This article identifies factors associated with patient‐reported satisfaction with multiple myeloma therapy and the treatment‐related time burden and indirect costs among patients with relapsed or refractory multiple myeloma and their caregivers. Improved understanding of these variables will inform treatment decisions across this complex treatment landscape.
Keywords: Relapsed/refractory multiple myeloma, Treatment satisfaction, Time burden, Indirect treatment costs
Abstract
Background.
Therapy choices in relapsed/refractory multiple myeloma (RRMM) should consider patient satisfaction with treatment, because it is associated with adherence to therapy, health outcomes, and medical safety. The primary objective of this pilot cross‐sectional observational study was to ascertain factors associated with patient‐reported treatment satisfaction in RRMM.
Patients and Methods.
Patients with a self‐reported diagnosis of RRMM recruited from PatientsLikeMe, MyelomaCrowd, and Facebook were administered an electronic survey that included questions on demographics and clinical history, treatment experience, economic burden, and standardized patient‐reported outcome measures, including the Treatment Satisfaction Questionnaire for Medication, Eastern Cooperative Oncology Group performance status (ECOG PS) measure, and Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0. Univariable and multivariable analyses were used to identify predictors of patient‐perceived treatment satisfaction.
Results.
One hundred sixty patients with RRMM participated in the study, with a median of two prior relapses and 66.3% reporting the most recent relapse within the last 12 months. ECOG PS ≥2 was associated with lower patient‐reported global satisfaction and perceived effectiveness of current treatment. In addition to shorter time spent receiving therapy, orally administered treatment was the strongest predictor of higher satisfaction with treatment convenience. For patients receiving an injectable drug‐containing regimen versus an all‐oral regimen, respectively, time spent receiving multiple myeloma‐directed therapy was higher (12.6 vs. 4.0 hours per month), and total monthly indirect costs were $1,033 and $241.
Conclusion.
Poor ECOG PS was linked to reduced treatment satisfaction and perceived effectiveness of current therapy, whereas an all‐oral regimen was associated with increased treatment convenience satisfaction.
Implications for Practice.
This study suggests that attributes including better Eastern Cooperative Oncology Group performance status, less time spent receiving treatment, and oral route of treatment administration lead to higher patient‐perceived satisfaction with relapsed/refractory multiple myeloma (RRMM) treatment. Oral route of administration was also associated with less time spent receiving treatment and reduced economic burden for patients. Increased attention to these factors in shared treatment decision making is warranted to help identify individual patient needs, preferences, and expectations for RRMM treatments, to resolve dissatisfaction issues, and to improve the experience of patients with RRMM.
Introduction
Multiple myeloma (MM) is a neoplasm of clonal plasma cells accounting for 1.8% of all cancers. MM is the second most common hematologic malignancy, with an estimated 30,280 new myeloma cases and 12,590 deaths in the U.S. in 2017 [1], [2]. In recent years, with novel therapies and a better understanding of the biology of MM, more patients achieve lasting remission, and there is an increased overall survival rate [3], [4]. However, the majority of MM cases present with patterns of regression and remission followed by relapse [5].
Management of relapsed/refractory MM (RRMM) is challenging because of the need to consider patient‐, disease‐, and treatment‐related factors. Current treatment modalities for RRMM comprise a spectrum of drugs with diverse mechanisms of action, including proteasome inhibitors (bortezomib, carfilzomib, and ixazomib), immunomodulatory agents (lenalidomide, thalidomide, and pomalidomide), monoclonal antibodies (elotuzumab and daratumumab), a histone deacetylase inhibitor (panobinostat), and more traditional treatments (e.g., alkylating agents and corticosteroids) [4], [6]. Autologous stem cell transplantation remains an important option for patients with MM [4], but eligibility is limited by older age and comorbidities. Many treatment regimens incorporate double and triple drug combinations [6].
A recent systematic review revealed a paucity of evidence‐based data describing the impact of therapies on symptoms and quality of life in patients with RRMM, identifying an unmet need to better understand patient burden in this population [7]. Findings from two targeted literature reviews suggested that the total cost of illness in patients with RRMM is driven by treatment choice, as well as symptoms, direct costs, productivity loss, and burden on caregivers [8].
Although direct costs of RRMM have been reported, less is known about patient satisfaction with RRMM therapy, the indirect costs of RRMM, and the impact of disease on caregivers [7], [9], [10].
The objectives of this descriptive study were to identify factors associated with patient‐reported satisfaction with MM therapy and the treatment‐related time burden and indirect costs among patients with RRMM and their caregivers. An improved understanding of these variables will help to facilitate care decisions across the complex RRMM treatment landscape.
Subjects, Materials, and Methods
Study Design and Participants
In this cross‐sectional observational study, participants were recruited between December 2016 and July 2017. The study consisted of a self‐administered electronic survey questionnaire investigating treatment experience, work productivity, and burden of illness in patients with a self‐reported diagnosis of MM. Participants were recruited from (a) PatientsLikeMe (www.patientslikeme.com), an online patient research network that provides a forum for sharing real‐world health experiences in order to improve patient outcomes, allow patients to track their own conditions, and gather crowdsourced data that can be used for disease research; (b) the patient advocacy group MyelomaCrowd; and (c) posts distributed via the Takeda Oncology Facebook page. Interested patients received a link to the survey via e‐mail, private message, or social media. Informed consent for the study was obtained electronically before patients began the electronic survey questionnaire.
Patients were eligible to participate in this study if they were aged ≥18 years, residing in the U.S., and currently receiving treatment for RRMM. Patients were classified as having RRMM if they reported ever changing their MM treatment because of disease progression or recurrence. Patients with concomitant amyloidosis or other cancers in the past 5 years were excluded. Respondents were not remunerated for their participation. The study received ethical approval from the New England Institutional Review Board.
Patient‐Reported Measures
The patient self‐reported survey included questions related to study eligibility criteria, demographic characteristics, treatment background and satisfaction, and time burden associated with MM therapy over the past month prior to study completion.
The survey consisted of patient‐reported outcome measures, including the Treatment Satisfaction Questionnaire for Medication (TSQM‐9) [11], Eastern Cooperative Oncology Group (ECOG) performance status (PS) [12], and the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0 (WPAI:SHP) [13].
The TSQM‐9 is a generic measure to assess treatment satisfaction with medication [11]. The TSQM‐9 includes nine items scored on a five‐ or seven‐point Likert‐type scale that cover three domains corresponding to distinct aspects related to the satisfaction of patients with their treatment (global satisfaction, effectiveness, and convenience). Higher scores on the TSQM‐9 domains indicate higher global satisfaction, better perceived effectiveness, and better convenience [11]. The adapted patient‐reported version of the ECOG PS was used in this study; it consists of a single item evaluating the current performance status of oncology patients [12]. ECOG PS is widely used to quantify the functional status of patients with cancer, and it is an important factor that is used to determine prognosis in oncology [14]. ECOG PS describes functional impairment at the patient level and a patient's ability to participate in self‐care and daily activities, as well as their physical ability. The single item question includes five numerical responses scored on a scale ranging from 0 to 4, with 0 denoting fully active without restriction and 4 defined as completely disabled and unable to carry on any self‐care [12]. The WPAI:SHP measures absenteeism (work time missed), presenteeism (impairment at work), work productivity loss (absenteeism plus presenteeism), and activity impairment and was adapted specifically for MM. In the current patient survey, the WPAI:SHP included one question on current employment status, two questions that assessed the number of hours missed from work because of MM, one question on the number of hours actually worked, one question on the impact of MM on work productivity, and one question on the impact of MM on non‐work‐related daily activities. WPAI:SHP outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity (i.e., worse outcomes).
Customized MM therapy‐related time burden questions included the number of monthly MM‐related treatment visits, the number of visits when the patient was accompanied by a caregiver, the average time to get to the treatment visit, and the average amount of time spent at the treatment visit(s).
Analysis
Continuous variables are summarized as means ± SD and medians and interquartile ranges, whereas categorical measures are summarized as counts and percentages.
Primary outcomes of interest corresponding to each of the three domains of the TSQM‐9 and characteristics associated with greater patient perception of global satisfaction, treatment effectiveness, and convenience (TSQM‐9 domains) were assessed in univariable models.
To identify factors independently associated with each of the three domains of the TSQM‐9, variables of interest and those with p < .1 in univariable analysis of the outcome of interest were assessed using general linear models with a stepwise selection algorithm with an entry and retention p value cutoff of .1 after adjusting for disease status (number of prior relapses, timing of prior relapse) and prior treatment history (prior treatment experience [injectable only vs. any oral therapy exposure or missing], stem cell transplant history). The following potential factors were considered in the model selection: age (continuous), race (white or missing vs. nonwhite), ECOG PS (0–1 vs. 2+), living situation (alone vs. not alone), educational status (less than college or missing vs. college or above), number of agents in current therapy (1 vs. 2 vs. 3+), current treatment administration mode (oral [oral] vs. injectable with or without oral [injectable]), and monthly patient time spent receiving therapy (travel and doctor's visit time). Interactions between current treatment administration mode and monthly patient time spent receiving therapy and between number of agents and administration mode in current therapy were tested for each outcome but were not significant.
The effect sizes on the TSQM‐9 domain were calculated using Cohen's f2 [15]. The global effect size was defined as ratio between the proportion of variation in dependent variable explained by the independent variables and the unexplained variation. In addition, a variation of Cohen's f2 was used to measure the local effect size of each individual independent variable in the multivariate analysis, which reflects the variance uniquely explained by the variable of interest while accounting for other variables. According to Cohen's guideline, the thresholds for defining a small, medium, and large effect size are f2 ≥ 0.02, f2 ≥ 0.15, and f2 ≥ 0.35, respectively.
Time burden was calculated based on responses to the customized MM therapy questions. Average travel time burden per month was defined as the number of doctor's visits per month multiplied by the average time for round‐trip travel. Average time spent at a doctor's visit per month was defined as the number of doctor's visits per month multiplied by the average time spent at the visit(s). The total time burden placed on patients as a result of monthly doctor's visits was defined as the sum of the average travel time per month and the average time spent at the doctor's visit per month. The total time burden placed on caregivers as a result of monthly doctor's visits was defined as the number of doctor's visits accompanied by caregivers multiplied by the average time for round‐trip travel plus average time spent at the doctor's visit. Monthly estimates of indirect treatment‐related costs were obtained from publicly available data sources (supplemental online Table 1) and combined with survey responses to determine economic burden. A review of the patient and treatment characteristics with the greatest predictive correlation to TSQM‐9 outcomes was used to guide further subgroup analyses. Because current treatment administration mode was the strongest predictor of the TSQM‐9 outcomes, resource utilization and economic burden of illness outcomes were compared between patients currently receiving oral versus injectable therapy. Monthly costs and time burden were compared by fitting generalized linear models (GLMs) with gamma distribution and a log link, and GLMs with a Poisson distribution and a log link were used for comparison of number of doctor's visits per month. Multivariable analyses to adjust for confounders between time burden, economic burden of illness outcomes, and current mode of administration were adjusted for age, ECOG PS, number of prior relapses, timing of prior relapse, prior stem cell transplant history, and number of agents in current therapy (1 vs. 2 vs. 3+). All data analysis was conducted in SAS 9.4 (SAS Institute Inc., Cary, NC). Unless otherwise stated, all analyses were two‐tailed with a significance level of .05.
Results
Population Characteristics
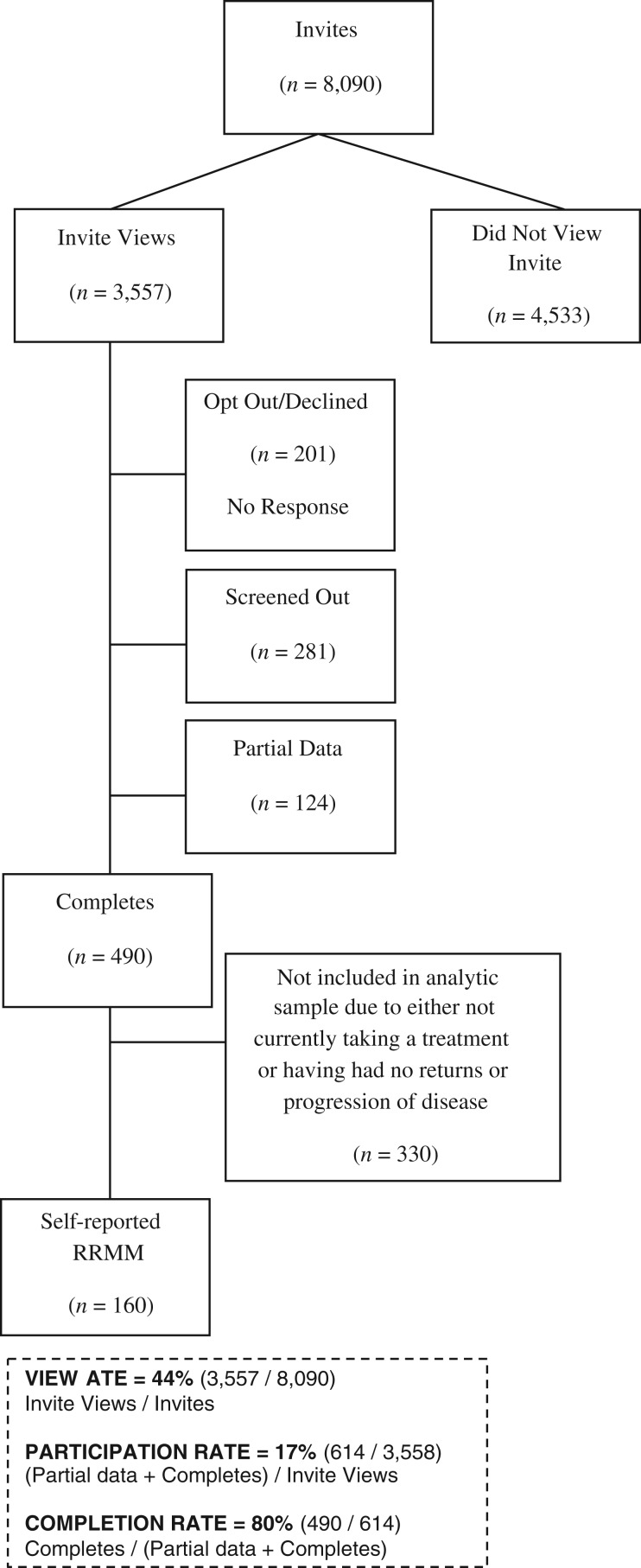
The demographic and clinical characteristics of the 160 patients with RRMM participating in the study (Fig. 1) are summarized in Table 1. Overall, the median age was 62 years, 61% were female, 85% were white, and 71% had an ECOG PS of 0–1. The majority of patients were not living alone (83%), and 44% had at least a college education. Forty‐eight percent were unemployed (retired or homemaker), followed by 29% employed and 23% medically unable to work. The median ages of these three groups were 68, 55, and 58 years, respectively. The vast majority of patients (>90%) reported having medical and pharmacy insurance coverage.
Figure 1.
Flow diagram of study sample.
Abbreviation: RRMM, relapsed/refractory multiple myeloma.
Table 1. Demographic characteristics of patients with relapsed/refractory multiple myeloma.
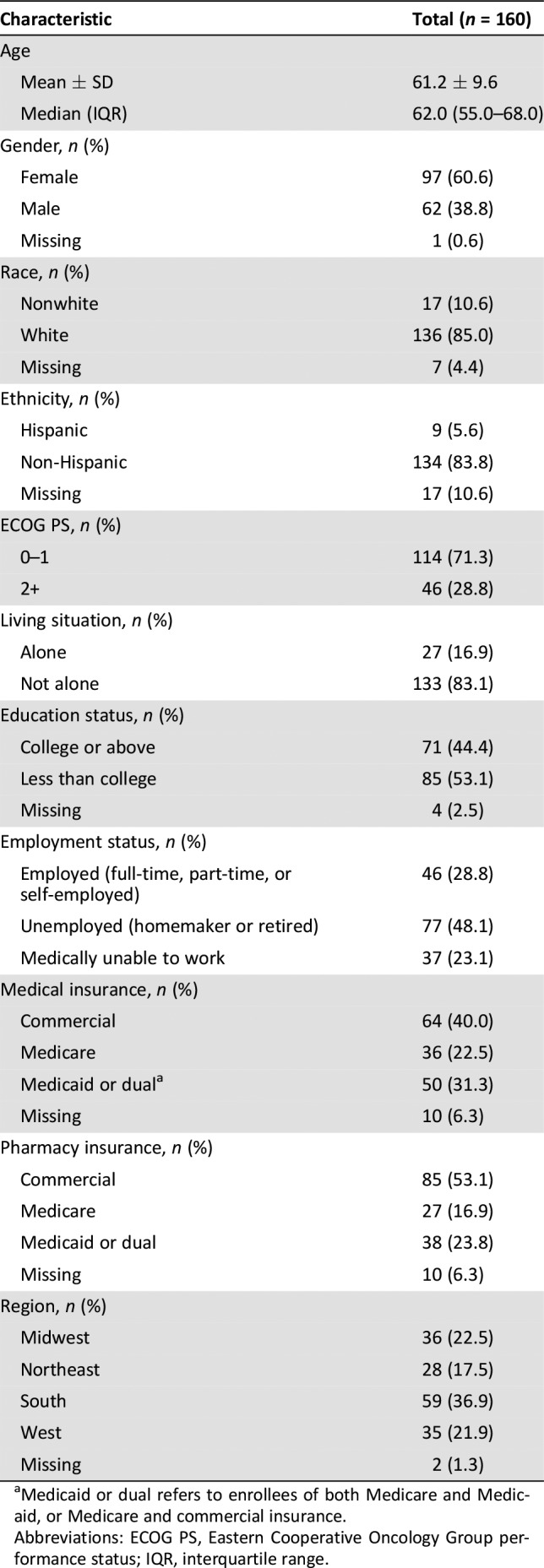
Medicaid or dual refers to enrollees of both Medicare and Medicaid, or Medicare and commercial insurance.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range.
Patient‐reported current and previous treatment characteristics are shown in Table 2. The majority of patients had prior stem cell transplant (66%) and a median of two prior relapses, with 66% reporting the most recent relapse within the last 12 months. Most patients had previous experience with an orally administered treatment with or without injectable therapy (81%). The majority of patients (65%) were currently treated with a doublet regimen or monotherapy. One third of patients (n = 55) were currently receiving therapy consisting of orally administered agents only.
Table 2. Baseline treatment characteristics among patients with relapsed/refractory multiple myeloma.
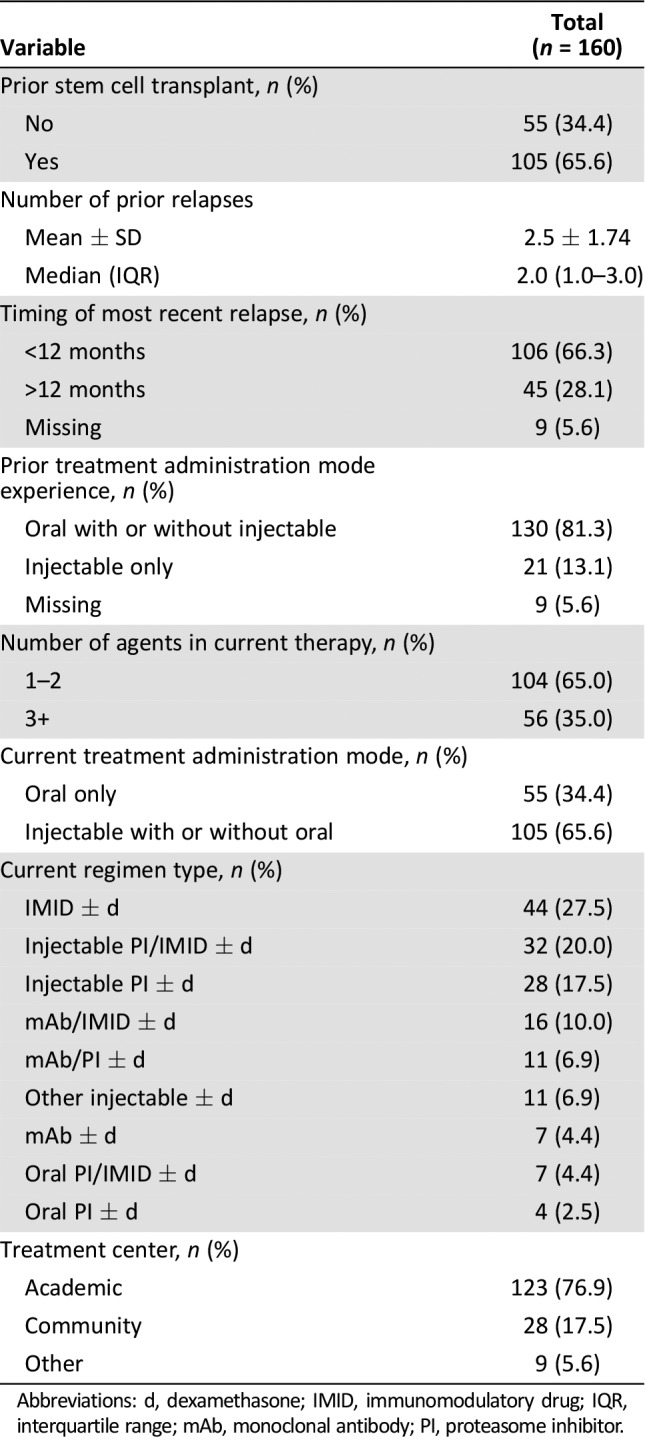
Abbreviations: d, dexamethasone; IMID, immunomodulatory drug; IQR, interquartile range; mAb, monoclonal antibody; PI, proteasome inhibitor.
Predictors of Treatment Satisfaction – TSQM‐9 Scales
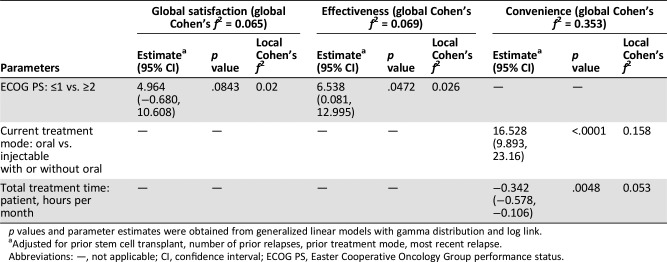
Univariable model results and final selected multivariable model results after adjustment for prior treatment history and disease status are listed in supplemental online Tables 1 and 3. In multivariable analyses, ECOG PS was the single most important factor associated with global satisfaction with MM treatment. Patients with ECOG PS ranging from 0 to 1 had a mean predicted score of 54 compared with 49 among those with ECOG PS ≥2 (Table 3; p = .0843). Likewise, ECOG PS was significantly associated with the effectiveness scale; patients with an ECOG PS of 0–1 had on average seven‐point higher scores than those with ECOG PS ≥2 (Table 3; p = .0472). Interestingly, patient‐perceived global satisfaction and effectiveness with current therapy were not influenced by number of agents or by the route of administration in current therapy. In contrast, current treatment administration mode was predictive of treatment convenience. Patients treated with an all‐oral regimen reported 17‐point higher scores on the convenience scale compared with patients who were receiving at least one injectable agent in current treatment (Table 3; p < .0001). In addition, amount of time patients spent per month in receiving therapy was a significant predictor of convenience, with longer time leading to lower scores on the convenience scale (Table 3; p = .0048). In tandem, the global effect size for the two predictors of the convenience scale was large, but small for the global satisfaction and effectiveness scales.
Table 3. Predictors of treatment satisfaction – Treatment Satisfaction Questionnaire for Medication scales.
p values and parameter estimates were obtained from generalized linear models with gamma distribution and log link.
Adjusted for prior stem cell transplant, number of prior relapses, prior treatment mode, most recent relapse.
Abbreviations: —, not applicable; CI, confidence interval; ECOG PS, Easter Cooperative Oncology Group performance status.
Patient Work and Activity Impairment
Patient work and activity impairment are summarized in Table 4. Patients who were employed (n = 46) reported a median of 30% work impairment while working and 40% overall work impairment because of MM, as measured by the WPAI:SHP questionnaire. The median level of activity impairment as a result of their MM for the overall cohort was 40%.
Table 4. Work and activity impairment among patients with relapsed/refractory multiple myeloma.
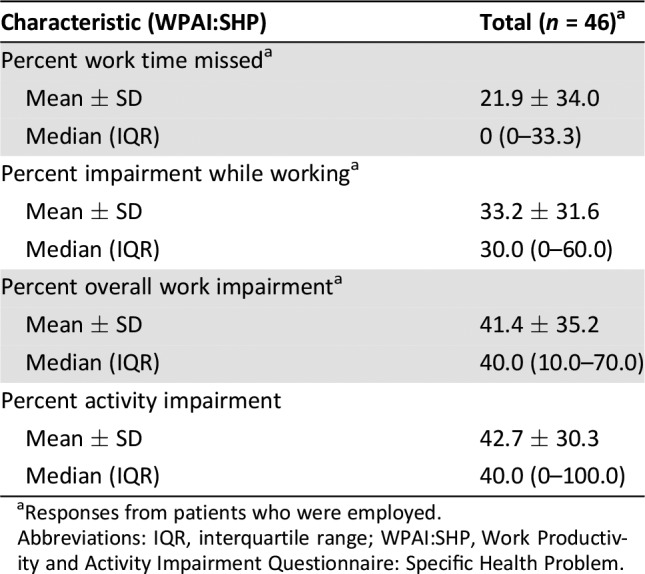
Responses from patients who were employed.
Abbreviations: IQR, interquartile range; WPAI:SHP, Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
Patient and Caregiver Time Burden
Patient and caregiver burdens related to time spent receiving MM treatment results are listed in Table 5. Patients in the injectable group reported a significantly higher monthly time burden of MM management for both themselves and caregivers when compared with the oral group (adjusted mean: 12.6 vs. 4.0 hours per month for patients; 7.3 vs. 3.6 hours per month for caregivers). This higher burden was due to more doctor's visits (adjusted mean: 3.2 vs. 0.9 hours per month), more time required to travel to treatment centers (adjusted mean: 5.1 vs. 2.7 hours per month), and more time spent at doctor's office (adjusted mean: 8.1 vs. 2.5 hours per month) each month compared with the oral group (p < .01 for all comparisons).
Table 5. Patient and caregiver monthly burden of multiple myeloma management by route of administration.
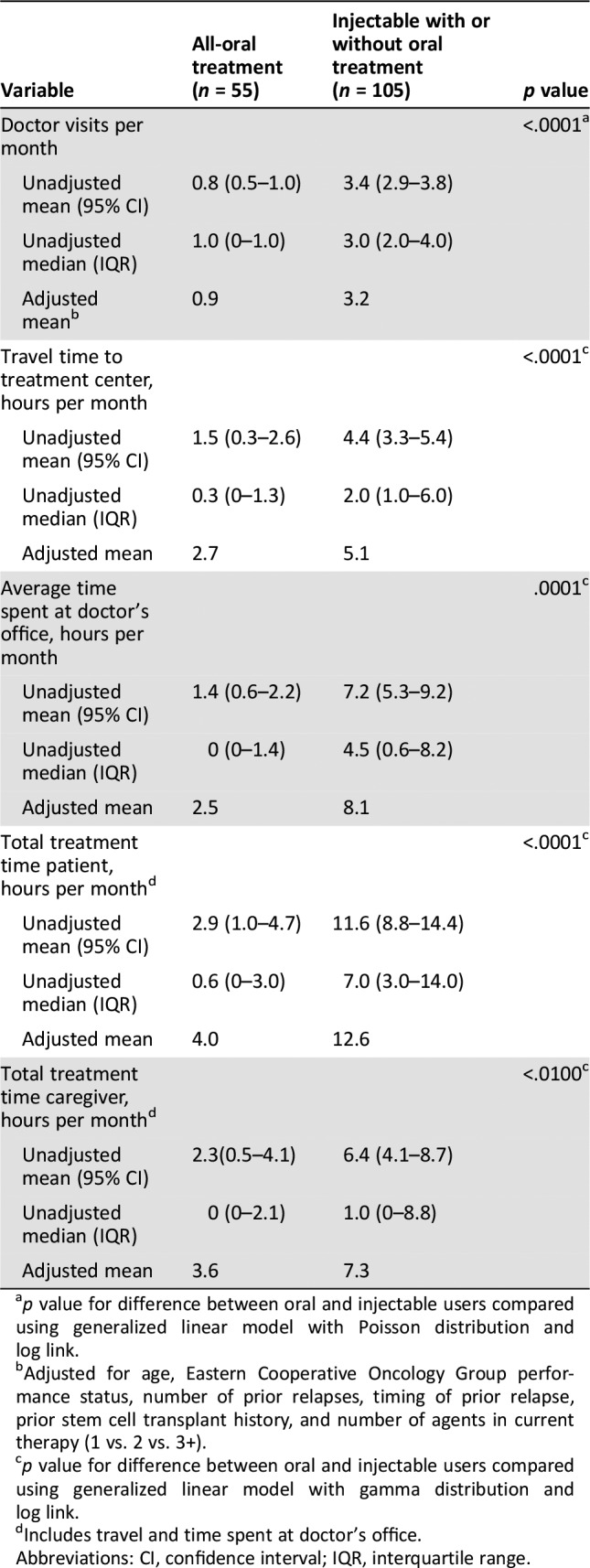
p value for difference between oral and injectable users compared using generalized linear model with Poisson distribution and log link.
Adjusted for age, Eastern Cooperative Oncology Group performance status, number of prior relapses, timing of prior relapse, prior stem cell transplant history, and number of agents in current therapy (1 vs. 2 vs. 3+).
p value for difference between oral and injectable users compared using generalized linear model with gamma distribution and log link.
Includes travel and time spent at doctor's office.
Abbreviations: CI, confidence interval; IQR, interquartile range.
Indirect Costs of MM Therapy
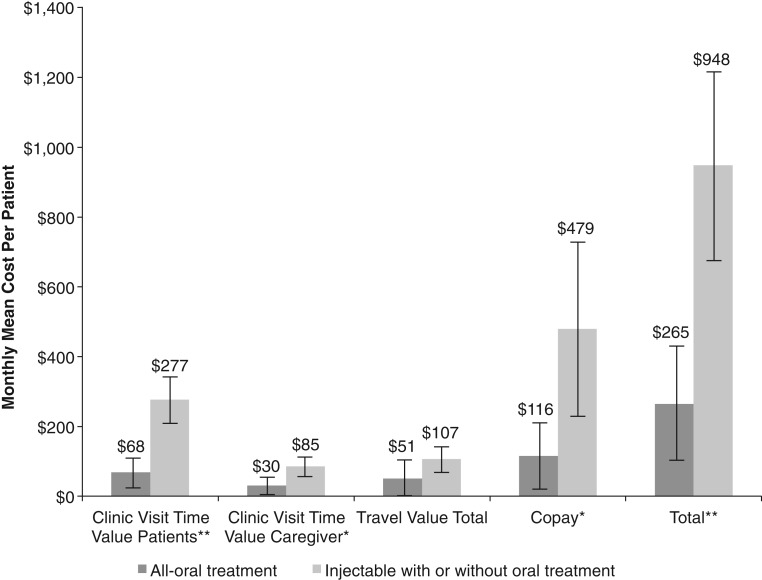
The estimated economic burden of treatment by route of administration is shown in Figure 2. Total unadjusted monthly costs were higher in the injectable compared with the oral group ($948 vs. $265, p < .0010). Monthly mean cost for doctor visit copay ($479 vs. $116, p < .0100), patient monthly time value receiving MM therapy ($277 vs. $68, p < .0010), and caregivers monthly time value ($85 vs. $30, p < .0100) were significantly higher for the injectable than oral group. MM drug copays, however, were not collected. After adjusting for age, ECOG PS, number of prior relapses, timing of prior relapse, prior stem cell transplant history, and number of agents in current therapy (1 vs. 2 vs. 3+), the mean monthly costs in the injectable versus the oral group were $1,033 versus $241 (total costs), $508 versus $109 (doctor visit copay), $301 versus $65 (patient monthly time value receiving therapy), $98 versus $32 (caregiver monthly time value receiving therapy), and $124 versus $46 (total travel value; p < .05 for all comparisons).
Figure 2.
Monthly mean costs for multiple myeloma treatment per patient per route of administration. Error bars represent 95% confidence intervals. Missing values were imputed by group mean. *p < .0100, **p < .0010.
Discussion
The present pilot study identified attributes associated with patient‐reported satisfaction with their current therapy and described the treatment‐related time burden and indirect costs of patients with RRMM and their caregivers. Findings showed that worse performance status was independently correlated with lower patient‐reported global satisfaction and patient‐perceived effectiveness of their current treatment. Surprisingly, the number of agents or route of administration of current therapy was not associated with patient‐reported global satisfaction or patient‐perceived effectiveness of treatment. However, the use of oral therapy was associated with increased perceived treatment convenience, whereas the use of therapies containing an injectable agent was associated with increased time burden for patients and caregivers and higher indirect costs compared with all‐orally administered therapies.
Findings revealed that in patients with RRMM, performance status was the most important predictor of global satisfaction and patient‐perceived effectiveness of current treatment. This link is in accordance with previous reports in cohorts of patients with other malignancies. In an exploratory analysis of data from a multicenter, open‐label, long‐term study of fentanyl pectin nasal sprays, patients with breakthrough pain in cancer who had higher performance score (lower ECOG) reported higher satisfaction with treatment [16]. In patients with advanced cancer in the Swiss oncology network, PS was a significant predictor of patients’ satisfaction with treatment decisions [17]. Similarly, in patients undergoing ambulatory chemotherapy or radiotherapy in two oncology centers in France, patients’ global health was significantly associated with satisfaction with health care providers and other aspects of care organization and services [18]. If confirmed in prospective clinical trials, these findings suggest that increased attention to shared treatment decision making among patients with worse performance status is warranted to help identify individual patient needs, preferences, and expectations for RRMM treatments.
In our analysis, use of orally administered drugs in current RRMM therapy was a predictor of perceived treatment convenience and decreased time burden. In various studies, patients with cancer have reported preference for oral therapy if efficacy is not compromised, with improved convenience mentioned as one of the main advantages when compared with injectable therapy [19], [20], [21]. In RRMM, relapse or disease progression is associated with an increase in the frequency of hospital and clinic visits, as well as an increase in time spent at the hospital and in clinics [22]. Orally administered cancer drugs may reduce the number and duration of outpatient visits and positively impact the overall time burden associated with treatment [20], [23]. In addition, a literature review suggests that greater treatment satisfaction is associated with better treatment compliance and improved persistence [24]. Patients with RRMM require long‐term treatment, and those who are satisfied with their treatments are more likely to participate in the management of their disease, adhere to therapy, and achieve the best possible outcomes [9], [19], [25], [26], [27], [28]. Whether convenience impacts adherence in MM needs to be confirmed in future studies.
In the present study, higher indirect costs were observed for patients on regimens containing at least one injectable drug compared with all‐oral RRMM regimens. In accordance with these findings, evidence suggests that the majority of patients with MM (71%) report at least some financial burden associated with their disease [29], and for patients with RRMM, the leading drivers of indirect costs are out‐of‐pocket expenses and costs associated with frequent office visits [29], [30], [31]. Previous studies of patients with cancer show that indirect costs are influenced by route of administration of therapies. In an analysis of U.S. patients with cancer using claims data extracted between 2004 and 2013, outpatient services, doctor visits, and absenteeism were higher when therapy was administered by physicians compared with self‐administered therapy [32]. The present study did not examine out‐of‐pocket costs related to MM drug copays, which may further impact patient burden. A recent analysis of commercially insured patients with MM reported monthly mean patient out‐of‐pocket expenditures for MM‐directed drug therapy of $81, with $42 and $39 per month spent on injectable and oral therapies, respectively [33]. In another study, 36% of patients with MM reported applying for financial assistance, and 21% borrowed money to pay for medications [29].
The present analysis was associated with several limitations. First, the small sample size and lack of randomization in treatment assignment led to possible selection bias in treatment administration to individual patients. Second, the nature of the data collection relied solely on patient self‐report without clinical validation of patient‐, disease‐, and treatment‐related factors that may confound patient‐reported outcomes, as well as the possibility of recall or confirmation bias. Third, this is the first study to use the TSQM‐9 scale to investigate satisfaction with current treatment for patients with RRMM. This scale provides a general measure of patients’ satisfaction with medication and was previously validated across different types of medication and diverse patient populations, including patients with cancer; however, it has not been validated in RRMM [11], [34]. Fourth, included patients may not be representative of the general population of patients with RRMM, as patients in online communities are likely to be a self‐selected sample of patients who are predominantly female and educated. Fifth, financial analyses were performed for the U.S., and findings may not be generalizable to other countries. Finally, this was a cross‐sectional study reflecting the patient experience of a month of the RRMM treatment process, which usually requires continuous therapies with multiple regimens. Furthermore, patients had required a change in their treatment because of disease progression or recurrence, which places some limits on the generalizability of the effects of treatment characteristics on treatment satisfaction [4], [6].
Conclusion
Limitations notwithstanding, this pilot study showed that patient ECOG PS, and not the number of agents or route of administration, was associated with patient‐reported global satisfaction and perceived effectiveness of their current treatment for patients with RRMM. The data suggest that an oral regimen is associated with a higher level of convenience for patients with RRMM and lower health care resource use than an injectable regimen, although MM drug copays were not available. The use of oral drugs could alleviate some of the RRMM treatment‐associated burden for both patients and caregivers, including time and costs. Despite the increasing number of therapeutic choices available for RRMM and routine mention of taking into account patient preferences in treatment selection, there is a dearth of evidence‐based data on these patient preferences in RRMM. The present study begins to bridge the gap between patient satisfaction and the burden of RRMM treatment to better inform treatment decision making.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Ajai Chari, Dorothy Romanus, Pronabesh DasMahapatra, Michael Hoole, Maria Lowe, Chris Curran, Scott Campbell, Jill A. Bell
Provision of study material or patients: Ajai Chari, Dorothy Romanus, Pronabesh DasMahapatra, Michael Hoole, Maria Lowe, Chris Curran, Scott Campbell, Jill A. Bell
Collection and/or assembly of data: Ajai Chari, Dorothy Romanus, Pronabesh DasMahapatra, Michael Hoole, Maria Lowe, Chris Curran, Scott Campbell, Jill A. Bell
Data analysis and interpretation: Ajai Chari, Dorothy Romanus, Pronabesh DasMahapatra, Michael Hoole, Maria Lowe, Chris Curran, Scott Campbell, Jill A. Bell
Manuscript writing: Ajai Chari, Dorothy Romanus, Pronabesh DasMahapatra, Michael Hoole, Maria Lowe, Chris Curran, Scott Campbell, Jill A. Bell
Final approval of manuscript: Ajai Chari, Dorothy Romanus, Pronabesh DasMahapatra, Michael Hoole, Maria Lowe, Chris Curran, Scott Campbell, Jill A. Bell
Disclosures
Ajai Chari: Takeda (RF, H); Dorothy Romanus: Takeda (E, OI); Michael Hoole: PatientsLikeMe (E, OI), Abbvie, Accorda, Actelion, Alexion, Amgen, AstraZeneca, Avanir, Biogen, Boehringer Ingelheim, Celgene, EMD, Genentech, Genzyme, Janssen, Johnson & Johnson, Merck, Neuraltus, Novartis, Otsuka, Permobil, Pfizer, Sanofi, Shire, Takeda, Teva, UCB (RF—institutional); Maria Lowe: Abbvie, Accorda, Actelion, Alexion, Amgen, AstraZeneca, Avanir, Biogen, Boehringer Ingelheim, Celgene, EMD, Genentech, Genzyme, Janssen, Johnson & Johnson, Merck, Neuraltus, Novartis, Otsuka, Permobil, Pfizer, Sanofi, Shire, Takeda, Teva, UCB, Kaiser Permanente, the Robert Wood Johnson Foundation, Sage Bionetworks, The AKU Society, University of Maryland (RF); Chris Curran: Abbvie, Accorda, Actelion, Alexion, Amgen, AstraZeneca, Avanir, Biogen, Boehringer Ingelheim, Celgene, EMD, Genentech, Genzyme, Janssen, Johnson & Johnson, Merck, Neuraltus, Novartis, Otsuka, Permobil, Pfizer, Sanofi, Shire, Takeda, Teva, UCB, Kaiser Permanente, the Robert Wood Johnson Foundation, Sage Bionetworks, The AKU Society, the University of Maryland (RF); Scott Campbell: Takeda (E, OI); Jill A. Bell: Takeda (E, OI). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Turesson I, Bjorkholm M, Blimark CH et al. Rapidly changing myeloma epidemiology in the general population: Increased incidence, older patients, and longer survival. Eur J Haematol 2018;101:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel, RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet 2015;385:2197–2208. [DOI] [PubMed] [Google Scholar]
- 4.Chim CS, Kumar SK, Orlowski RZ et al. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2017;32:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genadieva‐Stavric S, Cavallo F, Palumbo A. New approaches to management of multiple myeloma. Curr Treat Options Oncol 2014;15:157–170. [DOI] [PubMed] [Google Scholar]
- 6.Boudreault JS, Touzeau C, Moreau P. Triplet combinations in relapsed/refractory myeloma: Update on recent phase 3 trials. Expert Rev Hematol 2017;10:207–215. [DOI] [PubMed] [Google Scholar]
- 7.Sparano F, Cavo M, Niscola P et al. Patient‐reported outcomes in relapsed/refractory multiple myeloma: A systematic review. Support Care Cancer 2018:26:2075–2090. [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Kay G, O'Rourke J et al. Burden of illness associated with multiple myeloma. Poster presented at: 21st Congress of the European Hematology Association, Copenhagen, Denmark, June 9–12, 2016; Abstract E1443.
- 9.Efficace F, Boccadoro M, Palumbo A et al. A prospective observational study to assess clinical decision‐making, prognosis, quality of life and satisfaction with care in patients with relapsed/refractory multiple myeloma: The CLARITY study protocol. Health Qual Life Outcomes 2018;16:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez‐McQuire S, Yong K, Leleu H et al. Healthcare resource utilization among patients with relapsed multiple myeloma in the UK, France, and Italy. J Med Econ 2018;21:450–467. [DOI] [PubMed] [Google Scholar]
- 11.Bharmal M, Payne K, Atkinson MJ et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM‐9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E, Artz D, Dulko D et al. Patient online self‐reporting of toxicity symptoms during chemotherapy. J Clin Oncol 2005;23:3552–3561. [DOI] [PubMed] [Google Scholar]
- 13.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–365. [DOI] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 15.Cohen JE. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 16.Torres LM, Revnic J, Knight AD et al. Relationship between onset of pain relief and patient satisfaction with fentanyl pectin nasal spray for breakthrough pain in cancer. J Palliat Med 2014;17:1150–1157. [DOI] [PubMed] [Google Scholar]
- 17.Hitz F, Klingbiel D, Ribi K. Predictors of satisfaction with treatment decision, decision‐making preferences, and main treatment goals in patients with advanced cancer. Support Care Cancer 2013;21:3085–3093. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TV, Anota A, Bredart A et al. A longitudinal analysis of patient satisfaction with care and quality of life in ambulatory oncology based on the OUT‐PATSAT35 questionnaire. BMC Cancer 2014;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eek D, Krohe M, Mazar I et al. Patient‐reported preferences for oral versus intravenous administration for the treatment of cancer: A review of the literature. Patient Prefer Adherence 2016;10:1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borner M, Scheithauer W, Twelves C et al. Answering patients’ needs: Oral alternatives to intravenous therapy. The Oncologist 2001;6:12–16. [DOI] [PubMed] [Google Scholar]
- 21.Fallowfield L, Atkins L, Catt S et al. Patients’ preference for administration of endocrine treatments by injection or tablets: Results from a study of women with breast cancer. Ann Oncol 2006;17:205–210. [DOI] [PubMed] [Google Scholar]
- 22.Hulin C, Hansen T, Heron L et al. Living with the burden of relapse in multiple myeloma from the patient and physician perspective. Leukemia Res 2017;59:75–84. [DOI] [PubMed] [Google Scholar]
- 23.Weingart SN, Brown E, Bach PB et al. NCCN task force report: Oral chemotherapy. J Natl Compr Canc Netw 2008;6:S1–14. [PubMed] [Google Scholar]
- 24.Barbosa CD, Balp MM, Kulich K et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012;6:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta D, Rodeghier M, Lis CG. Patient satisfaction with service quality as a predictor of survival outcomes in breast cancer. Support Care Cancer 2014;22:129–134. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs JM, Pensak NA, Sporn NJ et al. Treatment satisfaction and adherence to oral chemotherapy in patients with cancer. J Oncol Pract 2017;13:e474–e485. [DOI] [PubMed] [Google Scholar]
- 27.Leleu X, Kyriakou C, Vande Broek I et al. Prospective longitudinal study on quality of life in relapsed/refractory multiple myeloma patients receiving second‐ or third‐line lenalidomide or bortezomib treatment. Blood Cancer J 2017;7:e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hari P, Romanus D, Palumbo A et al. Prolonged duration of therapy is associated with improved survival in patients treated for relapsed/refractory multiple myeloma in routine clinical care in the United States. Clin Lymphoma Myeloma Leuk 2018;18:152–160. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin JA, Coleman EA, Sullivan E et al. Personal financial effects of multiple myeloma and its treatment. Cancer Nurs 2013;36:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huntington SF, Weiss BM, Vogl DT et al. Financial toxicity in insured patients with multiple myeloma: A cross‐sectional pilot study. Lancet Haematol 2015;2:e408–e416. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Abouzaid S, Liebert R et al. Assessing the effect of adherence on patient‐reported outcomes and out of pocket costs among patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2018;18:210–218. [DOI] [PubMed] [Google Scholar]
- 32.Seabury SA, Frasco MA, van Eijndhoven E et al. The impact of self‐ and physician‐administered cancer treatment on work productivity and healthcare utilization. Res Social Adm Pharm 2018;14:434–440. [DOI] [PubMed] [Google Scholar]
- 33.Seal B, Schwartz S, Jhaveri M et al. Out of pocket economic burden among commercially insured patients newly diagnosed with multiple myeloma in the U.S. Poster presented at: 21st Congress of the European Hematology Association, Copenhagen, Denmark, June 9–12, 2016.
- 34.Atkinson MJ, Sinha A, Hass SL et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]