Cancer patients often seek complementary and alternative medicine options to treat their disease. This article focuses on how complementary and alternative medicine options are discussed in medical centers in the United States.
Keywords: Oncology, Complementary medicine, Alternative medicine, Patient‐centered care
Abstract
Background.
Little is known about how complementary and alternative medicine (CAM) is discussed in cancer care across varied settings in the U.S.
Methods.
In two practices affiliated with one academic medical center in southern California (SoCal), and one in the upper Midwest (UM), we audio‐recorded patient‐clinician interactions in medical oncology outpatient practices. We counted the frequency and duration of CAM‐related conversations. We coded recordings using the Roter Interaction Analysis System. We used chi‐square tests for bivariate analysis of categorical variables and generalized linear models for continuous variables to examine associations between dialogue characteristics, practice setting, and population characteristics with the occurrence of CAM discussion in each setting followed by multivariate models adjusting for clinician clustering.
Results.
Sixty‐one clinicians and 529 patients participated. Sixty‐two of 529 (12%) interactions included CAM discussions, with significantly more observed in the SoCal university practice than in the other settings. Visits that included CAM were on average 6 minutes longer, with CAM content lasting an average of 78 seconds. In bivariate tests of association, conversations containing CAM included more psychosocial statements from both clinicians and patients, higher patient‐centeredness, more positive patient and clinician affect, and greater patient engagement. In a multivariable model including significant bivariate terms, conversations containing CAM were independently associated with higher patient‐centeredness, slightly longer visits, and being at the SoCal university site.
Conclusion.
The frequency of CAM‐related discussion in oncology varied substantially across sites. Visits that included CAM discussion were longer and more patient centered.
Implications for Practice.
The Institute of Medicine and the American Society of Clinical Oncology have called for more open discussions of complementary and alternative medicine (CAM). But little is known about the role population characteristics and care contexts may play in the frequency and nature of those discussions. The present data characterizing actual conversations in practice complements a much larger literature based on patient and clinician self‐report about CAM disclosure and use. It was found that CAM discussions in academic oncology visits varied significantly by practice context, that the majority were initiated by the patient, and that they may occur more when visit time exists for lifestyle, self‐care, and psychosocial concerns.
Introduction
Patients with cancer seek out and use complementary and alternative medicine (CAM) at twice the rate of the general population [1], [2], [3]. As a primary cancer treatment, CAM alone could delay effective conventional treatment or cause harm [4]. As an adjunct to conventional therapy, CAM interventions may reduce symptoms and mitigate treatment side effects [5], [6], [7], [8], [9], [10]. Little is known about how CAM is discussed across the cancer continuum and the extent to which population characteristics and care contexts may influence these discussions.
A single‐center study in Australia suggested that 24%–29% of oncology visits contained a CAM discussion [11], [12]. Koenig found that 34% of visits at one center in San Francisco included CAM discussion [13]. Yet CAM use may vary by racial and ethnic subgroups as well as region and care setting [14], [15], [16], [17]. In our analysis of one upper Midwest (UM) practice, CAM discussions occurred in 11% of visits; CAM discussions were more likely to occur in visits that were longer, with more patient‐centered communication [18].
Here we compare data collected from two contrasting southern California (SoCal) academic oncology practices with data from the UM practice. We expected that CAM discussions would be relatively infrequent (<20%) and associated with longer visits and higher levels of patient‐centered conversation. We wondered if CAM discussion might also vary by patient factors like race and ethnicity and subjective health literacy.
Ethical Review
This study was reviewed and approved by the University of Southern California School of Medicine, Mayo Clinic, and Johns Hopkins Bloomberg School of Public Health institutional review boards.
Materials and Methods
Study Design
We conducted a cross‐sectional, audio‐recorded observational study of outpatient medical oncology visits with clinicians and patients from across the cancer continuum, as described elsewhere [19]. For the purposes of this study we defined complementary and alternative medicine based on the contemporary definition when the study was conceptualized (2009) as a “group of diverse medical and health care systems, practices, and products that are not generally considered to be part of conventional medicine” [20].
Setting and Populations
In three practice settings, two affiliated with one academic medical center in SoCal and one in the UM, we recruited patients and clinicians in medical oncology practices. The southern California settings included a university practice and a county hospital. The university practice is staffed by specialized medical oncologists and nurse practitioners. It is a National Cancer Institute (NCI)‐designated comprehensive cancer center caring for local and regional tertiary referral populations. The southern California county practice, also affiliated with that same academic medical center, is a public safety net hospital staffed by medical oncology fellows who primarily provide care to those who are uninsured or receiving Medicaid. At the time of data collection, the SoCal university site had a modest pilot CAM program for patients with limited services on site and none at the county hospital. The upper Midwest practice is an NCI‐designated comprehensive cancer center seeing local, regional, national, and international referral populations and is staffed by medical oncologists, nurse practitioners, and medical oncology fellows. At the time of data collection, it had a full‐service academic clinic for CAM that included acupuncture and massage.
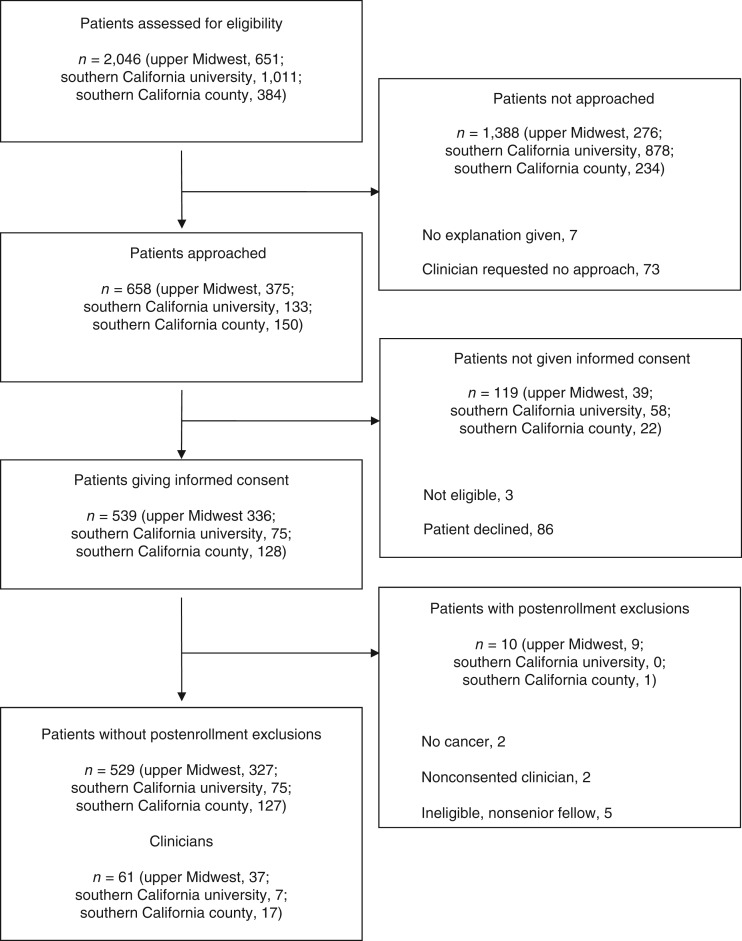
Clinicians at all sites were invited to participate in a communication study. Our interest in characterizing communication about CAM was not disclosed to patients or clinicians. In clinics where consented clinicians practiced, study coordinators identified and invited patients at the time of appointment check‐in to participate in an audio‐recorded communication study. Clinicians could veto a given patient's recruitment at their discretion. This study design feature was included to engender clinician comfort and participation, given the intimacy and sensitivity of some situations that arise in oncology practice. Exclusion criteria included language other than English or Spanish and current enrollment in hospice. Figure 1 shows the accrual process.
Figure 1.
Patient and clinician recruitment and selection process at southern California and upper Midwest academic oncology practices.
We approached 89 clinicians. Of these, 65 consented, and data were collected on 61. We screened 2,046 patients. Of these, 658 were approached, and 539 consented to participate. Ten enrolled patients met prespecified exclusion criteria discovered after consent. We stopped recruiting patients once we had achieved the target of 8 to 12 interactions per recruited clinician. This left 529 unique interactions for analysis across the three sites.
Measures
Immediately prior to the visit, patients rated their subjective health literacy regarding (a) help needed to read hospital materials, (b) difficulty with written information, (c) confidence filling out forms, and (d) trouble understanding spoken information (Cronbach's alpha = .84) [21], [22], [23]. Immediately after the recorded visit, patients rated provider communication with five items adapted from the Consumer Assessment of Healthcare Providers and Systems [24]. After each visit, clinicians indicated the patient's point on the cancer continuum (initial diagnosis, early or mid‐treatment, remission, recurrent, or end stage), and their primary tumor type. Patient questionnaires were translated from English to Spanish following a forward‐backward‐reconciliation procedure [25].
We analyzed visit recordings using the Roter Interaction Analysis System, a taxonomy characterizing all articulated thoughts by each speaker into mutually exclusive and exhaustive codes including psychosocial information exchange, expression and response to emotion, facilitation, and partnering as well as overall positive and negative affect [26]. We defined patient‐centeredness as the extent to which psychosocial, lifestyle, emotional, and facilitative exchanges occurred relative to medically focused and directive exchanges [18]. We also collected duration of session in minutes.
Data Management
Study staff compiled self‐reported and clinical variables in a Research Electronic Data Capture (REDCap) database [27]. Then, a study coordinator (C.F.) flagged audio‐recordings for any CAM content using accepted definitions. Unclear instances of potential CAM were resolved by consensus with senior CAM experts (J.T. and G.G.).
Statistical Analysis
Using Stata (StataCorp, College Station, TX, 2015), we used the Kruskal‐Wallis test for continuous values and cluster‐adjusted chi‐square test or Fisher's exact test for categorical variables to compare CAM communication characteristics in each of the SoCal practices with those in the UM practice in bivariate tests of association (<.05 significance). We calculated a “patient‐centeredness” score—a ratio of psychosocial, lifestyle, emotional, and facilitative exchanges relative to medically focused and directive exchanges. A higher score indicates greater visit patient‐centeredness [18], [28], [29], [30]. Patient ratings of communication quality were summarized using a percent‐top‐box score approach—the proportion of the sample endorsing the highest ordinal response option across all five communication items [24]. We applied “cut scores” to classify patients’ health literacy as inadequate (scores ranging from 4 to 12), marginal (scores ranging from 13 to 16), and adequate (scores ranging from 17 to 20) [21], [22], [23]. We collapsed race and ethnicity into non‐Hispanic white, non‐Hispanic black, non‐Hispanic Asian, non‐Hispanic other, and Hispanic or Latino of any race.
Using significant variables from unadjusted associations, a multivariable logistic model was conducted and adjusted for clustering by clinician. Given our interest in the roles of care context and differences in populations served, all models were adjusted by practice setting (UM, SoCal cancer center, and SoCal county). We tested for the interaction between practice setting and each characteristic using the likelihood ratio test (<.05 significance), although this analysis was only exploratory.
Results
Participant Characteristics
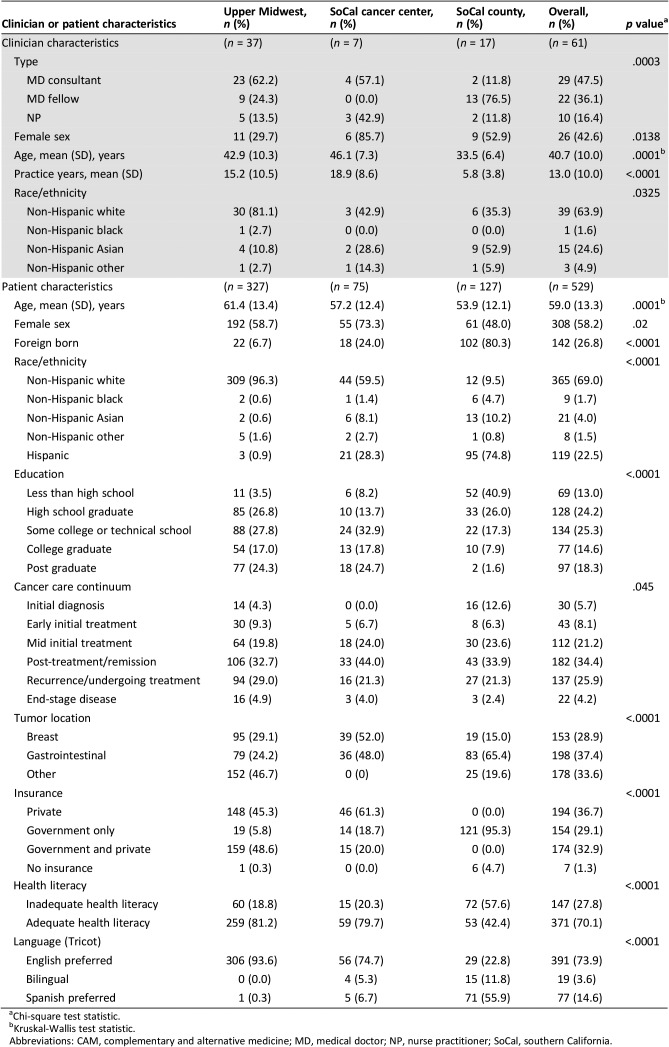
Among the 61 participating clinicians, 29 were attending faculty medical oncologists, 22 senior medical oncology fellow physicians, and 10 nurse practitioners. Clinician characteristics are shown in Table 1 (top). Sites varied by proportion of clinician training level (nurse practitioner, attending physician, fellow), gender, mean age, mean years in practice, and self‐reported race/ethnicity (Table 1, top). The university practices in both UM and SoCal had similarly high participation by attending oncologists (62% and 57%), but only two (11%) attending oncologists were included in the SoCal county site. In contrast, fellows comprised the majority of clinicians in SoCal county (76%) with none in the SoCal university site and 24% at UM.
Table 1. Characteristics of 529 patients and 61 clinicians audio‐recorded at three academic oncology practice contexts in southern California and the upper Midwest.
Chi‐square test statistic.
Kruskal‐Wallis test statistic.
Abbreviations: CAM, complementary and alternative medicine; MD, medical doctor; NP, nurse practitioner; SoCal, southern California.
Table 1 (bottom) also displays differences in patient demographics across the study sites. Among the 529 recruited patients, 327 (61%) were from the UM site, 75 (14%) in SoCal university practice, and 127 (25%) in the SoCal county practice (Table 1). A greater proportion of patients in the SoCal university practice were female (73%). Almost one quarter (n = 119, 23%) were Hispanic or Latino, comprising 28% of the SoCal university practice sample and 75% of SoCal county practice. Eighteen percent of patients were either bilingual or preferred Spanish. The most common educational attainment was “some college” (25%), with a much larger proportion (41%) of the SoCal county site participants reporting education less than high school. Twenty‐eight percent of patient participants had “inadequate” health literacy, over half (58%) of whom were seen at the SoCal county site.
Patients spanned the cancer continuum. Most were “post‐treatment/survivorship” (34%), “recurrence” (26%) or “mid‐initial treatment” (21%), although distribution of patients across the continuum varied somewhat by site. For instance, the SoCal university site had the highest proportion of post‐initial treatment patients (44%). A large proportion of patients had gastrointestinal cancers (37%), followed by breast cancer (29%). Patients with breast cancer constituted 52% of the SoCal university‐recruited patients. Sites varied by insurance type, health literacy, and preferred language. The UM site had the lowest rate of government‐only insurance, highest health literacy, and almost universal preference for English. In contrast, the SoCal county site had the highest rate of government‐only insurance, lowest health literacy, and a majority preference for Spanish.
CAM Conversation Frequency, Characteristics, and Associations
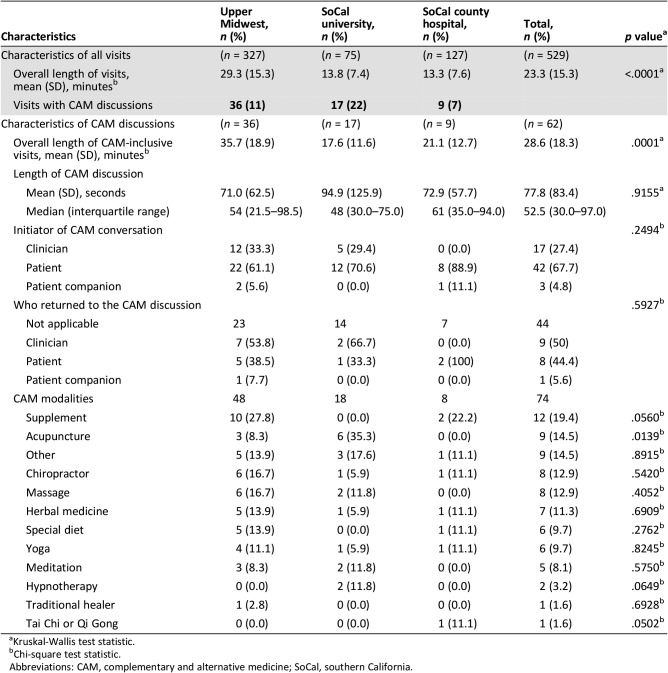
We previously reported that 36 of 327 (11%) consultations at the UM site contained some mention of CAM [18]. Table 2 shows significant variation in CAM discussion from the UM site with the SoCal sites. At the SoCal university site, 17 of 75 (22%) consultations included CAM, and at the SoCal county site, 9 of 128 (7%) consultations included CAM (p = .03). Intraclass correlation tests of 0.05 (95% confidence interval [CI], 0–0.11) showed a weak clustering effect by clinician.
Table 2. Characteristics of CAM conversations among 529 patients and 61 clinicians at three academic oncology practice contexts in southern California and the upper Midwest.
Kruskal‐Wallis test statistic.
Chi‐square test statistic.
Abbreviations: CAM, complementary and alternative medicine; SoCal, southern California.
Table 2 also shows mean appointment length in minutes across sites; UM visits were more than twice as long as SoCal sites (UM, 29.3; SoCal university, 13.8; and SoCal county, 13.3; p < .0001). When CAM was discussed, those visits were longer by several minutes at every site. These differences were especially striking in the SoCal county hospital (7.8‐minute difference), followed by 6.4‐minute longer visits at UM site and 3.8‐minute longer visits at the SoCal university clinic. Within these visits, explicit CAM conversation lasted just over a minute on average (mean, 77 seconds). The site with the most frequent CAM conversations (SoCal university) also had the longest average time devoted to CAM (mean, 95 seconds); this was also the site with the shortest overall visit length (mean, 17.6 minutes) for CAM‐containing conversations (Table 2). There were no significant differences in CAM discussion across study sites (p = .92).
Among the 62 total instances of CAM discussion, the patient initiated the conversation 42 times (68%). Patients and clinicians were equally likely to be the one who returned to the topic later in the conversation. At the SoCal county site, clinicians never initiated or returned to the topic of CAM. The most frequent forms of CAM discussed included supplements (19%), acupuncture (15%), chiropractic (13%), massage (13%), herbal medicine (11%), and yoga (10%). The SoCal university practice focused more on mind‐body modalities like yoga, meditation, and hypnotherapy. (See supplemental online Appendix 1 for other visit characteristics.)
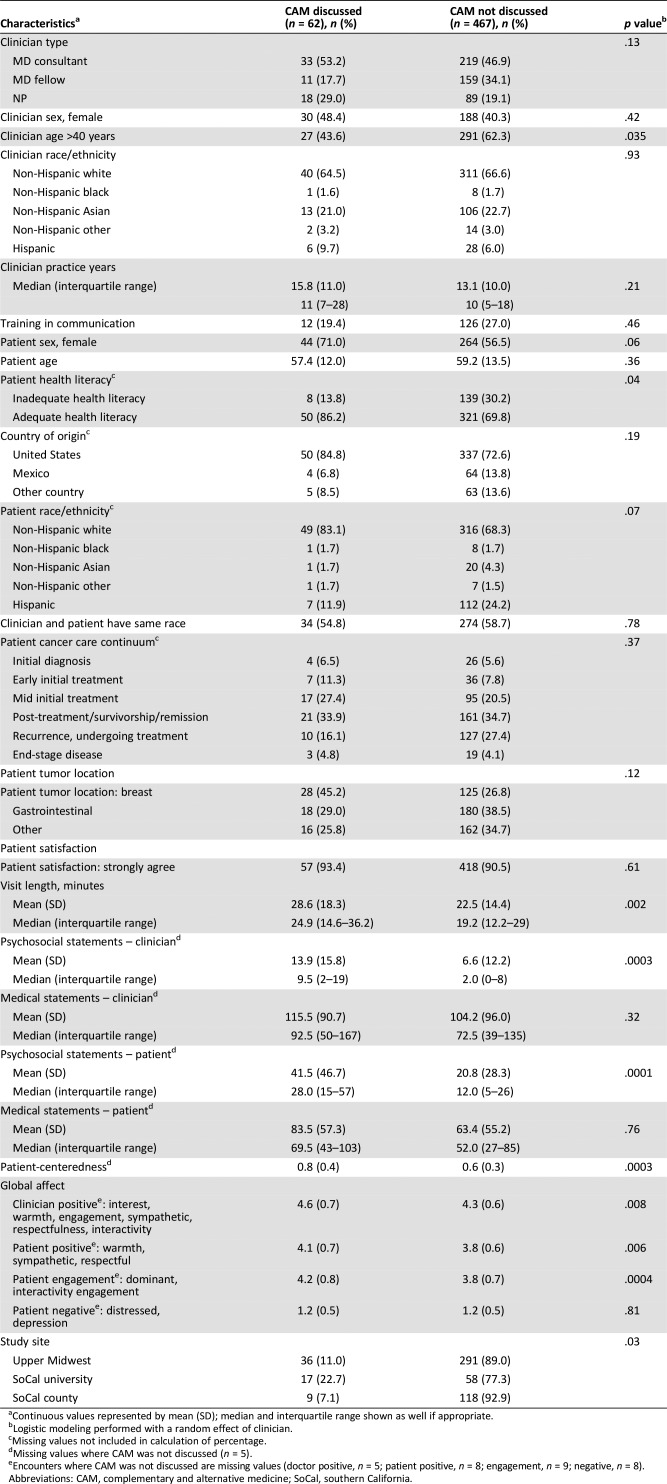
We found that dialogue containing CAM included consistently longer visit times (p = .002), more clinician and patient psychosocial statements (p = .0003 and p = .0001, respectively), greater patient engagement scores (p = .0004), greater positive global clinician and patient affect (p = .008 and p = .006, respectively), and greater patient‐centeredness (p = .003; Table 3). These bivariate associations were consistent across all three study sites. When we tested for a practice site interaction with several key variables—visit times, clinician psychosocial statements, patient psychosocial statements, patient engagement score, positive global clinician affect, patient positive global affect, and patient‐centeredness—no significant interaction effects were found.
Table 3. Unadjusted associations between patient, clinician, visit, conversation characteristics, and practice context with CAM discussion among 529 patients and 61 clinicians at three academic oncology practice contexts in southern California and the upper Midwest.
Continuous values represented by mean (SD); median and interquartile range shown as well if appropriate.
Logistic modeling performed with a random effect of clinician.
Missing values not included in calculation of percentage.
Missing values where CAM was not discussed (n = 5).
Encounters where CAM was not discussed are missing values (doctor positive, n = 5; patient positive, n = 8; engagement, n = 9; negative, n = 8).
Abbreviations: CAM, complementary and alternative medicine; SoCal, southern California.
In multivariable analyses of CAM discussion and significant covariates identified above, higher patient‐centeredness (odds ratio [OR], 3.57; 95% CI, 1.66–7.68) and longer visits (OR, 1.04; 95% CI, 1.02–1.06) were each independently associated with CAM discussions (Table 4). For every 0.1 increase in the patient‐centeredness ratio, conversations were 3.5 times more likely to include mentions of CAM. Positive clinician affect, positive patient affect, and patient health literacy did not remain significant in multivariable models. These findings persisted after controlling for study site.
Table 4. Multivariable associations among key patient, clinician, visit, conversation characteristics, and practice context with CAM discussion at three academic oncology practices in southern California and the upper Midwest.
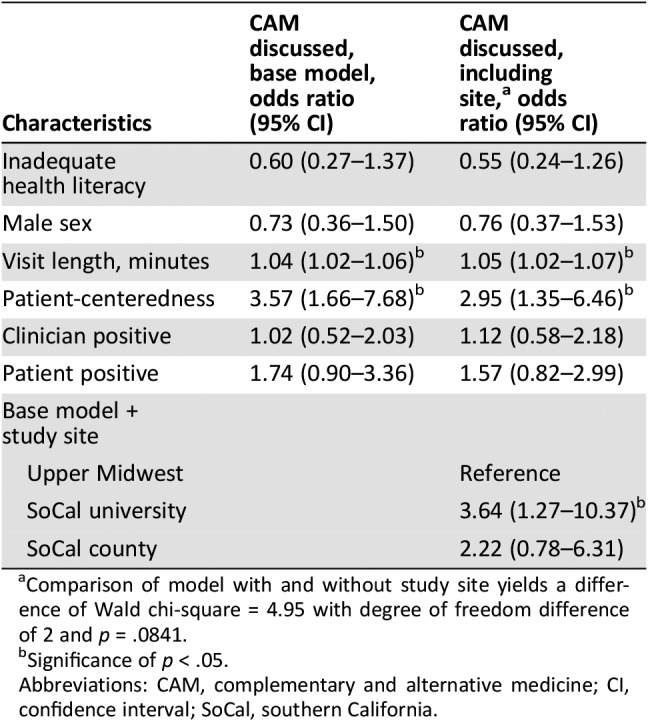
Comparison of model with and without study site yields a difference of Wald chi‐square = 4.95 with degree of freedom difference of 2 and p = .0841.
Significance of p < .05.
Abbreviations: CAM, complementary and alternative medicine; CI, confidence interval; SoCal, southern California.
Compared with the UM site, the SoCal university practice was strongly and independently associated with CAM discussion (OR, 3.64; 95% CI, 1.27–10.37), whereas the SoCal county practice did not differ significantly from the UM site (OR, 2.22; 95% CI, 0.78–6.31). When comparing the SoCal university practice with the SoCal county in the multivariable model, the confidence intervals showed no difference in CAM discussions (OR, 1.64; 95% CI, 0.50–5.41 for SoCal county compared with SoCal university).
Discussion
We found that CAM discussions in academic oncology visits varied significantly by practice context. The most striking differences existed between the two southern California sites; 23% of visits in the SoCal university practice included CAM, whereas only 7% in the SoCal county practice and 11% in the UM did. We observed a consistent positive association across all study sites between visit discussion of CAM, patient‐centeredness, and visit duration; regardless of study site, CAM inclusive visits were more patient centered and longer than visits that were not.
The nearly three‐fold difference in rates of CAM discussion between the two southern California sites is intriguing. It could be that the pressures and clinical time constraints of caring for a poor, urban, and immigrant patient population at the SoCal county site creates barriers to CAM discussion. Cross‐cultural differences in the definition of CAM or discomfort with disclosing CAM to a clinician with whom a patient is already struggling to communicate may further inhibit discussion of CAM. It is also important to acknowledge that institutional availability of CAM may influence the extent to which it gets discussed. Although we had no direct measure of it, at the time the study was initiated, our UM site had the most mature and extensive CAM clinical program. The SoCal university site had some pilot programs in CAM for breast cancer patients, and the SoCal county site had no such programs.
In a region where CAM practice may be normative among the majority population (SoCal), and in a practice setting with less time and resource constraints, the SoCal university patients may have felt encouraged to engage in these discussions. Meanwhile, in a Midwestern context where the underlying cultural appetite among patients for CAM may be somewhat less ubiquitous (UM), notwithstanding longer visits, those visits do not necessarily lead to a more CAM conversation. We also wonder whether socioeconomic constraints of the SoCal county patients may have hindered their ability to pay for CAM not covered by insurance, like acupuncture, making discussion of it moot. It is also possible that difference in care philosophy across categories of clinicians explains some variability in whether CAM is discussed. For instance, one might postulate that nurse practitioners may have a more holistic treatment philosophy. Unfortunately, or sample size of nurse practitioners (n = 10) was not sufficient to explore such associations.
Others have found that half to three quarters of CAM discussions were initiated by patients [11], [12], [13]. In our study, the UM and SoCal university settings showed patients raising the topic in 61% and 71% of instances, respectively. In the SoCal county site, the topic of CAM was initiated by patients in eight of the nine instances. The remaining discussion was initiated by a patient companion. Thus, our findings corroborate prior, single‐center observations regarding who initiates CAM discussions.
Our characterizing of actual conversations in practice complements a much larger literature based on patient and clinician self‐report about CAM disclosure and use. Survey data suggest that patients do not disclose CAM use to clinicians and that this failure may be due to clinicians’ lack of inquiry, patients’ anticipated disapproval from their clinician, clinicians’ disinterest or inability to help, and patients’ perception that it is irrelevant to care [31], [32]. Our observations across sites in our study lends additional support to these self‐reports. In nononcology Latino populations near our SoCal sites (Tijuana and San Diego), for instance, CAM use also goes undisclosed in chronic disease care, and clinicians acknowledge a lack of preparedness for CAM conversations [33].
The Institute of Medicine and the American Society of Clinical Oncology have called for more open CAM discussions [34], [35]. Our data provide insights regarding how that hope might become a reality. We have shown that CAM discussion in oncology care is highly associated with patient‐centered communication, even during brief office visits. If time prohibits patient‐centered communication about CAM in short oncology visits, consultative help from experts with these skills may need to be in place for patients needs to be met.
We found that CAM‐containing conversations were several minutes longer despite the CAM exchange itself lasting only about a minute. CAM conversations may occur more when visit time exists for lifestyle, self‐care, and psychosocial concerns.
However, the larger effect size of patient‐centeredness compared with visit length in our multivariable model suggests a CAM conversation does not necessarily take a lot of time. In fact, the site with the highest patient‐centeredness and most CAM discussions also had short visit lengths (SoCal university). This suggests that the relationship between time, practice context, and the patient‐centeredness of conversations deserves more detailed examination. For instance, it is possible that shorter visit lengths along with higher patient‐centeredness scores may merely reflect less participatory discussion of biomedical content, based on our definition of patient‐centeredness. Or some clinicians may have strategies to facilitate nonbiomedical concerns in a shorter period of time, inflating the patient‐centeredness ratio. Or some clinicians may be more facile at accommodating a conversation that allows the patients concerns to be voiced more clearly while still achieving the necessary biomedical talk.
This study has several limitations. It was conducted at a limited number of sites. Moreover, this analysis did not address whether the CAM conversations adequately addressed patients’ needs. Few black patients were included in any of our sites [30]. Moreover, the fact that clinicians could (and sometimes did) veto a given patient's recruitment introduces the possibility of a biased sampling. Although Hawthorne effects are possible in this setting, the fact that our interest in CAM was not disclosed to either patients or clinicians strengthens confidence in our results. Other factors limit generalizability, including our allowing physicians to veto approaching individual patients, although this happened in a minority of cases (73 out of 658 instances). It is possible that dynamics with vetoed patients or patients who declined to participate were fundamentally different and might somewhat have changed the associations we observed. However, our overall participation rate was high (529 out of 658). The association between greater patient‐centeredness and CAM discussion is only correlative.
Conclusion
Although complementary and alternative medicine is an important topic for most patients with cancer, whether patients discuss it with their cancer care team seems to relate to time, patient‐centeredness of conversation, and practice context. Ultimately, future studies should assess whether having a good conversation about CAM in oncology leaves patients feeling heard and being better off. Future studies might test strategies to better identify patients’ informational and conversational needs about CAM, bolster clinician confidence in navigating CAM with patients, or expand access to CAM experts in routine cancer care in both academic and community settings.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was supported by R01 AT006515 to J.T. from the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health. This publication was also made possible by CTSA grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author Contributions
Conception/design: Jon Tilburt, Kathleen J. Yost, Heinz‐Josef Lenz, María Luisa Zúñiga, Victor Montori, Barbara A. Koenig, Gail Geller, Susan Larson, Debra L. Roter
Provision of study material or patients: Heinz‐Josef Lenz, Cara Fernandez, Aminah Jatoi
Collection and/or assembly of data: Thomas O'Byrne, Megan E. Branda, Cara Fernandez, Ashok Kumbamu, Susan Larson, Debra L. Roter
Data analysis and interpretation: Jon Tilburt, Kathleen J. Yost, Thomas O'Byrne, Megan E. Branda, Amelia Barwise, Ashok Kumbamu, Gail Geller, Debra L. Roter
Manuscript writing: Jon Tilburt, Heinz‐Josef Lenz, María Luisa Zúñiga, Aaron L. Leppin, Brittany Kimball, Amelia Barwise, Ashok Kumbamu, Victor Montori, Barbara A. Koenig, Gail Geller, Susan Larson, Debra L. Roter
Final approval of manuscript: Jon Tilburt, Kathleen J. Yost, Heinz‐Josef Lenz, María Luisa Zúñiga, Thomas O'Byrne, Megan E. Branda, Aaron L. Leppin, Brittany Kimball, Cara Fernandez, Aminah Jatoi, Amelia Barwise, Ashok Kumbamu, Victor Montori, Barbara A. Koenig, Gail Geller, Susan Larson, Debra L. Roter
Disclosures
The authors indicated no financial relationships.
References
- 1.Gansler T, Kaw C, Crammer C et al. A population‐based study of prevalence of complementary methods use by cancer survivors: A report from the American Cancer Society's studies of cancer survivors. Cancer 2008;113:1048–1057. [DOI] [PubMed] [Google Scholar]
- 2.Mao JJ, Farrar JT, Xie SX et al. Use of complementary and alternative medicine and prayer among a national sample of cancer survivors compared to other populations without cancer. Complement Ther Med 2007;15:21–29. [DOI] [PubMed] [Google Scholar]
- 3.Yates JS, Mustian KM, Morrow GR et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer 2005;13:806–811. [DOI] [PubMed] [Google Scholar]
- 4.Werneke U, Earl J, Seydel C et al. Potential health risks of complementary alternative medicines in cancer patients. Br J Cancer 2004;90:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bindemann S, Soukop M, Kaye SB. Randomised controlled study of relaxation training. Eur J Cancer 1991;27:170–174. [DOI] [PubMed] [Google Scholar]
- 6.Classen C, Butler LD, Koopman C et al. Supportive‐expressive group therapy and distress in patients with metastatic breast cancer: A randomized clinical intervention trial. Arch Gen Psychiatry 2001;58:494–501. [DOI] [PubMed] [Google Scholar]
- 7.Edelman S, Bell DR, Kidman AD. A group cognitive behaviour therapy programme with metastatic breast cancer patients. Psychooncology 1999;8:295–305. [DOI] [PubMed] [Google Scholar]
- 8.Foley E, Baillie A, Huxter M et al. Mindfulness‐based cognitive therapy for individuals whose lives have been affected by cancer: A randomized controlled trial. J Consult Clin Psychol 2010;78:72–79. [DOI] [PubMed] [Google Scholar]
- 9.Listing M, Reisshauer A, Krohn M et al. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psychooncology 2009;18:1290–1299. [DOI] [PubMed] [Google Scholar]
- 10.Walker LG, Walker MB, Ogston K et al. Psychological, clinical and pathological effects of relaxation training and guided imagery during primary chemotherapy. Br J Cancer 1999;80:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schofield PE, Juraskova I, Butow PN. How oncologists discuss complementary therapy use with their patients: An audio‐tape audit. Support Care Cancer 2003;11:348–355. [DOI] [PubMed] [Google Scholar]
- 12.Juraskova I, Hegedus L, Butow P et al. Discussing complementary therapy use with early‐stage breast cancer patients: Exploring the communication gap. Integr Cancer Ther 2010;9:168–176. [DOI] [PubMed] [Google Scholar]
- 13.Koenig CJ, Ho EY, Trupin L et al. An exploratory typology of provider responses that encourage and discourage conversation about complementary and integrative medicine during routine oncology visits. Patient Educ Counsel 2015;98:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green RR, Santoro N, Allshouse AA et al. Prevalence of complementary and alternative medicine and herbal remedy use in Hispanic and non‐Hispanic white women: Results from the Study of Women's Health Across the Nation. J Altern Complement Med 2017;23:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith VM, Kronenfeld JJ, Rivers PA et al. Assessing the effects of race and ethnicity on use of complementary and alternative therapies in the USA. Ethn Health 2005;10:19–32. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie ER, Taylor L, Bloom BS et al. Ethnic minority use of complementary and alternative medicine (CAM): A national probability survey of CAM utilizers. Altern Ther Health Med 2003;9:50–56. [PubMed] [Google Scholar]
- 17.Bausell RB, Lee WL, Berman BM. Demographic and health‐related correlates to visits to complementary and alternative medical providers. Med Care 2001;39:190–196. [DOI] [PubMed] [Google Scholar]
- 18.Roter DL, Yost KJ, O'Byrne T et al. Communication predictors and consequences of Complementary and Alternative Medicine (CAM) discussions in oncology visits. Patient Educ Counsel 2016;99:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimball BC, James KM, Yost KJ et al. Listening in on difficult conversations: An observational, multi‐center investigation of real‐time conversations in medical oncology. BMC Cancer 2013;13:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Complementary and Alternative Medicine . What is CAM? 2009. http://nccam.nih.gov/health/whatiscam/overview.htm. Accessed May 9, 2010.
- 21.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–594. [PubMed] [Google Scholar]
- 22.Chew LD, Griffin JM, Partin MR et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haun J, Noland‐Dodd V, Varnes J et al. Testing the BRIEF health literacy screening tool. Fed Pract 2009;26:24–31. [Google Scholar]
- 24.Hospital Consumer Assessment of Healthcare Providers and Systems . http://www.hcahpsonline.org/SummaryAnalyses.aspx. Accessed November 28, 2017.
- 25.Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross‐cultural validation of health status questionnaires. Eval Health Prof 2005;28:212–232. [DOI] [PubMed] [Google Scholar]
- 26.Roter D, Larson S. The Roter interaction analysis system (RIAS): Utility and flexibility for analysis of medical interactions. Patient Educ Counsel 2002;46:243–251. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R et al. Research Electronic Data Capture (REDCap) ‐ A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roter DL, Stewart M, Putnam SM et al. Communication patterns of primary care physicians. JAMA 1997;277:350–356. [PubMed] [Google Scholar]
- 29.Mead N, Bower P. Measuring patient‐centredness: A comparison of three observation‐based instruments. Patient Educ Counsel 2000;39:71–80. [DOI] [PubMed] [Google Scholar]
- 30.Cooper LA, Roter DL, Johnson RL et al. Patient‐centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med 2003;139:907–915. [DOI] [PubMed] [Google Scholar]
- 31.Davis EL, Oh B, Butow PN et al. Cancer patient disclosure and patient‐doctor communication of complementary and alternative medicine use: A systematic review. The Oncologist 2012;17:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson MA, Masse LC, Nanny K et al. Discrepant views of oncologists and cancer patients on complementary/alternative medicine. Support Care Cancer 2004;12:797–804. [DOI] [PubMed] [Google Scholar]
- 33.Munoz FA, Servin AE, Kozo J et al. A binational comparison of HIV provider attitudes towards the use of complementary and alternative medicine among HIV‐positive Latino patients receiving care in the US‐Mexico border region. AIDS Care 2013;25:990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute of Medicine . Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 35.The physician and unorthodox cancer therapies. J Clin Oncol 1997;15:401–406. [DOI] [PubMed] [Google Scholar]