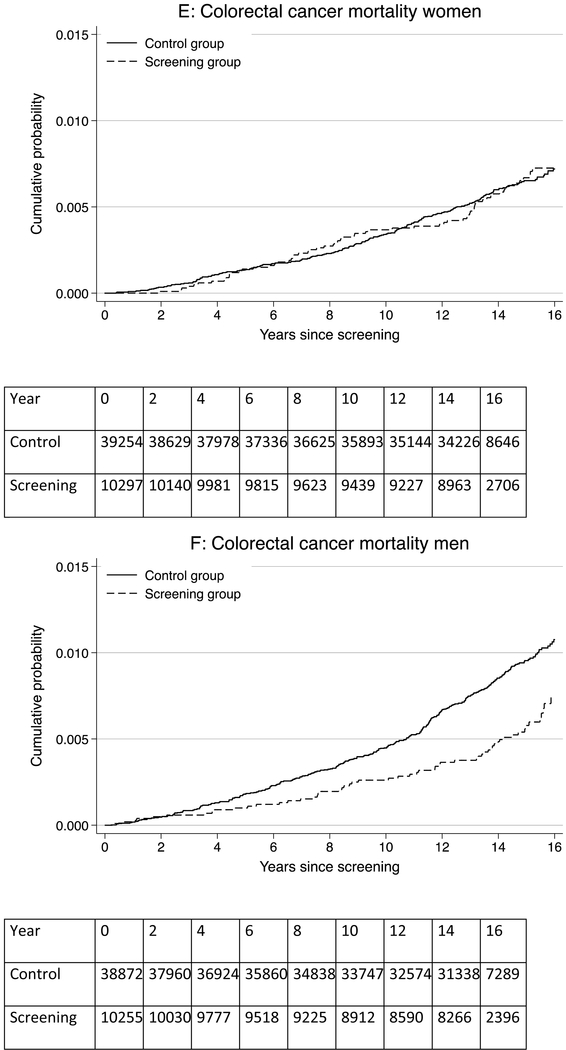
Figure 1: Risk of colorectal cancer, rectosigmoid cancer, and colorectal cancer mortality for men and women in the screening and control groups.
Panel A: colorectal cancer risk in women; Panel B: colorectal cancer risk in men; Panel C: rectosigmoid cancer risk in women; Panel D: rectosigmoid cancer risk in men; Panel E: colorectal cancer mortality risk in women; Panel F: colorectal cancer mortality risk in men
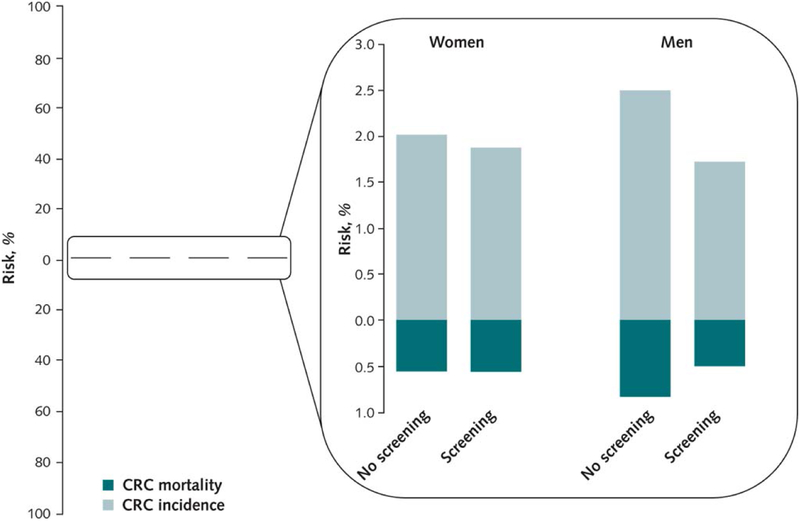
Figure 2:
15-year risks of colorectal cancer (light-blue) and colorectal cancer mortality (dark blue) with and without screening for men and women
Colorectal cancer incidence in men
During 669,827 person-years of observation in men, 186 colorectal cancers were diagnosed in the screening group and 962 in the control group. The CRC incidence rate per 100,000 person-years was 131.4 in the screening group and 196.9 in the control group, corresponding to 65.5 fewer cases per 100,000 person-years (95% CI −36.9 to −80.8) in the screening group and a HR of 0.66 (95% CI 0.56–0.77) (Table 1; Figure 1). The 15-year CRC risk was 1.72% in the screening group and 2.50% in the control group; risk difference −0.78% (95% CI −1.08% to −0.48%) (Figure 2).
The HR was 0.59 (95% CI 0.48–0.73) for cancer in the distal colon and 0.81 (95% CI 0.63–1.04) in the proximal colon (Table 1). The cancer incidence rate was lower in the screening group than in the control group in both older and younger age groups, and for sigmoidoscopy with or without FOBT.
Colorectal cancer mortality in women
A total of 290 women died of colorectal cancer during follow-up (723,871 person-years of observation); 65 in the screening group and 225 in the control group. The CRC mortality rate per 100,000 person-years was 43.7 in the screening group and 43.3 in the control group, corresponding to 0.4 more deaths per 100,000 person-years (95% CI −11.7 to 12.6) in the screening group and a HR of 1.01 (95% CI 0.77–1.33) (Table 1, Figure 1). The 15-year CRC mortality risk was 0.60% in the screening group and 0.59% in the control group; risk difference 0.01% (95% CI −0.16% to 0.18%) (Figure 2). There was little difference in CRC mortality rates regardless of tumor location (distal versus proximal colon) or screening modality (sigmoidoscopy screening alone or additional FOBT).
Colorectal cancer mortality in men
A total of 362 men died of colorectal cancer during follow-up (681,785 person-years of observation); 57 in the screening group and 305 in the control group. The CRC mortality rate per 100,000 person-years was 40.0 in the screening group and 63.3 in the control group, corresponding to 23.2 fewer deaths per 100,000 person-years (95% CI −32.8 to −8.7) in the screening group and a HR of 0.63 (95% CI 0.47–0.83) (Table 1, Figure 1). The 15 year CRC mortality risk was 0.49% in the screening group and 0.81% in the control group; risk difference −0.33% (95% CI −0.49 to −0.16) (Figure 2).
The HR was 0.65 (95% CI 0.45–0.93) for distal colorectal cancer death and 0.60 (95% CI 0.37–0.96) for proximal colon cancer death (Table 1). For sigmoidoscopy screening alone, the HR was 0.64 (95% CI 0.43–0.93), as compared with 0.62 (95% CI 0.42–0.91) for sigmoidoscopy with FOBT.
Results for men and women combined are shown in Appendix Table 1. Cumulative CRC incidence and mortality plots for screening attenders, non-attenders and controls are shown in Appendix Figures 2 and 3.
All-cause mortality
We observed 17,242 deaths (17.5%) during follow-up, 6,998 among women (14.1%) and 10,244 among men (20.9%). The all-cause mortality rates in women were 1056.4 and 1047.5 per 100,000 person-year in the screening and control groups, respectively (HR 1.02; 95% CI 0.96–1.09). For men, the all-cause mortality rates were 1572.0 and 1638.1 per 100,000 person-year in the screening and control groups, respectively (HR 0.96; 95% CI 0.91–1.00)
Per protocol analyses
Among compliers with screening, we estimated that the 14-year risk difference for CRC incidence was −0.27% (95% CI −0.72% to 0.18%) in women and −1.19% (95% CI −1.65% to −0.72%) in men (Appendix Table 1). Further, we estimated that the 14-year risk difference for CRC mortality was −0.03% (95% CI −0.27% to 0.21%) in women and −0.52% (95% CI −0.77% to −0.26%) in men.
Discussion
Our findings indicate that once-only sigmoidoscopy screening reduces the risk of colorectal cancer incidence over a 17-year period by 34% in men, but not in women. For colorectal cancer mortality, we observed a 37% reduction in men, but little or no reduction in women.
We and others have shown that sigmoidoscopy screening lowers CRC incidence and mortality for 10 to 12 years after screening (3–5, 10). The previous reports also indicated that there might be a smaller effect in women as compared with men, but the differences between men and women varied between studies and between the two endpoints (CRC incidence and mortality). The heterogeneity of the results may have been due to short follow-up time in the previous reports, which might have prevented finding differences in effectiveness between men and women. Different colonoscopy referral thresholds might also have contributed to the heterogeneity between the sex-specific effect in the trials since the distribution of cancers in the large bowel is different for men and women and also between different age-groups. (6) Another source of heterogeneity is the difference in surveillance recommendations because more intensive surveillance of individuals during follow-up might add to the effectiveness of the screening intervention.
In a recent pooled analysis of three of the four randomized trials - but with shorter follow-up than in the present paper - men invited to sigmoidoscopy screening had 24% reduced incidence of colorectal cancer and women younger than 60 years had 29% reduced incidence of colorectal cancer. There was no screening effect in older women (6).
Our study, with longer follow-up and more events than previously reported, found a strong effect of sigmoidoscopy screening in men, but little or no effect in women. Furthermore, our study found that the effect in men lasted beyond what we have previously reported, and that there was a strong trend towards reduction in all-cause mortality in men screened by sigmoidoscopy. In comparison, The UK Flexi Scope trial found that once-only sigmoidoscopy screening was effective in both men and women after 17 years follow-up, although less in women, which is consistent with our findings (2). The reduction in CRC incidence in the UK trial was 1.05% in men and 0.42% in women, and the reduction in CRC mortality was 0.36% in men and 0.17% in women.
It is unclear why sigmoidoscopy screening has limited or no effect in women. Because CRC is more prevalent in men than women, the CRC incidence and mortality rates were higher in the male control group than in the female control group, while men and women had comparable incidence and mortality rates for colorectal cancer in the screening groups. (Appendix Figures 2C and 3C). This could indicate that a maximum of risk reduction can be achieved by screening irrespective of sex. Alternatively, these findings may reflect that women who attended screening may have a different colorectal cancer risk profile as compared with men (screening attendance rates per se in our study were higher for women than men; Appendix Figures 2 and 3). Further, men have a higher prevalence of adenomas at sigmoidoscopy screening as compared to women and accordingly, men were more often referred for colonoscopy. In NORCCAP, screening reduced both proximal and distal cancer and cancer death in men, but not in women. Finally, the quality of the screening examination and follow-up colonoscopy in screening-positive individuals may have been different in men and women: The intubation depth at sigmoidoscopy was slightly shorter in women (44 cm in women, 49 cm in men,), and the caecum intubation rate for screening-positive women was lower than for men (87% versus 93%), while the quality of bowel preparation was similar for men and women (8). We cannot rule out or confirm any of these possible explanations, although we find it unlikely that they explain the observed differences.
Differences in transition rates and sojourn time of preclinical CRC are also unlikely to explain our finding, as similar estimates have been found in women and men (11). Finally, women have a higher risk of developing proximal colon cancer than men, and proximal colon cancer often show more aggressive disease course compared to distal colon cancer. (12) This may affect the effectiveness of sigmoidoscopy screening in women, but this does not explain the lack of effect of screening also in the distal colon. (6)
Our finding that sigmoidoscopy is not effective in women may have implications for future screening programs using sigmoidoscopy, such as in the United Kingdom, Italy and Norway. Also for biennial FOBT-screening, a lower effect has been reported in women than in men (13).
Until now, guidelines have not been gender-specific. This may be appropriate to consider. Colonoscopy may be a better choice of screening method in women compared to sigmoidoscopy, as suggested by others (14). This is, however, uncertain at the present time as randomized trials on the effectiveness of colonoscopy screening are still ongoing (15).
Our study comprises two screening arms; sigmoidoscopy alone, and sigmoidoscopy with FOBT. We did not find meaningful differences between these two screening modalities with regard to colorectal cancer incidence or mortality (Table 1, Appendix Table 1). Thus, according to our results, adding once-only FOBT to sigmoidoscopy screening does not appear to improve screening efficiency. Only repeated FOBT has been shown to be effective.(16).
We found a possible minor beneficial effect of sigmoidoscopy screening on all-cause mortality in men (HR 0.96; 95% CI 0.91–1.00), and this has been confirmed in a recent meta-analysis. (17) This is an important observation that may have impact on screening acceptance. The meta-analyses, however, did not report results for men and women separately.
Current guidelines recommend repeat sigmoidoscopy screening at 5- to 10-year intervals (1). We show that once-only sigmoidoscopy screening has a sustained effect for a follow-up of 15 years. An extension to 12 or 15 year intervals would result in substantial savings for the health care system. Also, it reduces the individual burden of screening, including patient discomfort and the risk of adverse events and complications. Sigmoidoscopy is generally well tolerated, but still is an invasive procedure. Up to 10% of individuals experience moderate or severe pain during sigmoidoscopy screening, which is more common in women (18–20).
Even though this is a large, population-based carefully conducted intervention trial, there are some limitations to keep in mind. NORCCAP was originally designed for 55–64 year old men and women combined. Even if sex-specific analyses were pre-specified, the decision to report results separately for men and women was done after a positive interaction between sex and randomization group had been detected. Still, we believe that taking the observed heterogeneity between men and women into account, presenting the combined results as the main finding would be misleading. We consider that a sex-stratified meta-analysis would be highly desirable, but it is important to be aware that combining data from NORCCAP and the other flexible sigmoidoscopy screening trials may imply that results are no longer generalizable. NORCCAP, due to its population-based design, evaluates the effectiveness of sigmoidoscopy screening in a population, while the other sigmoidoscopy screening trials by including volunteers are efficacy trials. Another limitation is that we did not have access to data on CRC treatment in the study population, but given the structure of the health-care system in Norway with universal coverage, it is unlikely that access to treatment should differ between the screening and control group. However, different treatment schemes for CRC are unlikely to have any effect on CRC incidence, and there was heterogeneity between men and women also for CRC incidence. Finally, we did not have information about socioeconomic status or ethnicity in the screening and control group, but due to the randomized design of the NORCCAP trial, these covariates are expected to be equally distributed between the groups.
In conclusion, men experienced a long-lasting effect of sigmoidoscopy screening with absolute risk reductions of colorectal cancer risk by 0.78% (from 2.50% to 1.72%) and cancer mortality by 0.33% (from 0.81% to 0.49%). For women, we could not detect an effect of sigmoidoscopy screening neither on colorectal cancer incidence, nor mortality. Our results may have implications for future screening recommendations, but also for the design of future trials where sex-stratified evaluations and sample-size calculations should be considered. We further believe that communicating absolute, and not relative, risk reductions as in the present paper and illustrated in figure 2 would be preferable during shared decision making with patients.
Supplementary Material
Acknowledgments
Funding/Grant Support
The NORCCAP trial was funded by research grants from the Norwegian Government and the Norwegian Cancer Society. Work with the present manuscript was funded by research grants from the Norwegian Cancer Society, the Research Council of Norway, The South-East Regional Health Authority of Norway, The Fulbright Foundation, Sorlandet Hospital Kristiansand, and the National Institutes of Health.
Appendix Figure 1.
Study flow chart.
CRC = colorectal cancer; FOBT = fecal occult blood testing.
Appendix Figure 2.
Risks for CRC, rectosigmoid cancer, and death from CRC for women and men in the screening and control groups.
CRC = colorectal cancer.
Appendix Figure 3.
Fifteen-year risks for CRC and death from CRC with and without screening for women and men.
CRC = colorectal cancer.
Table.
Age-Adjusted Hazard Ratios and Rate Differences for CRC Incidence and Mortality in the Screening Versus Control Group in Women and Men
| Women | Men | P value for Heterogeneity for Women vs. Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening group
(n = 10 297) |
Control group
(n = 39 254) |
Screening group
(n = 10 255) |
Control group
(n = 38 872) |
||||||||||
| Cases, n | Cases per 100 000 Person-Years, n | Cases, n | Cases per 100 000 Person-Years, n* | Hazard Ratio (95% CI) |
Rate Difference per 100 000 Person-Years (95% CI) | Cases, n | Cases per 100 000 Person-Years, n | Cases, n | Cases per 100 000 Person-Years, n* | Hazard Ratio (95% CI) |
Rate Difference per 100 000 Person-Years (95% CI) | ||
| CRC Incidence | 207 | 140.1 | 789 | 153.6 | 0.92 (0.79–1.07) | −13.5 (−35.4 to 8.5) | 186 | 131.4 | 962 | 196.9 | 0.66 (0.57–0.78) | −65.5 (−80.8 to – 36.9) | 0.004 |
| Person-years of observation | 147,762 | 556,457 | - | - | 141,510 | 528,317 | - | - | |||||
| Location† | |||||||||||||
| Distal | 89 | 60.2 | 389 | 74.3 | 0.81 (0.64–1.02) | −14.1 (−28.9 to 1.10) | 105 | 74.2 | 611 | 124.3 | 0.59 (0.48–0.73) | −50.1(−62.0 to −28.6) | 0.006 |
| Proximal | 113 | 76.5 | 383 | 76.1 | 1.01 (0.82–1.25) | 0.35 (−15.7 to 16.4) | 78 | 55.1 | 326 | 67.6 | 0.81 (0.63–1.04) | −12.5(−24.3 to 3.5) | 0.312 |
| Age group | |||||||||||||
| 50–54 y | 39 | 81.0 | 237 | 94.2 | 0.86 (0.61–1.21) | −13.2 (−41.3 to 14.9) | 38 | 81.9 | 317 | 126 | 0.65 (0.46–0.91) | −44.1(−73.6 to -14.6) | 0.25 |
| 55–64 y | 168 | 168.7 | 552 | 181.1 | 0.93 (0.78–1.11) | −12.4 (−42.1 to 17.2) | 148 | 155.6 | 645 | 233.1 | 0.67 (0.56–0.80) | −77.5 (−108.4 to–46.7) | 0.009 |
| Screening method | |||||||||||||
| Sigmoidoscopy | 104 | 140.6 | 789 | 153.6 | 0.92 (0.75–1.13) | −12.9 (−41.2 to 17.0) | 85 | 119.9 | 962 | 196.9 | 0.60 (0.48–0.75) | −77.0 (−91.9 to −35.7) | 0.002 |
| Sigmoidoscopy + FOBT | 103 | 139.5 | 789 | 153.6 | 0.91 (0.74–1.11) | −14.0 (−42.7 to 15.8) | 101 | 142.9 | 962 | 196.9 | 0.72 (0.59–0.89) | −54.0 (−72.0 to −10.7) | 0.274 |
| CRC mortality | 65 | 43.7 | 225 | 43.3 | 1.01 (0.77–1.33) | 0.43 (−11.7 to 12.6) | 57 | 40 | 305 | 63.3 | 0.63 (0.47–0.83) | −23.2(−32.8 to −8.7) | 0.014 |
| Person-years of observation | 148,705 | 575,166 | - | - | 142,370 | 539,415 | - | - | |||||
| Location† | |||||||||||||
| Distal | 33 | 22.2 | 99 | 18.8 | 1.17 (0.79–1.73) | 3.36 (−4.9 to 12.0) | 35 | 24.6 | 180 | 37.3 | 0.65 (0.45–0.93) | −12.8(−20.7 to −1.9) | 0.023 |
| Proximal | 27 | 18.2 | 114 | 22.2 | 0.83 (0.54–1.26) | −4.0 (−12.0 to 4.0) | 20 | 14.1 | 112 | 23.3 | 0.60 (0.37–0.96) | −9.2 (−15.5 to −1.1) | 0.43 |
| Age group | |||||||||||||
| 50–54 y | 12 | 24.8 | 70 | 27.7 | 0.90 (0.49–1.65) | −2.86 (−18.3 to 12.6) | 8 | 17.2 | 88 | 34.8 | 0.49 (0.24–1.02) | −17.6 (−31.6 to −3.7) | 0.21 |
| 55–64 y | 53 | 52.8 | 155 | 50.5 | 1.05 (0.77–1.42) | 2.3 (−14.0 to 18.6) | 49 | 51.1 | 217 | 77.7 | 0.66 (0.48–0.90) | −26.6 (−44.2 to −8.9) | 0.038 |
| Screening method | |||||||||||||
| Sigmoidoscopy | 35 | 47.1 | 225 | 43.3 | 1.09 (0.76–1.56) | 3.78 (−12.9 to 20.5) | 29 | 40.7 | 305 | 63.3 | 0.64 (0.43–0.93) | −22.6 (−34.6 to −1.9) | 0.010 |
| Sigmoidoscopy + FOBT | 30 | 40.4 | 225 | 43.3 | 0.94 (0.64–1.37) | −2.93 (−17.9 to 13.2) | 28 | 39.3 | 305 | 63.3 | 0.62 (0.42–0.91) | −23.9 (−35.3 to −3.3) | 0.084 |
| All-cause mortality | 1571 | 1056.4 | 5427 | 1047.5 | 1.02 (0.96–1.07) | 8.92 (−49.3 to 66.6) | 2238 | 1572.0 | 8006 | 1638.1 | 0.96 (0.91–1.00) | −66.1 (−137.5 to 5.77) | 0.11 |
| Age group | |||||||||||||
| 50–54 y | 315 | 652.4 | 1651 | 654.0 | 1.00 (0.88–1.13) | −1.5 (−80.2 to 77.1) | 463 | 995.3 | 2528 | 1001.8 | 0.99 (0.90–1.10) | −6.5 (−105.2 to 92.2) | 0.96 |
| 55–64 y | 1256 | 1251.4 | 3776 | 1230.0 | 1.02 (0.95–1.08) | 21.4 (−58.2 to 101.0) | 1775 | 1853.0 | 5478 | 1964.0 | 0.94 (0.89–1.00) | −111.0 (−211.7 to −10.3) | 0.08 |
CRC = colorectal cancer; FOBT = fecal occult blood testing.
Rates are age-standardized.
The sum of cases of distal and proximal CRC is lower than the total values because the location of some cases of cancer was unknown.
Footnotes
Disclosures: All authors report no conflicts of interest.
Reproducible research statement: Protocol: Available in the supplementary appendix. Data code: Available from Dr M Loberg (e-mail: magnus.loberg@medisin.uio.no). Data set: May be available after written agreement with the NORCCAP steering committee (e-mail: geir.hoff@kreftregisteret.no).
References
- 1.USPSTF, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–75. [DOI] [PubMed] [Google Scholar]
- 2.Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah U, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine. 2012;366(25):2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103(17):1310–22. [DOI] [PubMed] [Google Scholar]
- 6.Holme O, Schoen RE, Senore C, Segnan N, Hoff G, Loberg M, et al. Effectiveness of flexible sigmoidoscopy screening in men and women and different age groups: pooled analysis of randomised trials. BMJ. 2017;356:i6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretthauer M, Gondal G, Larsen K, Carlsen E, Eide TJ, Grotmol T, et al. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia: attendance rates in the NORCCAP study (Norwegian Colorectal Cancer Prevention). Scandinavian Journal of Gastroenterology. 2002;37(5):568–73. [DOI] [PubMed] [Google Scholar]
- 8.Gondal G, Grotmol T, Hofstad B, Bretthauer M, Eide TJ, Hoff G. The Norwegian Colorectal Cancer Prevention (NORCCAP) screening study: baseline findings and implementations for clinical work-up in age groups 50–64 years. Scandinavian Journal of Gastroenterology. 2003;38(6):635–42. [DOI] [PubMed] [Google Scholar]
- 9.Hoff G, Sauar J, Hofstad B, Vatn MH. The Norwegian guidelines for surveillance after polypectomy: 10-year intervals. Scandinavian Journal of Gastroenterology. 1996;31(9):834–6. [DOI] [PubMed] [Google Scholar]
- 10.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Altenhofen L, Katalinic A, Lansdorp-Vogelaar I, Hoffmeister M. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol. 2011;174(10):1140–6. [DOI] [PubMed] [Google Scholar]
- 12.Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21(17):5167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–14. [DOI] [PubMed] [Google Scholar]
- 14.Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. New England Journal of Medicine. 2005;352(20):2061–8. [DOI] [PubMed] [Google Scholar]
- 15.Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015;64(6):982–90. [DOI] [PubMed] [Google Scholar]
- 16.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007(1):CD001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swartz AW, Eberth JM, Josey MJ, Strayer SM. Re-analysis of All-Cause Mortality in the U.S. Preventive Services Task Force 2016 Evidence Report on Colorectal Cancer Screening. Ann Intern Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robb KA, Lo SH, Power E, Kralj-Hans I, Edwards R, Vance M, et al. Patient-reported outcomes following flexible sigmoidoscopy screening for colorectal cancer in a demonstration screening programme in the UK. J Med Screen. 2012;19(4):171–6. [DOI] [PubMed] [Google Scholar]
- 19.Larsen IK, Grotmol T, Bretthauer M, Gondal G, Huppertz-Hauss G, Hofstad B, et al. Continuous evaluation of patient satisfaction in endoscopy centres. Scandinavian Journal of Gastroenterology. 2002;37(7):850–5. [PubMed] [Google Scholar]
- 20.Senore C, Ederle A, Fantin A, Andreoni B, Bisanti L, Grazzini G, et al. Acceptability and side-effects of colonoscopy and sigmoidoscopy in a screening setting. J Med Screen. 2011;18(3):128–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.