This article reports on differences between men and women as related to immune‐related adverse events incidence and the potential link with response to therapy in patients with metastatic melanoma and non‐small cell lung cancer treated with anti‐PD1 agents.
Keywords: Non‐small cell lung cancer, Melanoma, Sex, Immunotherapy, Immune‐related adverse events
Abstract
Background.
Immune‐related adverse events (irAEs) have emerged as a serious clinical issue in the use of immune checkpoint inhibitors (ICIs). Risk factors for irAEs remain controversial. Therefore, we studied sex differences in irAEs in patients treated with anti‐programmed cell death protein 1 (PD‐1) therapy.
Materials and Methods.
All patients with metastatic melanoma and non‐small cell lung cancer (NSCLC) treated with anti‐PD‐1 therapy at Mayo Clinic Rochester and Florida from 2015 to 2018 were reviewed. Kaplan‐Meier method and log‐rank test was used for time‐to‐event analysis.
Results.
In 245 patients with metastatic melanoma, premenopausal women were more likely to experience irAEs (all grades) compared with postmenopausal women and men (67% vs. 60% vs. 46%), primarily because of an increase in endocrinopathies (33% vs. 12% vs. 10%, respectively). In patients with NSCLC (231 patients), women (all ages) were also more likely to develop irAEs of all grades (48% vs. 31%). Women with NSCLC were more likely to develop pneumonitis (11% vs. 4%) and endocrinopathies (14% vs. 5%). No differences in grade ≥3 toxicities were seen across sexes in both cohorts, but women were more likely to receive systemic steroids for the treatment of irAEs compared with men. Better progression‐free‐survival was observed in women with NSCLC and irAEs (10 months vs. 3.3 months) compared with women without irAEs.
Conclusion.
Women with metastatic melanoma and NSCLC are more likely to experience irAEs compared with men. We also observed differences between sexes in the frequency of certain irAEs. Larger studies are needed to investigate the mechanisms underlying these associations.
Implications for Practice.
The results of this study suggest that women may be at a higher risk for immune‐related adverse events (irAEs) compared with men when treated with anti‐programmed cell death protein 1 therapy. In addition, women were more likely to develop certain irAEs, including endocrinopathies and pneumonitis. Close follow‐up of women undergoing treatment with immune checkpoint inhibitors will allow clinicians to diagnose these treatment‐related complications early, potentially reducing their associated morbidity and mortality. In addition, a possible association between irAEs and response to therapy was observed.
摘要
背景。免疫相关不良事件 (irAE)在需要使用免疫检查点抑制剂 (ICI)时是一种严重的临床问题。有关 irAE 风险因素的讨论仍存在争议。因此,我们研究了采用抗程序性细胞死亡蛋白1 (PD‐1) 治疗患者的 irAE 性别差异。
材料和方法。本文回顾了 2015 年至 2018 年罗彻斯特与佛罗里达州梅奥诊所所有接受抗‐PD‐1 治疗的转移性黑色素瘤与非小细胞肺癌 (NSCLC) 患者的临床资料。采用 Kaplan‐Meier 法和时序检验法进行时间事件分析。
结果。245 例转移性黑色素瘤患者中,绝经前女性比绝经后女性和男性 (67% vs. 60% vs. 46%) 出现 irAE(所有等级)的可能性更高,主要原因为内分泌疾病的增加(分别为 33% vs. 12% vs. 10%)。NSCLC 患者中(231 例),女性(所有年龄段)发生各种等级 irAE 的可能性更高 (48% vs. 31%)。NSCLC 女性患者出现肺炎 (11% vs. 4%) 和内分泌疾病 (14% vs. 5%) 的可能性更高。两组患者的 ≥3 级毒副反应未显示出性别上的差异,但女性因 irAE 而接受全身类固醇治疗的可能性较男性更高。NSCLC 并 irAE 女性患者的无进展生存期(10 个月 vs. 3.3 个月)优于无 irAE 的女性患者。
结论。转移性黑色素瘤和NSCLC的女性患者发生 irAE 的概率高于男性。结果还发现,某些 irAE 的频率在不同性别之间存在差异。需要更大规模的研究调查这些关联背后的机制。
实践意义:本项研究结果表明,与接受抗程序性细胞死亡蛋白 1 治疗的男性相比,女性发生免疫相关不良事件 (irAE) 的风险更高。此外,女性更容易出现内分泌疾病和肺炎等某些特定irAE。对接受免疫检查点抑制剂治疗的女性患者进行密切随访,有助于临床医生尽早诊断此类与治疗相关的并发症,从而降低相关病损率和死亡率。此外,结果显示,irAE 与治疗反应之间可能具有一定的关联。
Introduction
Immune checkpoint inhibitors (ICIs) have changed the treatment landscape in oncology. These agents were first introduced for the treatment of metastatic melanoma and advanced non‐small cell lung cancer (NSCLC) [1], [2] and have now become a primary treatment modality for a number of cancers, resulting in prolonged survival for some patients. The primary target of ICIs include cytotoxic T‐lymphocyte associated protein 4 (CTLA‐4), programmed cell death protein 1 (PD‐1), and programmed cell death ligand 1 (PD‐L1). Interestingly, the target of these drugs is not the tumor, as with conventional cytotoxic chemotherapy and targeted therapy; rather, they work through the activation of cellular immunity by the blockage of negative regulators such as CTLA‐4 and the PD‐1‐PD‐L1 axis [3]. Despite important clinical benefits, checkpoint inhibition is associated with a unique spectrum of side effects defined as immune‐related adverse events (irAEs).
Immune‐related adverse events have emerged as a serious clinical problem in the use of ICIs. They include dermatologic, gastrointestinal, hepatic, endocrine, and other less common inflammatory events [4]. These adverse events are believed to emerge from immunologic hyper‐activation of normal tissues and are usually treated with immunosuppressive agents such as corticosteroids or tumor necrosis factor‐alpha antagonists [5], [6]. Although rare, fulminant and even fatal toxicities may occur with immune checkpoint inhibitors [7], [8], [9], and therefore, prompt recognition, identification of possible risk factors, and early treatment are vital.
Studies have suggested symptomatic brain metastasis [10] and high body mass index (BMI) [11] as clinical risk factors for the development of irAEs. Moreover, several biomarkers have been linked with irAEs, including high levels of interleukin (IL) 17 in patients with melanoma receiving ipilimumab [12], eosinophilia [13], and certain immunologically relevant genes [14], but additional studies are necessary before any of these biomarkers can be used in the clinic.
No studies have explored possible differences between sexes in the incidence of irAEs, despite many lines of evidence describing differences in immune responses between women and men [15], [16], higher incidence of autoimmune disorders in women [17], [18], and the effect of sex hormones on the immune system from CD4 T cells regulation to the enhancement of immunoglobulins production [19], [20], [21]. In addition, irAEs and their association with response to therapy remain unclear. Therefore, we studied differences between sexes in irAEs and their potential link with response to therapy in patients with metastatic melanoma and NSCLC treated with anti‐PD‐1 agents.
Materials and Methods
With approval of the Mayo Clinic Institutional Review Board and following Health Insurance Portability and Accountability Act regulations, we conducted a retrospective review of all patients with metastatic melanoma or NSCLC treated with anti‐PD‐1 agents at Mayo Clinic in Rochester, Minnesota, and Jacksonville, Florida, from January 1, 2015, to May 31, 2018.
Records were individually reviewed by nonblinded study investigators. Diagnosis of NSCLC or melanoma was made according to the 2010 World Health Organization and the American Joint Committee on Cancer histologic stage classification of NSCLC and melanoma. In order to be included in the study, the following characteristics were required: initial diagnosis of metastatic melanoma or NSCLC had to be made at our institution or confirmed by our Department of Laboratory Medicine and Pathology; age >18 years; follow‐up at our institution after initial treatment (minimum five outpatient visits after the time of diagnosis, including disease assessment by imaging); and accurately recorded vital status, time of progression, and time of death. Data from patients that discontinued therapy because of irAEs were collected and included until last available follow‐up or death.
Patients were excluded based on the following criteria: diagnosis of uveal melanoma, receipt of immune checkpoint inhibitors at an outside facility, history of autoimmune disorders, taking more than 10 mg of prednisone daily before the initiation of anti‐PD‐1 therapy, or history of previous treatment with anti‐CTLA‐4, anti‐PD‐1, or anti‐PD‐L1 agents. We excluded patients receiving anti‐PD‐L1 therapy in order to have a more homogenous group and better define adverse events in patients receiving nivolumab and pembrolizumab.
In the case of NSCLC, patients with a history of recent thoracic radiation (≤30 days from initiation of an immune checkpoint inhibitor) were also excluded from the final analysis. Thoracic radiation is a known risk factor for pneumonitis; the interval of 30 days from radiation was selected in order to reduce the confounding effect of radiation to the cases of immune‐related pneumonitis.
The study sample was divided into two tumor types: NSCLC and melanoma. We analyzed patients’ demographics and tumor clinicopathologic features. Immune‐related adverse events features include the type of irAEs and irAEs grades as recorded in the patient's chart and confirmed by investigator assessment following the Common Terminology Criteria for Adverse Events grading scale in managing immune‐mediated adverse events [22], [23]. Additionally, if patients were referred to a subspecialist (i.e., pulmonology), irAEs grades were recorded and compared with original grading. We also extracted the treatment for the irAEs and the rates of therapy discontinuation due to irAEs. Progression‐free survival (PFS) and overall survival (OS) were calculated in months.
We considered women ≥52 years of age to be postmenopausal in consensus with prior epidemiology studies [24], [25], [26]. Although some studies have considered >50 years as the age cutoff for menopause, this population may contain perimenopausal women, potentially confounding the results. In addition, chemotherapy is known to induce early menopause in women. Using chronological age to separate women into pre‐ and postmenopausal cohorts may have placed patients in the premenopausal cohort despite having prolonged amenorrhea after cytotoxic therapy [27]. However, the median age for menopause in the U.S. has been reported at 51.4 years and is a reflection of complete, or near complete, ovarian follicular depletion, with resulting hypoestrogenemia and high follicle‐stimulating hormone (FSH) concentrations [28], [29].
Response assessment varied by tumor type. This was evaluated by positron emission tomography‐computed tomography (PET‐CT) scans in the melanoma cohort and by conventional CT scans in the NSCLC cohort. A patient was classified as a responder in the presence of complete response or partial response as per RECIST 1.1 at their first tumor assessment [30], with most patients (93%) having their restaging scans at 10–12 weeks after the initiation of anti‐PD‐1 therapy. Further restaging imaging was not included in the analysis.
Statistical Analysis
Data analysis was performed using JMP statistical software (JMP for Windows, version 14; SAS Institute Inc., Cary, NC). Patient characteristics and irAEs were compared by one‐way analysis for continuous variables, and the chi‐square test was used for categorical variables. Fisher's exact test was used for small samples (expected values <5). Multinomial logistic regression analysis was used to compare clinical and pathologic characteristics among the patients with irAEs. For progression‐free survival calculations, the date of diagnosis was defined as the starting point, and the date of disease progression was defined as the endpoint. Time‐to‐event was calculated using the Kaplan‐Meier method for patients both with and without irAEs, and univariate comparison between the two groups was carried out by using the log‐rank test. Multivariable regressions were used to examine the effect of sex in the risk of developing irAEs. Variables considered in the multivariable model included all relevant clinical and pathologic factors (age, sex, race/ethnicity, performance status, smoking history, mutational status, histology [squamous and nonsquamous NSCLC], stage, presence of liver, brain, or bone metastases, lines of therapy, previous systemic therapy, and PD‐L1 tumor expression level in NSCLC). Regarding previous systemic therapy, for patients with metastatic melanoma, we recorded previous exposure to granulocyte‐macrophage‐colony‐stimulating factor (GM‐CSF) in the metastatic setting, and for patients with NSCLC, previous therapies were subdivided by platinum‐based, taxol monotherapy, and other. All previous systemic therapies were included in the multivariable model.
In this exploratory retrospective analysis of our data set, we considered p values <.05 to be significant.
Results
Melanoma Cohort
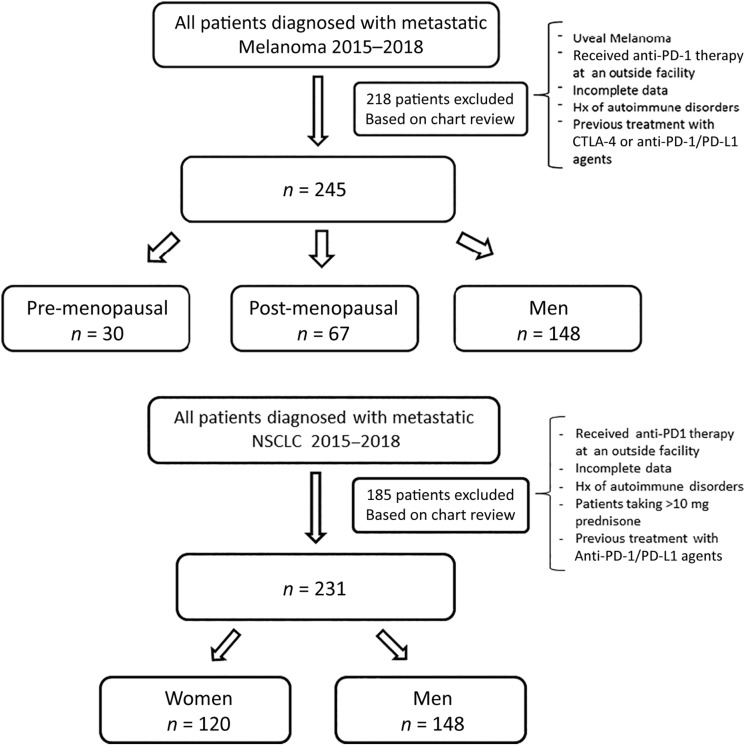
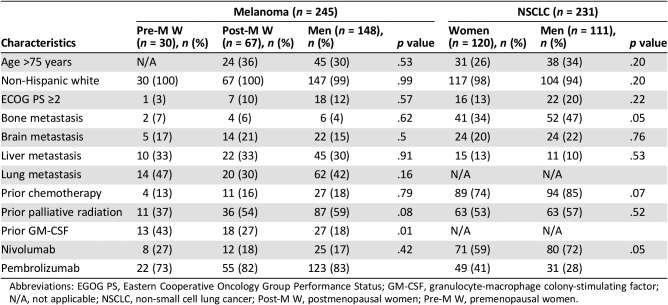
A total of 463 patients with metastatic melanoma were identified; 218 patients were excluded because of incomplete data, receiving anti‐PD‐1 therapy at an outside facility, or prior treatment with ipilimumab. For the analysis, 245 patients were included: 148 (60%) were men, 30 (12%) were premenopausal women (<52 years of age), and 67 (27%) were postmenopausal women (Fig. 1). Baseline characteristics were similar among the three groups (Table 1). Premenopausal women were more likely to have received prior treatment with GM‐CSF (43% vs. 27% in postmenopausal women and 18% in men, p < .01). No interval time differences were observed from the last dose of GM‐CSF and first dose of anti‐PD‐1 agent between sexes. Rates of prior radiation and chemotherapy were comparable across the groups.
Figure 1.
Consolidated Standards of Reporting Trials diagram depicting the criteria used to include and classify patients in the analysis (melanoma and non‐small cell lung cancer).
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte associated protein 4; NSCLC, non‐small cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death ligand 1.
Table 1. Patients’ baseline characteristics.
Abbreviations: EGOG PS, Eastern Cooperative Oncology Group Performance Status; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; N/A, not applicable; NSCLC, non‐small cell lung cancer; Post‐M W, postmenopausal women; Pre‐M W, premenopausal women.
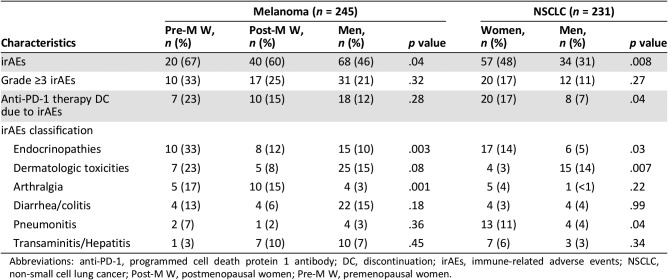
Regarding irAEs, premenopausal women were more likely to develop irAEs compared with postmenopausal women and men (67% vs. 60% vs. 46%, p < .04). We observed differences in the type of irAEs developing in each group. Specifically, premenopausal women were more likely to develop endocrinopathies and arthralgia compared with postmenopausal women and men (Table 2). Higher rates of grade ≥3 irAEs in premenopausal women were observed, but this was not statistically significant (33% for premenopausal women vs. 25% in postmenopausal women and 21% in men, p = .32). All observed cases of myositis (n = 4) and hypophysitis (n = 4) were reported in premenopausal women. The anti‐PD‐1 agent was permanently discontinued because of irAEs in 23% of premenopausal women compared with 12% of men (Table 2).
Table 2. Immune‐related adverse events by sex and tumor type.
Abbreviations: anti‐PD‐1, programmed cell death protein 1 antibody; DC, discontinuation; irAEs, immune‐related adverse events; NSCLC, non‐small cell lung cancer; Post‐M W, postmenopausal women; Pre‐M W, premenopausal women.
In this cohort, premenopausal women were more likely to receive intravenous (IV) steroids for the treatment of irAEs compared with postmenopausal women and men (47% vs. 19% vs. 32%, respectively, p < .0001), despite similar rates of grade 3 and 4 irAEs between groups. The remaining patients with grade 3 and 4 irAEs received treatment with oral steroids.
In a multivariate analysis of age, sex, performance status, previous treatments, and presence of distant metastases, sex was the only variable associated with higher risk for irAEs (odds ratio [OR]: 1.12, 95% confidence interval [CI]: 1.08–1.20, p < .035).
Non‐Small Cell Lung Cancer Cohort
In this cohort, 416 patients were initially identified, of whom 185 were excluded because of incomplete data, use of steroids, or receipt of anti‐PD‐1 therapy at another facility. We included 231 patients, of whom only 6 women met the age criteria for premenopausal classification (<52 years of age; Fig. 1). Because of this small sample, patients were divided by sex only: 120 (52%) were women, and 111 (48%) were men. Baseline characteristics were overall comparable among groups (Table 1). Men were more likely to be current or former smokers (>100 cigarettes in a lifetime) compared with women (63% [70] vs. 48% [57], p < .02). Adenocarcinoma was most commonly seen in women (77% [92] vs. 66% [73], p < .02). Regarding mutational status, three (1.3%) women were epidermal growth factor receptor mutated; no cases of ALK and ROS1 mutated NSCLC were identified. No differences in prior lines of treatments and the distribution of anti‐PD‐1 agents (pembrolizumab and nivolumab) were observed across sexes.
Women were more likely to experience irAEs compared with men (48% vs. 31%, p < .008; OR: 2.04, 95% CI: 1.19–3.51). Women were also more likely to develop pneumonitis (11% vs. 4%, p < .04) and endocrinopathies (14% vs. 5%, p < .03; Table 2). More cases of diabetic ketoacidosis were recorded in women (six vs. one). However, dermatologic toxicities (14% vs. 3%, p < .007) were reported more often in men. Similar to the melanoma cohort, no differences in grade ≥3 irAEs were seen. Among patients with irAEs (all grades), women were more likely to be prescribed oral steroids (63% vs. 41%, p < .02), despite no differences in grade ≥3 irAEs, including higher rates of oral steroids prescription for some grade 2 irAEs. Rates of treatment with IV steroids were 30% in women and 24% in men (p = .47). In 17% of women, the anti‐PD‐1 agent was discontinued because of irAEs (men 7%, p < .04; Table 2).
In a multivariable analysis of age, sex, race/ethnicity, performance status, smoking history, histologic subtype, tumor PD‐L1 expression >1%, lines of therapy, prior radiation, previous systemic therapy, and presence of liver or bone metastasis, sex was the only clinical characteristic associated with increased risk for irAEs in patients with NSCLC (OR: 1.38, 95% CI: 1.20–1.65, p < .001).
No difference in the rates of reported irAEs was observed when dividing patients by tumor PD‐L1 expression (>1% vs. <1% or >50% vs. <50%).
Less than 7% of patients (melanoma and NSCLC) were enrolled in a clinical trial receiving anti‐PD‐1 agents as monotherapy. No differences in the rates and frequency of irAEs were observed in this subgroup of patients.
Immune‐Related Adverse Events and Response to Therapy
Immune‐related adverse events were associated with a better response to therapy and improved PFS in several subgroups of patients. In melanoma, when all patients were considered, patients that experienced irAEs were more likely to have a radiographic response during their first disease assessment by imaging (complete or partial response as per PET/CT scan assessment) to anti‐PD‐1 agents regardless of sex (68% vs. 44%, p < .002). When patients were divided by sex and menopausal status, the differences were not statistically significant. There was a trend toward better PFS in men with irAEs compared with men without irAEs (16.5 months vs. 9.7 months, p = .05). We did not observe this in premenopausal or postmenopausal women with metastatic melanoma, which could be due to our small sample size (30 and 67 patients, respectively). Immune‐related adverse events were associated with better OS (34.3 months vs. 30.8 months, p < .02) in patients with metastatic melanoma.
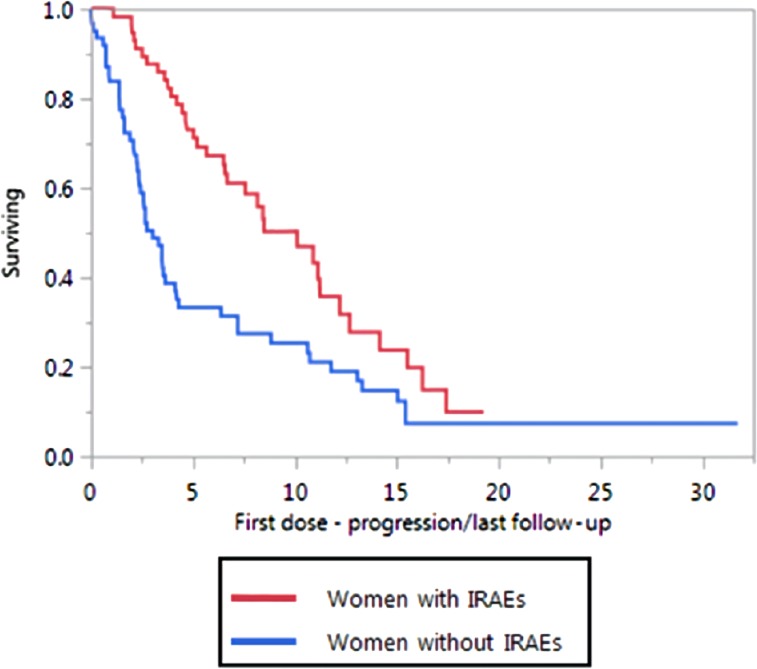
In the NSCLC cohort, women with irAEs were more likely to have a radiographic response during their first disease assessment (10–12 weeks from initiation of therapy) compared with women without irAEs (78% vs. 23%, p < .0001), although this was not observed in men (37% vs. 26%, p = .22). Better PFS was observed in women with irAEs (10 months vs. 3.3 months, p < .0006) compared with women without irAEs (Fig. 2). Men with irAEs had a slightly better PFS (6.2 vs. 4.9 months, p = .09), but this was not statistically significant. Immune‐related adverse events were not associated with better OS in patients with NSCLC.
Figure 2.
Progression‐free survival in women with non‐small cell lung cancer with irAEs and without irAEs.
Abbreviation: irAEs, immune‐related adverse events.
Discussion
We observed higher rates of irAEs in women in this retrospective review. In our melanoma cohort, we observed higher rates of irAEs in premenopausal women compared with postmenopausal women and men. Women with NSCLC were also more likely to experience irAEs. Women were more likely to develop endocrinopathies and arthralgias compared with men. No differences in the grade ≥3 irAEs were recorded between sexes, but women were more likely to receive oral or intravenous steroids compared with men, suggesting that women may be treated differently for these immunotherapy complications. One possible explanation for the discrepancy in the treatment of irAEs includes the differences in the type of irAEs experienced by each sex. Pneumonitis was more frequently seen in women; this is generally treated with oral or intravenous steroids. On the other hand, men had higher rates of dermatologic toxicities, which are usually treated with topical steroids.
The existing data regarding differences between sexes in response to ICIs are mixed. Conforti et al. [31] conducted a meta‐analysis of 20 immune checkpoint inhibitor clinical trials and reported that men have a larger treatment effect from ICIs compared with women. The pooled reduction of risk of death was double the size for male patients compared with female patients. However, a recent systematic review conducted by Wallis et al. [32] of 23 randomized ICI clinical trials demonstrated no statistically significant association between sexes and response to therapy. Although these studies are hypothesis generating, the use of patients’ individual data could provide us with more specific answers and include other factors such as comorbidities and health care delivery characteristics that were not accounted in these meta‐analyses. As discussed by McQuade et al. [33] an alternative explanation to these findings is that men have worse survival with the control treatments than women, and thus, their relative benefit from immunotherapy is more significant. Stratification of outcomes instead of the use of relative hazard ratios could provide a clearer picture of this phenomenon.
Several studies have described a correlation between hyper‐immunity and response to therapy, [34], [35], [36], and like in our study, irAEs were associated with improved PFS for both cohorts and better OS in the melanoma cohort. Owen et al. [34] reported that sex was not associated with an increased risk for irAEs, but this analysis was in an older population with a median age of 67 years. In contrast, our study included premenopausal women, who were found to have the highest rates of irAEs. In addition, our larger sample size may have allowed for identification of the described sex differences in irAEs. Over the past 3 decades, many studies have suggested differences between sexes in immune responses; this has been observed in vaccination, microbiology, and tumor‐specific studies [37], [38], [39], [40]. Women mount more vigorous antibody‐ and cell‐mediated immune responses than men following either infection or vaccination [41], [42], [43]. Also, women have a higher incidence of autoimmune diseases, and the onset of these conditions are common during the reproductive years, suggesting that the pathogenesis of irAEs could be influenced by sex hormones [44], [45], [46], [47], [48]. Studies in patients with endometriosis have reported a higher rate of autoimmune conditions in these patients, strengthening the link between autoimmunity and sex hormones [49].
Additionally, estradiol is associated with upregulation of CD4 T cells [50] and dendritic cells [51], increased survival of autoreactive B cells [52], and decreased tumor necrosis factor production [53]. These effects of estradiol on autoimmunity can partially explain our observations. In our analysis, sex was an independent risk factor for developing irAEs in patients with either melanoma or NSCLC.
Factors that we did not account for such as race/ethnicity [18], BMI [11], [54], and genetic predisposition to autoimmune disorders [55] could also be risk factors for developing irAEs.
We observed that women are more likely than men to receive systemic steroids for the treatment of irAEs despite no differences in the grade ≥3 irAEs. One study has suggested that the use of steroids can decrease the efficacy of ICIs in patients with NSCLC [56] as a result of possible IL‐2 suppression and the increase of immunosuppressive regulatory T cells [57], but data from other studies suggested that treating irAEs with steroids did not affect the outcome of patients with metastatic melanoma [58], [59]. Today, we lack a consensus regarding the effect of steroids on response to anti‐PD‐1 agents, but the variability observed in the treatment of women and men in our study brings attention to the need for a standardized process for treating irAEs. In 2018, the American Society of Clinical Oncology and the National Comprehensive Cancer Network released guidelines for the treatment of irAEs [6]. Consistent adherence to guidelines will enable patients to receive the best care, whether they are treated at tertiary centers or in community practices.
In our study, patients with metastatic melanoma with irAEs were more likely to respond to anti‐PD‐1 agents irrespective of sex (with better PFS and OS). In the case of patients with NSCLC, women with irAEs had better PFS and were more likely to respond to anti‐PD‐1 therapy. However, this was not seen in men with NSCLC. Immune‐related adverse events were not associated with better OS in patients with metastatic NSCLC. In concordance with our findings, several retrospective analyses have reported improved PFS and OS in patients with advanced NSCLC or melanoma that developed grade 2 or higher irAEs [34], [35], [36], [60], [61]. A prospective cohort of 43 patients with NSCLC treated with nivolumab demonstrated a higher objective response rate in the patients that experienced irAEs (37%) compared with those without irAEs (17%), as well as longer median PFS (6.4 vs. 1.5 months) [62]. To our knowledge, a strong relationship between irAEs and efficacy outcomes has not been reported in large prospective studies. Comprehensive and prospective studies are needed to enhance our understanding of these observations and to further determine if an association between irAEs and response to therapy exists.
Our current study is unique because it looks at sex as a biologic variable in the risk of developing irAEs. Our total sample size of 476 patients represents one of the most extensive studies from a single institution in regard to irAEs. Including two cancer types allowed us to confirm our findings and observe differences in the types of irAEs reported for patients with melanoma and NSCLC. These observed differences in the type and severity of irAEs between melanoma and NSCLC could in part be attributed to intrinsic tumor characteristics.
Our study has several limitations, including its retrospective design and the selection associated with care at a tertiary medical cancer. The relatively small sample size for premenopausal women in the melanoma and NSCLC cohorts also limits the strength of the conclusions. Furthermore, the use of chronologic age for menopausal status classification could include patients in their premenopausal period, but because of the retrospective nature of our study, we could not obtain hormonal levels. In our chart review, fewer than 5% of patients had recorded FSH or luteinizing hormone levels at the time of cancer diagnosis. Lastly, only including first tumor assessments may have excluded patients that experienced pseudoprogression or delayed responses to immunotherapy.
Conclusion
In our retrospective study of patients with melanoma and NSCLC, women were more likely to develop irAEs than men, and this effect was more prominent in premenopausal women than in postmenopausal women with metastatic melanoma. We also observed differences in the type of irAEs; women more commonly developed endocrinopathies, pneumonitis, and arthralgias. Variations in irAEs were also observed between patients with melanoma and those with NSCLC. Lastly, an association with irAEs and response to therapy was observed in patients with melanoma and women with NSCLC. Our findings could be attributed to sex‐based immunological differences that contribute to variations in the incidence of irAEs. Sex is a biological variable that should be considered when evaluating adverse events to anti‐PD‐1 agents. Larger prospective studies are needed to confirm these results and investigate the etiologic mechanisms underlying these associations.
Author Contributions
Conception/design: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav, Elizabeth Ann L. Enninga, Haidong Dong, Aaron S. Mansfield, Rami Manochakian, Alex A. Adjei, Roxana S. Dronca
Provision of study material or patients: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav, Katherine P. Hoversten, Clay T. Reed, Andrea N. Sitek, Elizabeth Ann L. Enninga
Collection and/or assembly of data: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav, Katherine P. Hoversten, Clay T. Reed, Andrea N. Sitek, Elizabeth Ann L. Enninga, Jonas Paludo, Jesus Vera Aguilera
Data analysis and interpretation: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav, Katherine P. Hoversten, Clay T. Reed, Andrea N. Sitek, Elizabeth Ann L. Enninga, Jonas Paludo, Jesus Vera Aguilera, Konstantinos Leventakos, Yanyan Lou, Lisa A. Kottschade, Haidong Dong, Aaron S. Mansfield, Rami Manochakian, Alex A. Adjei, Roxana S. Dronca
Manuscript writing: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav, Katherine P. Hoversten, Clay T. Reed, Andrea N. Sitek, Elizabeth Ann L. Enninga, Jonas Paludo, Jesus Vera Aguilera, Konstantinos Leventakos, Yanyan Lou, Lisa A. Kottschade, Haidong Dong, Aaron S. Mansfield, Rami Manochakian, Alex A. Adjei, Roxana S. Dronca
Final approval of manuscript: Narjust Duma, Azzouqa Abdel‐Ghani, Siddhartha Yadav, Katherine P. Hoversten, Clay T. Reed, Andrea N. Sitek, Elizabeth Ann L. Enninga, Jonas Paludo, Jesus Vera Aguilera, Konstantinos Leventakos, Yanyan Lou, Lisa A. Kottschade, Haidong Dong, Aaron S. Mansfield, Rami Manochakian, Alex A. Adjei, Roxana S. Dronca
Disclosures
Aaron S. Mansfield: Novartis (RF), Genentech, Abbvie, Bristol‐Myers Squibb, Trovagene (H); Rami Manochakian: Takeda, Guardant Health (SAB); Roxana S. Dronca: Merck (RF), Elsevier (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.La‐Beck NM, Jean GW, Huynh C et al. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacotherapy 2015;35:963–976. [DOI] [PubMed] [Google Scholar]
- 2.Soria JC, Marabelle A, Brahmer JR et al. Immune checkpoint modulation for non‐small cell lung cancer. Clin Cancer Res 2015;21:2256–2262. [DOI] [PubMed] [Google Scholar]
- 3.Weber J. Immune checkpoint proteins: A new therapeutic paradigm for cancer—Preclinical background: CTLA‐4 and PD‐1 blockade. Semin Oncol 2010;37:430–439. [DOI] [PubMed] [Google Scholar]
- 4.Naidoo J, Page D, Li B et al. Toxicities of the anti‐PD‐1 and anti‐PD‐l1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2015;27:559–574. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang DY, Salem JE, Cohen JV et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta‐analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzzubbo S, Javeri F, Tissier M et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer 2017;73:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Varricchi G, Galdiero MR, Marone G et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017;2:e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumenil C, Massiani MA, Dumoulin J et al. Clinical factors associated with early progression and grade 3‐4 toxicity in patients with advanced non‐small‐cell lung cancers treated with nivolumab. PloS One 2018;13:e0195945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eun Y, Kim I, Kim H et al. SAT0715 risk factors of immune‐related adverse events in patients treated with anti‐PD‐1 antibody pembrolizumab. Ann Rheum Dis 2018;77(suppl 2):1205. [Google Scholar]
- 12.Callahan M, Yang A, Tandon S et al. Evaluation of serum IL‐17 levels during ipilimumab therapy: Correlation with colitis. J Clin Oncol 2011;29(suppl 15):2505a. [Google Scholar]
- 13.Shahabi V, Berman D, Chasalow SD et al. Gene expression profiling of whole blood in ipilimumab‐treated patients for identification of potential biomarkers of immune‐related gastrointestinal adverse events. J Transl Med 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler K, Harmankaya K, Kuk D et al. Correlation of absolute and relative eosinophil counts with immune‐related adverse events in melanoma patients treated with ipilimumab. J Clin Oncol 2014;32(suppl 15):9096a. [Google Scholar]
- 15.Fish EN. The X‐files in immunity: Sex‐based differences predispose immune responses. Nat Rev Immunol 2008;8:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oertelt‐Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev 2012;11:A479–A485. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson DL, Gange SJ, Rose NR et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84:223–243. [DOI] [PubMed] [Google Scholar]
- 18.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev 2003;2:119–125. [DOI] [PubMed] [Google Scholar]
- 19.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015;294:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 1999;103:282–288. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol 2011;40:66–73. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute 2009;4. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed January 5, 2019.
- 23.Kumar V, Chaudhary N, Garg M et al. Current diagnosis and management of immune‐related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sowers M, La MP. Menopause: Its epidemiology and potential association with chronic diseases. Epidemiol Rev 1995;17:287–302. [DOI] [PubMed] [Google Scholar]
- 25.Hill K. The demography of menopause. Maturitas 1996;23:113–127. [DOI] [PubMed] [Google Scholar]
- 26.Phipps AI, Ichikawa L, Bowles EJ et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas 2010;67:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cathcart‐Rake EJ, Ruddy KJ, Gupta R et al. Amenorrhea after lung cancer treatment. Menopause 2019;26:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casper RF. Clinical manifestations and diagnosis of menopause. Available at https://www.uptodate.com/contents/clinical‐manifestations‐and‐diagnosis‐of‐menopause. Accessed December 21, 2018.
- 29.Santoro N. Perimenopause, an issue of obstetrics and gynecology clinics. Philadelphia, PA: Elsevier Health Sciences, 2011. [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 31.Conforti F, Pala L, Bagnardi V et al. Cancer immunotherapy efficacy and patients' sex: A systematic review and meta‐analysis. Lancet Oncol 2018;19:737–746. [DOI] [PubMed] [Google Scholar]
- 32.Wallis CJ, Butaney M, Satkunasivam R et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: A systematic review and meta‐analysis. JAMA Oncol 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuade JL, Daniel CR, Hess KR et al. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol 2018;19:e376. [DOI] [PubMed] [Google Scholar]
- 34.Owen DH, Wei L, Bertino EM et al. Incidence, risk factors, and effect on survival of immune‐related adverse events in patients with non‐small‐cell lung cancer. Clin Lung Cancer 2018;19:e893–e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricciuti B, Genova C, De Giglio A et al. Impact of immune‐related adverse events on survival in patients with advanced non‐small cell lung cancer treated with nivolumab: Long‐term outcomes from a multi‐institutional analysis. J Cancer Res Clin Oncol 2019;145:479–485. [DOI] [PubMed] [Google Scholar]
- 36.Haratani K, Hayashi H, Chiba Y et al. Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol 2018;4:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.vom Steeg LG and Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog 2016;12:e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010;10:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capone I, Marchetti P, Ascierto PA et al. Sexual dimorphism of immune responses: A new perspective in cancer immunotherapy. Front Immunol 2018;9:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin PY, Sun L, Thibodeaux SR et al. B7‐h1–dependent sex‐related differences in tumor immunity and immunotherapy responses. J Immunol 2010;185:2747–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol 2003;38:13–22. [DOI] [PubMed] [Google Scholar]
- 42.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: When a chromosome makes the difference. Nat Rev Immunol 2010;10:594–604. [DOI] [PubMed] [Google Scholar]
- 43.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626. [DOI] [PubMed] [Google Scholar]
- 44.Botticelli A, Onesti CE, Zizzari I et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017;8:99336–99346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quintero OL, Amador‐Patarroyo MJ, Montoya‐Ortiz G et al. Autoimmune disease and gender: Plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 2012;38:J109–J119. [DOI] [PubMed] [Google Scholar]
- 46.Borchers AT, Naguwa SM, Keen CL et al. The implications of autoimmunity and pregnancy. J Autoimmun 2010;34:J287–J299. [DOI] [PubMed] [Google Scholar]
- 47.Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis 2004;10:2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun 2007;28:1–6. [DOI] [PubMed] [Google Scholar]
- 49.Sinaii N, Cleary SD, Ballweg M et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum Reprod 2002;17:2715–2724. [DOI] [PubMed] [Google Scholar]
- 50.Pernis AB. Estrogen and CD4+ T cells. Curr Opin Rheumatol 2007;19:414–420. [DOI] [PubMed] [Google Scholar]
- 51.Bengtsson ÅK, Ryan EJ, Giordano D et al. 17β‐estradiol (E2) modulates cytokine and chemokine expression in human monocyte‐derived dendritic cells. Blood 2004;104:1404–1410. [DOI] [PubMed] [Google Scholar]
- 52.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17β‐estradiol impairs negative selection of high‐affinity DNA‐reactive B cells at more than one developmental checkpoint. J Immunol 2006;176:2703–2710. [DOI] [PubMed] [Google Scholar]
- 53.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- 54.Versini M, Jeandel PY, Rosenthal E et al. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun Rev 2014;13:981–1000. [DOI] [PubMed] [Google Scholar]
- 55.Taneja V, Mangalam A, David CS. Chapter 27 ‐ genetic predisposition to autoimmune diseases conferred by the major histocompatibility complex: Utility of animal models In: Rose NR, Mackay IR, eds. The Autoimmune Diseases. 5th ed Boston: Academic Press, 2014:365–380. [Google Scholar]
- 56.Ferrara R, Lai WV, Hendriks LE et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 57.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL‐2‐induced JAK‐STAT signaling by glucocorticoids. Proc Natl Acad Sci 2000;97:9573–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horvat TZ, Adel NG, Dang TO et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber JS, Larkin JM, Schadendorf D et al. Management of gastrointestinal (GI) toxicity associated with nivolumab (NIVO) plus ipilimumab (IPI) or IPI alone in phase II and III trials in advanced melanoma (MEL). J Clin Oncol 2017;35(suppl 15):9523a. [Google Scholar]
- 60.Toi Y, Sugawara S, Kawashima Y et al. Association of immune‐related adverse events with clinical benefit in patients with advanced non‐small‐cell lung cancer treated with nivolumab. The Oncologist 2018;23:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teraoka S, Fujimoto D, Morimoto T et al. Early immune‐related adverse events and association with outcome in advanced non–small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol 2017;12:1798–1805. [DOI] [PubMed] [Google Scholar]