This review focuses on checkpoint and BRAF inhibitors, exploring outcomes based on clinical and disease characteristics to identify trends that might inform treatment decisions for the management of melanoma.
Keywords: Melanoma, Molecular targeted therapy, BRAF, Immunotherapy
Abstract
Background.
Immune checkpoint inhibitors, along with BRAF and MEK inhibitors, have dramatically changed the management of and outlook for patients with metastatic melanoma. Analyses of long‐term follow‐up data and subanalyses based on disease characteristics may inform clinical decision making.
Methods.
Reports of clinical trials in metastatic melanoma published between January 1, 2012, and August 30, 2018, were identified using PubMed (terms: melanoma AND [dabrafenib OR trametinib OR vemurafenib OR cobimetinib OR encorafenib OR ipilimumab OR nivolumab OR pembrolizumab]) and were systematically reviewed. Relevant congress proceedings were also assessed. Efficacy data from key phase III trials were analyzed and trends identified.
Results.
Substantial improvements in objective response rates, progression‐free survival, and overall survival were documented across 14 identified publications. Subgroup findings supported that patients with lower disease burden derive greater benefit than patients with more advanced disease, limiting the value of disease burden in the clinical decision‐making process. However, these agents consistently conferred benefits despite the presence of poor prognostic features. Several clinically relevant questions remain, including how best to sequence immune checkpoint inhibitors and combination targeted therapy.
Conclusion.
This research, coupled with ongoing investigations, including those on predictive biomarkers, suggests that the treatment decision‐making process is likely to become more nuanced.
Implications for Practice.
The management of melanoma has been rapidly advancing with new classes of agents, including immune checkpoint and BRAF inhibitors. With long‐term follow‐up, their impact on response rates and survival outcomes is well documented. Additional findings from subgroup analyses suggest that patients with lower disease burden derive greater benefit, yet both consistently confer benefit in patients with higher disease burden. Currently, there is a paucity of data to guide first‐line treatment selection between immunotherapy and BRAF‐targeted therapy in clinical practice or to estimate their impact when sequenced. Gaining these insights will facilitate a more nuanced management approach.
Introduction
Since 2011, a number of systemic agents have been approved for the treatment of unresectable or metastatic melanoma. These agents include several checkpoint inhibitors—namely, the anti‐cytotoxic T‐lymphocyte associated protein‐4 (anti‐CTLA‐4) antibody ipilimumab and the anti‐programmed death‐1 (anti‐PD‐1) antibodies nivolumab and pembrolizumab—as well as the BRAF inhibitors vemurafenib and dabrafenib and the mitogen‐activated extracellular signal‐regulated kinase (MEK) inhibitors trametinib and cobimetinib [1], [2]. Additional agents that have completed phase III clinical trials for advanced melanoma include the BRAF inhibitor encorafenib, the MEK inhibitor binimetinib, and the oncolytic virus talimogene laherparepvec (T‐VEC) [3], [4], [5], [6], [7].
This systematic review focuses on checkpoint inhibitors and BRAF inhibitors because these agents have long‐term data and subanalyses based on disease characteristics, allowing for a comprehensive assessment of their clinical impact. Owing to the large number of currently approved agents and the anticipation of forthcoming approvals, the review focuses on exploring outcomes based on clinical and disease characteristics to identify trends that might help inform clinical treatment decisions. Of note, given the volume of available efficacy data, safety data are beyond the scope of this review and not discussed herein.
Materials and Methods
A comprehensive literature search was performed in Medline using PubMed (filters: clinical trial, humans, English, and January 1, 2012, to December 31, 2018) and the following terms: melanoma AND (dabrafenib OR trametinib OR vemurafenib OR cobimetinib OR encorafenib OR ipilimumab OR nivolumab OR pembrolizumab). Additional PubMed searches were performed for the same time frame to identify any publications that had been omitted because of filter use. Publications on prospective phase I/II, II, or III trials involving patients with metastatic cutaneous melanoma were reviewed. However, phase III trials are discussed in the results because their relatively higher patient numbers allowed for interpretation of subgroup analyses. Publications with the following characteristics were excluded: safety, quality of life, or economic focus or data from expanded access or similar patient programs; case reports; single‐center or single‐institution studies; and combined analyses across trials; of note, this analysis was supplemented with phase II studies of patients with brain metastases, as this is a subset of patients with a significant unmet need. Evaluation included any of the searched agents in combination with other targeted systemic therapies (except for dabrafenib plus trametinib, vemurafenib plus cobimetinib, nivolumab plus ipilimumab, and encorafenib plus binimetinib) or with other treatment modalities (e.g., radiotherapy, surgery, intratumoral therapy, chemotherapy). A few studies identified with these parameters reported primary results before the January 1, 2012 cutoff; in these cases, the primary studies were added to the analysis. Congress proceedings were manually searched, specifically American Society of Clinical Oncology (ASCO) 2012 to 2018 annual meetings, key European Society for Medical Oncology‐sponsored congresses (2014, 2016, 2017, and 2018 annual meetings); European Cancer Congress 2013 and 2015, and Society for Melanoma Research 2012 to 2016 annual meetings.
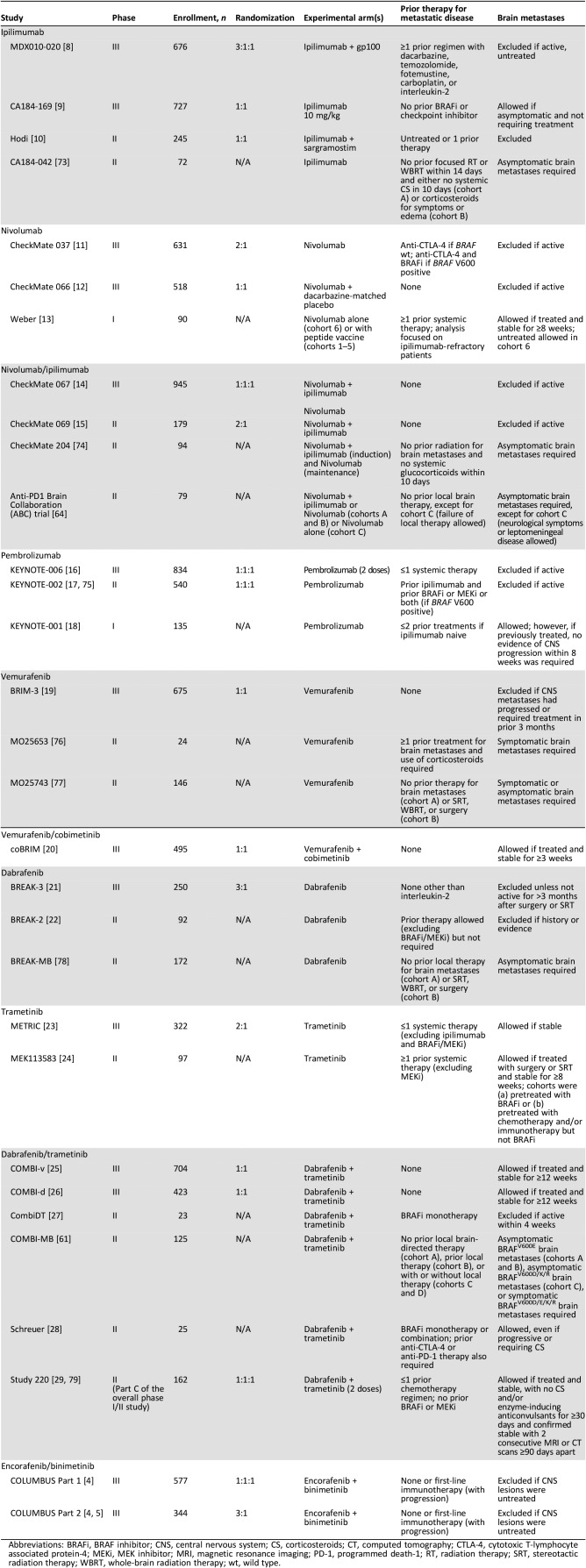
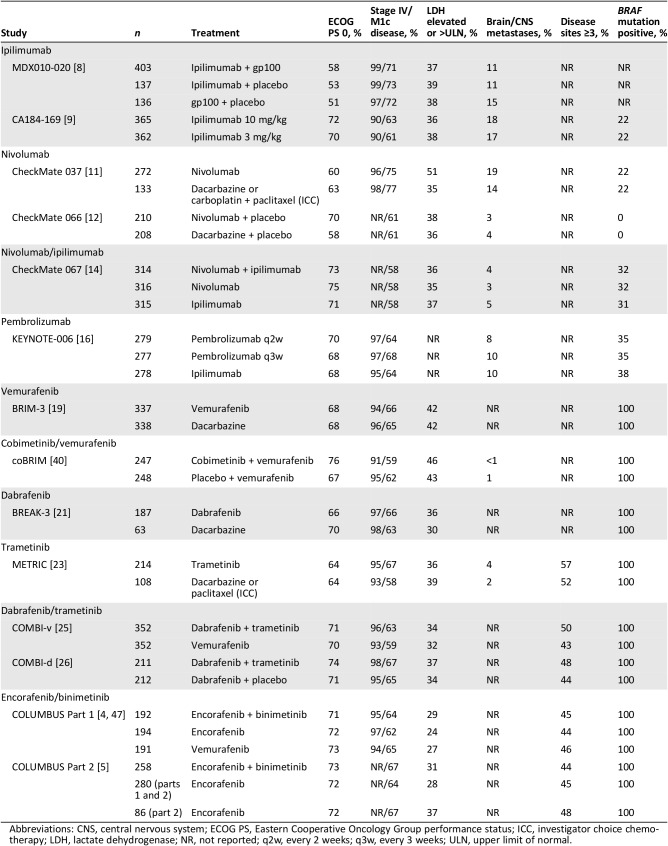
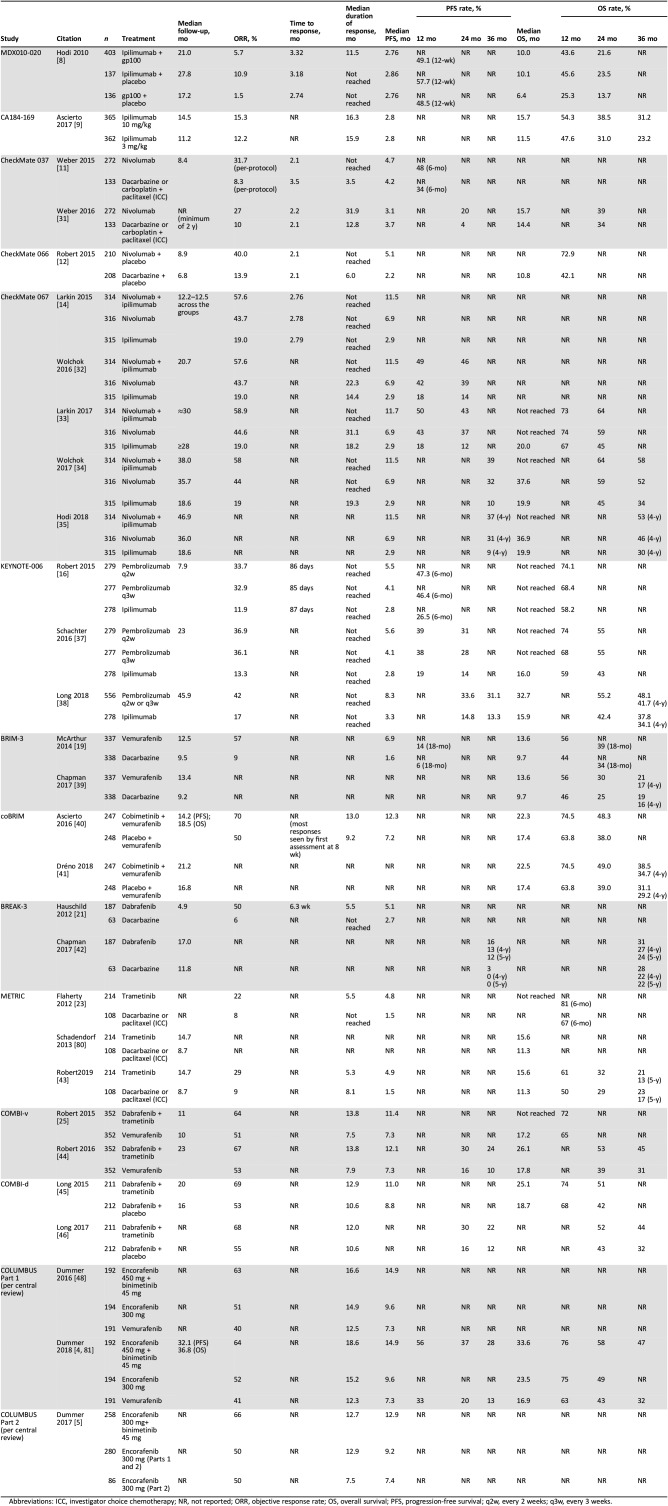
Results
Fourteen randomized phase III trials of immune checkpoint inhibitors, BRAF inhibitors, or MEK inhibitors alone or in combination in previously untreated and/or pretreated melanoma were identified among the studies of all phases (Table 1) [5], [6], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. Key study design aspects are summarized in Table 1, with key baseline characteristics in Table 2, overall efficacy results in Table 3 (results of phase II trials in brain metastases are found in supplemental online Table 1), and subgroup findings for progression‐free survival (PFS), overall survival (OS), and objective response rate (ORR) highlighted in supplemental online Tables 2, 3, and 4, respectively. Selected subgroup findings are summarized and discussed below, with a focus on those based on tumor characteristics of programmed death‐ligand 1 (PD‐L1) expression and BRAF mutation status, as well as clinical characteristics of baseline lactate dehydrogenase (LDH) levels and Eastern Cooperative Oncology Group performance status (ECOG PS).
Table 1. Study designs.
Abbreviations: BRAFi, BRAF inhibitor; CNS, central nervous system; CS, corticosteroids; CT, computed tomography; CTLA‐4, cytotoxic T‐lymphocyte associated protein‐4; MEKi, MEK inhibitor; MRI, magnetic resonance imaging; PD‐1, programmed death‐1; RT, radiation therapy; SRT, stereotactic radiation therapy; WBRT, whole‐brain radiation therapy; wt, wild type.
Table 2. Baseline characteristics for phase III trials.
Abbreviations: CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ICC, investigator choice chemotherapy; LDH, lactate dehydrogenase; NR, not reported; q2w, every 2 weeks; q3w, every 3 weeks; ULN, upper limit of normal.
Table 3. Key efficacy findings from phase III trials.
Abbreviations: ICC, investigator choice chemotherapy; NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; q2w, every 2 weeks; q3w, every 3 weeks.
Checkpoint Inhibitors
Ipilimumab.
Previously Treated Patients.
MDX010‐020 evaluated ipilimumab as second‐line or later treatment in stage III/IV melanoma, randomizing patients to receive ipilimumab plus gp100 peptide vaccine, ipilimumab plus gp100‐matched placebo, or gp100 plus ipilimumab‐matched placebo (supplemental online Table 2). The three arms were well balanced for ECOG PS, stage M1c disease, elevated LDH, and history of brain metastases (Table 2). No difference in the primary endpoint of OS was detected between the two ipilimumab groups, which improved OS relative to gp100 peptide vaccine alone; of the three treatments, ipilimumab monotherapy had the highest rates of ORR and 12‐month PFS (Table 3) [8].
Previously Treated or Untreated Patients.
The CA184‐169 trial evaluated ipilimumab 3 mg/kg versus ipilimumab 10 mg/kg in patients with previously untreated or treated unresectable stage III/IV melanoma (excluding patients treated with BRAF or immune checkpoint inhibitors; Table 1) [9]. Baseline characteristics were generally well balanced between treatment arms (Table 2). Median OS favored ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg (median OS, 15.7 vs. 11.5 months; hazard ratio [HR], 0.84; p = .04; Table 3) [9].
Subgroup analysis of OS demonstrated a larger benefit with 10 mg/kg versus 3 mg/kg in patients with BRAF‐mutant tumors (HR, 0.65) than in patients with BRAF‐wild‐type tumors (HR, 0.92). Similarly, greater risk reduction was observed with 10 mg/kg in patients with ECOG PS 0 (HR, 0.80) than in those with ECOG PS 1 (HR, 1.00) and patients with baseline LDH ≤2 × upper limit of normal (ULN; HR, 0.84) than in those with baseline LDH >2 × ULN (HR, 0.97; supplemental online Table 3).
Nivolumab.
Previously Treated Patients.
Checkmate 037 evaluated nivolumab monotherapy as second‐line or later treatment of stage IIIC/IV melanoma (including patients with a BRAF mutation), randomizing patients to receive nivolumab or investigator choice chemotherapy (ICC) with dacarbazine monotherapy or paclitaxel plus carboplatin (Table 1) [11]. The two arms were well balanced for ECOG PS of 0, stage M1c disease, history of brain metastases, and BRAF mutation status; 51% of patients in the nivolumab group versus 35% in the ICC group had elevated LDH levels at baseline (Table 2). Nivolumab conferred significant benefits over ICC in the primary endpoint of ORR [11], [31] and improvement in 2‐year PFS rate, but without significant prolongation of median PFS or OS (Table 3) [31].
Across prespecified subgroups (supplemental online Table 4), the ORR with nivolumab was more than twice as high in PD‐L1‐positive versus PD‐L1‐negative disease (43.6% vs. 20.3%), whereas response was numerically higher in BRAF‐wild‐type versus BRAF‐mutant tumors (BRAF wild type, 34.0%; BRAF mutant, 23.1%) [11].
Previously Untreated Patients.
CheckMate 066 evaluated nivolumab monotherapy in previously untreated stage III/IV melanoma (excluding patients with a BRAF mutation), randomizing patients to receive nivolumab plus dacarbazine‐matched placebo or dacarbazine plus nivolumab‐matched placebo (Table 1) [12]. The proportion of patients with ECOG PS 0 was higher in the nivolumab arm (70% vs. 58% with dacarbazine), with the groups well matched for baseline stage M1c disease, elevated LDH, and history of brain metastases (Table 2). Nivolumab conferred significant benefits over dacarbazine in the primary endpoint of 1‐year OS and secondary efficacy outcomes of median PFS and ORR (Table 3) [12].
In the analyses of prespecified subgroups (supplemental online Table 3), median OS was not reached with nivolumab, irrespective of PD‐L1 status or baseline LDH level, and it was not reached in patients with a history of brain metastases (of note, there were too few patients with brain metastases to calculate) or in those with ECOG PS 0. In the subset with ECOG PS 1, median OS was 12.7 months, translating into a 36% reduction in the risk of death with nivolumab versus dacarbazine versus a 68% reduction in the ECOG PS 0 subset. For ORR (supplemental online Table 4), subgroup data were available for PD‐L1 status, with rates of 52.7% in PD‐L1‐positive disease versus 33.1% in PD‐L1‐negative or indeterminate disease [12].
Nivolumab/Ipilimumab.
CheckMate 067 evaluated nivolumab plus ipilimumab in previously untreated stage III/IV melanoma (excluding patients with unknown BRAF mutation status), randomizing patients to receive the combination, nivolumab monotherapy, or ipilimumab monotherapy (Table 1). The three arms were well balanced for ECOG PS, stage M1c disease, elevated LDH levels, history of brain metastases, and BRAF mutation status (Table 2). Both the combination and nivolumab monotherapy were significantly more effective than ipilimumab monotherapy in the two coprimary endpoints of PFS and OS and in ORR (Table 3) [14], [32], [33], [34], [35].
Three‐ and 4‐year results are available for the prespecified subgroups in CheckMate 067 (supplemental online Tables 2–4), demonstrating numerically higher PFS and OS rates with the combination versus nivolumab monotherapy across most subgroups, with survival outcomes favoring nivolumab‐containing therapies versus ipilimumab monotherapy in all subgroups [32], [34], [35]. For PFS, certain subgroups fared better with the combination than with nivolumab monotherapy, particularly those with a PD‐L1 level ≥10% (4‐year PFS rate, 48% with combination vs. 37% with nivolumab; HR, 0.67) and BRAF mutation‐positive disease (39% vs. 23%; HR, 0.62; supplemental online Table 2). Four‐year PFS rate in combination‐treated patients was similar between BRAF‐mutant (39%) and BRAF‐wild‐type (35%) disease. The combination was associated with PFS rates of 42% to 45% in patients with the most favorable LDH levels (ULN or lower), disease burden (Q1 or lower [31 mm]), and lesion site (limited to one) categories, with corresponding rates that were slightly lower with nivolumab monotherapy (35%–37%) but markedly lower with ipilimumab monotherapy (11%–14%). The more mature OS data suggest that nivolumab plus ipilimumab may confer improved outcomes over nivolumab alone in patients with low PD‐L1‐expressing tumors (HR 0.68 with the combination vs. nivolumab in patients with PD‐L1 expression <1%), whereas the two regimens were associated with similar OS in patients with PD‐L1 expression ≥1% (HR, 0.98; supplemental online Table 3). OS results by BRAF subgroup were consistent with the PFS results, with a notable reduction in the risk of death in patients with BRAF‐mutant disease (HR 0.70 vs. 0.92 in BRAF‐wild‐type disease with the combination vs. nivolumab alone, although the trial was not powered for this comparison) [35]. In addition, results by region and BRAF mutation status showed that 2‐year OS in each arm in EU patients with BRAF‐wild‐type disease was lower than in EU patients with BRAF‐mutant disease and U.S. patients with BRAF‐wild‐type disease (possibly reflecting differences in the extent of advanced disease) [36].
Pembrolizumab.
KEYNOTE‐006 evaluated pembrolizumab monotherapy in previously treated or untreated (one or fewer prior systemic therapy for advanced disease) stage III/IV melanoma, randomizing patients to receive pembrolizumab every 2 weeks, pembrolizumab every 3 weeks, or ipilimumab every 3 weeks (Table 1). The three arms were well balanced (Table 2). In this study, pembrolizumab in both arms was superior to ipilimumab in the coprimary endpoints of PFS and OS and the secondary efficacy outcome of ORR [16], with higher 2‐ and 3‐year OS and PFS rates per updated results (Table 3) [37], [38].
In KEYNOTE‐006, PFS benefits in either pembrolizumab arm versus the ipilimumab arm were seen across subgroups based on BRAF mutation status, baseline ECOG PS, and PD‐L1 status (supplemental online Table 2) [16]. Risk reductions for progression and death were similar between the various categories within a subgroup. The risk reductions for death with pembrolizumab were similar irrespective of BRAF status and ECOG PS but were more favorable in patients with PD‐L1‐positive versus ‐negative disease (supplemental online Table 3) [16].
Summary of Key Findings: Checkpoint Inhibitors
Single‐agent checkpoint inhibitors show a clear benefit over chemotherapy for patients with metastatic melanoma, and these benefits appear to be consistent across patient subgroups.
Anti‐PD‐1 monotherapies (nivolumab and pembrolizumab) demonstrate an efficacy benefit over ipilimumab monotherapy that appears consistent over the several subgroups analyzed. Benefit for anti‐PD‐1 therapy appears greater for patients with elevated PD‐L1 expression; however, the relevance of this finding is debatable in consideration of standard practice.
Single‐agent checkpoint inhibitors show a clear benefit over chemotherapy for patients with metastatic melanoma, and these benefits appear to be consistent across patient subgroups.
The combination of nivolumab plus ipilimumab is significantly more effective in improving ORR, PFS, and OS relative to ipilimumab monotherapy in the first‐line setting, with benefit maintained at 4 years. Certain patient subgroups may derive greater benefit from the combination of nivolumab plus ipilimumab versus nivolumab monotherapy, particularly those who have low expression of PD‐L1.
Targeted Agents
Vemurafenib.
BRIM‐3 evaluated vemurafenib in previously untreated stage IIIC/IV melanoma harboring a BRAF V600 mutation, randomizing patients to receive vemurafenib or dacarbazine (Table 1). The two arms were well balanced for ECOG PS, stage M1c disease, and elevated LDH levels (Table 2). Vemurafenib conferred significant benefit over dacarbazine in the coprimary endpoints of PFS and OS and the secondary efficacy outcome of ORR [19], with the OS benefit maintained in the recently published final analysis (Table 3) [39].
Analysis of OS based on ECOG PS showed a median OS in the vemurafenib arm of 16.8 months in patients with ECOG PS 0 and 10.0 months in patients with ECOG PS 1 (supplemental online Table 3). Median OS in the vemurafenib arm was 18.1 months in patients with normal baseline LDH and 9.6 months in patients with elevated LDH [39].
Vemurafenib/Cobimetinib.
coBRIM evaluated vemurafenib plus cobimetinib in previously untreated stage IIIC/IV melanoma harboring a BRAF V600 mutation, randomizing patients to receive the combination or vemurafenib with placebo (Table 1). The two arms were well balanced for ECOG PS, stage M1c disease, elevated LDH, and history of brain metastases (Table 2). Vemurafenib plus cobimetinib plus conferred significant benefit over vemurafenib alone in the primary endpoint of PFS and the secondary outcomes of OS and ORR (Table 3) [40], [41].
For PFS and OS, HRs favored the combination in the prespecified subgroups, which included BRAF mutation type, baseline LDH, and ECOG PS (supplemental online Tables 2 and 3). HRs for PFS were similar based on BRAF mutation type, ECOG PS, and baseline LDH levels. For median OS, the most pronounced risk reductions were seen in the patients with ECOG PS 1 (47% reduction) or normal baseline LDH (41% reduction) (supplemental online Table 2) [40].
Dabrafenib.
BREAK‐3 evaluated dabrafenib monotherapy in previously untreated stage III/IV melanoma harboring a BRAF V600E mutation, randomizing patients to receive dabrafenib or dacarbazine (Table 1). The two arms were well balanced for ECOG PS, stage M1c disease, and elevated LDH (Table 2). Dabrafenib conferred significant benefit over dacarbazine in the primary endpoint of PFS [21], with updated results presented at the ASCO 2017 annual meeting reporting 5‐year outcomes, at which time PFS was 12% with dabrafenib and 0% with dacarbazine (Table 3) [42].
PFS was analyzed by baseline LDH levels (supplemental online Table 2) [42]. The results have been consistent over ongoing follow‐up, with PFS of 21% and 6% in patients with LDH levels at the ULN or lower and greater than the ULN, respectively, at 3 years; 17% and 4%, respectively, at 4 years; and 16% and 4%, respectively, at 5 years [42].
Trametinib.
METRIC evaluated trametinib monotherapy in previously treated or untreated (one or fewer prior chemotherapy regimen for advanced or metastatic disease) stage IIIC/IV melanoma harboring a BRAF V600E or V600K mutation, randomizing patients to receive trametinib or ICC with dacarbazine or paclitaxel (Table 1). The two arms were well balanced for baseline characteristics and disease history (Table 2). Trametinib conferred significant benefit over ICC in the primary endpoint of PFS and the secondary outcome of 6‐month OS [23]; a recently published update reported OS after 5‐year follow‐up, at which time the median was 15.6 months with trametinib versus 11.3 months with ICC and OS rates were 13% versus 17%, with a high rate of crossover from chemotherapy to trametinib and some differences in postprogression therapies between the arms (Table 3) [43]. PFS benefit with trametinib over ICC was observed in subgroups based on ECOG PS and LDH (supplemental online Table 2) [23].
Dabrafenib/Trametinib.
COMBI‐v evaluated dabrafenib plus trametinib in previously untreated stage IIIC/IV melanoma harboring a BRAF V600E or V600K mutation, randomizing patients to receive the combination or vemurafenib monotherapy (Table 1). The two arms were well balanced for ECOG PS, stage M1c disease, elevated LDH levels, and at least three disease sites (Table 2) [25]. At the published interim analysis [25] and subsequently presented update [44], dabrafenib plus trametinib conferred significant benefit over vemurafenib in the primary endpoint of OS and secondary outcomes of median PFS and ORR (Table 3).
In COMBI‐v, HRs for OS favored the combination in the prespecified subgroups (which included BRAF mutation subtype, baseline LDH levels, number of disease sites, and ECOG PS), except in the ECOG PS 1 subgroup (supplemental online Table 3). HRs for OS were 1.03 for patients with an ECOG PS of 1 and 0.53 for patients with ECOG PS of 0 [25]. With dabrafenib plus trametinib, median OS was not reached in patients with LDH levels at the ULN or lower but was 10.8 months in those with LDH levels higher than the ULN [44]. HRs for PFS all favored the combination in the prespecified subgroups; the HRs were similar to the risk reductions for OS (except, in contrast to the OS results, a 25% reduction in the risk of progression or death was seen in patients with an ECOG PS of 1; supplemental online Table 2) [25].
COMBI‐d evaluated dabrafenib plus trametinib in previously untreated stage IIIC/IV melanoma harboring a BRAF V600E or V600K mutation, randomizing patients (1:1) to receive the combination or dabrafenib with placebo (Table 1). The two arms were well balanced for ECOG PS of 0, stage M1c disease, elevated LDH levels, and at least three disease sites (Table 2). Dabrafenib plus trametinib conferred significant benefit over dabrafenib alone in the primary endpoint of PFS [45] and secondary outcomes of OS and ORR; 3‐year updated PFS and OS rates were published in 2017 (Table 3) [46].
Subgroup findings for COMBI‐d shared some similarities with those from COMBI‐v; however, in COMBI‐d, the benefit of the combination was similar irrespective of baseline ECOG PS (supplemental online Table 2). Whereas HRs for PFS favored the combination in most prespecified subgroups, the HR for dabrafenib plus trametinib versus dabrafenib alone was 1.02 for no more than two disease sites versus 0.60 for at least three disease sites in the initially reported data [26]. However, with longer follow‐up, 3‐year PFS and OS rates were highest with the combination in patients with both LDH levels ≤ULN or lower and fewer than three disease sites (PFS, 38% with combination vs. 16% with dabrafenib; OS, 62% vs. 45%; supplemental online Tables 2 and 3) [26], [46].
Encorafenib/Binimetinib.
COLUMBUS is a 2‐part study of encorafenib plus binimetinib in previously treated (with first‐line immunotherapy) or untreated stage III/IV melanoma harboring a BRAF V600 mutation. In part 1, patients were randomized to receive binimetinib with encorafenib 450 mg/day (COMBO450), encorafenib 300 mg/day, or vemurafenib; in part 2, patients were randomized to receive binimetinib with encorafenib 300 mg/day (COMBO300) or encorafenib 300 mg/day (Table 1). In both parts, the arms were well balanced (Table 2). In part 1, COMBO450 significantly improved PFS and OS over vemurafenib (but not encorafenib monotherapy) [4]; in part 2, COMBO300 demonstrated significantly improved PFS and ORR versus encorafenib monotherapy (therefore showing that binimetinib directly contributes to the efficacy of the combination; Table 3) [4], [5], [47].
Subgroup data are available from part 1 of COLUMBUS, showing reductions in the risk of progression or death for encorafenib plus binimetinib versus vemurafenib of 53% and 27% for those with LDH levels lower than the ULN and at the ULN or higher, respectively (supplemental online Table 2) [48]. Updated subgroup data showed reductions in the risk of death for COMBO450 versus vemurafenib of 49% and 5% for those with LDH levels at the ULN or lower and higher than the ULN, respectively (supplemental online Table 3) [4], [47].
Summary of Key Findings: Targeted Therapy
Single‐agent vemurafenib, dabrafenib, and trametinib are significantly more effective than single‐agent dacarbazine in BRAF V600‐mutant advanced melanoma.
The combination of BRAF and MEK inhibitors has been shown to improve ORR, PFS, and OS versus targeted monotherapy in patients with BRAF V600‐mutant advanced melanoma. Benefit is consistent across all subgroups.
Benefit for combination targeted therapy appears greatest among patients with favorable prognostic factors, such as normal LDH levels, ECOG status, and fewer than three sites of metastasis.
Discussion and Future Directions
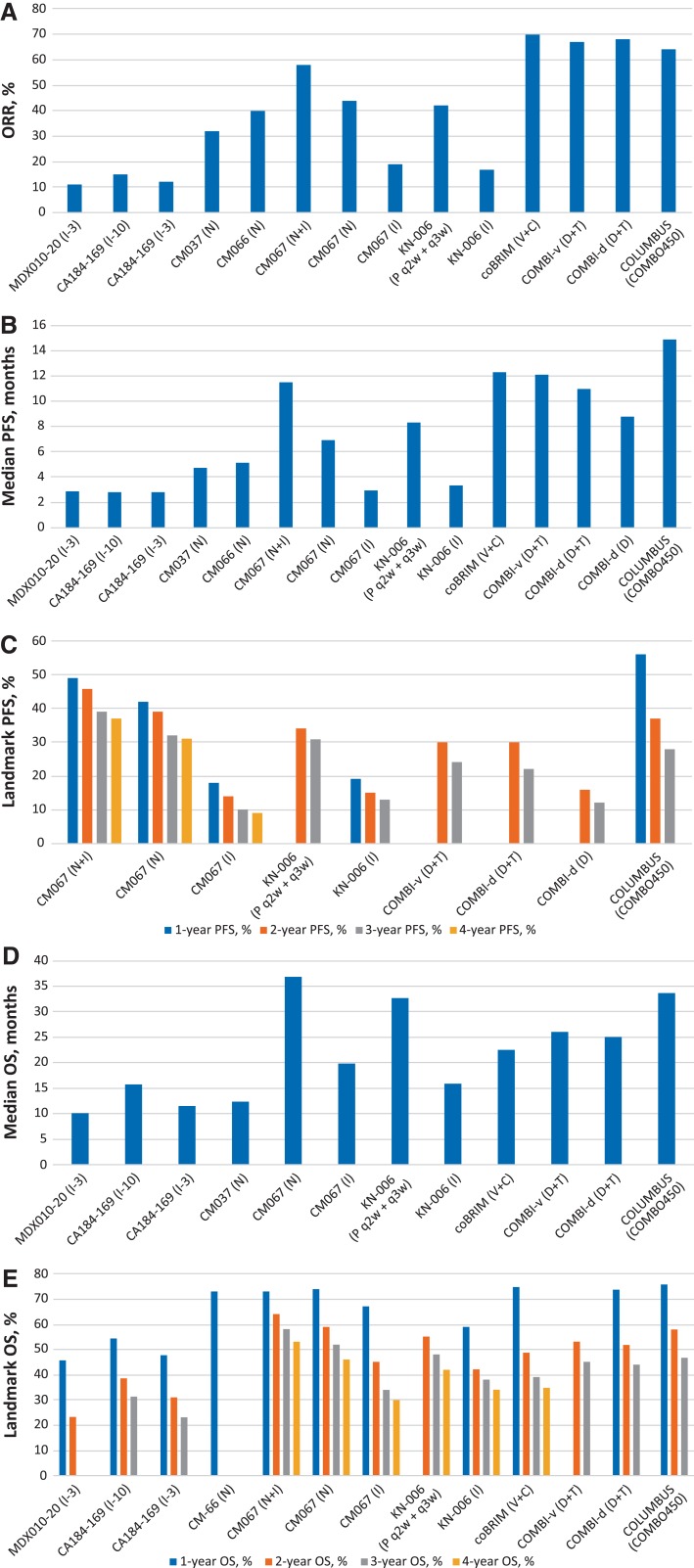
The field of systemic therapeutics has advanced rapidly over the past 7 years, from a time when chemotherapy and interleukin‐2 were the only treatment options to the current status, with several BRAF‐directed and checkpoint immunotherapies available. The impact of these treatments is clear, with increasing ORR, PFS, and OS over time, with key results specific to BRAF‐MEK combination therapy and checkpoint immunotherapy in Figure 1. Despite the growing list of effective therapies, data from their trials do not address a number of important questions about the clinical management of patients with advanced melanoma, including but not limited to how best to (1) choose first‐line therapy, (2) rechallenge with the same drug in later lines of therapy, and (3) manage populations with rare types of melanoma. Beyond these caveats, the field is developing rapidly with further combinations of these agents and the integration of novel therapeutics.
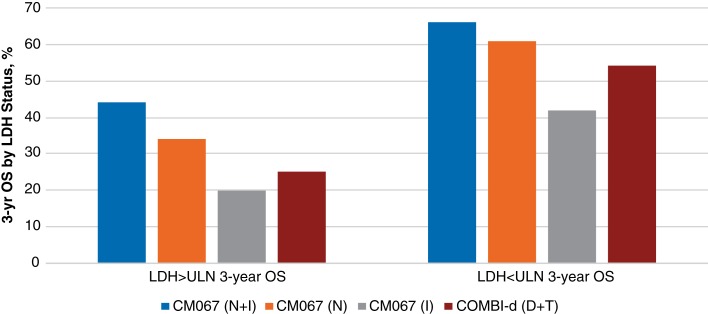
Figure 1.
Key efficacy data for BRAF‐MEK combination therapy and checkpoint immunotherapy in metastatic melanoma.
Abbreviations: C, cobimetinib; COMBO450, encorafenib 450 mg daily plus binimetinib 45 mg twice daily; D, dabrafenib; I, ipilimumab; I‐3, ipilimumab 3 mg/kg; I‐10, ipilimumab 10 mg/kg; N, nivolumab; ORR, overall response rate; OS, overall survival; P, pembrolizumab; PFS, progression‐free survival; Pt, part; q2w, every 2 weeks; q3w, every 3 weeks; T, trametinib; V, vemurafenib.
Multiple regimens are now approved as first‐line therapy for melanoma; however, there is a paucity of randomized data to guide treatment selection between immunotherapy and BRAF‐targeted therapy. The only data surrounding frontline therapy suggest that BRAF/MEK combination therapy is superior to and less toxic than BRAF inhibitor monotherapy [5] and, somewhat similarly, that anti‐PD‐1 monotherapy has higher efficacy and less toxicity than anti‐CTLA‐4 monotherapy [16], [37], whereas a combination of an anti‐PD‐1 and an anti‐CTLA‐4 is more efficacious than either checkpoint inhibitor alone but has greater toxicity [14], [32], [33], [34]. No prospective clinical trial data have addressed the question of BRAF‐directed therapy versus immunotherapy in the frontline setting, although an ongoing randomized trial is attempting to address it (NCT02224781), and there are several relevant retrospective analyses. Using regression tree analyses to assess hierarchical effect on treatment outcomes with dabrafenib and trametinib, Long et al. [46], [49] demonstrated that the clinical factors most highly associated with long‐term benefit with BRAF‐MEK inhibition included normal LDH levels (consistent with the 3‐year OS results from COMBI‐d [Fig. 2]), fewer than three sites of metastatic melanoma, and ECOG PS of 0. This is potentially in contrast to the most common clinical use of BRAF‐MEK inhibition: targeted therapy for large disease volume or rapidly progressive disease. At the same time, however, OS data from CheckMate 067 (Fig. 2 and supplemental online Table 3) [34], [35] and the findings of other studies of immunotherapies have also suggested that greater benefit is associated with lower disease burden, making this a less useful clinical discriminator [50], [51]. Instead, treatment choice should likely be dictated by other factors, such as route of administration (intravenous vs. oral), schedule of treatments and assessments, and toxicity profile (and health care provider comfort level in managing adverse events).
The only data surrounding frontline therapy suggest that BRAF/MEK combination therapy is superior to and less toxic than BRAF inhibitor monotherapy and, somewhat similarly, that anti‐PD‐1 monotherapy has higher efficacy and less toxicity than anti‐CTLA‐4 monotherapy, whereas a combination of an anti‐PD‐1 and an anti‐CTLA‐4 is more efficacious than either checkpoint inhibitor alone but has greater toxicity.
Figure 2.
Three‐year overall survival by LDH status with BRAF‐MEK combination therapy and checkpoint immunotherapy in metastatic melanoma.
Abbreviations: D, dabrafenib; I, ipilimumab; LDH, lactate dehydrogenase; N, nivolumab; OS, overall survival; P, pembrolizumab; T, trametinib; ULN, upper limit of normal.
To assess the efficacy of sequencing BRAF inhibitors before or after immunotherapy treatments, only retrospective data are available. Such observations have predominately suggested that there may be a detriment to the ORR with immunotherapy (specifically ipilimumab) if it is administered after BRAF inhibition, but the reverse sequence does not influence the ORR with BRAF inhibition [52], [53]. Recently, however, a retrospective series of 78 patients with BRAF V600‐mutant melanoma receiving a BRAF‐MEK combination after PD‐1‐based therapy showed an 83% rate of BRAF‐MEK dose modification, 31% rate of adverse event‐related hospitalization, and median BRAF‐MEK therapy and OS durations of 5.8 and 15.6 months, respectively [54]. Overall, the available retrospective data sets are small, and their findings should be considered on a less urgent level relative to patient‐specific factors and preferences. In addition, translational investigations have suggested potential overlap in resistance mechanisms between BRAF‐targeted agents and immunotherapies [55], [56], [57], [58]. Some have suggested this as a rationale for sequencing immunotherapy before BRAF‐directed treatment or, alternatively, for the use of triplet BRAF‐MEK‐PD‐1 combinations. Specific to immunotherapy, prospective phase II [59] and phase III data [14], [32], [33], [34] strongly suggest that treatment with an anti‐PD‐1 antibody should be universally prioritized over an anti‐CTLA‐4 antibody.
An intriguing consideration related to treatment sequencing is the possibility of rechallenge with previously used systemic therapies. The mechanisms of resistance to BRAF inhibitors, as understood from accumulating basic and preclinical research, suggest that resistance to BRAF inhibitors indicates a reactivation of the mitogen‐activated protein kinase pathway (independent of BRAF) or activation of alternative pathways and may be reversible after withholding and then reinitiating therapy [28]. In a phase II study by Schreuer et al. [28], 32% of patients (8/25) with BRAF inhibitor‐resistant stage IIIC/IV melanoma achieved a partial response when treated with dabrafenib plus trametinib. An analysis of the MDX010‐20 trial also supports the potential benefit of retreatment with ipilimumab, with the investigators reporting post‐retreatment ORRs of 13% with ipilimumab plus gp100 and 38% with ipilimumab plus placebo [60].
Although patients with brain metastases or uncontrolled brain metastases were typically excluded from the included studies, recent phase II data showing the intracranial activity of BRAF‐MEK and combination checkpoint inhibition in such patients are noteworthy and are changing the approach to managing brain metastases in clinical practice (supplemental online Table 1) [61], [62], [63], [64]. For example, in the COMBI‐MB trial of dabrafenib plus trametinib in BRAF‐mutant melanoma brain metastases, investigator‐determined intracranial response rates were 44% to 59% across the four patient cohorts (which differed in mutation type, prior local brain therapy, and symptoms), with median durations of 4.5 to 8.3 months [61]. In the context of increasing numbers of reports of long‐term adverse events following radiation, such as radionecrosis [65], these data raise the possibility that some patients may be better served by proceeding with systemic therapy (either targeted or immunotherapy) before considering radiation [66].
The complex mechanisms involved in primary and acquired resistance to molecularly and immune‐targeted therapies for metastatic melanoma are only beginning to be understood [56], [67], [68], [69], [70]. Recent findings with anti‐PD‐1 checkpoint inhibitors implicate the involvement of pathways associated with interferon receptor signaling (as evidenced by identification of loss‐of‐function mutations in Janus kinase 1 and 2), as well as antigen presentation [68]. Emerging data also suggest that immune evasion may play a role in acquired resistance to BRAF/MEK inhibitors, given observations of CD8+ T‐cell depletion and exhaustion that may suggest cross‐resistance to subsequent anti‐PD‐1/PD‐L1 therapy [69].
As follow‐up continues in most of the studies discussed, new investigations are underway to determine the potential of several combinations for treating metastatic melanoma. These include combinations in which pembrolizumab is combined with T‐VEC (NCT02965716) and PD‐1 or PD‐L1 inhibitors are combined or sequenced with BRAF‐MEK inhibitors (NCT02967692, NCT02902029). At the same time, the benefits of BRAF‐MEK and immune checkpoint inhibitors are being translated into earlier use in the adjuvant setting [71], [72], where they are improving recurrence‐free survival. Finally, biomarkers for treatment selection and monitoring are rapidly progressing, which may help guide treatment selection in each patient. The totality of this research suggests that just beyond the near‐term horizon, a much more nuanced and individual patient‐level treatment decision‐making process will be necessary to choose between the therapies already available and those yet to come.
Conclusion
Major advances in metastatic melanoma have been accomplished via dual BRAF and MEK inhibition as well as immune checkpoint blockade targeting programmed death receptor 1 alone and in combination cytotoxic T‐lymphocyte–associated antigen 4. The optimal therapy for an individual patient remains unclear, however, as the therapies have not been compared head to head. Here, a comprehensive clinical trial data summation is presented for the therapeutic utility for each approach and context is provided for the general practitioner to consider when choosing therapy for previously untreated metastatic melanoma.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The author would like to thank William Fazzone, Ph.D., of ArticulateScience LLC, for editorial support funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ). Neither Novartis Pharmaceuticals Corporation nor ArticulateScience LLC influenced the content of this manuscript, and the author did not receive financial compensation for authorship.
Disclosures
Jason J. Luke: Aduro, Astellas, AstraZeneca, Bayer, Bristol‐Myers Squibb, Castle, CheckMate, Compugen, EMD Serono, IDEAYA, Immunocore, Janssen, Jounce, Merck, NewLink, Novartis, RefleXion, Spring Bank, Syndax, Tempest, Vividion, WntRx (C/A), 7 Hills, Actym, Alphamab Oncology, Array, BeneVir, Mavu, Tempest (SAB), AbbVie, Boston Biomedical, Bristol‐Myers Squibb, Celldex, Compugen, Corvus, EMD Serono, Delcath, Five Prime, FLX Bio, Genentech, Immunocore, Incyte, Leap, MedImmune, Macrogenics, Novartis, Pharmacyclics, Merck, Tesaro, Xencor (RF—institutional), Array, CheckMate, Evelo, Palleon (RF—scientific research agreement), TTC Oncology (other—data and safety monitoring board), Array, AstraZeneca, Bayer, BeneVir, Bristol‐Myers Squibb, Castle, CheckMate, EMD Serono, IDEAYA, Immunocore, Janssen, Jounce, Merck, NewLink, Novartis, RefleXion (other—travel funding).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Luke JJ, Flaherty KT, Ribas A et al. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14(8):463–482. [DOI] [PubMed] [Google Scholar]
- 2.Garbe C, Eigentler TK, Keilholz U et al. Systematic review of medical treatment in melanoma: Current status and future prospects. The Oncologist 2011;16:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andtbacka RH, Kaufman HL, Collichio F et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–2788. [DOI] [PubMed] [Google Scholar]
- 4.Dummer R, Ascierto PA, Gogas HJ et al. Overall survival in patients with BRAF‐mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol 2018;19:1315–1327. [DOI] [PubMed] [Google Scholar]
- 5.Dummer R, Ascierto PA, Gogas H et al. Results of COLUMBUS part 2: A phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus ENCO in BRAF‐mutant melanoma. Ann Oncol 2017;28(suppl 5):1215OA. [Google Scholar]
- 6.Dummer R, Schadendorf D, Ascierto PA et al. Binimetinib versus dacarbazine in patients with advanced NRAS‐mutant melanoma (NEMO): A multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol 2017;18:435–445. [DOI] [PubMed] [Google Scholar]
- 7.Ott PA, Hodi FS. Talimogene laherparepvec for the treatment of advanced melanoma. Clin Cancer Res 2016;22:3127–3131. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascierto PA, Del Vecchio M, Robert C et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol 2017;18:611–622. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, Lee S, McDermott DF et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 2014;312:1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 13.Weber JS, Kudchadkar RR, Yu B et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab‐refractory or ‐naive melanoma. J Clin Oncol 2013;31:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 17.Hamid O, Puzanov I, Dummer R et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory advanced melanoma. Eur J Cancer 2017;86:37–45. [DOI] [PubMed] [Google Scholar]
- 18.Hamid O, Robert C, Daud A et al. Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. N Engl J Med 2013;369:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McArthur GA, Chapman PB, Robert C et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation‐positive melanoma (BRIM‐3): Extended follow‐up of a phase 3, randomised, open‐label study. Lancet Oncol 2014;15:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin J, Ascierto PA, Dréno B et al. Combined vemurafenib and cobimetinib in BRAF‐mutated melanoma. N Engl J Med 2014;371:1867–1876. [DOI] [PubMed] [Google Scholar]
- 21.Hauschild A, Grob JJ, Demidov LV et al. Dabrafenib in BRAF‐mutated metastatic melanoma: A multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2012;380:358–365. [DOI] [PubMed] [Google Scholar]
- 22.Ascierto PA, Minor D, Ribas A et al. Phase II trial (BREAK‐2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31:3205–3211. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty KT, Robert C, Hersey P et al. Improved survival with MEK inhibition in BRAF‐mutated melanoma. N Engl J Med 2012;367:107–114. [DOI] [PubMed] [Google Scholar]
- 24.Kim KB, Kefford R, Pavlick AC et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF‐mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013;31:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Karaszewska B, Schachter J et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–39. [DOI] [PubMed] [Google Scholar]
- 26.Long GV, Stroyakovskiy D, Gogas H et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–1888. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, McQuade JL, Panka DJ et al. Clinical, molecular, and immune analysis of dabrafenib‐trametinib combination treatment for BRAF inhibitor‐refractory metastatic melanoma: A phase 2 clinical trial. JAMA Oncol 2016;2:1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreuer M, Jansen Y, Planken S et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600‐mutant melanoma: An open‐label, single arm, dual‐centre, phase 2 clinical trial. Lancet Oncol 2017;18:464–472. [DOI] [PubMed] [Google Scholar]
- 29.Flaherty KT, Infante JR, Daud A et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ascierto PA, Schadendorf D, Berking C et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: A non‐randomised, open‐label phase 2 study. Lancet Oncol 2013;14:249–256. [DOI] [PubMed] [Google Scholar]
- 31.Weber J, Minor D, D'Angelo S et al. Overall survival in patients with advanced melanoma who received nivolumab vs investigator's choice chemotherapy in the phase 3 CheckMate 037 trial. Oral presentation at the Annual Society for Melanoma Research Meeting; 2016.
- 32.Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Updated results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment‐naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). J Clin Oncol 2016;34(suppl 15):9505A. [Google Scholar]
- 33.Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Overall survival (OS) results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment‐naïve patients with advanced melanoma (CheckMate 067). Presentation at the 107th Annual Meeting of the American Association for Cancer Research; 2017; abstract CT075.
- 34.Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, Chiarion‐Sileni V, Gonzalez R et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4‐year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 36.Grob JJ, Schadendorf D, Wagstaff J et al. Regional differences in overall survival (OS) in patients with advanced melanoma (MEL) who received nivolumab (NIVO) combined with ipilimumab (IPI) or NIVO alone in a phase 3 trial (CheckMate 067). Ann Oncol 2017;28(suppl 5):1222PDA. [Google Scholar]
- 37.Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival analysis of KEYNOTE‐006. J Clin Oncol 2016;34(suppl 15):9504A. [Google Scholar]
- 38.Long GV, Schachter J, Ribas A et al. 4‐year survival and outcomes after cessation of pembrolizumab (pembro) after 2‐years in patients (pts) with ipilimumab (ipi)‐naive advanced melanoma in KEYNOTE‐006. J Clin Oncol 2018;36(suppl 15):9503A. [Google Scholar]
- 39.Chapman PB, Robert C, Larkin J et al. Vemurafenib in patients with BRAFV600 mutation‐positive metastatic melanoma: Final overall survival results of the randomized BRIM‐3 study. Ann Oncol 2017;28:2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascierto PA, McArthur GA, Dréno B et al. Cobimetinib combined with vemurafenib in advanced BRAFV600‐mutant melanoma (coBRIM): Updated efficacy results from a randomised, double‐blind, phase 3 trial. Lancet Oncol 2016;17:1248–1260. [DOI] [PubMed] [Google Scholar]
- 41.Dréno B, Ascierto PA, McArthur GA et al. Efficacy and safety of cobimetinib (C) combined with vemurafenib (V) in patients (pts) with BRAFV600 mutation‐positive metastatic melanoma: Analysis from the 4‐year extended follow‐up of the phase 3 coBRIM study. J Clin Oncol 2018;36(suppl 15):9522A. [Google Scholar]
- 42.Chapman PB, Ascierto PA, Schadendorf D et al. Updated 5‐y landmark analyses of phase 2 (BREAK‐2) and phase 3 (BREAK‐3) studies evaluating dabrafenib monotherapy in patients with BRAF V600‐mutant melanoma. J Clin Oncol 2017;35(suppl 15):9526A. [Google Scholar]
- 43.Robert C, Flaherty K, Nathan P et al. Five‐year outcomes from a phase 3 METRIC study in patients with BRAF V600 E/K‐mutant advanced or metastatic melanoma. Eur J Cancer 2019;109:61–69. [DOI] [PubMed] [Google Scholar]
- 44.Robert C, Karaszewska B, Schachter J et al. Three‐year estimate of overall survival in COMBI‐v, a randomized phase 3 study evaluating first‐line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/k‐mutant cutaneous melanoma. Ann Oncol 2016;27(suppl 6):LBA40A. [Google Scholar]
- 45.Long GV, Stroyakovskiy D, Gogas H et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF‐mutant melanoma: A multicentre, double‐blind, phase 3 randomised controlled trial. Lancet 2015;386:444–451. [DOI] [PubMed] [Google Scholar]
- 46.Long GV, Flaherty KT, Stroyakovskiy D et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/k‐mutant melanoma: Long‐term survival and safety analysis of a phase 3 study. Ann Oncol 2017;28:1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dummer R, Ascierto PA, Gogas H et al. Overall survival in COLUMBUS: A phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) vs vemurafenib (VEM) or enco in BRAF‐mutant melanoma. J Clin Oncol 2018;36(suppl 15):9504A. [Google Scholar]
- 48.Dummer R, Ascierto PA, Gogas HJ et al. Results of COLUMBUS part 1: A phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in BRAF‐mutant melanoma. Oral presentation at the Annual Society for Melanoma Research Meeting; 2016.
- 49.Long GV, Grob JJ, Nathan P et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016;17:1743–1754. [DOI] [PubMed] [Google Scholar]
- 50.Huang AC, Postow MA, Orlowski RJ et al. T‐cell invigoration to tumour burden ratio associated with anti‐PD‐1 response. Nature 2017;545:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joseph RW, Gangadhar TC, Puzanov I et al. Model‐based analysis of the relationship between pembrolizumab (MK‐3475) exposure and efficacy in patients with advanced or metastatic melanoma. J Clin Oncol 2015;33(suppl 15):3068A. [Google Scholar]
- 52.Ackerman A, Klein O, McDermott DF et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 2014;120:1695–1701. [DOI] [PubMed] [Google Scholar]
- 53.Atkins MB, Larkin J. Immunotherapy combined or sequenced with targeted therapy in the treatment of solid tumors: Current perspectives. J Natl Cancer Inst 2016;108:djv414. [DOI] [PubMed] [Google Scholar]
- 54.Saab KR, Mooradian MJ, Wang DY et al. Tolerance and efficacy of BRAF plus MEK inhibition in patients with melanoma who previously have received programmed cell death protein 1‐based therapy. Cancer 2019;125:884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song C, Piva M, Sun L et al. Recurrent tumor cell‐intrinsic and ‐extrinsic alterations during MAPKi‐induced melanoma regression and early adaptation. Cancer Discov 2017;7:1248–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson DB, Menzies AM, Zimmer L et al. Acquired BRAF inhibitor resistance: A multicenter meta‐analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer 2015;51:2792–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hugo W, Zaretsky JM, Sun L et al. Genomic and transcriptomic features of response to anti‐PD‐1 therapy in metastatic melanoma. Cell 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi H, Hugo W, Kong X et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber JS, Gibney G, Sullivan RJ et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): An open‐label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert C, Schadendorf D, Messina M et al. MDX010‐20 investigators . Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res 2013;19:2232–2239. [DOI] [PubMed] [Google Scholar]
- 61.Davies MA, Saiag P, Robert C et al. Dabrafenib plus trametinib in patients with BRAFV600‐mutant melanoma brain metastases (COMBI‐MB): A multicentre, multicohort, open‐label, phase 2 trial. Lancet Oncol 2017;18:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tawbi HA, Forsyth PAJ, Algazi AP, et al. Efficacy and safety of nivolumab (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the brain: Results of the phase II study CheckMate 204. J Clin Oncol 2017;35(suppl 15):9507A. [Google Scholar]
- 63.Long GV, Atkinson V, Menzies AM et al. A randomized phase II study of nivolumab or nivolumab combined with ipilimumab in patients (pts) with melanoma brain metastases (mets): The Anti‐PD1 Brain Collaboration (ABC). J Clin Oncol 2017;35(suppl 15):9508A. [Google Scholar]
- 64.Long GV, Atkinson V, Lo S et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol 2018;19:672–681. [DOI] [PubMed] [Google Scholar]
- 65.Kohutek ZA, Yamada Y, Chan TA et al. Long‐term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 2015;125:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman PB. Changing the standard of care for treating melanoma brain metastases. Lancet Oncol 2018;19:589–591. [DOI] [PubMed] [Google Scholar]
- 67.Sharma P, Hu‐Lieskovan S, Wargo JA et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaretsky JM, Garcia‐Diaz A, Shin DS et al. Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med 2016;375:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hugo W, Shi H, Sun L et al. Non‐genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 2015;162:1271–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson GL, Stuhlmiller TJ, Angus SP et al. Molecular pathways: Adaptive kinome reprogramming in response to targeted inhibition of the BRAF‐MEK‐ERK pathway in cancer. Clin Cancer Res 2014;20:2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber J, Mandala M, Del Vecchio M et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 72.Long GV, Hauschild A, Santinami M et al. Adjuvant dabrafenib plus trametinib in stage III BRAF‐mutated melanoma. N Engl J Med 2017;377:1813–1823. [DOI] [PubMed] [Google Scholar]
- 73.Margolin K, Ernstoff MS, Hamid O et al. Ipilimumab in patients with melanoma and brain metastases: An open‐label, phase 2 trial. Lancet Oncol 2012;13:459–465. [DOI] [PubMed] [Google Scholar]
- 74.Tawbi HA, Forsyth PA, Algazi A et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): A randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dummer R, Goldinger SM, Turtschi CP et al. Vemurafenib in patients with BRAF(V600) mutation‐positive melanoma with symptomatic brain metastases: Final results of an open‐label pilot study. Eur J Cancer 2014;50:611–621. [DOI] [PubMed] [Google Scholar]
- 77.McArthur GA, Maio M, Arance A et al. Vemurafenib in metastatic melanoma patients with brain metastases: An open‐label, single‐arm, phase 2, multicentre study. Ann Oncol 2017;28:634–641. [DOI] [PubMed] [Google Scholar]
- 78.Long GV, Trefzer U, Davies MA et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF‐mutant melanoma metastatic to the brain (BREAK‐MB): A multicentre, open‐label, phase 2 trial. Lancet Oncol 2012;13:1087–1095. [DOI] [PubMed] [Google Scholar]
- 79.Long GV, Eroglu Z, Infante J et al. Long‐term outcomes in patients with BRAF V600‐mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 2018;36:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schadendorf D, Flaherty KT, Hersey P et al. Overall survival (OS) update on METRIC (NCT01245062), a randomized phase 3 study to assess efficacy of trametinib (T) compared with chemotherapy (C) in patients (pts) with BRAFV600E/K mutation‐positive (+) advanced or metastatic melanoma (MM). Pigment Cell Melanoma Res 2013;26:997A. [Google Scholar]
- 81.Dummer R, Ascierto PA, Gogas HJ et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF‐mutant melanoma (COLUMBUS): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2018;19:603–615. [DOI] [PubMed] [Google Scholar]