This article reports the results of a phase II trial that was conducted to evaluate the efficacy and safety of continuous
Keywords: Gastrointestinal stromal tumors, Regorafenib, Therapeutics, Drug administration schedule
Abstract
Background.
Regorafenib at the standard intermittent dosing schedule proved effective in the GRID trial for refractory gastrointestinal stromal tumors (GISTs). However, this dosing schedule requires frequent dose reduction, and the progression of GISTs or tumor‐related symptoms during the off‐treatment period has also been noted in some patients. Therefore, we conducted this phase II trial to evaluate the efficacy and safety of regorafenib at a lower dose on a continuous dosing schedule.
Methods.
Patients with measurable, metastatic, or recurrent GISTs who failed to respond to both imatinib and sunitinib were eligible for this study. Regorafenib 100 mg p.o. daily was administered continuously. The primary endpoint was disease control rate (DCR: complete response plus partial response [PR] plus stable disease [SD]) lasting for at least 12 weeks using RECIST version 1.1.
Results.
The best response was PR in 2 (8%), SD in 16 (64%), and progressive disease in 6 (24%) patients. DCR lasting for at least 12 weeks was 64% (16 of 25). The median progression‐free survival was 7.3 months (95% confidence interval, 5.9–8.6), and the 1‐year survival rate was 64.5%. Ten patients (40%) experienced grade 3–4 toxicities, including hand‐foot skin reaction (n = 4, 16%) and elevation of alanine aminotransferase (n = 2, 8%). Only six patients (24%) needed dose modification with a relative dose intensity of 95.0% for eight cycles in all patients.
Conclusion.
Regorafenib at a lower dose on a continuous schedule might be an alternative treatment in patients with GISTs after failure of imatinib and sunitinib. Clinical trial identification number. NCT02889328
Implications for Practice.
Regorafenib at the standard intermittent dosing schedule proved effective in the GRID trial for refractory gastrointestinal stromal tumors (GISTs). However, this dosing schedule requires frequent dose reduction, and the progression of GISTs or tumor‐related symptoms during the off‐treatment period has been noted in some patients. This study was to evaluate the efficacy and safety of regorafenib at a lower dose on a continuous dosing schedule. With good efficacy and acceptable safety profiles, regorafenib at a lower, continuously administered dose might be an alternative treatment in patients with GISTs after imatinib and sunitinib. Rechallenge of regorafenib may slow the disease progression.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common sarcomas of the mesenchymal tissue of gastrointestinal tract [1], [2]. Most of these tumors are characterized by mutation in the KIT or platelet‐derived growth factor receptor α (PDGFRA) genes [3], [4]. Because of receptor tyrosine kinase dependence, GISTs can be treated with tyrosine kinase inhibitors such as imatinib, which has activity against KIT, PDGFRA, and ABL [1], [5].
Localized resectable GISTs can be cured with surgical resection. In patients with unresectable or metastatic GISTs, imatinib showed long‐term efficacy and has been the standard first‐line therapy [2], [6]. In patients with progression or intolerance to imatinib, sunitinib is the approved second‐line therapy with a median time to progression of approximately 7 months in a randomized phase III trial [7]. Although many novel agents have been tested in the setting of failure of both imatinib and sunitinib, only regorafenib has been approved as the standard third‐line therapy after a randomized phase III trial, which showed a median progression‐free survival (PFS) of approximately 5 months in the regorafenib arm [8]. Current guidelines suggest treatment strategies of GISTs based on the results of these studies [9], [10].
Although third‐line regorafenib therapy of 160 mg once daily for 3 weeks followed by 1 week off demonstrated significant benefits in patients with GISTs who had failed both imatinib and sunitinib, dose modification was frequently required because of various toxicities. In the GRID study, 72% of patients needed dose modification. In addition, there are concerns that tumors and tumor‐related symptoms may progress during the off‐treatment period. In our previous study, approximately 26% of patients experienced an exacerbation of their cancer‐related symptoms during the rest period in the intermittent regorafenib regimen [11]. Therefore, continuous administration of regorafenib at a lower dose could be a feasible and effective measure in preventing disease flare‐up during the off‐treatment period. The present study assessed the efficacy and safety of a continuous daily dosing schedule of regorafenib in patients with GISTs after the failure of imatinib and sunitinib.
Subjects, Materials, and Methods
Patients
The eligibility criteria for this study included age ≥20 years; histologically confirmed metastatic or advanced GISTs; prior failure (disease progression or intolerance) of at least imatinib and sunitinib; no prior use of regorafenib; an Eastern Cooperative Oncology Group (ECOG) performance status ≤1; resolution of all toxic effects of prior treatments; presence of at least one measurable lesion; adequate bone marrow, hepatic, and renal function as assessed in a laboratory test; and life expectancy ≥12 weeks. Women of childbearing potential and men had to agree to use adequate contraception until at least 8 weeks after the last regorafenib administration.
Study Design and Procedures
This was a nonrandomized, open‐label, single arm, phase II study conducted at Asan Medical Center, University of Ulsan, Seoul, South Korea. The patients received regorafenib 100 mg orally (p.o.) daily continuously every 4 weeks (28 days). Each 100 mg dose consisted of two 40 mg tablets and one 20 mg tablet. The 40 mg tablet was a commercially available tablet, and the 20 mg formulation was a tablet specifically designed for this study. Patients received a total of 100 mg of regorafenib once daily in the morning. Patients continued regorafenib treatment until disease progression as defined by RECIST version 1.1, unacceptable toxicity, or consent withdrawal. For patients experiencing disease progression, further administration was allowed if a clinical benefit was evident according to the treating physician.
Medication was delayed or the dose was reduced in case of clinically significant hematological and other toxicities that were possibly, probably, or definitely related to protocol therapy. Dose reduction was allowed over two steps of 80 mg daily first and then 60 mg daily. Dose reduction lower than 60 mg daily was not allowed. If the dose needed to be reduced lower than 60 mg, treatment would be discontinued. Dose reescalation was allowed after resolution of toxicities.
Baseline and follow‐up images were evaluated using triphasic or dynamic computed tomography (CT) scans. A pretreatment baseline CT scan was performed within 2 weeks prior to the start of regorafenib medication. Follow‐up CT scans were performed every 8 weeks (± 7 days) until disease progression or death. Disease measurement and assessments of progression were performed using RECIST version 1.1. Hematologic adverse events were investigated at every cycle (every 4 weeks) through blood sampling. Adverse events were assessed before commencing each treatment cycle (every 4 weeks) using the U.S. National Cancer Insitute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Blood was collected for plasma free‐circulating tumor DNA assay before regorafenib treatment, every 3 months, and at the time of disease progression.
The primary endpoint of this study was disease control rate (DCR) lasting for at least 12 weeks according to RECIST version 1.1. The secondary endpoint was PFS, overall survival (OS), response rate, and adverse events determined using CTCAE version 4.03. As an exploratory endpoint, we investigated the correlation between the mutation or entity of serum free‐circulating tumor DNA and clinical outcomes, which will be reported later.
The study protocol was approved by the institutional review board, and all participants provided written informed consent before enrolment.
Statistical Analysis
The sample size was estimated using Simon single‐stage design. In the GRID and RIGHT trials, DCR at 12 weeks with intermittent regorafenib and imatinib rechallenge were 53% and 32%, respectively [8], [12]. The current study needed 25 patients to detect a 25% difference in the DCR at least 12 weeks between the null hypothesis of 30% and the alternative hypothesis of 55% using a significance level of .1 and power of 90%. If 11 or more patients achieved disease control by at least 12 weeks, the null hypothesis would be rejected.
PFS was defined as the time from the first day of treatment until the confirmation of disease progression or death as a result of any cause. OS was defined as the time from the first day of treatment until death as a result of any cause. The second PFS was defined as the time from the confirmation of progression with regorafenib until the progression with further regorafenib treatment or death. The Kaplan‐Meier method was used to calculate the PFS and OS. All calculations were performed using SPSS computer software version 21.0 (IBM Corporation, Armonk, NY).
Results
Patient Characteristics
From September 2016 to August 2017, 25 patients were enrolled with a median age of 60 (range, 42–74) years, and male patients were dominant (84%). All patients had an ECOG performance status of 1. The small bowel was the most common primary site (n = 15, 60%). The KIT exon 11 mutation was the most common (n = 16, 64%) primary mutation. The liver and peritoneum were the most common site of metastasis (n = 19, 76%). All patients had a history of failure of prior treatment with imatinib and sunitinib, and one patient was treated with nilotinib. The median treatment duration of imatinib 400 mg/day and sunitinib was 25.0 months (range, 1.8–86.3 months) and 8.3 months (range, 0.7–37.5 months), respectively (Table 1).
Table 1. Baseline characteristics.
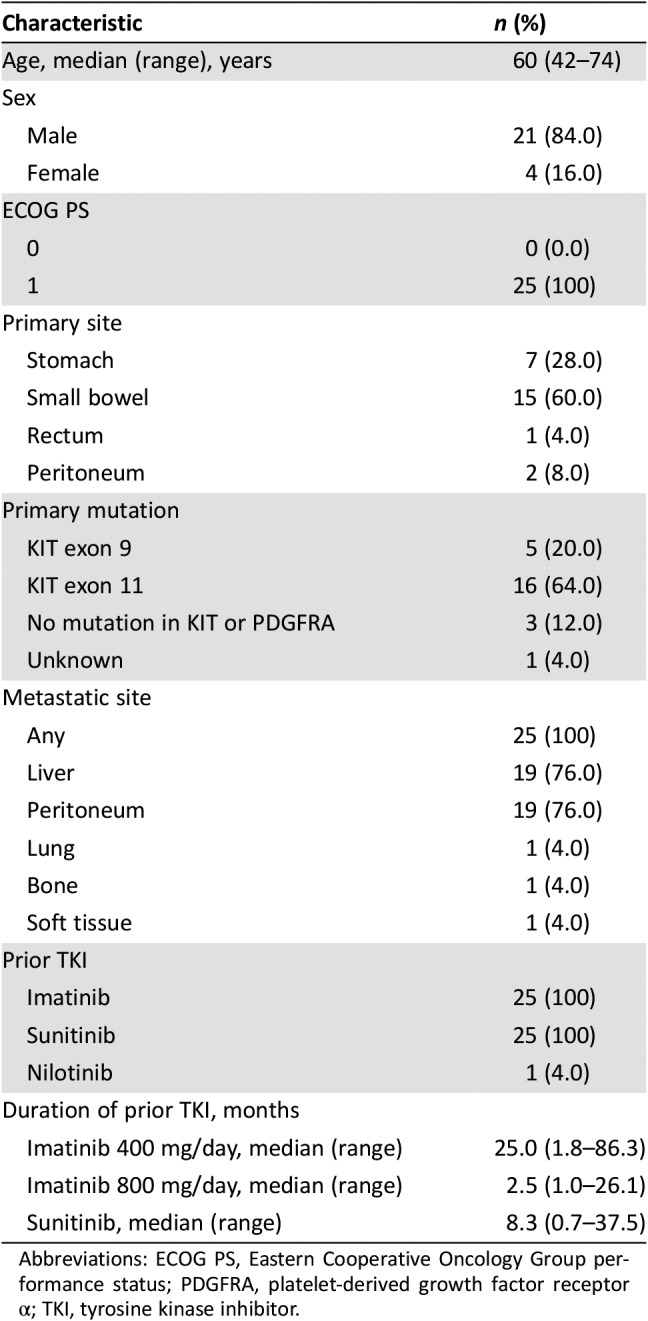
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PDGFRA, platelet‐derived growth factor receptor α; TKI, tyrosine kinase inhibitor.
Efficacy
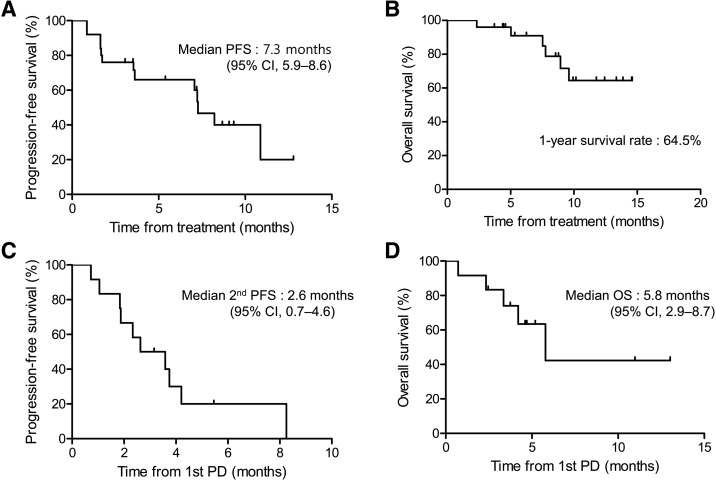
DCR lasting for at least 12 weeks was 64%. The best response was partial response in 2 patients (8.0%), stable disease (SD) in 14 patients (56.0%), and PD in 8 patients (32.0%). None achieved a complete response (Table 2). With a median follow‐up of 8.6 months (range, 2.3–14.6 months), the median PFS was 7.3 months (95% confidence interval [CI], 5.9–8.6 months; Fig. 1A), and the median OS was not reached with a 1‐year survival rate of 64.5% (Fig. 1B). There was no report of exacerbation of tumor‐related symptoms during continuous dosing schedule.
Table 2. Disease control rate lasting at least 12 weeks from the start of regorafenib.
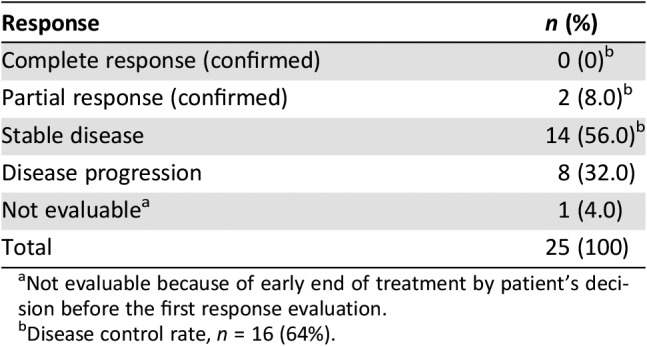
Not evaluable because of early end of treatment by patient's decision before the first response evaluation.
Disease control rate, n = 16 (64%).
Figure 1.
Progression‐free survival and overall survival. PFS (A) and OS (B) from the start of regorafenib (n = 25). PFS (C) and OS (D) from the first PD in patients who continued to receive regorafenib after the first PD (n = 12).
Abbreviations: CI, confidence interval; OS, overall survival; PD, progressive disease; PFS, progression‐free survival.
Safety
Before the disease progression, during a median of six treatment cycles (range, 2–16), all patients experienced drug‐related adverse events. There was no report of grade 3–4 hematologic toxicities during blood tests at a 4‐week interval, and there was no report of febrile neutropenia. In nonhematologic toxicities, ten patients (40%) experienced grade 3 toxicities, and there were no reports of grade 4 toxicities or treatment‐related deaths. Ten patients reported grade 3 adverse events; most common were hand‐food skin reaction (16.0%) and elevation of alanine aminotransferase (8.0%; Table 3).
Table 3. Hematologic laboratory abnormalities and nonhematologic adverse event.
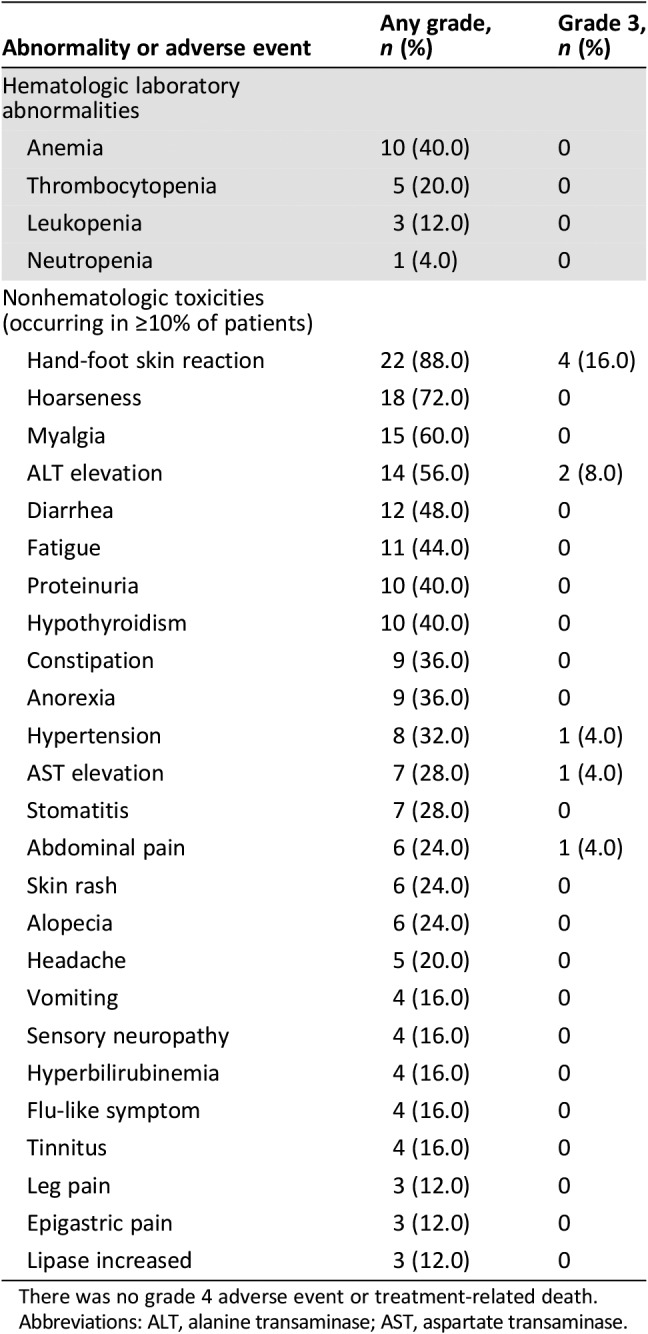
There was no grade 4 adverse event or treatment‐related death.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
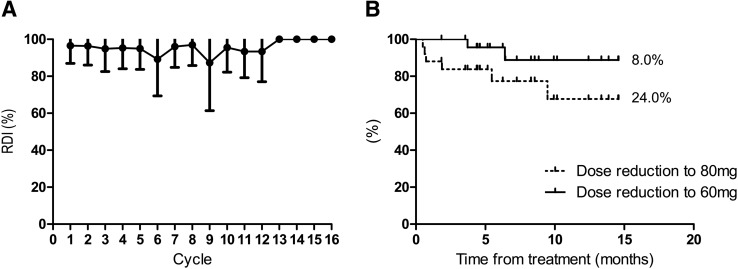
The relative dose intensity was 95.0% for the initial eight cycles before disease progression (Fig. 2A). Patients who needed a dose reduction to 80 and 60 mg per day before PD were 24.0% and 8.0%, respectively (Fig. 2B). Dose reduction to 80 mg was due to hand‐foot skin reaction (n = 4), hypertension (n = 1), and tinnitus (n = 1), and the reasons for dose reduction to 60 mg were hand‐foot skin reaction (n = 1) and increased ALT (n = 1). Serious adverse events (SAEs) were reported in five cases, two of which were possibly related to regorafenib treatment. One case was anal fistula, and the other was colonic fistula. In these two cases, all patients recovered with a temporary interruption of regorafenib.
Figure 2.
Relative dose intensity and time to dose reduction. (A): RDI until PD. (B): Time to dose reduction until PD.
Abbreviations: PD, progressive disease; RDI, relative dose intensity.
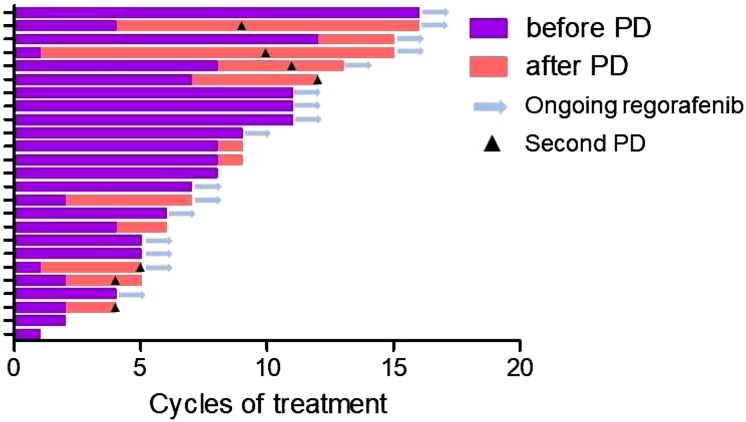
Further Administration of Regorafenib After PD
With a median of four treatment cycles (range, 1–12), 13 patients experienced PD, and 12 continued regorafenib treatment based on the physician's decision of evidence of benefits. Among them, seven patients showed the second PD with further treatment with regorafenib (Fig. 3). Among the patients who continued regorafenib beyond the PD with the assessable best response (n = 10), SD was observed in six patients (60%) and PD in four patients (40%). The median second PFS (Fig. 1C) and median OS (Fig. 1D) from the first PD were 2.6 months (95% CI, 0.7–4.6) and 5.8 months (95% CI, 2.9–8.7). Most of the adverse events were reported to be similar or less than those before PD, whereas fatigue was more frequent than it was before PD. Grade 3 toxicities were reported in three cases, including anemia, fatigue, and elevation of aspartate transaminase.
Figure 3.
Duration of regorafenib treatment in individual patients (n = 25).
Abbreviation: PD, progressive disease.
Discussion
In patients with GISTs who had failed both imatinib and sunitinib treatment, third‐line regorafenib therapy with 160 mg once daily for 3 weeks on treatment followed by 1 week off showed efficacy. However, tumors or tumor‐related symptoms may progress during the treatment off period. Son et al. [11] reported that 26% of patients experienced exacerbation of cancer‐related symptoms during the rest period. In this study, the exacerbation of symptoms occurred after stopping regorafenib and improved with the restart of regorafenib. All five patients who continued regorafenib during the rest period showed an improvement in their symptoms, and only one of them experienced a temporary exacerbation of drug‐related toxicity [11]. A Japanese study also reported a patient with rectal GISTs, who experienced pain during a 1‐week resting period from regorafenib and improved with continuous administration of regorafenib [12]. For patients with imatinib‐resistant or ‐intolerant GISTs, sunitinib had the same issues of severe toxicities, requiring frequent dose modification and exacerbation of tumor‐related symptoms during a rest period with the original intermittent dosing schedule (50 mg daily p.o. for 4 weeks followed by 2 weeks’ rest). The alternative continuous dosing schedule with a lower dose (37.5 mg daily p.o.) was established in a phase II study and is currently being used widely with comparable efficacy and a safety profile with an intermittent dosing schedule [13]. Based on the results of these studies, we hypothesized that a continuous dosing schedule of regorafenib might be feasible and effective in preventing exacerbation on the off‐treatment period. We investigated the 100 mg daily dose of regorafenib based on the results of previous dose escalation study and the possibility of causing frequent adverse effects with higher doses [14].
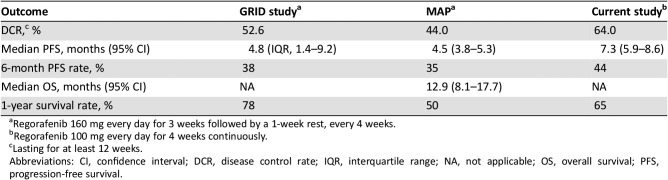
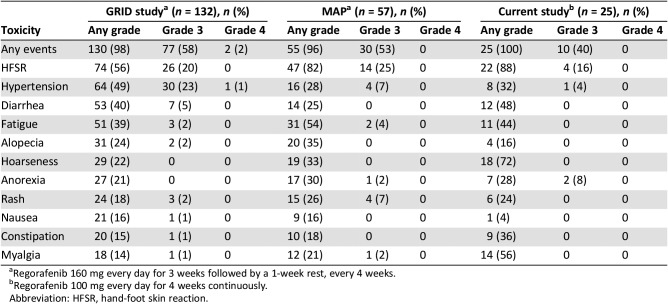
In the current study, with regorafenib 100 mg daily continuous dosing, DCR lasting for at least 12 weeks was 64% (16 of 25 patients), which met the primary endpoint. DCR values in the GRID study and MAP trial were 52.6% and 44% for at least 12 weeks, respectively [8], [11]. Thus, although this trial was an uncontrolled study and had somewhat different patient characteristics from previous studies, DCR of 100 mg of regorafenib continuous administration was comparable to that of the standard dosing schedule (160 mg daily for 3 weeks followed by a 1‐week rest). The median PFS was 7.3 months (95% CI, 5.9–8.6) in the current study, whereas the median PFS in the GRID study and MAP trial was 4.8 months and 4.5 months, respectively [8], [11]. The median PFS in the current study was not shorter than that of prior studies using 160 mg daily intermittent dosing schedule (Table 4).
Table 4. Treatment outcomes across studies.
Regorafenib 160 mg every day for 3 weeks followed by a 1‐week rest, every 4 weeks.
Regorafenib 100 mg every day for 4 weeks continuously.
Lasting for at least 12 weeks.
Abbreviations: CI, confidence interval; DCR, disease control rate; IQR, interquartile range; NA, not applicable; OS, overall survival; PFS, progression‐free survival.
In the current study, 40% of patients experienced grade 3 toxicities, and there was no report of grade 4 toxicities or treatment‐related death. Compared with the incidence of grade 3 or higher toxicities in the GRID study (58%) [8] and MAP trial (53%) [11], drug‐related adverse events in the current study with 100 mg daily continuous dosing were more favorable than those of prior studies with regorafenib 160 mg daily intermittent dosing, whereas patient characteristics differed from previous studies. In the current study, possible treatment‐related SAEs were reported in 8% of patients, and 24% needed dose modification. However, in the GRID study, SAEs and dose modifications were reported in 29% and 72% of patients, respectively [8], and dose modification was needed in 77% of patients in the MAP trial [11]. Some adverse events were reported more frequently in the current study than in previous studies. Myalgia was reported in 14 patients (56%) in our study, whereas it was reported in 18 of 132 patients (14%) in the GRID study and 12 of 57 patients (21%) in the MAP trial [8], [15]. Hoarseness (72%) and constipation (36%) were also reported more frequently in our study than they were in previous studies, although there was no report of grade 3 or higher toxicities (Table 5). These results may be due to different patient characteristics. And a much closer investigation of some adverse events known to be common based on previous studies may also affect these results. We acknowledged through previous studies that some adverse events including hoarseness and myalgia can occur with regorafenib, and we asked more specifically about these symptoms. Hypothyroidism was reported in ten patients (40%) in the current study, which were all grade 1 toxicity and improved without dose modification. Although there were no results of hypothyroidism in the GRID study or MAP trial, hypothyroidism has been reported at a rate of 31.4%–52% with a regorafenib intermittent dosing schedule in other studies [15], [16]. Therefore, the frequency of hypothyroidism in our study does not seem to be higher than that usually observed.
Table 5. Toxicities of regorafenib across studies.
Regorafenib 160 mg every day for 3 weeks followed by a 1‐week rest, every 4 weeks.
Regorafenib 100 mg every day for 4 weeks continuously.
Abbreviation: HFSR, hand‐foot skin reaction.
In the current study, patients experiencing disease progression were further administered regorafenib if the investigator determined that it was clinically beneficial. In patients who continued to receive regorafenib after the first PD, the median second PFS was 2.6 months (95% CI, 0.7–4.6). In the RIGHT study, which investigated the resumption of imatinib after failure of imatinib and sunitinib, imatinib rechallenge slowed disease progression with a median PFS of 1.8 months (95% CI, 1.7–3.6) according to a masked external central assessment and 1.8 months (95% CI, 1.7–2.7) by local investigator assessment [17]. Regorafenib seems to slow down the progression of the disease, similar to what was shown by imatinib in the RIGHT study. Therefore, rechallenge of regorafenib at a lower dose on a continuous schedule, which was favorable in toxicity and showed similar efficacy to that of the RIGHT study, can be a treatment option after failure with all standard treatments.
Conclusion
With good efficacy and acceptable safety profiles, regorafenib at a lower, continuously administered dose might be an alternative treatment in patients with GISTs after imatinib and sunitinib. Further administration of regorafenib after disease progression with regorafenib treatment may slow the disease progression.
Acknowledgments
This study was supported by a grant (HI14C0198) from the Korean Health Technology R&D through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea. This study was partly supported by Bayer. J.‐J.K. is currently affiliated with the Division of Hematology and Oncology, Pusan National University Yangsan Hospital, Yangsan, Korea.
Contributed equally.
Author Contributions
Conception/design: Yoon‐Koo Kang
Provision of study material or patients: Min‐Hee Ryu, Mo Youl Beck, Jung Eun Ma, Yoon‐Koo Kang
Collection and/or assembly of data: Jae‐Joon Kim, Min‐Hee Ryu, Changhoon Yoo
Data analysis and interpretation: Jae‐Joon Kim, Min‐Hee Ryu
Manuscript writing: Jae‐Joon Kim, Min‐Hee Ryu
Final approval of manuscript: Jae‐Joon Kim, Min‐Hee Ryu, Changhoon Yoo, Mo Youl Beck, Jung Eun Ma, Yoon‐Koo Kang
Disclosures
Min‐Hee Ryu: Bristol‐Myers Squibb, Ono Pharmaceutical, Merck Sharp & Dohme, Novartis, Taiho, Daehwa Pharmaceutical (C/A, H); Changhoon Yoo: Bayrer (H); Yoon‐Koo Kang: Novartis, Bayer, Blueprint (C). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973–983. [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, von Mehren M, Blanke CD et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CD, Berman JJ, Corless C et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459–465. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Demetri GD et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342–4349. [DOI] [PubMed] [Google Scholar]
- 5.Wozniak A, Gebreyohannes YK, Debiec‐Rychter M et al. New targets and therapies for gastrointestinal stromal tumors. Expert Rev Anticancer Ther 2017;17:1117–1129. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H, Fletcher C, Dimitrijevic S et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3:655–664. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, van Oosterom AT, Garrett CR et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006;368:1329–1338. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, Reichardt P, Kang YK et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ESMO/European Sarcoma Network Working Group . Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii21–iii26. [DOI] [PubMed] [Google Scholar]
- 10.Koo DH, Ryu MH, Kim KM et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat 2016;48:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son MK, Ryu MH, Park JO et al. Efficacy and safety of regorafenib in Korean patients with advanced gastrointestinal stromal tumor after failure of imatinib and sunitinib: A multicenter study based on the Management Access Program. Cancer Res Treat 2017;49:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajiura S, Hosokawa A, Nanjyo S et al. A case of a gastrointestinal stromal tumor of the rectum effectively treated with continuously‐administered regorafenib after failure of imatinib and sunitinib. Nihon Shokakibyo Gakkai Zasshi 2016;113:655–661. [DOI] [PubMed] [Google Scholar]
- 13.George S, Blay JY, Casali PG et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959–1968. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Tolcher AW, Patnaik A et al. Phase I dose‐escalation study of continuously administered regorafenib (BAY 73‐4506), an inhibitor of oncogenic and angiogenic kinases, in patients with advanced solid tumors. J Clin Oncol 2010;28:3035.20458031 [Google Scholar]
- 15.Pani F, Massidda M, Pusceddu V et al. Regorafenib‐induced hypothyroidism and cancer‐related fatigue: Is there a potential link? Eur J Endocrinol 2017;177:85–92. [DOI] [PubMed] [Google Scholar]
- 16.Sugita K, Kawakami K, Yokokawa T et al. Investigation of regorafenib‐induced hypothyroidism in patients with metastatic colorectal cancer. Anticancer Res 2015;35:4059–4062. [PubMed] [Google Scholar]
- 17.Kang YK, Ryu MH, Yoo C et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): A randomised, placebo‐controlled, phase 3 trial. Lancet Oncol 2013;14:1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]