The phase II CABOSUN trial compared cabozantinib with sunitinib as initial treatment in patients with advanced renal cell carcinoma of intermediate or poor risk. This article presents subgroup analyses by baseline patient characteristics.
Abstract
Cabozantinib treatment prolonged progression‐free survival (PFS) and improved objective response rate (ORR) compared with sunitinib in patients with advanced renal cell carcinoma (RCC) of intermediate or poor risk by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria in the phase II CABOSUN trial (NCT01835158). In the trial, 157 patients were randomized 1:1 to receive cabozantinib or sunitinib, stratified by IMDC risk group and presence of bone metastases. Here, PFS and ORR, both determined by independent radiology committee (IRC), were analyzed by subgroups of baseline characteristics. Cabozantinib treatment was generally associated with improved PFS and ORR versus sunitinib across subgroups, including in groups defined by IMDC risk group, bone metastases, age, and tumor burden. Clinical trial identification number. NCT01835158.
Introduction
Cabozantinib is an oral inhibitor of MET, AXL, and vascular endothelial growth factor (VEGF) receptors [1]. The phase II CABOSUN trial (Alliance for Clinical Trials in Oncology A031203) compared cabozantinib with sunitinib as initial treatment in patients with advanced renal cell carcinoma (RCC) of intermediate or poor International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk [2], [3]. The study met the primary endpoint of improved progression‐free survival (PFS) per investigator with cabozantinib versus sunitinib. Retrospective analysis of PFS per independent radiology committee (IRC) also showed significant prolongation of PFS with cabozantinib. Median PFS per IRC was 8.6 months (95% confidence interval [CI], 6.8 − 14.0) with cabozantinib versus 5.3 months (95% CI, 3.0 − 8.2) with sunitinib (hazard ratio [HR], 0.48; 95% CI, 0.31 − 0.74; two‐sided p = .0008), and objective response rate (ORR) per IRC was 20% (95% CI, 12.0 − 30.8) versus 9% (95% CI, 3.7 − 17.6), respectively. With the growing number of therapies for first‐line RCC [4], [5], information on efficacy based on patient characteristics may help to select optimal use. Here, PFS per IRC and ORR per IRC were analyzed by subgroups of baseline characteristics for CABOSUN.
Materials and Methods
The CABOSUN trial and retrospective analysis of PFS and ORR by IRC have been previously described [2], [3]. Eligible patients were ≥18 years of age with advanced or metastatic clear‐cell RCC without previous systemic treatment. Additional study requirements included intermediate‐ or poor‐risk disease per IMDC criteria, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and adequate organ function.
Patients were randomly assigned 1:1 to receive cabozantinib (60 mg once daily) or sunitinib (50 mg once daily for 4 weeks, followed by a 2‐week break), stratified by IMDC risk group (intermediate or poor) and bone metastases (yes or no). Tumor assessments by magnetic resonance imaging or computed tomography were performed at baseline and every 12 weeks thereafter until progression or until 5 years after randomization. MET tumor expression levels were analyzed by immunohistochemistry using archival or recently biopsied tumor tissue.
The primary endpoint was PFS per investigator; secondary endpoints were ORR per investigator, overall survival (OS), and safety [2], [3]. Tumor response and PFS were retrospectively assessed based on a blinded IRC review using Response Evaluation Criteria in Solid Tumors version 1.1. This analysis used a data cutoff of September 15, 2016. Subgroup analyses by stratification factors and MET tumor expression were prespecified and have been reported previously [2], [3]. Subgroup analyses focused on PFS and ORR, as the study was not powered for determination of the secondary endpoint of OS, and were post hoc. PFS and ORR subgroup analyses were per IRC. The Cox proportional hazards model and logistic regression analysis were performed; both analyses used treatment groups and the subgroups as independent variables. No adjustments for multiplicity were employed. HRs and odds ratios are unstratified and considered exploratory.
Results
A total of 157 patients were randomized to receive cabozantinib (n = 79) or sunitinib (n = 78). Baseline characteristics have been reported previously and were generally balanced between treatment groups [2], [3]. Eighty‐one percent of patients were intermediate risk and 19% were poor risk by IMDC criteria, 45% were ≥ 65 years, 78% were male, 54% were ECOG 1 or 2, and 36% had bone metastases. Tumor MET status was determined in 131 of 157 (83%) patients; of these, 47% were MET positive.
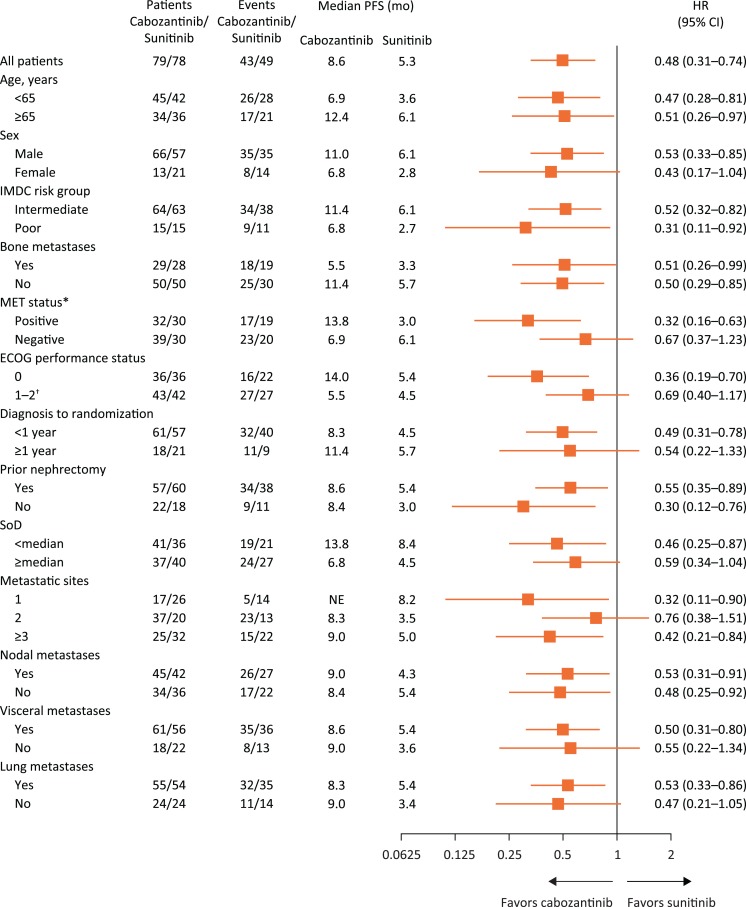
As of the data cutoff for PFS and ORR, median duration of follow‐up was 25.0 months (interquartile range, 21.9 − 30.9). The HR for PFS favored cabozantinib over sunitinib across all subgroups analyzed, including those defined by stratification factors (Fig. 1). Subgroups with characteristics suggesting poor prognosis generally had shorter median PFS for both cabozantinib and sunitinib compared with results for the overall population, including in subgroups defined by IMDC poor risk disease, ECOG status of 1 or 2, and bone metastases. For MET‐positive patients (n = 62), median PFS was 13.8 months (95% CI, 5.7–22.1) versus 3.0 months (95% CI, 2.5–5.4; HR 0.32 [95% CI, 0.16–0.63]) for cabozantinib versus sunitinib. For MET‐negative patients (n = 69), median PFS was 6.9 months (95% CI, 5.4–14.6) versus 6.1 months (95% CI, 3.6–9.6; HR, 0.67 [95% CI 0.37–1.23]), respectively. Kaplan‐Meier plots for subgroups based on stratification factors and MET status are shown in supplemental online Figures 1–3.
Figure 1.
Forest plot of progression‐free survival by subgroups. All analyses are per independent radiology committee. Hazard ratios are unstratified with the exception of the analysis for all patients. Metastatic sites are per investigator.
*Eight patients in the cabozantinib group and 18 patients in the sunitinib group had unknown MET status.
†Ten patients in the cabozantinib group and 10 patients in the sunitinib group were Eastern Cooperative Oncology Group (ECOG) 2.
Abbreviations: CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; NE, not estimable; PFS, progression‐free survival; SoD, sum of diameters.
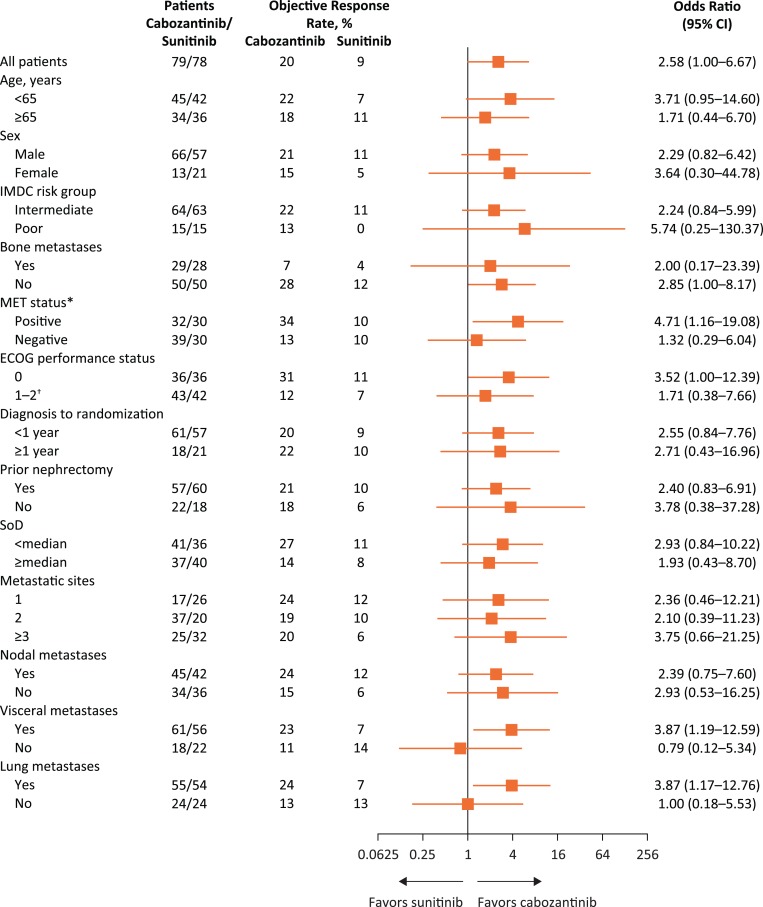
Odds ratios for ORR generally favored cabozantinib over sunitinib (Fig. 2). The MET‐positive subgroup had the numerically highest ORR with cabozantinib (34% with cabozantinib; 10% with sunitinib).
Figure 2.
Forest plot of objective tumor response by subgroups. All analyses are per independent radiology committee. Metastatic sites are per investigator.
*Eight patients in the cabozantinib group and 18 patients in the sunitinib group had unknown MET status.
†Ten patients in the cabozantinib group and 10 patients in the sunitinib group were Eastern Cooperative Oncology Group (ECOG) 2.
Abbreviations: CI, confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; SoD, sum of diameters.
Discussion and Summary
In the randomized phase II CABOSUN trial, cabozantinib treatment prolonged PFS and improved the ORR compared with sunitinib as initial treatment in patients with advanced RCC of intermediate or poor IMDC risk. Subgroup analyses of PFS per IRC by baseline characteristics favored cabozantinib over sunitinib (HR <1) in all groups analyzed, including groups defined by age, IMDC risk group, bone metastases, MET status, and tumor burden. Furthermore, analyses of ORR per IRC by baseline characteristics were generally consistent with those for the overall population.
The study was not designed to determine outcomes in subgroups, and results presented here are considered exploratory and hypothesis generating. In this context, positive MET status may be associated with a greater treatment benefit with cabozantinib versus sunitinib, although patients benefited with cabozantinib irrespective of MET status. Further prospective validation of this finding is warranted.
The treatment landscape for first‐line RCC is rapidly evolving, with approval of the combination of nivolumab and ipilimumab for patients with advanced RCC of intermediate or poor IMDC risk and ongoing trials of VEGF pathway and checkpoint inhibitor combinations [4], [5], [6], [7], [8]. Additional prospective clinical trials would be needed to better determine outcomes for cabozantinib compared with sunitinib or other first‐line therapies based on baseline characteristics. Nonetheless, results presented here suggest that cabozantinib treatment was generally associated with improved PFS and ORR versus sunitinib across subgroups of baseline characteristics, consistent with results for the overall population.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank the patients, their families, the investigators and site staff, the Alliance for Clinical Trials in Oncology, the Cancer Therapy Evaluation Program (John Wright), and the study teams participating in this trial. Medical writing support was provided by Julie C. Lougheed, Ph.D. (Exelixis), with editorial assistance by Fishawack Communications Inc. (Conshohocken, PA), which was funded by Exelixis.
Research reported in this publication was supported by National Institutes of Health (Award Numbers U10CA180821 and U10CA180882 [to the Alliance for Clinical Trials in Oncology], U10CA180791, U10CA180833, U10CA180850, U10CA180857, U10CA180867), and Exelixis, Inc. (Alameda, CA). TKC is supported in part by the Dana‐Farber/Harvard Cancer Center Kidney SPORE and Program, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at the Dana‐Farber Cancer Institute. Patient treatment at Memorial Sloan Kettering Cancer Center was supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). The content is the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. For more information on support for the Alliance for Clinical Trials in Oncology, see https://acknowledgments.alliancefound.org.
Footnotes
For Further Reading: Ai‐Ping Zhou, Yuxian Bai, Yan Song et al. Anlotinib Versus Sunitinib as First‐Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial. The Oncologist 2019;24:e702–e708.
Implications for Practice: This study evaluated the efficacy and safety of anlotinib for the first‐line treatment of metastatic renal cell carcinoma. Anlotinib, which was developed independently in China, is a new tyrosine kinase inhibitor inhibiting multiple kinases involved in angiogenesis and tumor proliferation. Results indicated that the efficacy of anlotinib is comparable to and the safety is better than that of sunitinib.
Disclosures
Daniel J. George: Axess Oncology, Bayer, BIOPHARM, Dendreon, Medivation, Sanofi (H), Acceleron Pharma, Astellas Pharma, Bayer, Bristol‐Myers Squibb, Celgene, Dendreon, Exelixis, Genentech, GlaxoSmithKline, Innocrin Pharma, Janssen, Medivation, Merck Sharp & Dohme, Myovant Sciences, Novartis, Pfizer, Sanofi (C/A), Bayer, Exelixis, Sanofi (Speaker's Bureau), Acerta Pharma (institutional), Astellas Pharma (institutional), Bayer (institutional), Bristol‐Myers Squibb (institutional), Dendreon (institutional), Exelixis (institutional), Genentech/Roche (institutional), Innocrin Pharma (institutional), Janssen Oncology (institutional), Millennium (institutional), Novartis (institutional), Pfizer (institutional) (RF), Bayer, Exelixis, Genentech/Roche, Medivation, Merck, Pfizer (Travel/Accommodations/Expenses); Colin Hessel: Exelixis (E, OI); Darren R. Feldman: Novartis, Seattle Genetics (RF); Milan Mangeshkar: Exelixis (E, OI); Christian Scheffold: Exelixis (E, IP, OI); Michael J. Morris: Advanced Accelerator Applications, Astellas Pharma, Bayer HealthCare Pharmaceuticals, Endocyte (C/A), Bayer HealthCare Pharmaceuticals (institutional), Endocyte (institutional), Progenics (institutional), Sanofi (institutional) (RF), Bayer HealthCare Pharmaceuticals, Endocyte (Travel/Accommodations/Expenses); Toni K. Choueiri: AstraZeneca, Bayer, Bristol‐Myers Squib, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Takeda (RF), AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol‐Myers Squib, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up‐to‐Date, NCCN, Analysis Group, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OnClive and PER), L‐path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, New England Journal of Medicine, Lancet Oncology (H), AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Heron Therapeutics, Genentech, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up‐to‐Date, NCCN, Analysis Group (CA), Dana‐Farber Cancer Institute may have received additional independent funding/royalties from drug companies potentially involved in research around the subject matter. Susan Halabi: Eisai, Ferring Pharmaceuticals (C/A); M. Dror Michaelson: Pfizer, Exelixis, Novartis (C/A); Olwen Hahn: Cardinal Health (institutional) (H), Pfizer (C/A), Cardinal Health (I) (Travel/Accommodations/Expenses); Eric J. Small: Fortis, Harpoon Therapeutics (OI), Janssen‐Cilag (H), Fortis, Gilead Sciences, Valeant Pharmaceuticals International (C/A), Janssen (institutional) (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yakes FM, Chen J, Tan J et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298–2308. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Halabi S, Sanford BL et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN trial. J Clin Oncol 2017;35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choueiri TK, Hessel C, Halabi S et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression‐free survival by independent review and overall survival update. Eur J Cancer 2018;94:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKay RR, Bossé D, Choueiri TK. Evolving systemic treatment landscape for patients with advanced renal cell carcinoma. J Clin Oncol 2018;36:3615–3623. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med 2017;376:354–366. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]