Therapeutic advances have improved prognosis for follicular lymphoma, although the disease is still incurable. This comprehensive clinical review considers prognostic approaches and the treatment landscape for follicular lymphoma, including emerging therapies and unmet needs.
Keywords: Follicular lymphoma, Non‐Hodgkin lymphoma, Antineoplastic agents, Neoplasms
Abstract
Follicular lymphoma (FL) is a heterogeneous disease with varying prognosis owing to differences in clinical, laboratory, and disease parameters. Although generally considered incurable, prognosis for early‐ and advanced‐stage disease has improved because of therapeutic advances, several of which have resulted from elucidation of the biologic and molecular basis of the disease. The choice of treatment for FL is highly dependent on patient and disease characteristics. Several tools are available for risk stratification, although limitations in their routine clinical use exist. For limited disease, treatment options include radiotherapy, rituximab monotherapy or combination regimens, and surveillance. Treatment of advanced disease is often determined by tumor burden, with surveillance or rituximab considered for low tumor burden and chemoimmunotherapy for high tumor burden disease. Treatment for relapsed or refractory disease is influenced by initial first‐line therapy and the duration and quality of the response. Presently, there is no consensus for treatment of patients with early or multiply relapsed disease; however, numerous agents, combination regimens, and transplant options have demonstrated efficacy. Although the number of therapies available to treat FL has increased together with an improved understanding of the underlying biologic basis of disease, the best approach to select the most appropriate treatment strategy for an individual patient at a particular time continues to be elucidated. This review considers prognostication and the evolving treatment landscape of FL, including recent and emergent therapies as well as remaining unmet needs.
Implications for Practice.
In follicular lymphoma, a personalized approach to management based on disease biology, patient characteristics, and other factors continues to emerge. However, application of current management requires an understanding of the available therapeutic options for first‐line treatment and knowledge of current development in therapies for previously untreated and for relapsed or refractory disease. Thus, this work reviews for clinicians the contemporary data in follicular lymphoma, from advances in characterizing disease biology to current treatments and emerging novel therapies.
Introduction
Follicular lymphoma (FL) is the most common indolent non‐Hodgkin lymphoma (NHL) in Western countries, with an annual incidence of 3.4–5 per 100,000 in Europe and the U.S. [1], [2]. Median age of diagnosis is 65 years [3], but a large proportion of cases also occurs in younger adults [4]. Although incurable, prognosis has improved for early‐ and advanced‐stage disease, largely attributed to therapeutic advances.
FL is a heterogeneous disease with varying prognosis, influenced by differences in clinical, laboratory, and disease parameters between patients. Spontaneous regressions might occur in about 5%–10% of patients [5]. Although many patients can be initially observed, most require therapy after a median of 3–4 years after diagnosis [6]. Approximately 20% of patients will have early relapse within 2 years following current first‐line therapy [7]. Thus, FL has a typically protracted course, with multiple remissions and relapses.
Although continued elucidation of the biologic and molecular basis of FL is leading to identification of new potential therapeutic avenues, the heterogeneity of FL presents challenges, including selection of appropriate management for individual patients. This review considers prognostic approaches and the FL treatment landscape, including recent and emergent therapies and remaining unmet needs.
Pathobiological Heterogeneity
The World Health Organization (WHO) FL grading system is formed on the basis of differing proportions of centroblasts per high‐powered field, with a greater proportion indicative of a more aggressive phenotype [8]. Grades 1–2 and 3A are considered histologically low‐grade and indolent disease [9], whereas grade 3B is considered biologically distinct from grades 1–3A [10], [11] and typically treated as an aggressive lymphoma [2], [12]. However, it is important to note that centers largely group grades 1–3A as low‐grade lymphoma given inter‐ and even intraobserver variability in scoring grade, whereas grade 3B remains a routinely distinguishable pathologic entity characterized by sheets of immunoblasts. Further highlighting the histologic heterogeneity, the 2016 WHO classification update includes entities (e.g., duodenal‐type disease) [13] that are distinguished from nodal FL by different immune‐microenvironment profiles [14].
FL transformation from an indolent to more aggressive disease is a well‐recognized complication during the natural disease history. Transformation is defined by histologic evidence of diffuse large B‐cell lymphoma (DLBCL) or other high‐grade morphology, usually accompanied by rapid progression of lymphadenopathy, extranodal disease outside the marrow, B symptoms, elevated serum lactate dehydrogenase, and, less commonly, hypercalcemia [15]. Histologic‐transformation risk has historically been reported in 3% of patients annually [16], [17] but is believed to be lower in the rituximab era [18], [19], [20].
An extensive catalog of genetic changes occurring in FL is available. An unexpected revelation was the high prevalence of mutations in genes encoding proteins regulating the epigenome through specific histone post‐translational modifications (KMT2D, CREBBP, EP300, EZH2) [21], [22], [23], [24]. Mutations in genes involved in cellular processes such as JAK‐STAT, BCR‐NFkB, mTOR signal transduction, cell‐cycle regulation, immune modulation, and cellular differentiation are also frequent [23], [24], [25], [26], [27], several of which are potential therapeutic targets and some of which are prognostically relevant [25], [28]. Furthermore, genetic profiles of FL tumors can evolve longitudinally over the disease course [23], [24], [29], [30], [31] and spatially within tumors at different sites of involvement [32].
In addition to genetic alterations, tumor cells exist within a milieu of nonmalignant cells making up the microenvironment, which is pivotal in contributing to disease development, maintenance, and progression. A seminal study identified that gene expression signatures derived from nonmalignant immune cells (T cells vs. macrophages) independently predicted clinical outcome in FL, underpinning the critical role of the tumor microenvironment [33]. It is envisaged that building a more in‐depth understanding of the biological basis of FL will inform newer approaches to targeting these tumors.
Risk Stratification
Recent data suggest the strongest predictor of long‐term FL outcomes is length of first remission after front‐line therapy. Patients with progression of disease within 24 months of completing induction chemoimmunotherapy (POD24), which made up 19% of patients in this data set, had poorer outcomes compared with those with longer remission durations (5‐year overall survival [OS]: 50% vs. 90%, respectively) even after adjustment for Follicular Lymphoma International Prognostic Index (FLIPI) score (discussed below) [7]. These results were validated in the University of Iowa and Mayo Clinic Molecular Epidemiology Resource data sets [7], and in the GALLIUM study and using the FLASH data set [34], [35]. Additionally, a longer duration from diagnosis to progression (i.e., event‐free survival of >12 months after diagnosis) did not result in excess mortality compared with the age‐ and sex‐matched general population [36].
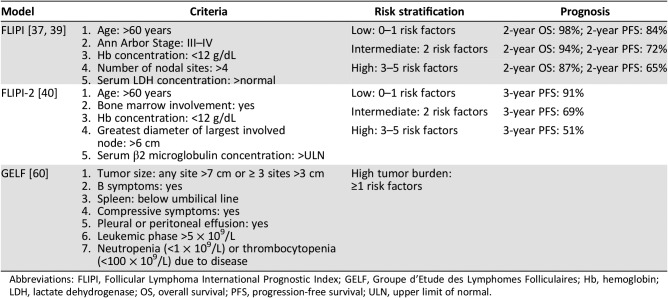
Based on retrospective survival data from patients diagnosed in the preimmunotherapy era, the FLIPI consists of five clinical parameters and categorizes patients into one of three risk groups (Table 1) [37]. The FLIPI has been validated in clinical trials of chemoimmunotherapy and in the National LymphoCare study observational cohort and was validated using OS and progression‐free survival (PFS) in the prechemoimmunotherapy era [38], [39]. FLIPI‐2 was subsequently developed as a more contemporary prognostic model using data reflective of treatment in the chemoimmunotherapy era [40]. It includes bone marrow involvement, nodal diameter, and β2‐microglobulin levels and was built on PFS in chemoimmunotherapy‐treated patients. A streamlined index has been proposed, the PRIMA‐PI, which uses a simplified algorithm of bone marrow involvement and β2‐microglobulin level to predict PFS [41]. Other tools to predict outcome in FL have been proposed, including total metabolic tumor volume (TMTV) as determined from baseline positron emission tomography (PET) [42], combining FLIPI‐2 with TMTV [43], and assessment of the presence of circulating peripheral blood lymphoma cells [44].
Table 1. Currently used clinical prognostic models for follicular lymphoma.
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; GELF, Groupe d'Etude des Lymphomes Folliculaires; Hb, hemoglobin; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression‐free survival; ULN, upper limit of normal.
The m7‐FLIPI, a clinicogenetic risk score derived from a combination of the mutation status of seven candidate genes (EZH2, ARID1A, MEF2B, EP300, FOXO1, CREBBP, CARD11) together with clinical parameters (FLIPI score and Eastern Cooperative Oncology Group performance status) stratifies patients into a low‐risk group (78% of patients) with a 5‐year failure‐free survival of 68% versus 25% in a high‐risk group (22% of patients) [45]. m7‐FLIPI was used to identify patients at risk of early relapse (POD24) using data from the German Low‐Grade Lymphoma Study Group trial and the British Columbia Cancer Agency population‐based registry [46]. They confirmed that 5‐year OS of patients with POD24 was significantly worse than that of patients without POD24 (26%–41% vs. 86%–91%, respectively; p < .0001) and that the m7‐FLIPI had a higher accuracy in predicting POD24 compared with FLIPI. Recently, a 23‐gene expression‐based prognostic tool was developed that again segregates patients into a low‐risk (5‐year PFS 73%) and high‐risk group (5‐year PFS 26%) [47]. Both of these tools were established and validated in retrospective cohorts of patients with FL with symptomatic, advanced stage, grade 1–3A disease requiring systemic first‐line therapy (commonly chemotherapy plus rituximab) [45], [47].
Metabolic responses to therapy have also been considered as a means of predicting subsequent outcomes in FL [7], [34], [48], [49], [50], [51], [52], [53]. For instance, end‐of‐induction PET was shown to be prognostic after first‐line FL therapy, correlating with outcome in prospective trials, retrospective analyses of prospective trials, and pooled analyses [49], [50], [51].
Metabolic responses to therapy have also been considered as a means of predicting subsequent outcomes in FL. For instance, end‐of‐induction PET was shown to be prognostic after first‐line FL therapy, correlating with outcome in prospective trials, retrospective analyses of prospective trials, and pooled analyses.
Choice of FL treatment is highly individualized, dependent on extent, burden, and progression of disease and associated symptoms; treatment decisions currently cannot be driven by available prognostic models, and work in this field is currently active. Although the current risk stratification models are predictive of outcomes, they do not have sufficient sensitivity or specificity to guide decision making and remain primarily research tools that are being examined in prospective clinical trials
FL Treatment
Newly Diagnosed Disease
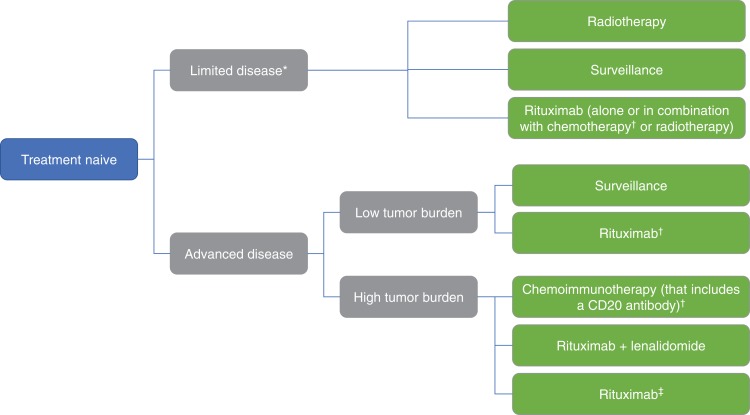
Assessment of a patient newly diagnosed with FL includes consideration of disease burden, lymphoma‐related symptoms, presence of comorbidities, patient preference, and age. Newly diagnosed FL can be broadly classified as limited or advanced‐stage disease and further classified according to degree of tumor burden; choice of management varies accordingly (Fig. 1).
Figure 1.
Treatment options in newly diagnosed follicular lymphoma. *Patients with limited disease but high tumor burden should be treated as per patients with advanced disease and high tumor burden. †With or without anti‐CD20 maintenance therapy. ‡For frail patients.
Localized/Limited‐Stage Disease.
Approximately 10%–15% of patients with FL have limited, nonbulky disease at diagnosis, which includes Ann Arbor stage I–II disease [2]. For Ann Arbor stage I or II (possibly confirmed by fluorodeoxyglucose‐PET) disease, use of 24‐Gy involved‐field radiotherapy (IFRT) administered in 12 fractions is potentially curative and is often the preferred treatment option for disease encompassed in a single appropriate radiation port, with no additional apparent efficacy benefit of higher doses [2], [12], [54]. By restricting the radiation field size to smaller volumes, IFRT limits radiation exposure to adjacent uninvolved tissue, thereby reducing risk of long‐term adverse effects [12]. A recent assessment of outcomes of definitive IFRT found that local relapses in or near irradiated fields are rare, and the vast majority of failures are distant [55]. Additionally, only about 25% of patients experience grade 1/2 and <1% have grade 3 toxicities. For palliation and local control alone, two fractions of 2 Gy may be sufficient [2], [56].
Based on experience in advanced disease, patients with mildly symptomatic, localized disease have been treated with rituximab monotherapy [2], [12], but this strategy should only be used if radiotherapy cannot be safely administered. Different rituximab treatment strategies (alone, in combination with chemotherapy, or together with radiation therapy) and comparison with radiotherapy alone in limited disease have been reported [57], [58]. Although combined modality approaches improved PFS compared with radiotherapy alone, no OS difference between treatment approaches was seen. Additionally, extrapolation from the experience in advanced‐stage disease, in which surveillance is standard, has been used as a rationale to support use of surveillance for management of limited‐stage disease in selected patients [59].
Advanced Disease.
Patients with advanced disease may be symptomatic or asymptomatic at diagnosis, and not all require immediate therapy. The Groupe d'Etude des Lymphomes Folliculaires criteria, which include tumor burden and clinical parameters, offer guidance for when treatment should be initiated (Table 1) [60].
Surveillance is routinely favored for advanced disease, with therapy typically reserved for patients with symptoms from or significant burden or rapid pace of disease [2]. For advanced disease but low tumor burden and no disease‐related symptoms, surveillance or rituximab monotherapy may be considered front‐line therapy [2], [12]. Rituximab monotherapy is a relevant consideration for such patients, particularly those who are frail and unsuitable for chemoimmunotherapy, as a notable proportion of patients (30%) with symptomatic, advanced disease treated with rituximab alone may not need further therapy for >10 years [20].
Studies have assessed the benefit of immediate rituximab treatment versus surveillance in asymptomatic, advanced‐stage disease [5], [20]. The largest showed a predictable PFS benefit of immediate rituximab therapy versus observation but no OS difference [5]. Additionally, the RESORT trial compared an extended rituximab schedule (four weekly doses followed by a single dose every 3 months until treatment failure) with a retreatment schedule (four weekly doses followed by retreatment at each disease progression) in stage III/IV and low tumor burden FL [61]. The retreatment strategy provided comparable disease control and delay of need for cytotoxic chemotherapy comparable to the extended schedule (median time to treatment failure: 3.9 vs. 4.3 years) and required cumulatively fewer rituximab doses, the latter of which is anticipated to result in benefits to the patient and health care system in terms of decreased direct and indirect costs. Importantly, this approach should only be used for patients with advanced stage but low tumor burden and not for patients who are candidates for chemoimmunotherapy.
Current standard of care (SOC) for advanced FL with high disease burden is chemoimmunotherapy regimens that include a CD20 antibody (e.g., rituximab, obinutuzumab) [2]. The addition of rituximab to combination chemotherapy regimens has resulted in improvement in clinical outcomes including objective response rates (ORR), complete responses (CR), time‐to‐progression, and OS in untreated, advanced‐stage FL compared with chemotherapy alone [62], [63], [64], [65], [66], [67], [68], [69]. Additionally, the 10‐year follow‐up of the PRIMA study showed that 2 years of rituximab maintenance therapy after first‐line chemoimmunotherapy significantly improved PFS and >50% of patients did not require second‐line treatment 10 years after chemoimmunotherapy [70], [71]. However, there continues to be no difference in OS with maintenance rituximab in this setting. Although these regimens remain SOC for first‐line FL therapy, no randomized studies have shown OS superiority of a particular chemotherapy backbone among the available options; however, the safety profiles differ between regimens [67], [72], [73], [74]. For instance, the StiL and BRIGHT trials showed that a bendamustine backbone is as effective as rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R‐CHOP) and has a better‐tolerated side‐effect profile especially with regard to hematological toxicities and infection rates [72], [73]. However, although bendamustine is favored as the chemotherapy backbone by many, there continues to be some variability in clinical practice [75], [76].
Obinutuzumab is a glycoengineered anti‐CD20 monoclonal antibody that has greater antibody‐dependent cellular toxicity and direct B‐cell killing compared with rituximab [77]. First‐line obinuzutuzumab in combination with chemotherapy was reported in the phase III GALLIUM study in indolent FL or marginal zone lymphoma [78]. Patients were randomized to induction treatment with obinutuzumab‐based or rituximab‐based chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP], cyclophosphamide, vincristine, prednisone [CVP], or bendamustine), and responding patients received ≤2 years of maintenance treatment with the same anti‐CD20 antibody received during induction. An improvement in 3‐year PFS was observed with obtinuzumab‐based therapy (80% vs. 73%; p = .001), with no difference in 3‐year OS. Post hoc analysis suggested that greater PFS benefit was seen in intermediate or high versus low FLIPI disease. ORR at the end of induction was similar in the two groups (89% vs. 87%), with PFS curves separating during maintenance therapy. Notably, obinutuzumab was associated with higher rates of infusion reactions (59% vs. 49%). Additionally, the side‐effect profile varied by chemotherapy regimen and treatment phase; bendamustine was associated with higher grade 3–5 infection and second neoplasm rates during maintenance treatment, and CHOP with higher grade 3–5 neutropenia rates during induction.
Recently, results of the RELEVANCE trial in advanced, untreated FL indicated similar efficacy with rituximab plus lenalidomide (an immunomodulatory agent) compared with rituximab plus chemotherapy [79]. As a primary objective of this study, superiority of lenalidomide in combination with rituximab versus rituximab‐based chemoimmunotherapy was not demonstrated (interim 3‐year PFS: 77% vs. 78%). Moreover, higher rates of grade 3/4 neutropenia (50% vs. 32%) and cutaneous reactions (7% vs. 1%) were observed in the lenalidomide‐containing arms. However, these results support consideration of rituximab and lenalidomide as a relevant first‐line therapeutic option for select patients.
Relapsed/Refractory Disease
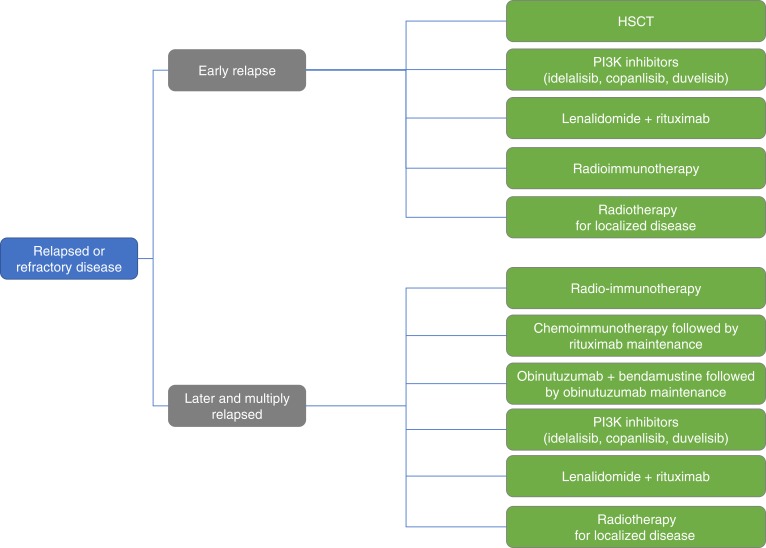
Even with therapeutic advances, FL remains largely characterized by multiple recurrences. Successive treatment lines will often be required in the disease course, and the choice of each treatment (Fig. 2) should aim to achieve disease control, promote quality of life (QoL), and minimize treatment‐related toxicity.
Figure 2.
Treatment options in relapsed or refractory follicular lymphoma.
Abbreviation: HSCT, hematopoietic stem cell transplant.
Later and Multiply Relapsed Disease.
Treatment of FL relapse or progression depends in part upon duration of remission with previous treatments [2], [12]. For patients with relapse or progression on first‐line treatment who have a longer duration of remission, options can include chemoimmunotherapy followed by rituximab maintenance.
Obinutuzumab in combination with bendamustine followed by obinutuzumab maintenance is also available for patients with rituximab‐refractory FL [80], [81]. Approval in this setting is based on the phase III GADOLIN trial of obinutuzumab plus bendamustine versus bendamustine alone in patients with CD20+ indolent NHL refractory to rituximab [82]. PFS was significantly longer with combination therapy than with bendamustine monotherapy (25.8 vs. 14.1 months; hazard ratio [HR] = 0.57; p < .001), as was OS (HR = 0.67; p = .027) [83]. Obinutuzumab in combination with bendamustine represents a validated strategy for rituximab‐refractory patients not previously treated with either agent; however, given the increased use of bendamustine as first‐line therapy, this option may be relevant to only a small patient subset.
The oral PI3K‐δ inhibitor idelalisib is included in treatment guidelines as a second‐line treatment option in relapsed/refractory FL [12], [84], although U.S. approval is for relapsed FL in patients who have received ≥2 prior systemic therapies [84]. Importantly, idelalisib in combination regimens has been associated with increased risk of serious and potentially fatal adverse events (AEs) such as colitis or pneumonitis. This led to early halting of several phase III clinical trials and a low clinical uptake of idelalisib [85].
Copanlisib is an intravenously administered pan‐class I PI3K inhibitor with higher potency against all class I isoforms than other clinically investigated PI3K inhibitors and specifically with heightened activity against the α and δ isoforms [86]. Copanlisib was licensed in the U.S. for patients with relapsed FL who have received ≥2 prior systemic therapies [87]. Accelerated approval was based on the phase II CHRONOS‐1 trial in patients with indolent BCL (73% had FL) relapsing after or refractory to ≥2 prior therapies and who had received prior treatment with rituximab and an alkylating agent [88]. After a median treatment duration of 6 months, ORR was 60.6% in the full population and 58.7% in patients with FL; overall median duration of response was 14.1 months, and median PFS was 12.5 months [89]. The safety profile differs from that of daily oral PI3K inhibitors, with low rates of liver toxicity, colitis, pneumonitis, and opportunistic infections [88], [89]. Unique toxicities associated with copanlisib included transient hypertension and hyperglyemia. Phase III trials of copanlisib in relapsed FL are ongoing, including in combination with rituximab in patients relapsing after their last rituximab‐containing therapy (NCT02367040), and in combination with chemoimmunotherapy (R‐CHOP or bendamustine, rituximab [BR]; NCT02626455) [90].
Duvelisib, an oral PI3K inhibitor targeting both the δ and γ catalytic subunits, recently gained U.S. approval for relapsed/refractory indolent BCL including FL after ≥2 prior lines of therapy based on results of the phase II DYNAMO trial [91], [92]. ORR was 46% and median PFS and OS were 8.4 and 18.4 months, respectively, among patients refractory to both rituximab and alkylating chemotherapy [92]. Grade ≥ 3 AEs were seen, including neutropenia (28%), diarrhea (15%), and infection (20%); 5% of patients had a fatal AE.
Early encouraging reports are emerging from the phase III AUGMENT trial (NCT01938001), comparing rituximab and lenalidomide (“R2”) with rituximab monotherapy in patients with indolent NHL who were previously treated and had rituximab‐sensitve relapsed disease [93]. R2 was recently reported to achieve improved PFS (39.4 vs. 14.1 months) and ORR (78% vs. 53%; both p < .0001) compared with rituximab alone, although no significant OS benefit is yet seen at a median follow‐up of 28.3 months.
Radioimmunotherapy (RIT) regimens, in which radioisotopes are conjugated to antibodies (commonly, anti‐CD20 agents), have been used for the treatment of relapsed/refractory FL [2], [94]. Response rates of approximately 60% and median PFS and OS of >4 years have been reported with RIT in patients who have received ≥1 prior therapy, with best responders those with minimal bone marrow involvement and low tumor burden [95], [96]. RIT provides a valuable less toxic alternative for a select group of patients, although complexities in delivery of this treatment and availability of numerous competing agents have limited its widespread adoption; currently, ibirtumomab tiuxetan is the only RIT remaining on the market for FL treatment [97].
Early Relapse.
Patients with FL experiencing early relapse after initial therapy are at high risk of poor outcomes. Currently, no single SOC exists for patients with POD24, and therapeutic approaches are generally intensification with standard agents or use of agents with novel mechanisms of action compared with front‐line therapy.
High‐dose chemotherapy with autologous hematopoietic stem cell transplantation (auto‐HSCT) can prolong PFS and OS and should be considered as consolidative therapy in early relapse [2], [12], [98]. Few prospective data are available regarding HSCT in the era of modern therapies, and its role continues to be elucidated [2], [12]. Retrospective data suggest that patients with POD24 benefit from auto‐HSCT (i.e., increased PFS and OS compared with those not receiving transplant) [98], [99], [100], [101]. A recent study compared auto‐HSCT with either matched‐sibling donor (MSD) or unrelated matched donor (UMD) allogeneic HSCT (allo‐HSCT) in patients with POD24 [102]. Findings suggest that outcomes are similar with either autologous or allogeneic transplant with MSD, whereas outcomes with UMD transplant were inferior, largely because of higher transplant‐related mortality. Currently, allo‐HSCT is largely restricted to fit patients who cannot achieve complete remission or have multiply relapsed disease.
In high‐risk FL and early relapse after initial chemoimmunotherapy, idelalisib was studied in a retrospective analysis of patients with POD24, with 59% of patients achieving lymph‐node response with idelalisib. The ORR was 57% and did not differ significantly whether patients had refractory or early relapsing disease (within 12 vs. 12–24 months) after initial therapy.
Data in early relapse are emerging for other agents and are particularly important for transplant‐ineligible patients. In high‐risk FL and early relapse after initial chemoimmunotherapy, idelalisib was studied in a retrospective analysis of patients with POD24, with 59% of patients achieving lymph‐node response with idelalisib [103]. The ORR was 57% and did not differ significantly whether patients had refractory or early relapsing disease (within 12 vs. 12–24 months) after initial therapy. Lenalidomide is being investigated in the phase III MAGNIFY trial in combination with rituximab in relapsed/refractory NHL, including FL [104]. In patients with POD24, the ORR was 48% compared with an ORR of 67% in all patients with FL included in the study; 1‐year PFS was 45% and 66%, respectively. Recently, exploratory assessments from the CHRONOS‐1 trial of copanlisib in patients with relapsed/refractory NHL who had received ≥2 prior therapies suggested that ORR was similar between patients with FL with and without POD24 (60.3% vs. 58.8%) [105]. Additionally, the ongoing phase II SWOG 1608 study is evaluating obinutuzumab‐based therapeutic combinations in early‐relapsing/refractory FL.
Investigational Therapies
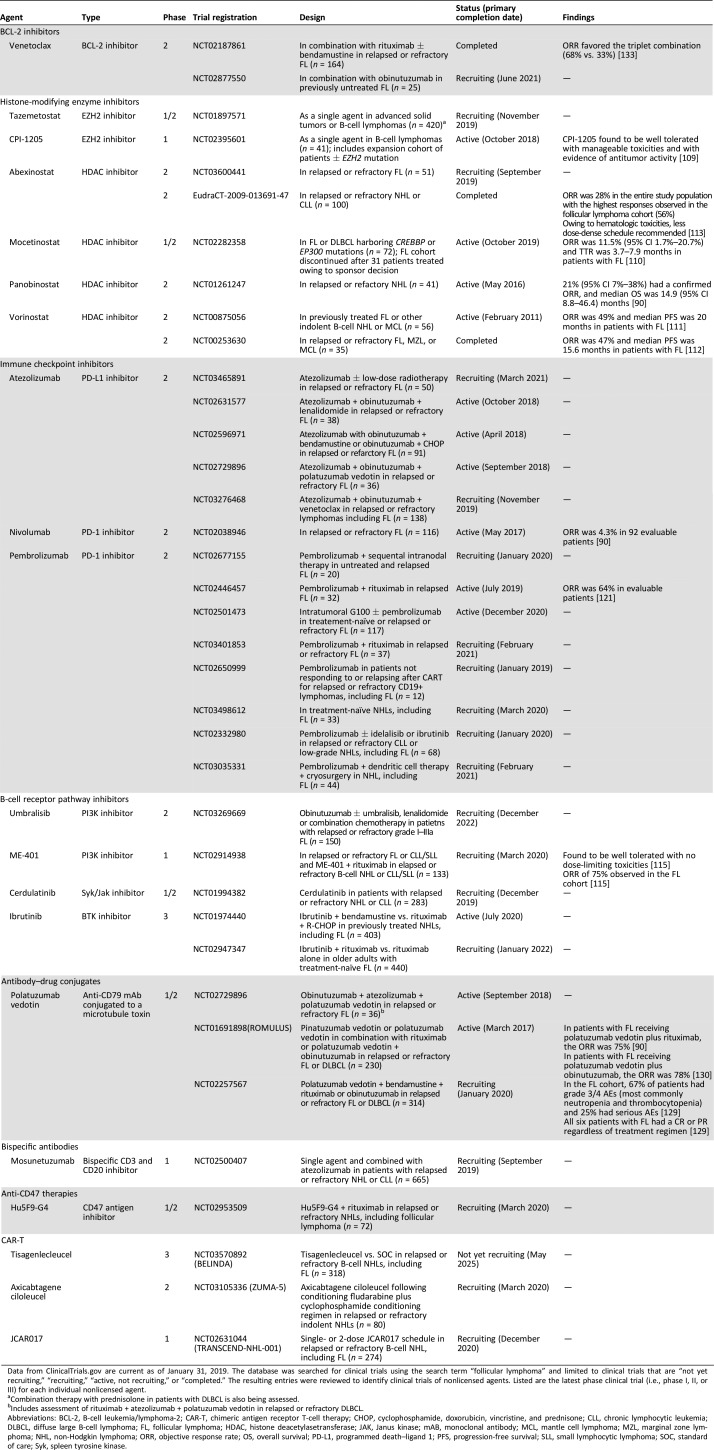
The armamentarium of treatment options is continually expanding, with numerous novel therapies attempting to exploit specific biological vulnerabilities of FL. Enrollment in clinical trials evaluating emerging therapies remains a high priority for patients with relapsed/refractory FL requiring treatment, especially those who are refractory to both rituximab and alkylating agents (double refractory). The following section summarizes some of these emerging therapies (Table 2).
Table 2. Select clinical trials of investigational therapies in follicular lymphoma.
Data from ClinicalTrials.gov are current as of January 31, 2019. The database was searched for clinical trials using the search term “follicular lymphoma” and limited to clinical trials that are “not yet recruiting,” “recruiting,” “active, not recruiting,” or “completed.” The resulting entries were reviewed to identify clinical trials of nonlicensed agents. Listed are the latest phase clinical trial (i.e., phase I, II, or III) for each individual nonlicensed agent.
Combination therapy with prednisolone in patients with DLBCL is also being assessed.
Includes assessment of rituximab + atezolizumab + polatuzumab vedotin in relapsed or refractory DLBCL.
Abbreviations: BCL‐2, B‐cell leukemia/lymphoma‐2; CAR‐T, chimeric antigen receptor T‐cell therapy; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; HDAC, histone deacetylasetransferase; JAK, Janus kinase; mAB, monoclonal antibody; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non‐Hodgkin lymphoma; ORR, objective response rate; OS, overall survival; PD‐L1, programmed death–ligand 1; PFS, progression‐free survival; SLL, small lymphocytic lymphoma; SOC, standard of care; Syk, spleen tyrosine kinase.
Drugs Targeting Epigenetic Modification
A genetic hallmark of FL is the presence of mutations in histone‐modifying enzymes [106]. The gene for one such enzyme, EZH2, is mutated in about 25% of cases [107]. Oral inhibitors of EZH2 (e.g., tazemetostat, CPI‐1205) are being examined in phase II studies in FL, with early analyses suggesting high response rates in EZH2‐mutated disease (ORR 82% for tazemetostat) [108], [109]. Several histone deacetylase inhibitors are also undergoing clinical investigation in FL to address loss of histone acetylation associated with CREBBP and EP300 mutations, including phase II trials with panobinostat [90], mocetinostat [110], vorinostat [110], [111], [112], and abexinostat [113].
Drugs Targeting Signaling Pathways
Chronic activation of B‐cell receptor (BCR) signaling is critical in FL progression, leading to activation of several signal transduction pathways (e.g., NF‐kB, PI3K‐AKT) promoting proliferation and survival of malignant B cells [106]. Mutations in genes encoding proteins in BCR pathways are also common [23], [24], [29], [30], [31]. In addition to PI3K already described, new PI3K inhibitors are under development in FL, including umbralisib (which also targets casein kinase‐1ε) [114] and ME‐401 [115].
Mutations in the BCR and NF‐κB signaling pathway can occur in patients with FL, affecting the caspase recruitment domain‐containing protein 11 (CARD11) commonly and Bruton tyrosine kinase (BTK) infrequently [116]. Ibrutinib is a small‐molecule BTK inhibitor with effects on apoptosis, cellular adhesion, migration, and the tumor microenvironment [117]. In the phase II DAWN trial in relapsed/refractory FL, single‐agent ibrutinib did not meet the lower bound ORR threshold for the primary endpoint (ORR 20.9%) [118]. Similar low ORR was reported in another phase II trial of ibrutinib in recurrent FL (37.5%), although interestingly, CARD11 mutations predicted resistance to ibrutinib [119]. Given limited monotherapy activity, combination ibrutinib regimens in FL are being investigated [90].
Drugs Targeting the Immune System
The programmed cell death‐1 (PD‐1) protein is widely expressed and found on activated T cells, B cells, and natural killer (NK) cells [120]. PD‐1 binding to programmed death ligand‐1 and ligand‐2 produces inhibitory signals leading to T‐cell apoptosis. Additionally, PD‐1 inhibits antitumor cytotoxicity through decreased NK cell‐mediated killing. Inhibitors of the PD‐1 pathway (i.e., immune checkpoint inhibitors) are undergoing clinical investigation in FL, including pembrolizumab [121], nivolumab [90], and atezolizumab [122]. Although single‐agent activity appears limited in FL [123], there may be synergy with other agents, including CD20 antibodies [124].
CD47 is a ubiquitously expressed penta‐transmembrane domain immunoglobulin‐like protein that associates with integrins and is a receptor for thrombospondin, an extracellular matrix protein [125]. CD47 is also involved in the macrophage interaction that inhibits phagocytosis, and is involved in several biologic processes (e.g., motility, adhesion, and migration of leukocytes, phagocytosis, recognition of “self”). Hu5F9‐G4 is a humanized antibody targeting CD47 that stimulates tumor phagocytosis and antitumor responses [126], and results in a phase I/IIb trial in relapsed/refactory FL have recently been reported (ORR and CR of 71% and 43%, respectively, in the seven patients in the FL cohort) [127].
Antibody‐drug conjugates, designed to provide targeted delivery of cytotoxic agents to antigen‐expressing tumor cells, are also being investigated in FL [128]. Phase I/II trials suggest polatuzumab vedotin is active in FL in combination with rituximab (ORR 75.0%) [90], in combination with rituximab and bendamustine (ORR 100%) [129], and in combination with obinutuzumab (ORR 78%) [130].
Another strategy for immune‐based therapy is development of biphenotypic antibodies that are able to bind to malignant lymphocytes as well as engage and activate cytotoxic T cells [90], [129]. Mosunetuzumab is one such biphenotypic antibody, with binding domains for both CD20 and CD3, and evaluation of the agent in FL is ongoing [131]. Additionally, tisagenlecleucel, an anti‐CD19 chimeric antigen receptor (CAR) T‐cell therapy in which autologous T cells are gene‐modified to carry antigen specificity for CD19, is licensed for the treatment of relapsed/refractory high‐grade B‐cell lymphoma and DLBCL arising from FL based on results from a single‐arm clinical trial [132]. Tisagenlecleucel is under investigation in a phase III trial in relapsed/refractory FL [90], [117]. Other CAR‐T therapies undergoing clinical investigation in FL include axicabtagene ciloleucel and JCAR017.
Remaining Unmet Needs
The protracted multiply relapsing clinical course of FL presents a fundamental challenge for how clinicians should best balance treatment efficacy, minimizing toxicity and preserving QoL.
Although the armamentarium of FL therapies has expanded, the optimal approach to selecting and sequencing treatments for an individual patient continues to be elucidated (Table 3).
Although several risk stratification tools exist with new models being developed, we continue to lack a clinically useful tool that can accurately identify patients with high‐risk disease who may benefit from individualized management to achieve longer remission, prevent transformation or resistance, and thereby further improve clinical outcomes.
Table 3. Unmet needs in follicular lymphoma.
Abbreviation: SOC, standard of care.
Conclusion
Significant strides have been made in outcomes for patients with FL. Our next priorities must tackle the subsets of patients that are early progressors or multiply relapsed by defining optimum strategies to improve survival. Successfully achieving this will require improved prognostication, understanding and integration of the disease biology, and delineating molecular determinants of response and resistance to existing and emergent therapies. Most notably, POD24 has been shown to be a powerful predictor of poor outcome, although it is not clear if it can become a standard surrogate endpoint to evaluate efficacy of investigational treatments. Finally, current FL treatment strategies are based on a “one size fits all” approach; specific genetic and epigenetic aberrations in an individual patient are not currently accounted for in their management. No genomic studies can be recommended currently with sufficient validation, although this is an area of ongoing investigation if we can identify biomarkers correlated with predictive or prognostic value. In the future, a personalized approach to treatment could help determine the most appropriate treatment for an individual patient based on specific patient, clinical, genetic, and epigenetic factors with our improved ability to marry disease biology to therapy.
Acknowledgments
This review article was written on behalf of LYMPHOMA CONNECT; for more information, please visit www.lymphomaconnect.info. LYMPHOMA CONNECT is supported by an Independent Educational Grant from Bayer. Bayer did not have a critical role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review and approval of the manuscript; or decision to submit the manuscript for publication.We thank Dr. Georg Lenz for his helpful comments in the preparation of this review. Medical writing assistance was provided by Tricia Newell, Ph.D., and Mark English, Ph.D., of COR2ED, Basel, Switzerland.
Author Contributions
Conception/design: Matthew J. Matasar, Stefano Luminari, Paul M. Barr, Stefan K. Barta, Alexey V. Danilov, Brian T. Hill, Tycel J. Phillips, Mats Jerkeman, Massimo Magagnoli, Loretta J. Nastoupil, Daniel O. Persky, Jessica Okosun
Manuscript writing: Matthew J. Matasar, Stefano Luminari, Paul M. Barr, Stefan K. Barta, Alexey V. Danilov, Brian T. Hill, Tycel J. Phillips, Mats Jerkeman, Massimo Magagnoli, Loretta J. Nastoupil, Daniel O. Persky, Jessica Okosun
Final approval of manuscript: Matthew J. Matasar, Stefano Luminari, Paul M. Barr, Stefan K. Barta, Alexey V. Danilov, Brian T. Hill, Tycel J. Phillips, Mats Jerkeman, Massimo Magagnoli, Loretta J. Nastoupil, Daniel O. Persky, Jessica Okosun
Disclosures
Matthew J. Matasar: Genentech, Roche, Bayer, Spectrum, Seattle Genetics, GlaxoSmithKline, Pharmacyclics (RF), Genentech, Roche, Jannsen, Bayer, Spectrum, Seattle Genetics, Rocket (H); Stefano Luminari: Roche, Celgene, Sandoz, Servier, Gilead (SAB); Paul M. Barr: Pharmacyclics/AbbVie, Gilead, Celgene, Seattle Genetics, Merck, Genentech, Janssen, Verastem, TG Therapeutics (C/A); Stefan K. Barta: Celgene, Merck, Seattle Genetics, Bayer, Takeda (RF), Janssen (H), Curis (travel support); Alexey V. Danilov: Takeda Oncology, Gilead Sciences, Verastem, TG Therapeutics, Bayer (RF), Genentech, Bristol‐Myers Squibb, AstraZeneca, Verastem, Gilead Sciences, Bayer, Celgene, Abbvie, TG Therapeutics, Teva Oncology, Pharmacyclics, Juno Therapeutics (H); Brian T. Hill: Kite, Genentech, Abbvie, Celgene, Takeda (RF); Gilead, Celgene, Genentech, Abbvie, Bayer, Pharmacyclics (H); Tycel J. Phillips: Abbvie, Pharmacyclics, Bayer (RF), Incyte, Pharmacyclics, Genentech, Gilead, Bayer, Seattle Genetics (H); Mats Jerkeman: Janssen, Celgene, Abbvie, Gilead (RF), Janssen, Gilead, Celgene, Abbvie (H); Massimo Magagnoli: AstraZeneca, Sanofi (H); Loretta J. Nastoupil: Bayer, Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, TG Therapeutics (H); Daniel O. Persky: Bayer, Debiopharm, Morphosys (C/A), Merck (RF); Jessica Okosun: Gilead Sciences, Epizyme (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Teras LR, DeSantis CE, Cerhan JR et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–459. [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Ghielmini M, Rule S et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27(suppl 5):v83–v90. [DOI] [PubMed] [Google Scholar]
- 3.Smith A, Crouch S, Lax S et al. Lymphoma incidence, survival and prevalence 2004‐2014: Sub‐type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer 2015;112:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conconi A, Lobetti‐Bodoni C, Montoto S et al. Life expectancy of young adults with follicular lymphoma. Ann Oncol 2015;26:2317–2322. [DOI] [PubMed] [Google Scholar]
- 5.Ardeshna KM, Qian W, Smith P et al. Rituximab versus a watch‐and‐wait approach in patients with advanced‐stage, asymptomatic, non‐bulky follicular lymphoma: An open‐label randomised phase 3 trial. Lancet Oncol 2014;15:424–435. [DOI] [PubMed] [Google Scholar]
- 6.Soumerai JD, Bantilan KS, Ni A et al. Intervention versus observation: What is the appropriate endpoint? Assessment of endpoints in patients with advanced stage follicular lymphoma who are initially observed. Blood 2016;128:1777–1777.27688782 [Google Scholar]
- 7.Casulo C, Byrtek M, Dawson KL et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National Lymphocare Study. J Clin Oncol 2015;33:2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow SH, Campo E, Harris NL et al. WHO classification of tumours of haematopoietic and lymphoid tissues In: Bosman FT, Jaffe ES, Lakhani SR. et al., eds. World Health Organization Classification of Tumours. Lyon, France: IARC, 2008. [Google Scholar]
- 9.Rimsza LM, Li H, Braziel RM et al. Impact of histological grading on survival in the SWOG S0016 follicular lymphoma cohort. Haematologica 2018;103:e151–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott G, Katzenberger T, Lohr A et al. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood 2002;99:3806–3812. [DOI] [PubMed] [Google Scholar]
- 11.Bosga‐Bouwer AG, van Imhoff GW, Boonstra R et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: T(14;18) and 3q27 are mutually exclusive. Blood 2003;101:1149–1154. [DOI] [PubMed] [Google Scholar]
- 12.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). B‐cell lymphomas (version 4.2018), 2018.
- 13.Swerdlow SH, Campo E, Pileri SA et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellmuth JC, Louissaint A Jr, Szczepanowski M et al. Duodenal type and nodal follicular lymphoma differ by their immune microenvironment rather than their mutation profiles. Blood 2018;132:1695–1702. [DOI] [PubMed] [Google Scholar]
- 15.Freedman AS. Biology and management of histologic transformation of indolent lymphoma. Hematology Am Soc Hematol Educ Program 2005:314–320. [DOI] [PubMed] [Google Scholar]
- 16.Montoto S, Davies AJ, Matthews J et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B‐cell lymphoma. J Clin Oncol 2007;25:2426–2433. [DOI] [PubMed] [Google Scholar]
- 17.Al‐Tourah AJ, Gill KK, Chhanabhai M et al. Population‐based analysis of incidence and outcome of transformed non‐Hodgkin's lymphoma. J Clin Oncol 2008;26:5165–5169. [DOI] [PubMed] [Google Scholar]
- 18.Wagner‐Johnston ND, Link BK, Byrtek M et al. Outcomes of transformed follicular lymphoma in the modern era: A report from the National Lymphocare Study (NLCS). Blood 2015;126:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Federico M, Caballero Barrigon MD, Marcheselli L et al. Rituximab and the risk of transformation of follicular lymphoma: A retrospective pooled analysis. Lancet Haematol 2018;5:e359–e367. [DOI] [PubMed] [Google Scholar]
- 20.Lockmer S, Ostenstad B, Hagberg H et al. Chemotherapy‐free initial treatment of advanced indolent lymphoma has durable effect with low toxicity: Results from two Nordic Lymphoma Group trials with more than 10 years of follow‐up. J Clin Oncol 2018:JCO1800262. [DOI] [PubMed] [Google Scholar]
- 21.Morin RD, Mendez‐Lago M, Mungall AJ et al. Frequent mutation of histone‐modifying genes in non‐Hodgkin lymphoma. Nature 2011;476:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasqualucci L, Dominguez‐Sola D, Chiarenza A et al. Inactivating mutations of acetyltransferase genes in B‐cell lymphoma. Nature 2011;471:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okosun J, Bodor C, Wang J et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 2014;46:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green MR, Kihira S, Liu CL et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A 2015;112:E1116–E1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung KJ, Johnson NA, Affleck JG et al. Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res 2010;70:9166–9174. [DOI] [PubMed] [Google Scholar]
- 26.Okosun J, Wolfson RL, Wang J et al. Recurrent mTORC1‐activating RRAGC mutations in follicular lymphoma. Nat Genet 2016;48:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildiz M, Li H, Bernard D et al. Activating STAT6 mutations in follicular lymphoma. Blood 2015;125:668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huet S, Xerri L, Tesson B et al. EZH2 alterations in follicular lymphoma: Biological and clinical correlations. Blood Cancer J 2017;7:e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green MR, Gentles AJ, Nair RV et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 2013;121:1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasqualucci L, Khiabanian H, Fangazio M et al. Genetics of follicular lymphoma transformation. Cell Rep 2014;6:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kridel R, Chan FC, Mottok A et al. Histological transformation and progression in follicular lymphoma: A clonal evolution study. PLoS Med 2016;13:e1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araf S, Wang J, Korfi K et al. Genomic profiling reveals spatial intra‐tumor heterogeneity in follicular lymphoma. Leukemia 2018;32:1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dave SS, Wright G, Tan B et al. Prediction of survival in follicular lymphoma based on molecular features of tumor‐infiltrating immune cells. N Engl J Med 2004;351:2159–2169. [DOI] [PubMed] [Google Scholar]
- 34.Maurer MJ, Jais JP, Ghesquieres H et al. Personalized risk prediction for event‐free survival at 24 months in patients with diffuse large B‐cell lymphoma. Am J Hematol 2016;91:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seymour JF, Marcus R, Davies A et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: Benefit of obinutuzumab in reducing the rate of early progression. Haematologica 2019;104:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer MJ, Bachy E, Ghesquieres H et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol 2016;91:1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solal‐Celigny P, Roy P, Colombat P et al. Follicular Lymphoma International Prognostic Index. Blood 2004;104:1258–1265. [DOI] [PubMed] [Google Scholar]
- 38.Buske C, Hoster E, Dreyling M et al. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high‐risk from intermediate‐ or low‐risk patients with advanced‐stage follicular lymphoma treated front‐line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) with respect to treatment outcome. Blood 2006;108:1504–1508. [DOI] [PubMed] [Google Scholar]
- 39.Nooka AK, Nabhan C, Zhou X et al. Examination of the Follicular Lymphoma International Prognostic Index (FLIPI) in the National Lymphocare Study (NLCS): A prospective US patient cohort treated predominantly in community practices. Ann Oncol 2013;24:441–448. [DOI] [PubMed] [Google Scholar]
- 40.Federico M, Bellei M, Marcheselli L et al. Follicular Lymphoma International Prognostic Index 2: A new prognostic index for follicular lymphoma developed by the International Follicular Lymphoma Prognostic Factor Project. J Clin Oncol 2009;27:4555–4562. [DOI] [PubMed] [Google Scholar]
- 41.Bachy E, Maurer MJ, Habermann TM et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood 2018;132:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meignan M, Cottereau AS, Versari A et al. Baseline metabolic tumor volume predicts outcome in high‐tumor‐burden follicular lymphoma: A pooled analysis of three multicenter studies. J Clin Oncol 2016;34:3618–3626. [DOI] [PubMed] [Google Scholar]
- 43.Cottereau AS, Versari A, Luminari S et al. Prognostic model for high‐tumor‐burden follicular lymphoma integrating baseline and end‐induction PET: A LYSA/FIL study. Blood 2018;131:2449–2453. [DOI] [PubMed] [Google Scholar]
- 44.Sarkozy C, Baseggio L, Feugier P et al. Peripheral blood involvement in patients with follicular lymphoma: A rare disease manifestation associated with poor prognosis. Br J Haematol 2014;164:659–667. [DOI] [PubMed] [Google Scholar]
- 45.Pastore A, Jurinovic V, Kridel R et al. Integration of gene mutations in risk prognostication for patients receiving first‐line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population‐based registry. Lancet Oncol 2015;16:1111–1122. [DOI] [PubMed] [Google Scholar]
- 46.Jurinovic V, Kridel R, Staiger AM et al. Clinicogenetic risk models predict early progression of follicular lymphoma after first‐line immunochemotherapy. Blood 2016;128:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huet S, Tesson B, Jais JP et al. A gene‐expression profiling score for prediction of outcome in patients with follicular lymphoma: A retrospective training and validation analysis in three international cohorts. Lancet Oncol 2018;19:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ladetto M, De Marco F, Benedetti F et al. Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R‐HDS) versus conventional (CHOP‐R) chemoimmunotherapy in high‐risk follicular lymphoma at diagnosis: The superior disease control of R‐HDS does not translate into an overall survival advantage. Blood 2008;111:4004–4013. [DOI] [PubMed] [Google Scholar]
- 49.Trotman J, Luminari S, Boussetta S et al. Prognostic value of PET‐CT after first‐line therapy in patients with follicular lymphoma: A pooled analysis of central scan review in three multicentre studies. Lancet Haematol 2014;1:e17–e27. [DOI] [PubMed] [Google Scholar]
- 50.Trotman J, Barrington SF, Belada D et al. Prognostic value of end‐of‐induction PET response after first‐line immunochemotherapy for follicular lymphoma (GALLIUM): Secondary analysis of a randomised, phase 3 trial. Lancet Oncol 2018;19:1530–1542. [DOI] [PubMed] [Google Scholar]
- 51.Trotman J, Fournier M, Lamy T et al. Positron emission tomography‐computed tomography (PET‐CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: Analysis of PET‐CT in a subset of PRIMA trial participants. J Clin Oncol 2011;29:3194–3200. [DOI] [PubMed] [Google Scholar]
- 52.Launonen A, Hiddemann W, Duenzinger U et al. Early disease progression predicts poorer survival in patients with follicular lymphoma (FL) in the GALLIUM study. Blood 2017;130:1490–1490. [Google Scholar]
- 53.Luminari S, Galimberti S, Versari A et al. Positron emission tomography response and minimal residual disease impact on progression‐free survival in patients with follicular lymphoma. A subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Haematologica 2016;101:e66–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowry L, Smith P, Qian W et al. Reduced dose radiotherapy for local control in non‐Hodgkin lymphoma: A randomised phase III trial. Radiother Oncol 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- 55.Brady JL, Binkley MS, Hajj C et al. Outcome of curative radiotherapy for localised follicular lymphoma in the era of 18 F‐FDG PET‐CT staging: An international collaborative study on behalf of ILROG. Hematol Oncol 2017;35:29–31. [Google Scholar]
- 56.Hoskin PJ, Kirkwood AA, Popova B et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): A randomised phase 3 non‐inferiority trial. Lancet Oncol 2014;15:457–463. [DOI] [PubMed] [Google Scholar]
- 57.Friedberg JW, Byrtek M, Link BK et al. Effectiveness of first‐line management strategies for stage I follicular lymphoma: Analysis of the National Lymphocare Study. J Clin Oncol 2012;30:3368–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mondello P, Steiner N, Wasle I et al. Radiotherapy for stage I/II follicular lymphoma (FL): Is it time for a re‐appraisal? Anticancer Res 2014;34:6701–6704. [PubMed] [Google Scholar]
- 59.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non‐Hodgkin's lymphoma: Long‐term follow‐up of no initial therapy. J Clin Oncol 2004;22:1454–1459. [DOI] [PubMed] [Google Scholar]
- 60.Brice P, Bastion Y, Lepage E et al. Comparison in low‐tumor‐burden follicular lymphomas between an initial no‐treatment policy, prednimustine, or interferon alfa: A randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 1997;15:1110–1117. [DOI] [PubMed] [Google Scholar]
- 61.Kahl BS, Hong F, Williams ME et al. Rituximab extended schedule or re‐treatment trial for low‐tumor burden follicular lymphoma: Eastern Cooperative Oncology Group protocol e4402. J Clin Oncol 2014;32:3096–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiddemann W, Kneba M, Dreyling M et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with chop alone: Results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood 2005;106:3725–3732. [DOI] [PubMed] [Google Scholar]
- 63.Marcus R, Imrie K, Belch A et al. CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood 2005;105:1417–1423. [DOI] [PubMed] [Google Scholar]
- 64.Marcus R, Imrie K, Solal‐Celigny P et al. Phase III study of R‐CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008;26:4579–4586. [DOI] [PubMed] [Google Scholar]
- 65.Herold M, Haas A, Srock S et al. Rituximab added to first‐line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group hematology and oncology study. J Clin Oncol 2007;25:1986–1992. [DOI] [PubMed] [Google Scholar]
- 66.Bachy E, Houot R, Morschhauser F et al. Long‐term follow up of the FL2000 study comparing CHVP‐interferon to CHVP‐interferon plus rituximab in follicular lymphoma. Haematologica 2013;98:1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luminari S, Ferrari A, Manni M et al. Long‐term results of the FOLL05 trial comparing R‐CVP versus R‐CHOP versus R‐FM for the initial treatment of patients with advanced‐stage symptomatic follicular lymphoma. J Clin Oncol 2018;36:689–696. [DOI] [PubMed] [Google Scholar]
- 68.Schulz H, Bohlius JF, Trelle S et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: A systematic review and meta‐analysis. J Natl Cancer Inst 2007;99:706–714. [DOI] [PubMed] [Google Scholar]
- 69.Salles G, Mounier N, de Guibert S et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA‐GOELAMS FL2000 study. Blood 2008;112:4824–4831. [DOI] [PubMed] [Google Scholar]
- 70.Salles G, Seymour JF, Offner F et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2011;377:42–51. [DOI] [PubMed] [Google Scholar]
- 71.Salles GA, Seymour JF, Feugier P et al. Long term follow‐up of the PRIMA study: Half of patients receiving rituximab maintenance remain progression free at 10 years. Blood 2017;130:486–486. [Google Scholar]
- 72.Rummel MJ, Niederle N, Maschmeyer G et al. Bendamustine plus rituximab versus chop plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: An open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet 2013;381:1203–1210. [DOI] [PubMed] [Google Scholar]
- 73.Flinn IW, van der Jagt R, Kahl BS et al. Randomized trial of bendamustine‐rituximab or R‐CHOP/R‐CVP in first‐line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014;123:2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Federico M, Luminari S, Dondi A et al. R‐CVP versus R‐CHOP versus R‐FM for the initial treatment of patients with advanced‐stage follicular lymphoma: Results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 2013;31:1506–1513. [DOI] [PubMed] [Google Scholar]
- 75.Friedberg JW, Taylor MD, Cerhan JR et al. Follicular lymphoma in the United States: First report of the National Lymphocare Study. J Clin Oncol 2009;27:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin P, Byrtek M, Dawson K et al. Patterns of delivery of chemoimmunotherapy to patients with follicular lymphoma in the United States: Results of the National Lymphocare Study. Cancer 2013;119:4129–4136. [DOI] [PubMed] [Google Scholar]
- 77.Golay J, Da Roit F, Bologna L et al. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 2013;122:3482–3491. [DOI] [PubMed] [Google Scholar]
- 78.Marcus R, Davies A, Ando K et al. Obinutuzumab for the first‐line treatment of follicular lymphoma. N Engl J Med 2017;377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 79.Morschhauser F, Fowler NH, Feugier P et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med 2018;379:934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gazyva: Obinutuzumab. South San Francisco, CA: Genetech, Inc, 2016.
- 81.Gazyvaro: obinutuzumab. Grenzach‐Wyhle, Germany: Roche Registration GmbH, 2018.
- 82.Sehn LH, Chua N, Mayer J et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab‐refractory indolent non‐hodgkin lymphoma (GADOLIN): A randomised, controlled, open‐label, multicentre, phase 3 trial. Lancet Oncol 2016;17:1081–1093. [DOI] [PubMed] [Google Scholar]
- 83.Cheson BD, Chua N, Mayer J et al. Overall survival benefit in patients with rituximab‐refractory indolent non‐Hodgkin lymphoma who received obinutuzumab plus bendamustine induction and obinutuzumab maintenance in the GADOLIN study. J Clin Oncol 2018;36:2259–2266. [DOI] [PubMed] [Google Scholar]
- 84.Zydelig: idelalisib. Foster City, CA: Gilead Sciences, Inc, 2018.
- 85.The Lancet Haematology. Straying from the target . Lancet Haematol 2016;3:e205. [DOI] [PubMed] [Google Scholar]
- 86.Krause G, Hassenruck F, Hallek M. Copanlisib for treatment of B‐cell malignancies: The development of a PI3K inhibitor with considerable differences to idelalisib. Drug Des Devel Ther 2018;12:2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aliqopa: copanlisib. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc, 2017.
- 88.Dreyling M, Santoro A, Mollica L et al. Copanlisib in patients with relapsed or refractory indolent B‐cell lymphoma (CHRONOS‐1). Hematol Oncol 2017;35:119–120. [Google Scholar]
- 89.Dreyling M, Santoro A, Mollica L et al. Long‐term efficacy and safety from the copanlisib CHRONOS‐1 study in patients with relapsed or refractory indolent B‐cell lymphoma. Presented at: American Society of Hematology Annual Meeting; 2018; San Diego.
- 90.US National Library of Medicine. Clinicaltrials.Gov. 2018. Available at https://clinicaltrials.gov. Accessed September 29, 2018.
- 91.Copiktra: Duvelisib. Needham, MA: Verastem, Inc, 2018.
- 92.Flinn IW, Miller CB, Ardeshna KM et al. Dynamo: A phase 2 study demonstrating the clinical activity of duvelisib in patients with relapsed refractory indolent non‐Hodgkin lymphoma. Blood 2016;128:1218–1218. [Google Scholar]
- 93.Leonard JP, Trneny M, Izutsu K et al. AUGMENT: A phase III randomized study of lenalidomide plus rituximab (R2) vs rituximab/placebo in patients with relapsed/refractory indolent non‐Hodgkin lymphoma. Presented at: American Society of Hematology Annual Meeting; 2018; San Diego.
- 94.Cheson BD. Radioimmunotherapy of non‐Hodgkin lymphomas. Blood 2003;101:391–398. [DOI] [PubMed] [Google Scholar]
- 95.Fisher RI, Kaminski MS, Wahl RL et al. Tositumomab and iodine‐131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low‐grade and transformed non‐Hodgkin's lymphomas. J Clin Oncol 2005;23:7565–7573. [DOI] [PubMed] [Google Scholar]
- 96.Witzig TE, Molina A, Gordon LI et al. Long‐term responses in patients with recurring or refractory B‐cell non‐Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer 2007;109:1804–1810. [DOI] [PubMed] [Google Scholar]
- 97.Zevalin: Ibritumomab tiuxetan. Irvine, CA: Spectrum Pharmaceuticals, Inc, 2013.
- 98.Casulo C, Friedberg JW, Ahn KW et al. Autologous transplantation in follicular lymphoma with early therapy failure: A National Lymphocare Study and Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant 2018;24:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jurinovic V, Metzner B, Pfreundschuh M et al. Autologous stem cell transplantation for patients with early progression of follicular lymphoma‐ retrospective analysis of 2 randomized trials of the German Low Grade Lymphoma Study Group. Blood 2016;128:3464–3464. [DOI] [PubMed] [Google Scholar]
- 100.Jurinovic V, Metzner B, Pfreundschuh M et al. Autologous stem cell transplantation for patients with early progression of follicular lymphoma: A follow‐up study of 2 randomized trials from the German Low Grade Lymphoma Study Group. Biol Blood Marrow Transplant 2018;24:1172–1179. [DOI] [PubMed] [Google Scholar]
- 101.Jimenez‐Ubieto A, Grande C, Caballero D et al. Autologous stem cell transplantation may be curative for patients with follicular lymphoma with early therapy failure who reach complete response after rescue treatment. Hematol Oncol 2018;36:765–772. [DOI] [PubMed] [Google Scholar]
- 102.Smith SM, Godfrey J, Ahn KW et al. Autologous transplantation versus allogeneic transplantation in patients with follicular lymphoma experiencing early treatment failure. Cancer 2018;124:2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gopal AK, Kahl BS, Flowers CR et al. Idelalisib is effective in patients with high‐risk follicular lymphoma and early relapse after initial chemoimmunotherapy. Blood 2017;129:3037–3039. [DOI] [PubMed] [Google Scholar]
- 104.Andorsky DJ, Yacoub A, Melear JM et al. Phase IIIb randomized study of lenalidomide plus rituximab (R2) followed by maintenance in relapsed/refractory NHL: Analysis of patients with double‐refractory or early relapsed follicular lymphoma (FL). J Clin Oncol 2017;35:7502–7502. [Google Scholar]
- 105.Santoro A, Mollica L, Leppa S et al. Outcomes for patients with high‐risk relapsed or refractory indolent B‐cell lymphoma treated with copanlisib in the CHRONOS‐1 study. Presented at: 61st ASH Annual Meeting; 2018; San Diego.
- 106.Huet S, Sujobert P, Salles G. From genetics to the clinic: A translational perspective on follicular lymphoma. Nat Rev Cancer 2018;18:224–239. [DOI] [PubMed] [Google Scholar]
- 107.Bodor C, Grossmann V, Popov N et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 2013;122:3165–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morschhauser F, Tilly H, Chaidos A et al. Interim update from a phase 2 multicenter study of tazemetostat, and EZH2 inhibitor, in patients with relapsed or refractory (R/R) follicular lymphoma (FL). Presented at: 23rd Congress European Hematology Association; 2018; Stockholm, Sweden. [Google Scholar]
- 109.Harb W, Abramson J, Lunning M et al. 42OA phase 1 study of CPI‐1205, a small molecule inhibitor of EZH2, preliminary safety in patients with B‐cell lymphomas. Ann Oncol 2018;29:mdy048.001‐mdy048.001.
- 110.Batlevi CL, Crump M, Andreadis C et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol 2017;178:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogura M, Ando K, Suzuki T et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B‐cell non‐Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2014;165:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kirschbaum M, Frankel P, Popplewell L et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non‐Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol 2011;29:1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ribrag V, Kim WS, Bouabdallah R et al. Safety and efficacy of abexinostat, a pan‐histone deacetylase inhibitor, in non‐Hodgkin lymphoma and chronic lymphocytic leukemia: Results of a phase II study. Haematologica 2017;102:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lampson BL, Brown JR. PI3Kdelta‐selective and PI3Kalpha/delta‐combinatorial inhibitors in clinical development for B‐cell non‐Hodgkin lymphoma. Expert Opin Investig Drugs 2017;26:1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soumerai JD, Pagel JM, Jagadeesh D et al. Initial results of a dose escalation study of a selective and structurally differentiated PI3Kδ inhibitor, ME‐401, in relapsed/refractory (R/R) follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). J Clin Oncol 2018;36:7549. [Google Scholar]
- 116.Krysiak K, Gomez F, White BS et al. Recurrent somatic mutations affecting B‐cell receptor signaling pathway genes in follicular lymphoma. Blood 2017;129:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheah CY, Fowler NH. Novel agents for relapsed and refractory follicular lymphoma. Best Pract Res Clin Haematol 2018;31:41–48. [DOI] [PubMed] [Google Scholar]
- 118.Gopal AK, Schuster SJ, Fowler NH et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: Results from the open‐label, multicenter, phase II dawn study. J Clin Oncol 2018;36:2405–2412. [DOI] [PubMed] [Google Scholar]
- 119.Bartlett NL, Costello BA, LaPlant BR et al. Single‐agent ibrutinib in relapsed or refractory follicular lymphoma: A phase 2 consortium trial. Blood 2018;131:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Granier C, De Guillebon E, Blanc C et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017;2:e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nastoupil L, Westin JR, Fowler MH et al. High complete response rates with pembrolizumab in combination with rituximab in patients with relapsed follicular lymphoma: Results of an open‐label, phase II study. Blood 2017;130:414. [Google Scholar]
- 122.Gong J, Chehrazi‐Raffle A, Reddi S et al. Development of PD‐1 and PD‐L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lesokhin AM, Ansell SM, Armand P et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase Ib study. J Clin Oncol 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Westin JR, Chu F, Zhang M et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open‐label, phase 2 trial. Lancet Oncol 2014;15:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spaargaren M. Lymphoma spread? Target CD47‐SIRPalpha! Blood 2011;118:4762–4764. [DOI] [PubMed] [Google Scholar]
- 126.Advani RH, Flinn I, Popplewell L et al. Activity and tolerabilty of the first‐in‐class anti‐CD47 antibody Hu5F9‐G4 with rituximab tolerated in relapsed/refractory non‐hodgkin lymphoma: Initial phase 1b/2 results. J Clin Oncol 2018;36(suppl 15):7504a. [Google Scholar]
- 127.Advani R, Flinn I, Popplewell L et al. CD47 blockade by Hu5F9‐G4 and rituximab in non‐Hodgkin's lymphoma. N Engl J Med 2018;379:1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang L, Fang Y, Kopecek J et al. A new construct of antibody‐drug conjugates for treatment of B‐cell non‐Hodgkin's lymphomas. Eur J Pharm Sci 2017;103:36–46. [DOI] [PubMed] [Google Scholar]
- 129.Herrera AF, Mtasar MJ, Assouline S et al. Polatuzumab vedotin combined with bendamustine (B) and rituximab (R) or obinutuzumab (G) in patients with relapsed or refractory (R/R) follicular lymphoma (FL) or diffuse large B‐cell lymphoma (DLBCL): Preliminary results of a phase Ib/II dose‐escalation study. Presented at: 58th American Society of Hematology (ASH) Annual Meeting; 2016; San Diego.
- 130.Phillips T, Brunvand M, Chen A et al. Polatuzumab vedotin combined with obinutuzumab for patients with relapsed or refractory non‐Hodgkin lymphoma: Preliminary safety and clinical activity of a phase Ib/II study. Blood 2016;128:622–622. [Google Scholar]
- 131.Budde LE, Sehn LH, Assouline S, et al. Mosunetuzumab, a full‐length bispecific CD20/CD3 antibody, displays clinical activity in relapsed/refractory B‐cell non‐hodgkin lymphoma (NHL): Interim safety and efficacy results from a phase 1 study. Presented at ASH Annual Meeting; 2018; San Diego.
- 132.Kymriah: tisagenlecleucel. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2018.
- 133.Zinzani PL, Topp MS, Yuen SLS et al. Phase 2 study of venetoclax plus rituximab or randomized ven plus bendamustine+rituximab (BR) versus BR in patients with relapsed/refractory follicular lymphoma: Interim data. Blood 2016;128:617. [Google Scholar]