The approval of PD‐1/PDL‐1 and CTLA‐4 specific therapies has revolutionized oncology; however, the sequencing of these drugs to treat MSI‐high metastatic colorectal cancer is still being determined. this article presents the case of a patient with Lynch syndrome who was treated with multiple sequential checkpoint inhibitors with prolonged disease control.
Abstract
Immune checkpoint blockade (ICB) is an approved therapy for advanced metastatic mismatch repair (MMR)‐deficient cancer regardless of tissue of origin. Although therapy is effective initially, recurrence rates are significant, and long‐term outcomes remain poor for most patients. It is not currently recommended to give sequential ICB for advanced MMR‐deficient colorectal cancer (CRC) or for patients with metastatic cancer from Lynch syndrome. The need for subsequent therapy options in advanced MMR‐deficient cancer beyond the first ICB regimen arises in clinical practice, and there are often no effective standard chemotherapies or other targeted therapies. We report the case of a Lynch syndrome patient with metastatic CRC and urothelial cancer who was treated sequentially with pembrolizumab (targeting PD1), atezolizumab (targeting PD‐L1), brief rechallenge with pembrolizumab, and finally the combination of ipilimumab (targeting CTLA‐4) and nivolumab (targeting PD1). Over a 28‐month period the patient experienced prolonged disease control with each different regimen the first time it was given, including metabolic response by positron emission tomography and computed tomography scanning and tumor marker reductions. The case suggests that some patients with advanced MMR‐deficient CRC may experience meaningful clinical benefit from multiple sequential ICB regimens, a strategy that can be further tested in clinical trials.
Key Points.
The case exemplifies clinical benefit from sequential immune checkpoint blockade in a patient with Lynch syndrome with advanced metastatic colorectal cancer and urothelial cancer.
Metabolic response, with decreased fluorodeoxyglucose avidity on positron emission tomography and computed tomography, and reductions in tumor markers, such as carcinoembryonic antigen, were helpful in this case to monitor disease status over a 28‐month period of therapy.
The concept of sequential immune checkpoint blockade in patients with advanced mismatch repair‐deficient cancer merits further study to determine which patients are most likely to benefit.
摘要
无论组织来源如何,免疫检查点阻断 (ICB) 都是一种已获批准的治疗晚期转移性错配修复 (MMR) 缺陷型癌症的方法。尽管治疗在最初阶段十分有效,但是,对于大多数患者而言,复发率较高且长期疗效较差。目前不推荐采用序贯ICB治疗晚期转移性MMR缺陷型结直肠癌 (CRC) 或林奇综合征转移癌患者。在临床实践中需要在首次ICB治疗方案之后对晚期转移性MMR缺陷型癌症实施后续治疗方案,通常不存在有效的标准化疗或其他靶向治疗。我们报告了一名患有转移性CRC和尿路上皮癌的林奇综合征患者的案例,该患者序贯接受帕博利珠单抗(靶向 PD1)治疗、阿特朱单抗(靶向 PD‐L1)治疗、短暂的帕博利珠单抗激发试验以及最终的易普利姆玛单抗(靶向 CTLA‐4)联合纳武单抗(靶向 PD1)治疗。在 28 个月内,患者在首次采用每种不同的治疗方案时经历了较长时间的疾病控制,包括通过正电子发射断层扫描和计算机断层扫描观察到的代谢反应以及肿瘤标志物减少。此病例表明,一些晚期转移性MMR缺陷型CRC患者可能会从多个序贯ICB治疗方案中获得重要的临床受益,该方案可以在临床试验中进一步测试。
要点
• 此病例证实了患有晚期转移性结直肠癌和尿路上皮癌的林奇综合征患者可以从序贯免疫检查点阻断中获得临床受益。
• 在此病例中,代谢反应、通过正电子发射断层扫描和计算机断层扫描观察到的氟脱氧葡萄糖亲和力降低以及肿瘤标志物(如癌胚抗原)减少均有助于监控 28 个月治疗期间的疾病情况。
• 在患有晚期错配修复缺陷型癌症的患者中,序贯免疫检查点阻断的概念值得进一步研究,以便判断哪些患者最有可能从中受益。
Background
Impaired mechanisms of DNA mismatch repair (MMR) either by mutation in or promoter methylation of essential genes lead to highly mutated repetitive DNA sequences (microsatellites) across the genome. Microsatellite instability (MSI) contributes to different tumor types. Although an inherited form of MMR deficiency (Lynch syndrome) accounts for ∼3% of colorectal cancers (CRCs), MMR deficiency accounts for ∼15% of all CRCs via somatic mutation [1].
MSI‐high (MSI‐H) CRC has a high tumor mutation burden, increasing neoantigen presentation [2]. Within the tumor microenvironment, MSI‐H CRC tumors are enriched with type 1 T helper cells and cytotoxic T lymphocytes, indicating an ongoing immune response [3]. However, this is counterbalanced by increased immune checkpoint expression with upregulation of PD‐1/PDL‐1 and CTLA‐4, inhibiting the immune response, thereby allowing tumor growth [2], [3]. Immune checkpoint inhibitors relieve this block and restore antitumor immune function. Trials of these drugs in patients with MSI‐H CRC previously treated with chemotherapy have yielded significant responses, and these drugs are approved by the U.S. Food and Drug Administration (FDA) to treat MSI‐high CRC in the second line [4], [5], [6].
Immune checkpoint therapies have been studied as a single line of treatment in MSI‐high metastatic CRC (mCRC). If checkpoint inhibition does not work or stops working, sequential treatment is not recommended. We present a case of a patient treated effectively with sequential PD‐1/PDL‐1 inhibitors as well as dual checkpoint inhibition beyond progression with good disease control. The patient agreed for his case to be published in the literature.
Patient Story
The patient was a 64‐year‐old man diagnosed with stage IIIA colon cancer 11 years prior to establishing care at our institution. Immunohistochemistry revealed absent MSH‐2 and MSH‐6 expression. The patient completed adjuvant chemotherapy and remained disease free until recurrence 10 years later with a 16.5‐cm mass in the liver, after which he was treated with FOLFIRI (leucovorin calcium, fluorouracil, irinotecan hydrochloride) and bevacizumab, followed by irinotecan and cetuximab at disease progression with interval growth in liver lesions and metastatic lymphadenopathy (for full details of his prior therapy, please refer to our earlier publication on this patient) [7]. Five months later, his disease progressed in the liver and lymph nodes with new hydroureteronephrosis bilaterally. Workup revealed localized urothelial carcinoma via right ureteral cytology, also lacking MSH‐2 and MSH‐6 expression.
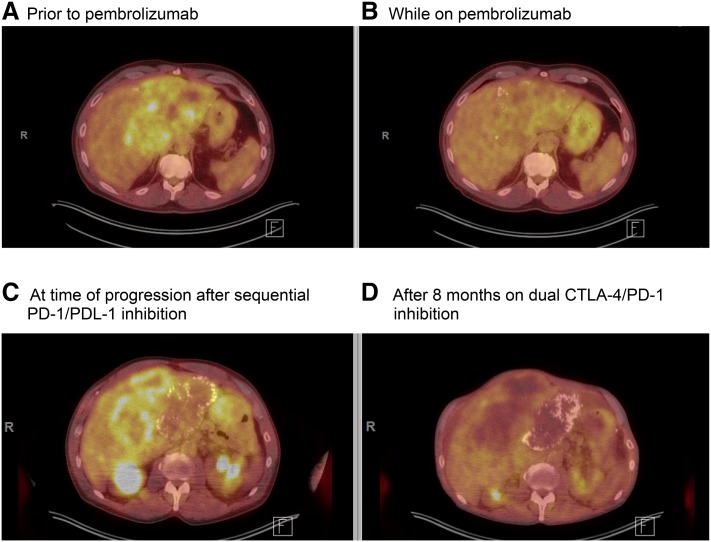
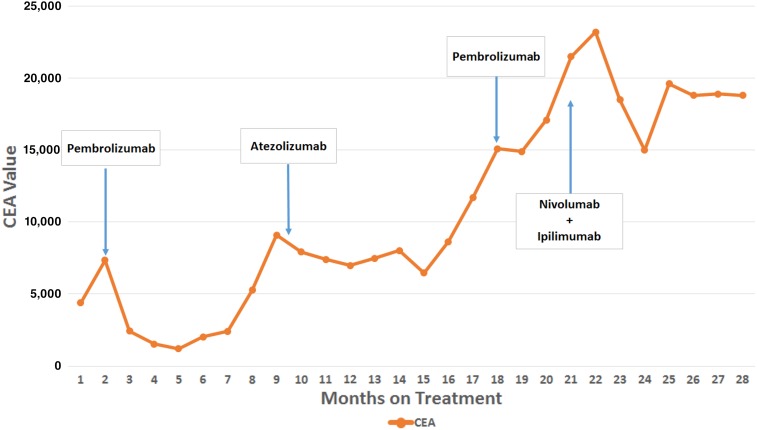
The patient established care in our clinic in 2016. Given the MMR deficiency evident in his colon tumors, we performed germline testing, which revealed an MSH‐2 mutation (IVS1 + 2T > G) in some but not all of his cells confirming the diagnosis of a mosaic attenuated Lynch syndrome, consistent with his later age of presentation. The patient was started on compassionate use pembrolizumab initially at a dose of 2 mg/kg, which was rotated to a flat dose of 200 mg every 3 (q3) weeks. Carcinoembryonic antigen (CEA) subsequently declined, and a partial response was seen on positron emission tomography and computed tomography (PET/CT; Fig. 1A–B) with decreased metabolic activity and improvement in the patient's abdominal pain. After 9 months of treatment, the patient's CEA rose, and increased fluorodeoxyglucose activity was noted in his liver metastasis on a PET/CT, along with activity in the ureters, bilaterally. Repeat cystoscopy demonstrated high‐grade T1 urothelial carcinoma of the bladder in addition to his ureteral urothelial carcinoma. Given the approval of atezolizumab for patients with urothelial carcinoma, the patient was treated with atezolizumab 1,200 mg q3 weeks with stability of his urothelial tumors on repeat cystoscopy and 8 months of CRC disease control before progressive disease in the liver. He was briefly retreated with pembrolizumab 200 mg q3 weeks for an additional 3 months, given that on re‐review of his CEA trends it was unclear if there was a decline on pembrolizumab just before atezolizumab started. However, after four additional cycles of pembrolizumab, the patient's right upper quadrant pain worsened, CEA rose from 15,100 to 21,500, and PET/CT imaging showed progression of his liver tumor (Fig. 1C). Therefore, pembrolizumab was discontinued.
Figure 1.
Positron emission tomography and computed tomography (PET/CT) throughout treatment. (A): PET/CT prior to initiating pembrolizumab. (B): PET/CT after 5 months of immunotherapy indicating a partial response with decrease in standardized uptake value (SUV) of liver mass from 6.5 down to 4.6. (C): PET imaging at time of progression after atezolizumab and pembrolizumab with maximum SUV of 6.5. (D): PET obtained after 8 months on ipilimumab plus nivolumab for four doses followed by nivolumab alone showed a response to combination therapy with a maximum SUV of 3.6.
The patient then began treatment with off‐label compassionate use nivolumab 3 mg/kg and ipilimumab 1 mg/kg q3 weeks based on a study published in the Journal of Clinical Oncology showing activity of combination PD‐1 and CTLA‐4 blockade in MSI‐H tumors [4]. With this combination, the patient's CEA level remained stable at ∼18,000, and PET/CT imaging revealed a metabolic response (Fig. 1D) with decline in uptake in his primary tumor as well in other metastatic implants and shrinkage of urothelial tumors. After four cycles of the combination, the patient continued single agent nivolumab 3 mg/kg once every 4 weeks with continued disease control for a total of 7 months on this therapy. At that time, a rise in bilirubin to 5.3 was noted, which was attributed to an immunotherapy‐related adverse event, and the patient was treated with high‐dose steroids. During his steroid taper, the patient chose to discontinue all therapy and subsequently passed away.
Discussion
The development and approval of PD‐1/PDL‐1 and CTLA‐4‐specific therapies have revolutionized oncology, yet the full utility of these drugs to treat mCRC is still being elucidated. Anti‐PD‐1 (nivolumab, pembrolizumab), anti‐PD‐L1 (avelumab, durvalumab, atezolizumab), and anti‐CTLA4 (ipilimumab) targeted drugs have been studied in mCRC. In patients with MSI‐H mCRC, immune checkpoint therapy has shown responses in a substantial proportion of patients. Overall response rates (ORR) with pembrolizumab or nivolumab range between 30% and 40%, with disease control achieved in >50% of treated patients, and these drugs are approved in the second line for MMR‐deficient/MSI‐H mCRC [5], [6]. In patients with MSI‐H mCRC, the addition of CTLA‐4 inhibitor ipilimumab to nivolumab increased ORR to 55% [4]. However, the utility of sequential immunotherapy after progression is unknown. For the five PD1/PDL‐1 inhibitors currently FDA approved, preclinical data suggest the drugs bind different epitopes on PD‐1/PDL‐1, and therefore it may be reasonable to sequence them [8]. The current literature on sequential PD‐1/PDL‐1 inhibition is mixed, with some reports showing continued progression of disease in patients who had previously responded to PD‐1 inhibition [9]. Although there are emerging data to add CTLA‐4 inhibition to a PD‐1 inhibitor upon progression on single checkpoint blockade in multiple tumor types other than colorectal carcinoma, this is not yet standard of care [10], [11], [12], [13], [14].
Our patient with Lynch syndrome was treated with sequential PD‐1/PDL‐1 inhibitors with response, followed by the combination of a CTLA‐4 and PD‐1 inhibitor at the time of progression with significant disease control. His disease was controlled by single as well as dual checkpoint inhibition, both on PET/CT imaging (Fig. 1) and by stabilization in his CEA (∼18,000 for 8 months while he was on dual immunotherapy; Fig. 2). Prolonged disease control suggests possible utility in sequencing immunotherapy medications in patients with MSI‐H mCRC, and studies are needed to help determine who may benefit from receiving multiple immunotherapy‐based treatments.
Figure 2.
CEA trend over time during treatment.
Abbreviation: CEA, carcinoembryonic antigen.
Footnotes
For Further Reading: Claudia Maletzki, Maja Hühns, Ingrid Bauer et al. Suspected Hereditary Cancer Syndromes in Young Patients: Heterogeneous Clinical and Genetic Presentation of Colorectal Cancers. The Oncologist 2019;24:877–882.
Abstract:
Colorectal cancer (CRC) is rare in young patients without a confirmed family history of cancer. Reports of an increased prevalence of POLD1/POLE mutations in young patients with colorectal cancer have raised awareness and support routine genetic testing for patients with early‐onset tumors. In cases of CRC without proven MMR‐germline mutation, molecular analyses are warranted to confirm or rule out other familial CRC syndromes. This article describes the cases of two young male patients, who presented with locally advanced and metastatic CRC, and reports the results of the germline mutational analyses done for both patients. These cases demonstrate the importance of special care and molecular diagnostic procedures for young patients with CRC.
Key Points:
• Patients with colorectal cancer who are younger than 50 years at initial diagnosis (early onset) should routinely undergo genetic testing.
• Early‐ and very‐early‐onset patients (younger than 40 years) with absence of microsatellite instability should be considered for tumor mutation burden testing and/or DNA polymerase proofreading mutation.
• The mutational signature of HSP110 within mismatch repair deficiency‐related tumors may help to identify patients likely to benefit from 5‐fluorouracil‐based chemotherapy.
• Intensified, maintained, and specific surveillance may help to reduce secondary tumor progression.
Author Contributions
Conception/design: Arthur Winer, Pooja Ghatalia, Nicole Bubes, Fern Anari, Asya Varshavsky, Vineela Kasireddy, Yang Liu, Wafik S. El‐Deiry
Provision of study material or patients: Arthur Winer, Pooja Ghatalia, Nicole Bubes, Fern Anari, Asya Varshavsky, Vineela Kasireddy, Yang Liu, Wafik S. El‐Deiry
Manuscript writing: Arthur Winer, Pooja Ghatalia, Nicole Bubes, Fern Anari, Asya Varshavsky, Vineela Kasireddy, Yang Liu, Wafik S. El‐Deiry
Final approval of manuscript: Arthur Winer, Pooja Ghatalia, Nicole Bubes, Fern Anari, Asya Varshavsky, Vineela Kasireddy, Yang Liu, Wafik S. El‐Deiry
Disclosures
The authors indicated no financial relationships.
References
- 1.Sinicrope FA. Lynch syndrome‐associated colorectal cancer. N Engl J Med 2018;379:764–773. [DOI] [PubMed] [Google Scholar]
- 2.Llosa NJ, Cruise M, Tam A et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter‐inhibitory checkpoints. Cancer Discov 2015;5:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozcan M, Janikovits J, von Knebel Doeberitz M et al. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology 2018;7:e1445453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overman MJ, Lonardi S, Wong KYM et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair‐deficient/microsatellite instability‐high metastatic colorectal cancer. J Clin Oncol 2018;36:773–779. [DOI] [PubMed] [Google Scholar]
- 5.Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghatalia P, Nagarathinam R, Cooper H et al. Mismatch repair deficient metastatic colon cancer and urothelial cancer: A case report of sequential immune checkpoint therapy. Cancer Biol Ther 2017;18:651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Lee HT, Shin W et al. Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat Commun 2016;7:13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martini DJ, Lalani AA, Bossé D et al. Response to single agent PD‐1 inhibitor after progression on previous PD‐1/PD‐L1 inhibitors: A case series. J Immunother Cancer 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehmi I. Ipilimumab with anti PD‐1 (nivolumab or pembrolizumab) after progression on first line anti‐PD‐1 therapy for advanced melanoma. J Clin Oncol 2018;36(suppl 15):e21552A. [Google Scholar]
- 11.Olson D, Luke JJ, Hallmeyer S et al. Phase II trial of pembrolizumab (pembro) plus 1 mg/kg ipilimumab (ipi) immediately following progression on anti‐PD‐1 Ab in melanoma (mel). J Clin Oncol 2018;36(suppl 15):9514A. [Google Scholar]
- 12.Gaughan EM, Petroni GR, Grosh WW et al. Salvage combination ipilimumab and nivolumab after failure of prior checkpoint inhibitor therapy in patients with advanced melanoma. J Clin Oncol 2017;35(suppl 15):e21009A. [Google Scholar]
- 13.Gardon EB, Spira AI, Goldberg SB et al. Safety and activity of durvalumab + tremelimumab in immunotherapy (IMT)‐pretreated advanced NSCLC patients. J Clin Oncol 2018;36(suppl 15):9041A. [Google Scholar]
- 14.Keegan NM, Funt SA, Kania EB et al. Durable clinical benefit from combination ipilimumab (IPI) and nivolumab (NIVO) in anti‐PD‐1 therapy resistant, platinum resistant metastatic urothelial carcinoma (mUC). J Clin Oncol 2019;37(suppl 7):481A.30620669 [Google Scholar]