Abstract
Over the past 15 years, fluorescence has become the dominant detection/sensing technology in medical diagnostics and biotechnology. Although fluorescence is a highly sensitive technique, where single molecules can readily be detected, there is still a drive for reduced detection limits. The detection of a fluorophore is usually limited by its quantum yield, autofluorescence of the samples and/or the photostability of the fluorophores; however, there has been a recent explosion in the use of metallic nanostructures to favorably modify the spectral properties of fluorophores and to alleviate some of these fluorophore photophysical constraints. The use of fluorophore-metal interactions has been termed radiative decay engineering, metal-enhanced fluorescence or surface-enhanced fluorescence.
Introduction
Fluorescence experiments are typically performed in sample geometries that are large relative to the size of the fluorophores and relative to the absorption and emission wavelengths. In this arrangement the fluorophores radiate into free space. Most of our knowledge and intuition about fluorescence is derived from the spectral properties observed in these free-space conditions. The presence of nearby metallic surfaces or particles can alter the free-space condition and can result in dramatic spectral changes. Remarkably, metal surfaces can increase or decrease the radiative decay rates of fluorophores and can increase the extent of resonance energy transfer [1–4]. These effects result from interactions of the excited-state fluorophores with free electrons in the metal, the so-called surface plasmon electrons, which in turn produce favorable effects on the fluorophore. The effects of metallic surfaces include fluorophore quenching at short distances (~0–5 nm), spatial variation of the incident light field (~0–15 nm), and changes in the radiative decay rates (~0–20 nm). The use of fluorophore-metal interactions in biotechnology has primarily been referred to as radiative decay engineering or metal-enhanced fluorescence (MEF).
The concept of modifying the radiative decay rate is unfamiliar to the majority of fluorescence spectroscopists. It is therefore informative to consider the novel spectral effects expected by increasing the radiative rate, Γ. The quantum yield (Q0) and lifetime of the fluorophore (τ0) in the free-space condition are given by:
| (1) |
| (2) |
where knr is the non-radiative rate. The presence of a nearby metal (m) surface increases the radiative rate by addition of a new rate Γm [1,2,5]. In this case, the quantum yield (Qm) and lifetime of the fluorophore (τm) near the metal surface are given by:
| (3) |
| (4) |
These equations result in unusual predictions for a fluorophore near to a metallic surface. From Equations (1)–(4), it can be seen that as the value of Γm increases, the quantum yield increases while the lifetime decreases. This is contrary to most observations where the fluorescence lifetime and quantum yield nearly always change in unison [1].
These effects have been predicted theoretically [6–7] and are analogous to the increases in Raman signals observed for surface-enhanced Raman scattering, except that the effects on fluorescence result from through-space interactions and do not require molecular contact between the fluorophores and the metal. This was first demonstrated by Cotton [8] and has been reviewed by Lakowicz [1].
As briefly mentioned in the abstract, the use of metallic nanostructures to favorably alter the intrinsic photophysical characteristics of fluorophores in a controllable manner is a fairly new concept, most of the work appearing in the literature within the past three years [1–5]. However, the historical origins of MEF can be traced back to the first observations of Drexhage in 1974 [9]. Here, the possibility of altering the radiative decay rate was demonstrated by the measurement of the decay times (lifetimes) of a europium complex positioned at various distances from a planar metal surface (i.e. mirror) [9]. The lifetime of the complex remained a single exponential, but oscillated with distance from the mirror. This effect is explained by the changes in phase of the reflected field from the mirror on the fluorophore. A decrease in lifetime is found when the reflected field is in phase with the fluorophore’s oscillating dipole (i.e. Equation (4)) and an increase is seen when the reflected field is out of phase with the fluorophore’s dipole.
In the present article we review the work carried out to date in this emerging area of biotechnology and describe the effects of different silver nanostructures, which have been prepared by various deposition methods, on the emission intensity and photostability of appropriately positioned fluorophores. The systems reviewed typically employ fluorophores that are used in many biological assays. The silver nanostructures usually consist of subwavelength-size nanoparticles of silver deposited on inert substrates. The proximity to silver nanostructures results in a preferential increase in intensity of low-quantum-yield fluorophores; the lifetimes decrease as the intensities increase. We subsequently discuss the use of MEF for its multifarious applications in biotechnology; for example, in immunoassays, enhanced ratiometric sensing and DNA detection.
Methodologies for the preparation of silver nanoparticles
A moderately extensive physics literature exists on noble metal nanostructures. For MEF more dramatic effects have been reported for non-continuous surfaces such as silver island and colloid films [1].
For use in medical and biotechnological applications, such as diagnostic or microfluidic devices, it would be useful to obtain MEF at desired locations in the measurement device (i.e. MEF on demand). In this regard, recent studies on MEF have reported the use of a relatively facile deposition of silver particles onto glass slides (i.e. silver island films, SiFs [2]). The possibility of using silver colloids [10] and anisotropic particles such as silver nanorods [11] has also been investigated. In addition to varying particle shapes, different techniques for silver deposition, such as light deposition and electroplating of silver onto electrodes and/or glass slides, have also been employed to obtain localized enhancement of fluorescence [12,13].
In a typical SiF preparation, silver islands are deposited onto a glass substrate, in a random fashion, by reducing a silver salt with the sequential addition of sodium hydroxide, ammonium hydroxide and glucose solutions while the glass substrate is immersed [2]. Atomic force microscopy studies have revealed that the SiFs are 30–80 nm in size with approximately 40% surface mass coverage [2]. The deposited SiFs display plasmon absorption maxima near 430 nm.
In contrast to SiFs, the preparation of colloidal suspensions of silver yields spherical silver particles of homogeneous size. The preparation of silver colloids is well established and many reports are available in the literature ([10] and references therein).
Anisotropic silver particles are predicted to enhance the emission of fluorescence owing to the increased local excitation fields around the edges of the particles [1]. Recently, a methodology for depositing silver nanorods with controlled size and loadings onto glass substrates has been reported [11]. In this method, silver nanorods are grown directly on glass substrates from small spherical silver colloids in the presence of a cationic surfactant. Silver nanorods deposited onto glass substrates display two distinct surface plasmon peaks: transverse and longitudinal, which typically appear at ~420 and ~650 nm, respectively. The length of the silver nanorods was determined by atomic force microscopy to be 100–1000 nm.
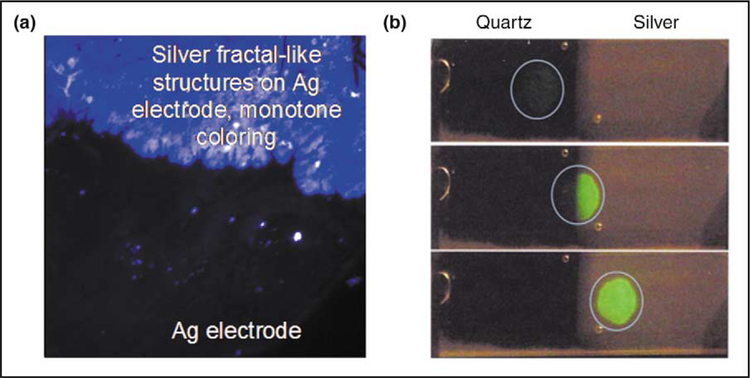
In a typical preparation of light-induced deposition of silver on glass slides, the silver-colloid-forming solution is prepared by adding trisodium citrate solution to a warmed silver nitrate solution in a closed chamber on a glass slide. The solution is then irradiated by a collimated HeCd laser [12]. Fractal-like silver nanostructures have also been deposited onto glass substrates (Figure 1a) [13]. In this case, commercially available silver electrodes are placed in deionized water 10 mm apart and a constant current is supplied across the electrodes for a short period of time by a constant current generator. All of these recent silver nanostructure preparations have resulted in attractive silvered surfaces for MEF applications. Some of these applications are described below.
Figure 1.
Fluorescence enhancement from silver nanostructures. (a) Silver fractal-like structures grown on silver electrodes [13]. (b) Photograph of fluorescein-labeled human serum albumin (molar ratio of fluorescein/ human serum albumin = 7) on quartz and on SiFs as observed with 430 nm excitation and a 480 nm long-pass filter. The excitation was progressively moved from the quartz side to the silver side. (Figure adapted from [15•] with permission.)
Applications of metal-enhanced fluorescence to biotechnology
Ultrabright over-labeled proteins: a new class of probes based on MEF
Proteins covalently labeled with fluorophores are widely used as reagents, for example, in immunoassays or for the immunostaining of biological specimens with specific antibodies. In these applications, fluorescein is one of the most widely used probes. An unfortunate property of fluorescein is self-quenching, which results from Förster resonance energy transfer between nearby fluorescein molecules (homotransfer) [14]. As a result, the intensity of a labeled protein does not increase with increased extents of labeling, but actually decreases [15•].
It has been reported that self-quenching can be largely eliminated by the close proximity to SiFs [15•, 16]; the emission intensity of fluoroscein isothiocyanate-labeled human serum albumin (FITC-HSA) with a labeling ratio (fluorophore per protein) of seven is approximately 17 times larger in the presence of SiFs than the emission on glass alone [15•]. It has been speculated that the decrease in self-quenching results from an increase in the rate of radiative decay, Γm. The difference in the intensity of heavily labeled human serum albumin on glass and on SiFs is shown pictorially in Figure 1b. The effect is dramatic, as seen from the nearly invisible intensity on quartz (left-hand side of the slides) and the bright image on the SiFs (right-hand side of the slides) in this unmodified photograph. These results suggest the possibility of ultrabright labeled proteins based on high labeling ratios, and the release of self-quenching using MEF [15•].
DNA hybridization
The detection of DNA hybridization [17] forms the basis of a wide range of biotechnological and diagnostic applications, such as gene chips [18,19], PCR [20,21] and fluorescence in situ hybridization [22]. In all these applications increased sensitivity is desirable, particularly for the detection of a small number of copies of biohazard agents. Also, it would be valuable to have a general approach to detect changes in fluorescence intensity upon hybridization. In general, the detectability of a fluorophore is determined by two factors: the extent of background emission from the sample and the photostability of the fluorophore. A highly photostable fluorophore can undergo about 106 excitation-relaxation cycles before photobleaching, yielding about 103–104 measured photons per fluorophore [23,24]. Background emission from samples can easily overwhelm weak emission signals.
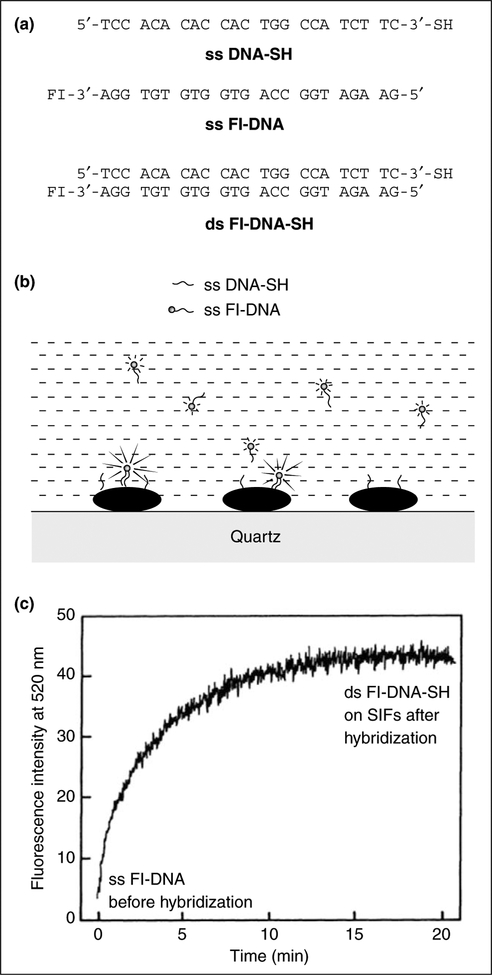
In a recent report, an approach is described that should provide a readily measurable change in fluorescence intensity in DNA hybridization formats [25•]. This approach increases the intensity relative to the background and increases the number of detected photons per fluorophore molecule by a factor of 10-fold or more. Figure 2a shows the sequence of the oligomers used in this study. The thiolated oligonucleotide single-stranded (ss) DNA-SH was used as the capture sequence, which bound spontaneously to the silver particles. The sample containing the silver-bound DNA was then positioned in a fluorometer followed by the addition of ss fluorescein-labeled DNA (Fl-DNA), in an amount approximately equal to the amount of silver-bound capture DNA (Figure 2b).
Figure 2.
The application of MEF to DNA hybridization. (a) Structures of the DNA oligomers: ss DNA-SH (single-stranded thiolated oligonucleotide); ss Fl-DNA (single-stranded fluorescent-labeled oligonucleotide).
(b) Schematic of the DNA oligomers bound to silver particles.
(c) The time-dependent hybridization of ss Fl-DNA to ss DNA-SH. (Figure adapted from [25•] with permission.)
The fluorescence intensity began to increase immediately upon mixing, and leveled off after about 20 min. This increase in intensity resulted from the localization of ss Fl-DNA near to the silver particles by hybridization with the capture DNA (Figure 2c). In the reported control experiments, ss DNA-SH was hybridized with ss Fl-DNA before deposition on silver particles; a similar 12-fold increase in intensity upon immobilization on silver, as compared with an equivalent amount of double-stranded Fl-DNA-SH in solution, was observed [25•].
Enhanced ratiometric fluorescence sensing
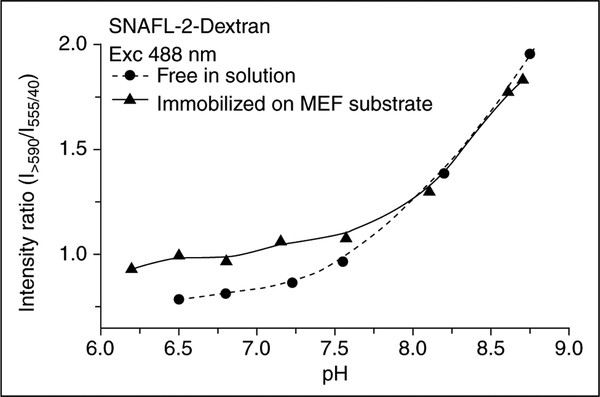
Enhanced ratiometric pH sensing using the pH-sensitive fluorophore seminaphthofluorescein carboxy SNAFL-2 and SiFs has also been recently reported [26]. Metallic surfaces can provide up to a 40-fold increase in probe fluorescence intensity as compared to non-metallic surfaces with the same probe coverage [26]. However, although the signal-to-noise ratio is significantly better for pH sensing, the emission wavelength ratiometric values are similar to those obtained in solution (i.e. the ratios are the same) owing to the fact that the emission from both the acidic and basic forms of the probe are enhanced to similar extents (Figure 3). This probably also reflects similar unmodified quantum yields of both forms. However, the substantial drop in probe lifetime precludes its utilization as a ratiometric lifetime sensor (see Equations (3) and (4)). Hence, it is fair to conclude that ratiometric lifetime sensing will not be favorable by this approach.
Figure 3.
The pH sensitivity of SNAFL-2 is retained in the presence of silver nanoparticles. The intensity on the MEF substrate is about 30 to 40 times brighter than the intensity of probe immobilized on glass slides; however, the ratios are similar. (Figure adapted from [26] with permission.)
Metal-enhanced multiphoton excitation
For multiphoton excitation of fluorescence, most of the excitation occurs at the focal point of the excitation where the local intensity is the highest. For a two-photon absorption process, the rate of excitation is proportional to the square of the incident intensity. This suggests that two-photon excitation could be enhanced by a factor of 3.8 × 108 [2,4]. Such an enhancement in the excitation rate is thought to provide selective excitation of fluorophores near to metal islands or colloids, even if the solution contains a considerable concentration of other fluorophores that could undergo two-photon excitation at the same wavelength, but which are more distal from the metal surface.
Recently, the enhanced and localized multiphoton excitation of Rhodamine B [27], Pacific Blue, Lissamine and Texas Red [28] fluorescence near metallic silver islands has been reported. A significant increase (up to 235-fold) in fluorescence emission intensity was seen for these fluorophores adjacent to metallic silver islands, accompanied by a reduction in lifetime as compared to that observed using one-photon excitation [27,28]. Given the now widespread use of multiphoton excitation in microscopy and medical imaging, this recent finding suggests the use of metallic nanostructures to both enhance and localize fluorescence.
Metal-enhanced planar immunoassays
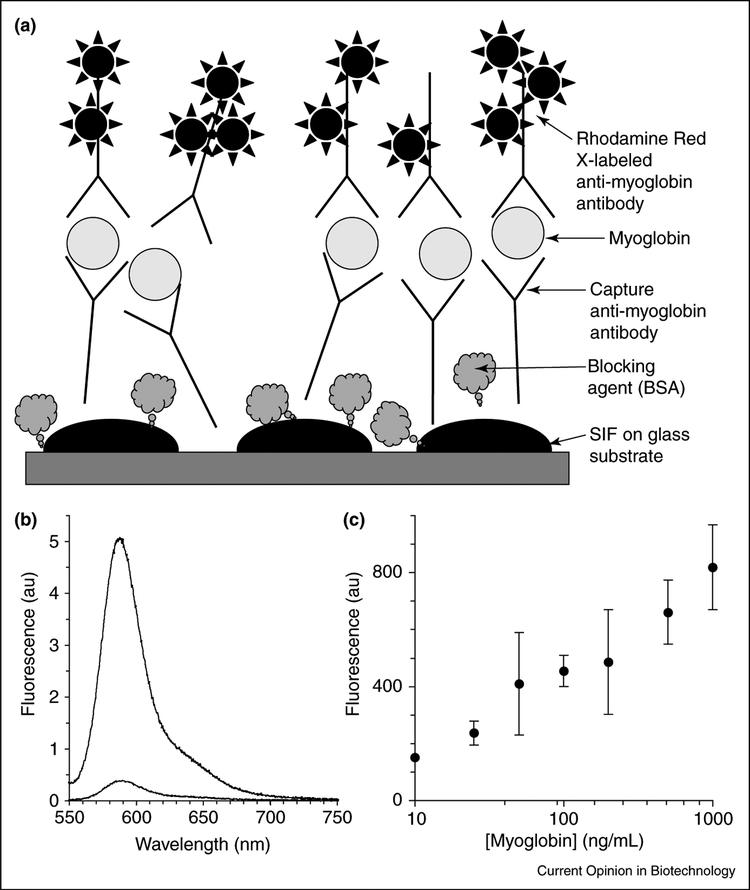
MEF has also been utilized in the development of an enhanced detection limit sandwich-format immunoassay for the cardiac marker myoglobin. In this important example of MEF, myoglobin was first captured on SiFs and glass surfaces coated with anti-myoglobin antibodies; the surfaces were then incubated with fluorophore-labeled anti-myoglobin antibodies (Figure 4a). A 10–15-fold increase in fluorescence emission was observed from the fluorophore-labeled antibody on the SiFs compared with that on the glass substrate alone (Figure 4b). Figure 4c shows that the detection range of myoglobin is between 10 and 1000 ng/mL. These results show that it is possible to detect myoglobin concentrations below 50 ng/mL with an immunoassay performed using SiFs, much lower than the clinical cut-off concentrations for myoglobin in healthy patients (100 ng/mL).
Figure 4.
Metal-enhanced planar immunoassays. (a) Schematic of a myoglobin-enhanced sandwich immunoassay on SiFs. (b) Fluorescence spectra of the Rhodamine Red-X-labeled anti-myoglobin antibody bound to the surface-immobilized myoglobin; the concentration of myoglobin was 100 ng/mL on SiFs and on glass. (c) Fluorescence emission of Rhodamine Red-X-labeled anti-myoglobin antibody at different myoglobin concentrations on SiFs (excitation 532 nm).
Metal-enhanced fluorescent probes
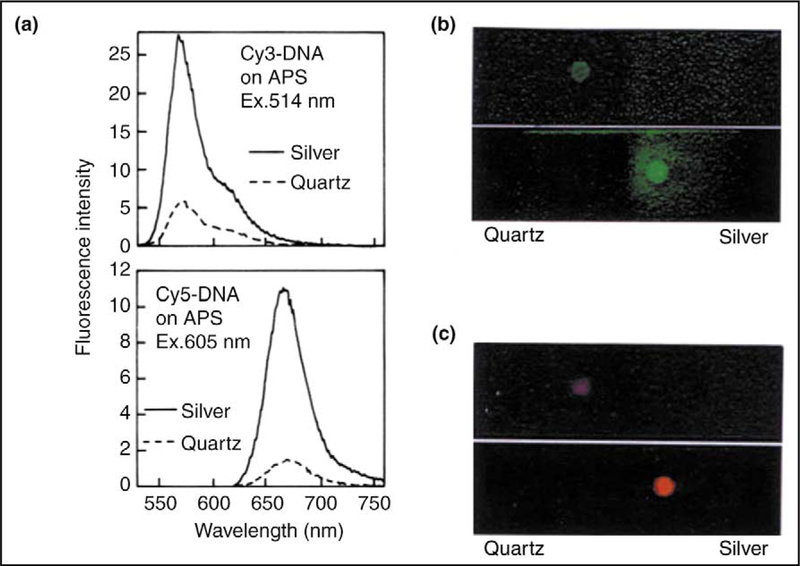
Fluorophores are essential in fluorescence-based technology and especially in DNA-based technologies. A recent report studied the effects of SiFs on the emission spectral properties of fluorophore-labeled DNA [17]. The emission spectra of Cy3-DNA and Cy5-DNA on glass and on SiFs are shown in Figure 5a. The emission intensity is increased two- to threefold on SiFs as compared with quartz slides (the control sample). The slightly larger increase in emission intensity for Cy5-DNA is consistent with previous observations of larger enhancements for low quantum yield fluorophores [17]. Photographs of the labeled oligomers on quartz and SiFs are also shown (Figures 5b,c). The emission from the labeled DNA on quartz is almost invisible, but is brightly visible on the SiFs. This difference in intensity results from an increase in the radiative decay rate of the fluorophore, which in turn results in an increase in the quantum yield (intensity) of the fluorophores [17].
Figure 5.
Metal-enhanced fluorescent probes. (a) Emission spectra of Cy3-DNA and Cy5-DNA between quartz plates, with and without SiFs. (b,c) Photographs of the corresponding fluorophores. Note that the photographs are taken through emission filters and, importantly, the increase in emission intensity is not due to an increased excitation scatter from the silvered plates. Abbreviation: APS, aminopropyl silane. (Figure adapted from [17] with permission.)
Reports of the effects of silver particles on metal-ligand complexes, which are deemed to be useful as luminescence probes in biochemistry owing to their long luminescent decay times, have been reported [29]. The emission spectral properties of [Ru(bpy)3]2+ on SiFs and glass yielded several fold higher intensities on SiFs, accompanied by shortened lifetimes. These results are consistent with an approximate 20-fold increase in radiative decay rate of the [Ru(bpy)3]2+ in close proximity to silver particles. It is likely, therefore, that silver particles might find use in increasing the detectability of emissions from other metal-ligand complexes.
Metal-enhanced solution assays
Nearly all of the work pertaining to MEF to date, has dealt with fluorophores in close proximity to planar surfaces. However, it might also be desirable to use metals as colloidal suspensions for medical imaging, as they are potentially injectable and silver and gold colloids are already widely used in medicine. For example, such approaches are used in retinal angiography and could benefit from an enhanced indocyanine green and fluorescein quantum yield and from increased photostability (see the article by Malicka et al. [16] and references therein).
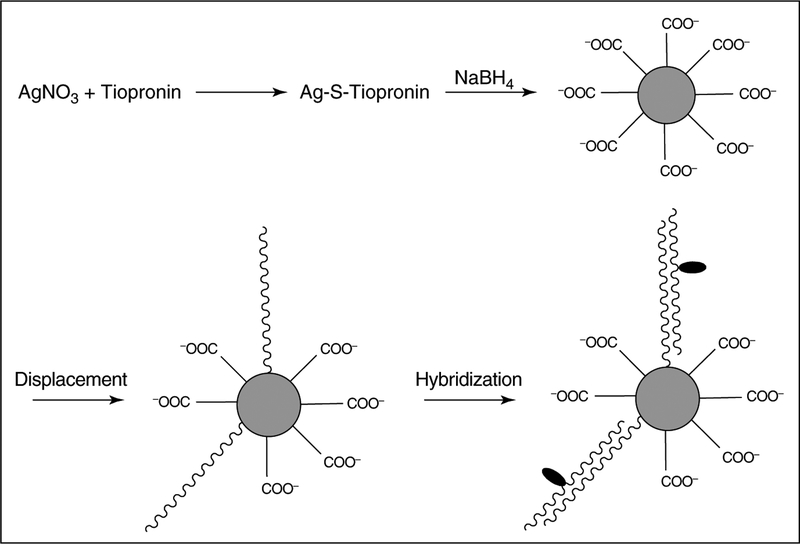
To illustrate the usefulness of MEF in solution-based biosensing applications we acknowledge two recent approaches. In the first approach, SiO2-coated silver colloids provide for a solution-based enhanced-fluorescence sensing platform with a three- to fivefold enhancement typically observed [30•]. In the second approach, oligonucleotide-modified silver particles and a fluorophore-labeled complementary oligonucleotide were employed (Figure 6) [31]. An increased emission from the fluorophore-labeled complementary oligonucleotide after hybridization in the presence of silver particles has been observed. This result suggests a possible approach to DNA detection based on the aggregation of metallic nanoparticles bound by fluorophore-labeled oligonucleotides [31].
Figure 6.
Metal-enhanced solution assays. One approach to the use of MEF in solution-based assays is to use oligonucleotide-modified silver particles in solution together with fluorophore-labeled complementary oligonucleotides. The schematic illustrates the preparation, displacement by thiolate oligonucleotides, and hybridization with fluorescein-labeled complementary oligonucleotides. The silver nanoparticles were protected with a tiopronin monolayer. Silver particles are shown as gray spheres and fluorophore molecules as black ovals. (Figure adapted from [31] with permission.)
Interpretation of metal-enhanced fluorescence in terms of a radiating plasmon model
Over the past five years, the exciting possibilities that metal-fluorophore interactions potentially have to offer to medical imaging and diagnostics have been realized. Although most of these approaches have been poised from a sensing perspective, and therefore phenomenological in nature, the descriptive nature of the underlying physics has been traditionally centered on excited distal fluorophores in resonance with surface plasmon electrons. That is, the fluorophore is excited directly and spontaneously emits; the spectral changes observed then result from the excited-state resonance interaction with the free electrons on the metallic surface.
More recently, however, the interpretation of the same results has shifted somewhat — to a model whereby non-radiative energy transfer occurs from distal excited fluorophores to the surface plasmon electrons. The surface plasmon electrons, in turn, radiate (under certain conditions) the photophysical characteristics of the coupling fluorophore. This new model has been referred to as the radiating plasmon model [32].
The extinction properties of metal particles can be expressed by a combination of both absorption (CA) and scattering (CS) factors, when the particles are spherical and have sizes comparable to the incident wavelength of light:
| (5) |
where K1 = 2πn1/λ0 and is the wave vector of the incident light in medium 1 and α is the polarizability of the sphere with radius r and is given by:
| (6) |
where εm is the complex dielectric constant of the metal. The first term represents the cross-section owing to absorption (CA) and the second term the cross-section owing to scattering (CS). Current interpretation of MEF is therefore underpinned by the scattering component of the metal extinction (i.e. the ability of fluorophore-coupled plasmons to radiate [32]).
Conclusions
We have reviewed recent fluorophore-metal architectures that offer enhanced spectral properties of the fluorophores, such as increased quantum yields, increased rates of excitation and energy transfer, and enhanced fluorophore photostability (reduced lifetimes). Metallic silver can be readily deposited by a variety of methods, on demand, using a range of approaches and in biologically inert environments. In our opinion these fluorophore-metal effects offer unique perspectives in clinical chemistry and biochemistry, providing for improved background suppression, increased detection limits and even localized excitation. MEF is an emerging technology that in several years is likely to be widespread throughout fluorescence-based applications of biotechnology.
Acknowledgements
The authors would like to thank the National Institutes of Health for financial support (National Center for Research Resources, RR-08119 and R21GM070929), Philip Morris USA Inc. and Philip Morris International, and the National Institute for Biomedical Imaging and Bioengineering (EB-1690). The authors also wish to thank the Medical Biotechnology Center, University of Maryland Biotechnology Institute for partial salary support to JRL, CDG and IG. Others involved in this work include Zygmunt Gryczynski, Joanna Lukomska, Jian Zhang, Ramachandram Badugu, Slawomir Makowiec, Kazimierz Nowacyzk, Meng Wu and Jun Huang.
Abbreviations
- MEF
metal-enhanced fluorescence
- SiF
silver island film
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Lakowicz JR: Radiative decay engineering: biophysical and biomedical applications. Anal Biochem 2001, 298:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, Gryczynski I: Radiative decay engineering 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal Biochem 2002, 301:261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakowicz JR, Shen Y, Gryczynski Z, D’Auria S, Gryczynski I: Intrinsic fluorescence from DNA can be enhanced by metallic particles. Biochem Biophys Res Com 2001, 286:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gryczynski I, Malicka J, Shen Y, Gryczynski Z, Lakowicz JR: Multiphoton excitation of fluorescence near metallic particles: enhanced and localized excitation. J Phys Chem B 2002, 106:2191–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geddes CD, Lakowicz JR: Metal-enhanced fluorescence. J Fluores 2002, 12:121–129. [Google Scholar]
- 6.Gersten J, Nitzan A: Spectroscopic properties of molecules interacting with small dielectric particles. J Chem Phys 1981, 75:1139–1152. [Google Scholar]
- 7.Weitz DA, Garoff S, Gersten JI, Nitzan A: The enhancement of Raman scattering, resonance Raman scattering and fluorescence from molecules absorbed on a rough silver surface. J Chem Phys 1983, 78:5324–5338. [Google Scholar]
- 8.Sokolov K, Chamanov G, Cotton TM: Enhancement of molecular fluorescence near the surface of colloidal metal films. Anal Chem 1998, 70:3898–3905. [DOI] [PubMed] [Google Scholar]
- 9.Drexhage KH: Interaction of light with monomolecular dye lasers In Progress in Optics. Edited by Wolfe E. North-Holland: Amsterdam; 1974:161–232. [Google Scholar]
- 10.Geddes CD, Cao H, Gryczynski I, Gryczynski Z, Fang J, Lakowicz JR: Metal-enhanced fluorescence due to silver colloids on a planar surface: potential applications of indocyanine green to in vivo imaging. J Phys Chem A 2003, 107:3443–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD: Fast and slow deposition of silver nanorods on to glass substrates. J Phys Chem B: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geddes CD, Parfenov A, Lakowicz JR: Photodeposition of silver can result in metal-enhanced fluorescence. Appl Spectrosc 2003, 57:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geddes CD, Parfenov A, Roll D, Fang J, Lakowicz JR: Electrochemical and laser deposition of silver for use in metal-enhanced fluorescence. Langmuir 2003, 19:6236–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster Th: Intermolecular energy migration and fluorescence. Ann Phys 1948, 2:55–75. [Google Scholar]
- 15.•.Lakowicz JR, Malicka J, D’Auria S, Gryczynski I: Release of the self-quenching of fluorescence near silver metallic surface. Anal Biochem 2003, 320:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of the release of self-quenching of fluorophores near-to metallic surfaces.
- 16.Malicka J, Gryczynski I, Geddes CD, Lakowicz JR: Metal-enhanced emission from indocyanine green: a new approach to in vivo imaging. J Biomed Opt 2003, 8:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakowicz JR, Malicka J, Gryczynski I: Silver particles enhance emission of fluorescent DNA oligomers. Biotechniques 2003, 34:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown PO, Botstein D: Exploring the new world of the genome with DNA microarrays. Nat Genet Supp 1999, 21:33–37. [DOI] [PubMed] [Google Scholar]
- 19.Schena M, Heller RA, Theriault TP, Konrad K, Lachenmeier E, Davis RW: Microarrays: biotechnology’s discovery platform for functional genomics. Trends Biotechnol 1998, 16:301–306. [DOI] [PubMed] [Google Scholar]
- 20.Komurian-Pradel F, Paranhos-Bacala G, Sodoyer M, Chevallier P, Mandrand B, Lotteau V, Andre P: Quantitation of HCV RNA using real-time PCR and fluorimetry. J Virol Methods 2001, 95:111–119. [DOI] [PubMed] [Google Scholar]
- 21.Walker NJ: A technique whose time has come. Science 2002, 296:557–559. [DOI] [PubMed] [Google Scholar]
- 22.Difilippantonio MJ, Ried T: Technicolor genome analysis In Topics in Fluorescence Spectroscopy, DNA Technology, Vol 7. Edited by Lakowicz JR. Kluwer Academic Publishers/Plenum Press: New York; 2003: 291–316. [Google Scholar]
- 23.Soper SA, Nutter HL, Keller RA, Davis LM, Shera EB: The photophysical constants of several fluorescent dyes pertaining to ultrasensitive fluorescence spectroscopy. Photochem Photobiol 1993, 57:972–977. [Google Scholar]
- 24.Ambrose WP, Goodwin PM, Jett JH, VanOrden A, Werner JH, Keller RA: Single molecule fluorescence spectroscopy at ambient temperature. Chem Rev 1999, 99:2929–2956. [DOI] [PubMed] [Google Scholar]
- 25.•.Malicka J, Gryczynski I, Lakowicz JR: DNA hybridization assays using metal-enhanced fluorescence. Biochem Biophys Res Commun 2003, 306:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of MEF to follow DNA hybridization.
- 26.Aslan K, Lakowicz JR, Geddes CD: Enhanced ratiometric pH sensing using SNAFL-2 on silver island films: metal-enhanced fluorescence sensing. J Fluores, 2005: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakowicz JR, Gryczynski I, Malicka J, Gryczynski Z, Geddes CD: Enhanced and localized multiphoton excited fluorescence near metallic silver islands: metallic islands can increase probe photostability. J Fluores 2002, 12:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maliwal BP, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR: Fluorescence properties of labeled proteins near silver colloid surfaces. Biopolymers 2003, 70:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gryczynski I, Malicka J, Holder E, DiCesare, Lakowicz JR: Effects of metallic silver particles on the emission properties of [Ru(bpy)3]2+. Chem Phys Lett 2003, 372:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.•.Aslan K, Lakowicz JR, Szmacinski H, Geddes CD: Metal-enhanced fluorescence solution-based sensing platform. J Fluores 2004, 14:677–679. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of MEF in solution with colloids that result in enhanced intensities upon aggregation.
- 31.Zhang J, Malicka J, Gryczynski I, Lakowicz JR: Oligonucleotide-displaced organic monolayer-protected silver nanoparticles and enhanced luminescence of their salted aggregates. Anal Biochem 2004, 330:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakowicz JR: Radiative decay engineering 5. Non-quenching metallic surfaces and plasmon emission. Anal Biochem 2005: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]