Abstract
Purpose of Review
Bone marrow adipocytes have emerged in recent years as key contributors to metastatic progression in bone. In this review, we focus specifically on their role as the suppliers of lipids and discuss pro-survival pathways that are closely linked to lipid metabolism, affected by the adipocyte-tumor cell interactions, and likely impacting the ability of the tumor cell to thrive in bone marrow space and evade therapy.
Recent Findings
The combined in silico, pre-clinical, and clinical evidence shows that in adipocyte-rich tissues such as bone marrow, tumor cells rely on exogenous lipids for regulation of cellular energetics and adaptation to harsh metabolic conditions of the metastatic niche. Adipocyte-supplied lipids have a potential to alter the cell’s metabolic decisions by regulating glycolysis and respiration, fatty acid oxidation, lipid desaturation, and PPAR signaling. The downstream effects of lipid signaling on mitochondrial homeostasis ultimately control life vs. death decisions, providing a mechanism for gaining survival advantage and reduced sensitivity to treatment.
Summary
There is a need for future research directed towards identifying the key metabolic and signaling pathways that regulate tumor dependence on exogenous lipids and consequently drive the pro-survival behavior in the bone marrow niche.
Keywords: Bone marrow adipocyte, Bone metastasis, Lipids, Apoptosis, Survival, Metabolism
Introduction
Bone marrow is a common host of several types of tumors, including secondary cancers of the breast, prostate, thyroid, kidney, lung, and bladder as well as hematological malignancies, such as multiple myelomas (MM) and leukemias [1–3]. A common feature of tumor cells that reside in bone is that their proliferation and survival are critically dependent on the interaction with the bone marrow microenvironment. Bone marrow adipocytes, which originate from mesenchymal stem cells, are a major component of bone marrow stroma [4•, 5]. Adipocyte-enriched bone marrow, known as yellow fat or bone marrow adipose tissue (BMAT), dramatically increases with age and can be prematurely augmented by obesity, caloric restriction, treatments with PPARγ agonists, or radiation [5–8]. Marrow adipocytes are a known source of hormones, adipokines, incretins, and growth factors, whose key effects on bone health, marrow adipogenesis, insulin sensitivity, inflammation, and tumorigenesis have been well described elsewhere [4•, 6, 8–10]. Importantly, they also produce and contain significant amounts of fat and play key roles in the regulation of energy metabolism in the bone [11]. Although multiple cell types within the marrow space are subject to metabolic effects of marrow fat cells, little is known about specific effects of adipocyte-supplied lipids on the behavior and metastatic progression of tumor cells that have colonized the bone.
Marrow Adipocytes as a Source of Lipids: Metabolic Effects on Tumor Cells
Adipocyte-Driven Lipolysis: a Source of Glycerol and Fatty Acids
Fat cells store lipids in the form of triglycerides in lipid droplets and, when necessary, break them down in the catabolic process of lipolysis into glycerol and free fatty acids (FFAs) [12, 13]. This event is driven by the activation of rate-limiting adipose triglyceride lipase (ATGL) [14, 15] and phosphorylation and activation of hormone-sensitive lipase (HSL) [16, 17]. Our recent studies have shown that both ATGL and HSL are upregulated in marrow adipocytes exposed to prostate carcinoma cells, an event coinciding with the release of FFA from adipocytes [18]. Increased activity of HSL and augmented levels of glycerol and FFA have also been demonstrated in adipocytes interacting with leukemic blasts [19••]. Furthermore, the phenomenon of lipid exchange has been suggested to occur between adipocytes and multiple myeloma cells [9], although its impact on MM progression and survival is still not well understood and needs further investigation.
The FFAs released by hydrolysis of adipocyte-derived triglycerides can be taken up by the tumor cells via lipid transporters such as fatty acid translocase (CD36; FAT) and fatty acid-binding protein 4 (FABP4). Indeed, significant overexpression of these regulators of lipid trafficking, along with enhanced lipid uptake, have been revealed upon exposure of leukemia, prostate, and ovarian cells to adipocytes [19••, 20, 21, 22•]. These findings are supported by our Oncomine data analyses, demonstrating highly increased expression of FABP4 and CD36, as well as HSL, in metastatic prostate and ovarian tumors [2, 21]. Furthermore, FABP4 knockdown or pharmacological targeting of its activity reduces prostate tumor cell invasion [20] or reverses adipocyte-induced pro-survival effects on leukemia cells [19••, 23••], further speaking to the pro-tumor effects of adipocyte-supplied lipids. CD36 is especially emerging as a key lipid transporter and regulator of the adipocyte-tumor cell metabolic interactions in ovarian cancer metastasis [21]. In breast cancer, CD36-mediated lipid uptake was recently shown to promote tumor cell proliferation [24]. Interestingly, a specific metabolic phenotype of leukemia cells responsible for their resistance to chemotherapy has been linked to the expression of CD36 [25]. These findings place fatty acid uptake and transport as potential targetable mechanisms for cancer therapy.
Warburg Effect vs. Oxidative Phosphorylation
The energy metabolism of the cell is regulated by the lipid and glucose pathways, which are tightly linked to each other [26]. Triglyceride breakdown by adipocytes generates glycerol, which has a potential to feed into the glycolytic pathway [27]. This can potentially affect the metabolic phenotype of the tumor, as cancer cells are known to favor glycolysis over oxidative phosphorylation (OXPHOS) to meet their energy demand and gain advantage in progression and response to therapy [28–30]. In fact, the defects in the OXPHOS pathway are thought to be key reasons for the attenuation of tumor cell apoptosis [31, 32]. Given the inefficiency of glycolysis over OXPHOS in terms of ATP production, it is surprising that cancer cells would turn to this pathway for energy to thrive and survive. However, if the glucose flux is high enough, it not only provides important intermediates for tumor growth, but the adenosine triphosphate (ATP) produced via high rates of glycolysis can outweigh OXPHOS [33]. Indeed, multiple reports have recently implicated aerobic glycolysis, known as the Warburg effect, in MM progression and survival [34–36]. In addition, increased glycolysis and low efficiency of OXPHOS were suggested as contributors to drug resistance in other hematological cancers [37–40]. Studies from our laboratory have shown that exposure to bone marrow adipocytes induces a metabolic switch to a glycolytic phenotype in prostate carcinoma cells [18]. On the other hand, recent growing evidence suggests that some cancer cells are capable of boosting their oxidative mitochondrial metabolism to gain survival advantage [41–43]. A study by Henkenius et al. showed that subpopulations of drug-resistant acute myeloid leukemia (AML) cells maintain their oxidative metabolism to escape therapy [44]. Increased mitochondrial biogenesis and respiration were also reported in drug-resistant and relapsed MM cells [45]. As we do not currently understand how adipocyte-supplied lipids affect glucose metabolism and respiration of a tumor cell, more studies exploring energy metabolism in adipocyte-rich tumor microenvironments such as bone marrow are warranted.
Fatty Acid Oxidation
Although most tumors will depend on a high rate of glucose uptake for their energetic needs [46], there is growing evidence that β-oxidation of fatty acids (FAO) can serve as a main source of energy for several types of cancers [47]. FAO is an essential source of nicotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide (FADH2), nicotinamide adenine dinucleotide phosphate (NADPH), and ATP and therefore a facilitator of survival advantage in cancer cells. The key rate-limiting enzyme in FAO, carnitine palmitoyltransferase I (CPTI), is overexpressed in many types of tumors, and its knockdown or pharmacological inhibition suppresses tumor cell growth and survival [48, 49]. An elegant study by Nieman et al. demonstrated that FAO can be effectively stimulated in ovarian cancer cells upon interaction with adipocytes and the augmented rates of β-oxidation support fast tumor growth [22•]. Furthermore, an increased fatty acid uptake and overexpression of enzymes involved in β-oxidation have also been demonstrated in prostate tumors [50, 51]. Similarly, human leukemia cells have been shown to depend on β-oxidation for their proliferation and survival [19••, 52]. High rates of fatty acid oxidation are known to produce large amounts of NADH and acetyl-coenzyme A, and thus inhibit mitochondrial oxidation, a phenomenon that appears to promote quiescence and “sternness” of AML cells, and consequently drive their resistance to therapy [25].
Adipocytes and Hypoxia
It is well recognized that bone marrow, in contrast to other organs, is naturally hypoxic and activity of hypoxia inducible factor 1α (HIF-1α) is critical for normal bone marrow hematopoiesis [53]. It is also becoming increasingly clear that the hypoxic niche plays an essential role in the biology and progression of tumors that colonize the bone marrow. High levels of HIF-1α expression and stability have been reported in bone marrow biopsies of MM patients [54, 55], and induction of hypoxic environment was shown to promote dissemination and colonization of new bone marrow areas by MM cells [56]. Importantly, numerous genes responsible for MM progression have been identified as downstream targets of HIF-1α [57]. Hypoxia has also been demonstrated to affect proliferation, differentiation, and the response of leukemia cells to therapy [58]. Notably, during hypoxia, an upregulation of genes critical for metastatic colonization and expansion of breast cancer in bone, such as parathyroid hormone-related protein (PTHrP), receptor activator of nuclear factor kappa-B (RANK), and its ligand (RANKL), has been demonstrated [59], and expression of HIF-1α was shown to promote tropism of breast cancer cells to the skeletal sites [60].
Hypoxia and oxidative stress often accompany states of increased adiposity [61], and under hypoxic conditions, deregulated adipocytes have been shown to reduce the expression of estrogen receptor in breast cancer cells and thus make them less sensitive to hormonal therapies [62]. Studies from our laboratory have shown that marrow adipocytes are capable of activating HIF-1α signaling in metastatic prostate cancer cells, a process associated with increased adipocyte lipolysis and enhanced lipid uptake by the tumor cells [18, 20]. Whether the adipocyte-induced HIF-1α activation in prostate tumor cells is a cause or a consequence of the observed lipid accumulation is not presently clear. It is well-known that hypoxic conditions diminish a cancer cell’s ability to synthesize its own lipids and the accumulation of lipid droplets has been linked to HIF-1α activation [63, 64]. It is likely that exposure of tumor cells to marrow adipocyte-supplied lipids stabilizes HIF-1α and activated downstream signaling, which promotes further lipid uptake, perpetuating the hypoxic phenotype in tumor cells [18]. Proteins that stabilize the integrity of lipid droplets, such as perilipin and adipose differentiation-related protein (ADRP), as well as lipid transporters, such as FABP4 and CD36, are often upregulated under hypoxic conditions [63, 64].
Importance of Lipid Desaturation
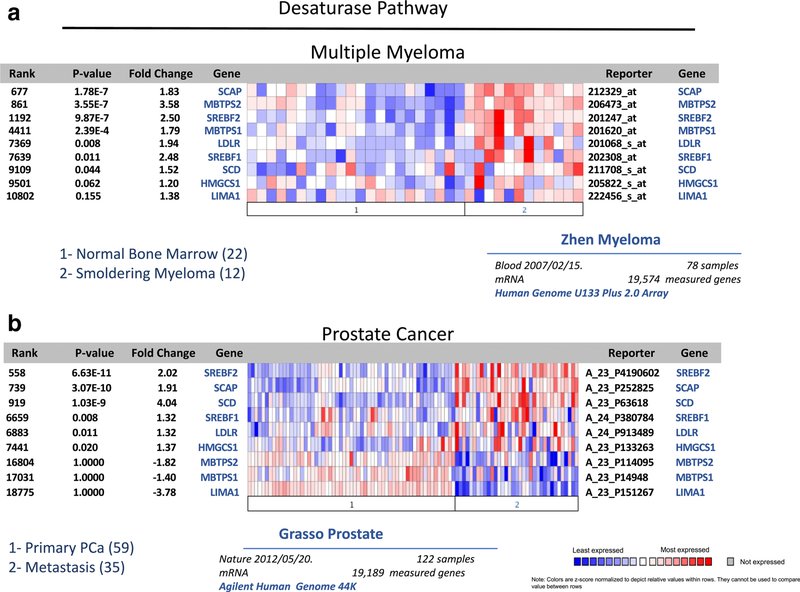
One of the key characteristics of hypoxia is that it can affect the ability of cancer cells to modify cellular lipids by regulating the activity of enzymes involved in FFA desaturation [65, 66•]. Indeed, multiple enzymes in the desaturase pathway appear to be overexpressed in both MM and metastatic prostate cancer (Fig. 1). Of particular interest in this context is the role of stearoyl-CoA desaturase (SCD), which catalyzes the formation of double bonds at the Δ9 position of palmitoyl-CoA and stearoyl-CoA to generate monounsaturated FFA. SCD is frequently overexpressed in cancers, and tumor cells rely on its activity for proliferation, growth, and survival [67••, 68]. However, as SCD-mediated desaturation reaction requires O2, the synthesis of monounsaturated FA is compromised under severe hypoxia [66•]. Consequently, when exposed to hypoxic conditions, tumor cells favor the scavenging of unsaturated lipids from the microenvironment as opposed to turning on the lipogenesis [65, 66]. On the other hand, to ensure the supply of unsaturated fatty acids and to compensate for reduced desaturase activity, tumor cells might upregulate SCD levels via sterol regulatory element-binding protein (SREBP) [68, 69]. Accordingly, our in silico analyses of Oncomine databases across several types of tumors reveal augmented SCD1 expression in metastatic tumors as compared to primary tumors or normal tissue (Table 1). Importantly, two other key desaturases, FADS1 and FADS2 (fatty acid desaturases 1 and 2), which are rate-limiting enzymes in conversion of polyunsaturated fatty acids (PUFA) and are main determinants of PUFA levels [70], are also significantly increased in several metastatic cancers as well as leukemias (Table 2). Unfortunately, little is known to date about desaturase expression patterns and the role in tumor survival and progression in bone, especially in the context of marrow adiposity. Given the critical roles of PUFA and their metabolites in biological processes, including the modulation of adipose tissue, inflammation, and cancer, studies delineating contribution of desaturases to tumor-induced bone disease are desperately needed.
Fig. 1.
Oncomine gene analysis comparing the expression of desaturase pathway genes [SCD, SCAP, SREBF1, SREBF2, MBTPS1, MBTPS2, LDLR, HMGCS1, LIMA1] in patient samples collected from metastatic vs. primary prostate cancer (a) and multiple myeloma vs. normal bone marrow (b). Data were ordered by “overexpression,” and the threshold was adjusted to P value < 1E−4; fold change, 2; and gene rank, top 10%
Table 1.
Oncomine (https://www.oncomine.org) gene analysis comparing the expression of stearoyl-CoA desaturase (SCD1) in patient samples collected from metastatic or primary sites across all cancers. Leukemia and multiple myeloma samples were compared to normal bone marrow. Data were ordered by “overexpression,” and the threshold was adjusted to P value < 1E4; fold change, 2; and gene rank, top 10%
| Gene | Fold change | P value | Dataset | Analysis |
|---|---|---|---|---|
| SCD1 | 3.22 | 1.49E−8 | Xu melanoma | Metastasis vs. primary |
| 4.042 | 1.03E−9 | Grasso prostate | Metastasis vs. primary | |
| 1.770 | 1.32E−6 | Jones renal | Metastasis vs. primary | |
| 1.450 | 3.85E−6 | Bittner ovarian | Metastasis vs. primary | |
| 3.667 | 4.40E−4 | Bhattacherjee lung | Metastasis vs. primary | |
| 2.399 | 0.084 | Weigelt breast | Metastasis vs. primary | |
| 1.259 | 2.90E−4 | Tothill ovarian | Metastasis vs. primary | |
| 2.051 | 0.052 | Garber lung | Metastasis vs. primary | |
| 1.711 | 0.047 | Jain endocrine (head and neck) | Metastasis vs. primary | |
| 1.406 | 0.004 | Anglesio ovarian | Metastasis vs. primary | |
| 1.600 | 9.884E−4 | Linn sarcoma | Metastasis vs. primary | |
| 3.517 | 0.003 | Riker melanoma | Metastasis vs. primary | |
| 2.040 | 0.040 | LaTulippe prostate | Metastasis vs. primary | |
| 1.294 | 0.030 | Bittner colon | Metastasis vs. primary | |
| 1.754 | 1.840E−4 | Durig leukemia | T cell ALL vs. normal | |
| 1.465 | 7.88E−19 | Haferlach leukemia | CML vs. normal | |
| 1.388 | 1.72E−17 | Haferlach leukemia | T cell ALL vs. normal | |
| 1.702 | 6.86E−7 | Andersson leukemia | AML vs. normal | |
| 1.465 | 1.20E−5 | Andersson leukemia | B cell ALL vs. normal | |
| 1.681 | 5.00E−4 | Andersson leukemia | T cell ALL vs. normal | |
| 2.605 | 0.037 | Rosenwald multi-cancer | CLL vs. normal | |
| 1.518 | 0.044 | Zhan myeloma 3 | Smoldering myeloma vs. normal |
Table 2.
Oncomine (https://www.oncomine.org) gene analysis comparing the expression of fatty acid desaturases (FADS1 and FADS2) in patient samples collected from metastatic or primary sites across all cancers. Leukemia and multiple myeloma samples were compared to normal bone marrow. Data were ordered by “overexpression,” and the threshold was adjusted to P value < 1E4; fold change, 2; and gene rank, top 10%
| Gene | Fold change | P value | Dataset | Analysis |
|---|---|---|---|---|
| FADS1 | 1.335 | 5.84E–7 | Yu prostate | Metastasis vs. primary |
| 1.953 | 3.31E–5 | Grasso prostate | Metastasis vs. primary | |
| 1.353 | 0.002 | Lapointe prostate | Metastasis vs. primary | |
| 1.343 | 0.003 | Bittner colon | Metastasis vs. primary | |
| 1.124 | 0.006 | Chen gastric | Metastasis vs. primary | |
| 1.164 | 0.008 | Bittner ovarian | Metastasis vs. primary | |
| 1.307 | 0.010 | Linn sarcoma | Metastasis vs. primary | |
| 1.201 | 0.024 | Graudens colon | Metastasis vs. primary | |
| 1.447 | 0.025 | Holzbeierlein prostate | Metastasis vs. primary | |
| 2.593 | 0.034 | Varambally prostate | Metastasis vs. primary | |
| 1.807 | 0.037 | Bittner lung | Metastasis vs. primary | |
| 1.570 | 0.040 | Segal sarcoma | Metastasis vs. primary | |
| 1.508 | 0.042 | Adib ovarian | Metastasis vs. primary | |
| 2.022 | 0.064 | Segal sarcoma 2 | Metastasis vs. primary | |
| 1.119 | 0.068 | Jones renal | Metastasis vs. primary | |
| 3.378 | 0.082 | Ramaswamy multi-cancer 2 | Metastasis vs. primary | |
| 2.831 | 0.092 | Ramaswamy multi-cancer | Metastasis vs. primary | |
| 2.066 | 2.11E–57 | Haferlach leukemia | T cell ALL vs. normal | |
| 1.459 | 4.32E–36 | Haferlach leukemia | B cell childhood ALL vs. normal | |
| 1.427 | 8.31E–34 | Haferlach leukemia | AML vs. normal | |
| 1.444 | 1.36E–28 | Haferlach leukemia | B cell ALL vs. normal | |
| 1.170 | 3.05E–4 | Haferlach leukemia | CML vs. normal | |
| FADS2 | 1.673 | 3.24E–6 | Linn sarcoma | Metastasis vs. primary |
| 1.377 | 1.12E–5 | Chandran prostate | Metastasis vs. primary | |
| 1.357 | 0.011 | Jones renal | Metastasis vs. primary | |
| 1.399 | 0.016 | Bittner colon | Metastasis vs. primary | |
| 2.604 | 0.037 | Varambally prostate | Metastasis vs. primary | |
| 1.409 | 0.050 | Lapointe prostate | Metastasis vs. primary | |
| 1.204 | 0.050 | Bittner ovarian | Metastasis vs. primary | |
| 2.151 | 0.081 | Liao liver | Metastasis vs. primary | |
| 1.363 | 0.098 | Holzbeierlein prostate | Metastasis vs. primary | |
| 1.490 | 1.22E–12 | Haferlach leukemia | T cell ALL vs. normal | |
| 1.118 | 0.007 | Haferlach leukemia | AML vs. normal | |
| 1.097 | 0.058 | Haferlach leukemia | CML vs. normal | |
| 4.258 | 1.84E–5 | Andersson leukemia | T cell ALL vs. normal | |
| 1.746 | 0.010 | Andersson leukemia | AML vs. normal | |
| 1.503 | 0.027 | Andersson leukemia | B cell ALL vs. normal | |
| 1.425 | 0.002 | Maia leukemia | B cell ALL vs. normal | |
| 2.522 | 0.004 | Zhan myeloma 3 | Smoldering myeloma vs. normal |
Supplying Ligands for PPAR Signaling
Lipids are strong ligands for peroxisome proliferator-activated receptors (PPARs), a family comprised of three members, PPARα, PPARβ/δ, and PPARγ, all playing a range of important functions in health and disease, including cancer [71]. The three receptors have some selectivity, as well as overlap, in the preference for specific lipids that include unsaturated FAs, branched chain FAs, oxidized FAs, nitro-FAs, eicosanoids, and phospholipids [72, 73].
PPARγ
The most well-described PPAR, especially in a context of adipose-rich organs, is PPARγ, a master regulator of adipogenesis [74]. PPARγ signaling has been credited with insulin-sensitizing and anti-inflammatory effects [73]; however, its overall function in tumor development and progression has been, at minimum, controversial [73]. A number of studies to date have reported that activating PPARγ inhibits tumorigenesis [75]. The inhibitory effects of PPARγ activity on MM growth have been shown to occur through the suppression of IL-6 production [76] and potentiation of cytotoxic effects of valproic acid [77] and HDAC inhibitors [78]. PPARγ activation has also been suggested as a treatment approach to overcome kinase resistance in CML [79]. At the same time, tumor-promoting effects of PPARγ ligands or receptor overexpression have been demonstrated in tumors of the bladder, breast, and prostate [80–83]. In fact, an elegant study utilizing a Sleeping Beauty screen by Ahmad et al. showed that PPARγ overexpression correlates with phosphatase and tensin homolog (PTEN) loss, a more aggressive phenotype, and it indicates poor prognosis in human prostate cancer [84•].
Recent studies by Boyd et al. have reported that induction of marrow adipogenesis by PPARγ agonists can actually repress leukemia growth [85]. Authors propose that since AML disrupts de novo adipogenesis in the red bone marrow and compromises the myelo-erythroid maturation, pharmacological stimulation of adipogenesis could serve as a means of enhancing healthy human myelo-erythroid cell production. However, the use of synthetic PPARγ agonists and consequent increases of adipogenesis in the bone marrow come at the cost of bone loss [7, 86, 87]. In addition, for cancers that have been shown to depend on adipocyte-supplied lipids, such as MM, AML, or prostate tumors, rosiglitazone (PPARγ agonist)-driven marrow adiposity has a potential to fuel their progression in bone. Transcriptional activity of PPARγ is driven by nucleocytoplasmic shuttling of its downstream target FABP4 [88]. Ligand delivery and binding promotes activation of the PPARγ receptor but it can also stimulate its elimination [89]. Our previous studies showed that exposure of prostate tumor cells to adipocyte-derived factors leads to PPARγ-driven FABP4 upregulation followed by PPARγ downregulation, coincident with more invasive behavior [20]. This is consistent with reports linking PPARγ suppression with disruption of metabolic oversight, initiation of inflammatory pathways, and malignant transformation [90–92].
PPARα
PPARα is a fatty acid sensor and a transcriptional activator of fatty acid β-oxidation through induction of fatty acid catabolic enzymes and transport proteins such as Acyl-CoA oxidase (ACO), CPT1, mitochondrial uncoupling proteins (UCP2 and UCP3), and repression of SREBP-1 and SREBP-2, Acetyl-CoA carboxylase (ACC), and fatty acid synthase (FAS) [93]. PPARα activity is thought to drive anti-cancer effects through the antagonism of major inflammatory pathways and governing metabolic equilibrium through its interaction with 5’ AMP-activated protein kinase (AMPK) [93]. PPARα is activated by the fatty acid released by adipocyte lipolysis and its activity is essential for liver function [94]. On the other hand, long-term exposure to PPARα ligands drives the development of liver tumors in mice, although this tumorigenic effect of PPARα activation appears to be absent in humans [95]. Paracrine interactions between adipocytes and chronic lymphocytic leukemia (CLL) cells have been shown to induce PPARα activity and promote FAO, resulting in resistance to glucocorticoid treatment [96], and selective antagonism of PPARα activity induces apoptosis in CLL cells [97]. Whether the bone marrow adipocytes would have similar effects on PPARα activity in tumors residing in bone is not known, as it has been reported that PPARα activity can be repressed by hypoxia [98]. Keeping PPARα inactive under hypoxic conditions in the marrow could potentially be a mechanism of survival for the tumors that do not rely on β-oxidation.
PPARβ/δ
Although PPARβ/δ is the least studied subtype of PPARs, its key involvement in regulation of lipid metabolism has been well established through the overexpression and knockout studies in mice (reviewed in [71]). Specifically, metabolic pathways regulated by this receptor include FAO and mitochondrial respiration, processes that impact the ability of cells to function in challenging environments. Indeed, PPARβ/δ activation has been shown to specifically promote breast cancer survival in harsh metabolic conditions [99], and its involvement was revealed as critical for CLL survival under energetic stress [100]. Links between PPARβ/δ overexpression and advanced stage of the disease with reduced patient survival have also been reported for other cancers [101]. PPARβ/δ has been demonstrated to be involved in the proliferation of AR-expressing tumors [102] and to interact with HIF-1α pathway, both implicated in tumor survival and resistance to therapy [99, 103–106].
Life vs. Death Decision-making: Anti-apoptosis and Pro-survival Signaling Driven by Marrow Adipocytes
There are multiple ways by which adipocyte-supplied lipids can affect tumor cell survival. In addition to serving as building blocks for newly synthesized membrane phospholipids and a source of energy via the β-oxidation pathway discussed previously, lipids are used for biosynthesis of pro-tumorigenic lipid signaling molecules such as phosphatidylinositol-3,4,5-triphosphate [PI(3,4,5)P3]. Here, we will focus specifically on their contribution to the levels of oxidative stress and ROS production, mitochondrial homeostasis, and cellular metabolism, as well as binding and the function of molecules that regulate survival and death pathways in the cell.
Oxidative Stress and ROS
The bone marrow niche is a harsh environment prone to stress conditions associated with hypoxia, nutrient depletion, and generation of reactive oxygen species (ROS). Importantly, these effects are exacerbated by skeletal aging and increased adiposity [107, 108]. Oxidative stress is a known inducer of adipogenesis, and its levels increase even more with fat accumulation in adipocytes [109, 110]. ROS levels affect multiple cell types in the bone marrow in several ways: they can compromise bone homeostasis and promote osteoporosis [108], modulate the function of immune cells [111], and can make cancer cells more susceptible to other stressors and promote apoptosis [112]. However, persistently high levels of ROS and consequent DNA damage and genomic instability have also been linked to the induction of pro-survival signaling in a number of cancers [113, 114]. Recent studies from our laboratory have shown that, when exposed to marrow adipocytes in vitro or in vivo, bone-trophic prostate and breast tumor cells show increased ROS levels along with augmented expression of oxidative stress enzyme, heme oxygenase 1 (HO-1) [115]. Importantly, induction of HO-1 and endoplasmic reticulum (ER) stress responses lead to the activation of pro-survival pathways involving a member of the inhibitor of apoptosis protein (IAP) family, Survivin [115]. Growing evidence links oxidative stress with the progression of other cancers in bone: in metastatic renal cell carcinoma, oxidative stress was recently implicated in tumor-induced immune suppression [116], and the induction of ROS was linked to aggressive phenotype in MM [117]. It is the magnitude of oxidative stress combined with the levels of the anti-oxidant enzymes that drive life vs. death decisions in most cancers, including myelomas and leukemias [118–120], and further studies are needed to understand the contribution of marrow adipose tissue to these events.
PI3K/Akt Pathway
PI(3,4,5)P3 is a lipid signaling molecule generated through the action of phosphoinositol-3-kinase (PI3K). PI3K activity results in the downstream phosphorylation of the serine-threonine kinase Akt, a major protein dysregulated in cancer [121, 122]. Akt is an activator of the mammalian target of rapamycin (mTOR), which controls many genes involved in cell proliferation, metabolism, and regulation of apoptosis [123]. A recent study showed that omental adipocytes in coculture with gastric cancer cells secrete increased amounts of oleic acid, a lipid that significantly increases the invasiveness and growth through the PI3K/Akt pathway [124]. The long-chain monosaturated oleate was also shown as a culprit in PI3K-dependent induction of proliferation of breast cancer cells [125]. In addition, interaction of prostate carcinoma cells with adipocytes was shown to activate the PI3K/Akt pathway, leading to downstream induction of epithelial-to-mesenchymal transition [126]. PI3K/Akt activity is highly dependent on lipid composition of the cell as both PPARγ and PPARβ/δ ligands can mediate pro-survival signals via Akt signaling [127]. Interestingly, growing evidence indicates that fatty acids, such as those supplied by the adipocytes, can change the membrane composition of a cancer cell and affect localization and signaling of PI3K/Akt, thus driving downstream pro-survival phenotype [128, 129].
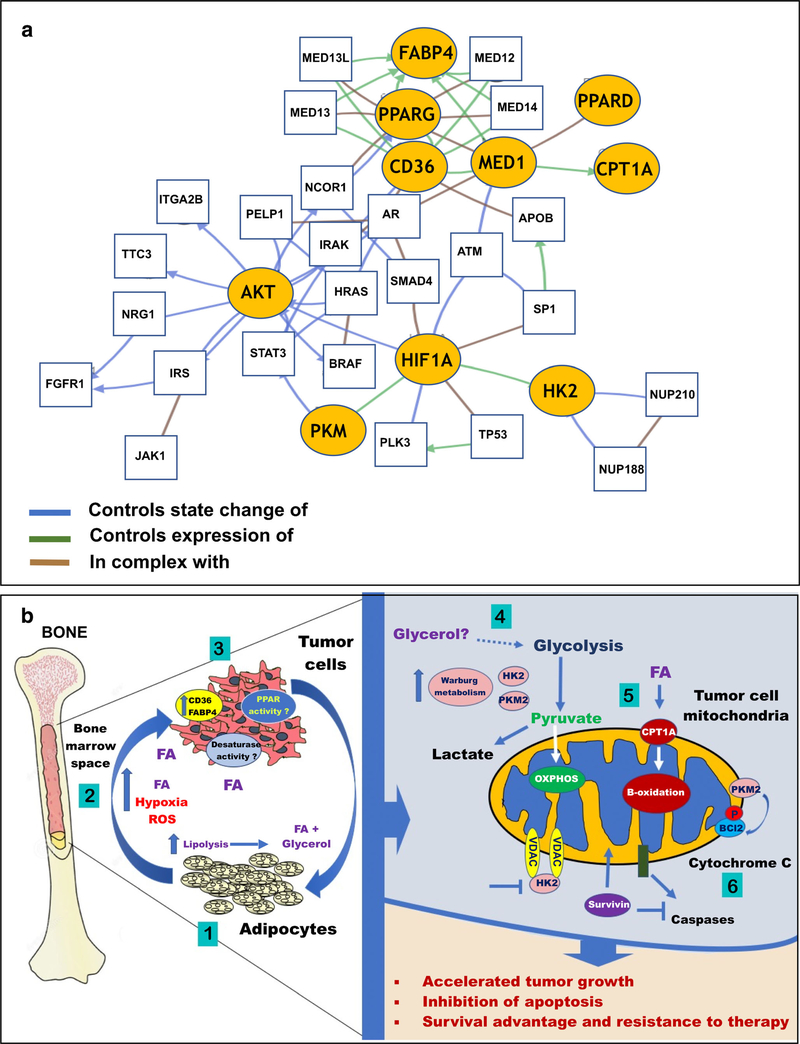
It is noteworthy that the aberrant PI3K signaling and Akt activation are often associated with the loss of phosphatase and tensin homolog (PTEN), one of the most commonly mutated or downregulated tumor suppressors in cancers [130]. PTEN loss is very common in prostate cancer and has been shown to be correlated with a poor clinical outcome, aggression of the disease, and clinical recurrence [131–133]. In MM, AML, and other myeloid malignancies, PTEN deletions occur in the advanced stage of the disease and are suggested to be associated with disease progression [134]. In addition, PTEN-null MM cells appear to depend on PI3K/Akt activation for cell survival and response to therapy [135]. Notably, an enzymatic PTEN activity is known to depend on posttranslational regulation including phosphorylation, acetylation, and oxidation [136]. Adipocyte-driven oxidative stress and ROS production can lead to phosphorylation and inactivation of PTEN, a process that is reversible by anti-oxidant treatment [137]. Intriguingly, PTEN has been reported to interact and form a complex with FABP4, a fatty acid chaperone known to be secreted by adipocytes and upregulated by tumor cells exposed to adipocyte-rich microenvironments [20, 22•, 138]. PTEN deletion in hepatocytes leads to adipogenic transformation resulting in steatohepatitis and hepatocellular carcinomas [139]. In line with these findings, a positive regulation of breast cancer cell proliferation by PI3K/Akt pathways appears to be dependent on FABP4 [140]. Whether this interaction is important for tumor survival in adipocyte-rich bone marrow remains to be investigated as there is clearly a cross-talk between PI3K/Akt axis and multiple regulators of cellular energetics (Fig. 2a).
Fig. 2.
a Network analysis showing interaction between signaling pathways involving AKT, HIF1A, CPT1A, PPARD, PPARG, HK2, PKM, FABP4, MED1, and CD36 using the Genomic Hallmarks of Prostate Adenocarcinoma (CPC-Gene, Nature 2017) dataset. Nodes with yellow ovals represent the selected genes, while genes with thin black rectangles represent co-expressed genes that are interacting with the selected genes. b Schematic diagram depicting the potential impact of adipocyte-tumor cell interactions in bone marrow on lipid signaling and downstream pathways promoting survival. An adipocyte-tumor cell cross-talk induces triglyceride lipolysis in adipocytes to glycerol and fatty acids (FA) (1), which leads to an activation of hypoxia signaling and ROS production (2). Exposure to adipocyte-supplied lipids induces expression of lipid transporters FABP4 and CD36 in tumor cells and modulates PPAR and desaturase activity (3). Adipocyte-supplied glycerol can feed into the glycolytic cycle (4) and stimulate Warburg phenotype in tumor cells as opposed to oxidative phosphorylation. Some tumors will rely on catabolism of adipocyte-supplied fatty acids through β-oxidation for growth and survival (5). Alterations in tumor metabolism will be associated with increased levels of HK2, PKM2, and Survivin, leading to the inhibition of mitochondrial pro-apoptotic machinery (6) and resulting in tumor survival and evasion of therapy
Hexokinase II
Mitochondria are at the heart of cellular life and death decisions. They drive metabolic functions such as FAO, TCA cycle, and respiration [141], but are also a regulatory site for apoptosis [142]. Within mitochondria, Bcl-2 family members, which bind to voltage-dependent anion channel (VDAC), regulate the release of proteins from the space between the inner and outer membrane and activate a pro-apoptotic cascade [142]. Intriguingly, some of the metabolic enzymes, such as Hexokinase 2 (HK2), can compete with VDAC binding of Bcl-2 family proteins and disrupt pro-apoptotic signaling [143, 144]. Being the first enzyme in the glycolysis pathway, the primary role of HK2 is the catabolism of glucose [145]. HK2 binds to the outer membrane of mitochondria and partners with VDAC proteins [146] and allows for closer proximity to the ATP sources that pass through the VDACs [147, 148]. In exchange, the mitochondria benefit from the ADP produced by HK2. Importantly, when bound to the mitochondria, HK2 can no longer be inhibited by its product glucose-6-phosphate [149, 150]. Upregulation of HK2 and its interaction with VDAC are thought to bring VDAC closer to the inner mitochondrial membrane and to enhance Warburg metabolism, a hallmark of many cancers [151], including metastatic prostate cancers in bone under conditions of increased marrow adiposity [18] (Fig. 2b).
Apart from its role in glucose metabolism, overexpression of HK2 has been shown to be protective against cell death induced by pro-oxidants [152, 153]. Although the mechanisms behind this protection are not well understood, it is the ability of HK2 to competitively inhibit VDAC association with pro-apoptotic factors such as Bcl-2 family members Bax and Bak that are proposed to be the culprit [143, 144]. HK2 expression appears to be transcriptionally regulated by Akt and mTOR [144] and is a direct target of HIF-1α activity [154]. In addition, both glycolysis and HK2 expression are induced by phosphorylation of PPARγ at Ser84 [155], suggesting the role of PPARγ in HK2 regulation [156]. Interestingly, HK2 appears to be constitutively overexpressed in MM cells, and the treatment with HK2 inhibitor 3-bromopyruvate (3BrPA) suppresses ATP production and induces apoptosis [157, 158]. Around 80% of total HK2 is reported to be bound to the mitochondrial VDAC [149]. Since aerobic glycolysis can be induced in metastatic tumor cells by bone marrow adipocytes [18], and there is increasing evidence that Warburg metabolism is highly operative in cancers that thrive in bone, including metastatic prostate cancer [18], MM [34, 159], and leukemias [160, 161], it is feasible to propose that marrow adipocytes are likely playing a role in HK2-driven regulation of tumor cell survival.
PKM2 Overexpression and Stabilization of Pro-survival Factors
Another glycolytic enzyme with capabilities of translocating to mitochondria and affecting life and death decisions is pyruvate kinase 2 (PKM2), the splice variant of PKM overexpressed in a variety of tumors and associated with the Warburg phenotype [162, 163••]. PKM2 overexpression has been shown to predict survival [34] and drive chemoresistance [164] in MM, and it has been linked to fatty acid metabolism and progression of AML [96]. A recent study by Liang et al. has demonstrated that PKM2 translocates to the mitochondria under oxidative stress conditions where it phosphorylates Bcl-2 and protects it from degradation, a process leading to the inhibition of apoptosis [163••]. It has also been shown that PKM2 is capable of enhancing the stability of NF-κB subunit p65, facilitating its binding to the Bcl-xL promoter, which could serve as an additional pro-survival mechanism for tumor cells [165]. PKM2 has been demonstrated to function as a coactivator of HIF-1α, driving the downstream transcription of HIF-1α target genes such as lactate dehydrogenase (LDHα), pyruvate dehydrogenase kinase 1 (PDK1), and glucose transporter 1 (GLUT1) [166]. In addition, important for its role in tumor cell growth and survival, phosphorylation of histone H3 by PKM2 drives the induction of many critical cell cycle genes including Cyclin D and c-MYC [167]. Notably, the nuclear localization of PKM2 is important for its role as a transcriptional co-activator of c-Src phosphorylated β-catenin [168] and a mediator of STAT3 phosphorylation [169]. Activation of these pathways promotes cellular survival and collectively can facilitate growth of cancer cells within the harsh bone microenvironment.
It is noteworthy that both PKM2 and HK2 have been shown to be induced by PPARγ agonists in PTEN-null fatty livers, a process that has been proposed as a link between liver steatosis and cancer [170]. Whether the exposure of tumor cells residing in the adipocyte-rich bone marrow to fat cell-supplied PPARγ ligands would cause upregulation of PKM2 and HK2 and drive tumor progression is not clear and warrants further investigations.
Survivin
Survivin is a member of the inhibitor of apoptosis protein (IAP) family, expressed in almost all cancers and implicated in tumor aggressiveness and chemoresistance [171]. Survivin is a bi-functional protein which functions as a regulator of mitosis and an inhibitor of apoptosis [172]. This pro-survival factor is known to be regulated by HIF-1α, and its function is required to maintain cell viability during hypoxia [173–175]. Our recent studies have shown that, in prostate cancer, Survivin levels are regulated by oxidative stress and are particularly high in metastatic tissues as compared to primary tumors [115], a result in line with previous studies implicating Survivin as a mediator of tumor metastasis to bone [176]. Further testimony to its potential role in bone metastatic disease are the reports on significantly higher Survivin levels in patients with skeletal lesions from breast cancer as compared to patients with non-metastatic or benign disease [177].
Tightly linked to the Survivin expression levels is its function. Survivin has been reported to have protective effects on tumor cells, and its role in chemoresistance has been specifically linked to its localization to mitochondria in response to stressors such as chemotherapeutics, ROS, or hypoxia [172]. Mitochondrial Survivin has been suggested to directly interfere with apoptosis pathways by binding to caspase-3 and caspase-7 and preventing activation of initiator caspase-9 and the induction of apoptosis [172, 178]. Consistent with these results, targeting Survivin to the mitochondria was demonstrated to be sufficient to increase colony formation in soft agar and accelerate in vivo tumor growth while, again, ablating an apoptotic response and facilitating cell survival [172]. Accordingly, overexpression of Survivin was shown to be a major contributor to multidrug resistance in MM [179] and a prognostic indicator of poor outcome in acute leukemias, especially AML and CLL [180, 181].
One important feature of Survivin is that it is regulated by the insulin-activated PI3K/mTOR signaling, and it plays important roles in adipocyte homeostasis [182]. Specifically, it is postulated that Survivin is a nutrient-sensitive molecule and a critical checkpoint against metabolic dysfunction in response to overnutrition [182]. This is intriguing, as a similar mechanism involving Survivin could be functional in tumor cells exposed to high levels of adipocyte-supplied lipids in bone marrow. Indeed, emerging reports link Survivin expression with modulation of tumor metabolism, indicating a crucial cross-talk between metabolic signatures and chemoresistance. Specifically, overexpression of Survivin in neuroblastoma cells shifts tumor metabolism from OXPHOS to aerobic glycolysis and induces resistance to forms of cell death that depend on the accumulation of ROS [183•]. Our own studies show that overexpression of Survivin occurs in tumor cells with enhanced Warburg phenotype and increased production of ROS [18, 115]. On the other hand, mitochondrial Survivin was recently reported to promote oxidative phosphorylation in prostate tumor cells [184]. Specifically, this anti-apoptotic protein was demonstrated to cooperate with the chaperone TRAP-1 and protect mitochondrial bioenergetics by maintaining succinate dehydrogenase SDH (folding) and activity of Complex II. Authors of this study proposed that the oxidative phosphorylation maintained by Survivin-TRAP-1 interaction provides concentrated “regional” energy source to support specific energy-intensive tasks [184]. Clearly, more studies are needed to understand the role of Survivin in metabolic adaptation and tumor survival in bone. Survivin-overexpressing tumors have recently been shown to be sensitive to glycolysis inhibitors [183•], thus elucidating that the molecular link between marrow adiposity, glycolytic phenotype, and Survivin expression in the tumor will almost certainly have clinical implications.
Conclusions
There is growing, compelling evidence that alterations in lipid metabolism and lipid signaling pathways are the key characteristics behind tumor growth, progression, and adaptation in metastatic environments. Tumor cells have high avidity for lipids and are likely to be very receptive to the abundance of exogenous fats in the adipose-rich tissues such as bone marrow. The resulting lipid-mediated cross-talk between marrow adipocytes and resident tumor cells alters cellular energetics, disrupts redox homeostasis, and profoundly affects signaling pathways that allow the cells to gain pro-survival advantage and thrive (Fig. 2). There is a need for continuous effort to identify key molecular mechanisms responsible for the oncogenic nature of adipocyte-supplied lipids. Although there have been many advances in cancer therapies in a context of tumor metabolism, understanding how to target tumor dependence on the lipids has a potential to fundamentally change therapeutic approaches for cancers that thrive in adipocyte-rich bone marrow.
Acknowledgments
Funding NIH/NCI 1 R01 CA181189 DOD W81XWH-14-1-0036 NIH/NCI 1F31CA203036
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Jonathan Diedrich, Mackenzie Herroon, Erandi Rajagurubandara, and Izabela Podgorski declare no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.NCI Metastatic Cancer 2018. [Available from: https://www.cancer.gov/types/metastatic-cancer.
- 2.Gusky Chkourko H, Diedrich J, MacDougald OA, Podgorski I. Omentum and bone marrow: how adipocyte-rich organs create tumour microenvironments conducive for metastatic progression. Obes Rev. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114(6):1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Paula FJA, Rosen CJ. Structure and function of bone marrow adipocytes. Compr Physiol. 2017;8(1):315–49.•Comprehensive review of bone marrow adipocyte properties and function in normal physiology and several pathologies.
- 5.Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004;23(2–3):183–8. [DOI] [PubMed] [Google Scholar]
- 6.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: new insights from an “old” molecule. Cell Cycle. 2010;9(18):3648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Hardij J, Bagchi DP, Scheller EL, MacDougald OA. Development, regulation, metabolism and function of bone marrow adipose tissues. Bone. 2018;110:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falank C, Fairfield H, Reagan MR. Signaling interplay between bone marrow adipose tissue and multiple myeloma cells. Front Endocrinol. 2016;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldhuis-Vlug AG, Rosen CJ. Clinical implications of bone marrow adiposity. J Intern Med. 2018;283(2):121–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7(5):373–8. [DOI] [PubMed] [Google Scholar]
- 13.Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19(1):3–9. [DOI] [PubMed] [Google Scholar]
- 14.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–7. [DOI] [PubMed] [Google Scholar]
- 15.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17(9):1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282(8):5726–35. [DOI] [PubMed] [Google Scholar]
- 17.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J LipidRes. 2002;43(10):1585–94. [DOI] [PubMed] [Google Scholar]
- 18.Diedrich JD, Rajagurubandara E, Herroon MK, Mahapatra G, Huttemann M, Podgorski I. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1alpha activation. Oncotarget. 2016;7(40):64854–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafat MS, Oellerich T, Mohr S, Robinson SD, Edwards DR, Marlein CR, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129(10):1320–32.••First study to demonstrate that acute myeloid leukemia cells induce lipolysis in marrow adipocytes to support and promote tumor progression.
- 20.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4(11):2108–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieman K, Kenny H, Penicka C, Ladanyi A, Buell-Gutbrod R, Zillhardt M, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503.•First study to demonstrate the role of FABP4 in metabolic regulation of tumor cells by adipocytes.
- 23.Tabe Y, Yamamoto S, Saitoh K, Sekihara K, Monma N, Ikeo K, et al. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Res. 2017;77(6):1453–64.••Important study demonstrating the potential therapeutic utility of inhibiting fatty acid oxidation in AML treatment.
- 24.Zhao J, Zhi Z, Wang C, Xing H, Song G, Yu X, et al. Exogenous lipids promote the growth of breast cancer cells via CD36. Oncol Rep. 2017;38(4):2105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell. 2016;19(1): 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parhofer KG. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. 2015;39(5): 353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53(6):482–91. [DOI] [PubMed] [Google Scholar]
- 28.Philip B, Ito K, Moreno-Sanchez R, Ralph SJ. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34(8):1699–707. [DOI] [PubMed] [Google Scholar]
- 29.Raja R, Kale S, Thorat D, Soundararajan G, Lohite K, Mane A, et al. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1alpha-mediated VEGF-dependent angiogenesis. Oncogene. 2014;33(16):2053–64. [DOI] [PubMed] [Google Scholar]
- 30.Zecchini V, Madhu B, Russell R, Pertega-Gomes N, Warren A, Gaude E, et al. Nuclear ARRB1 induces pseudohypoxia and cellular metabolism reprogramming in prostate cancer. EMBO J. 2014;33(12):1365–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav N, Kumar S, Marlowe T, Chaudhary AK, Kumar R, Wang J, et al. Oxidative phosphorylation-dependent regulation of cancer cell apoptosis in response to anticancer agents. Cell Death Dis. 2015;6:e1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra D, Liu JW, Tang DG. Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J Biol Chem. 2002;277(52):50842–54. [DOI] [PubMed] [Google Scholar]
- 33.Kuhnel A, Blau O, Nogai K, Blau I. The Warburg effect in multiple myeloma and its microenvironment. KEI Journals 2017. 1–16. [Google Scholar]
- 34.Panchabhai S, Schlam I, Sebastian S, Fonseca R. PKM2 and other key regulators of Warburg effect positively correlate with CD147 (EMMPRIN) gene expression and predict survival in multiple myeloma. Leukemia. 2017;31(4):991–4. [DOI] [PubMed] [Google Scholar]
- 35.Cheng JC, McBrayer SK, Coarfa C, Dalva-Aydemir S, Gunaratne PH, Carpten JD, et al. Expression and phosphorylation of the AS160_v2 splice variant supports GLUT4 activation and the Warburg effect in multiple myeloma. Cancer Metab. 2013;1 (1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lis P, Dylag M, Niedzwiecka K, Ko YH, Pedersen PL, Goffeau A, et al. The HK2 dependent “Warburg effect” and mitochondrial oxidative phosphorylation in cancer: targets for effective therapy with 3-bromopyruvate. Molecules. 2016;21 (12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song K, Li M, Xu X, Xuan LI, Huang G, Liu Q. Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol Lett. 2016;12(1):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauge M, Bruserud O, Hatfield KJ. Targeting of cell metabolism in human acute myeloid leukemia—more than targeting of isocitrate dehydrogenase mutations and PI3K/AKT/mTOR signaling? Eur J Haematol. 2016;96(3):211–21. [DOI] [PubMed] [Google Scholar]
- 39.Boag JM, Beesley AH, Firth MJ, Freitas JR, Ford J, Hoffmann K, et al. Altered glucose metabolism in childhood pre-B acute lymphoblastic leukaemia. Leukemia. 2006;20(10):1731–7. [DOI] [PubMed] [Google Scholar]
- 40.Kominsky DJ, Klawitter J, Brown JL, Boros LG, Melo JV, Eckhardt SG, et al. Abnormalities in glucose uptake and metabolism in imatinib-resistant human BCR-ABL-positive cells. Clin Cancer Res. 2009;15(10):3442–50. [DOI] [PubMed] [Google Scholar]
- 41.Le A, Stine ZE, Nguyen C, Afzal J, Sun P, Hamaker M, et al. Tumorigenicity of hypoxic respiring cancer cells revealed by a hypoxia-cell cycle dual reporter. Proc Natl Acad Sci USA. 2014;111 (34):12486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ertel A, Tsirigos A, Whitaker-Menezes D, Birbe RC, Pavlides S, Martinez-Outschoorn UE, et al. Is cancer a metabolic rebellion against host aging? In the quest for immortality, tumor cells try to save themselves by boosting mitochondrial metabolism. Cell Cycle. 2012;11(2):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitaker-Menezes D, Martinez-Outschoorn UE, Flomenberg N, Birbe RC, Witkiewicz AK, Howell A, et al. Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle. 2011;10(23):4047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henkenius K, Greene BH, Barckhausen C, Hartmann R, Marken M, Kaiser T, et al. Maintenance of cellular respiration indicates drug resistance in acute myeloid leukemia. Leuk Res. 2017;62: 56–63. [DOI] [PubMed] [Google Scholar]
- 45.Zhan X, Yu W, Franqui-Machin R, Bates ML, Nadiminti K, Cao H, et al. Alteration of mitochondrial biogenesis promotes disease progression in multiple myeloma. Oncotarget. 2017;8(67): 111213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heiden Vander MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Clinical implications of bone marrow adiposity. 2013;52(4):585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyl transferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogene. 2016;5:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(3):230–4. [DOI] [PubMed] [Google Scholar]
- 51.Zha S, Ferdinandusse S, Hicks JL, Denis S, Dunn TA, Wanders RJ, et al. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate. 2005;63(4): 316–23. [DOI] [PubMed] [Google Scholar]
- 52.Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120(1):142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu J, Van Valckenborgh E, Menu E, De Bruyne E, Vanderkerken K. Understanding the hypoxic niche of multiple myeloma: therapeutic implications and contributions of mouse models. Dis Model Mech. 2012;5(6):763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colla S, Storti P, Donofrio G, Todoerti K, Bolzoni M, Lazzaretti M, et al. Low bone marrow oxygen tension and hypoxia-inducible factor-lalpha overexpression characterize patients with multiple myeloma: role on the transcriptional and proangiogenic profiles of CD138(+) cells. Leukemia. 2010;24(11):1967–70. [DOI] [PubMed] [Google Scholar]
- 55.Martin SK, Diamond P, Williams SA, To LB, Peet DJ, Fujii N, et al. Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12 expression in multiple myeloma plasma cells. Haematologica. 2010;95(5):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borsi E, Terragna C, Brioli A, Tacchetti P, Martello M, Cavo M. Therapeutic targeting of hypoxia and hypoxia-inducible factor 1 alpha in multiple myeloma. Transl Res. 2015;165(6):641–50. [DOI] [PubMed] [Google Scholar]
- 58.Deynoux M, Sunter N, Herault O, Mazurier F. Hypoxia and hypoxia-inducible factors in leukemias. Front Oncol. 2016;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilkes DM. Implications of hypoxia in breast cancer metastasis to bone. Int J Mol Sci. 2016;17(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semenza GL. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863(3):382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–11. [DOI] [PubMed] [Google Scholar]
- 62.Yao-Borengasser A, Monzavi-Karbassi B, Hedges RA, Rogers LJ, Kadlubar SA, Kieber-Emmons T. Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol Rep. 2015;33(6):2689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, et al. Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9(1):349–65. [DOI] [PubMed] [Google Scholar]
- 64.Michalopoulou E, Bulusu V, Kamphorst JJ. Metabolic scavenging by cancer cells: when the going gets tough, the tough keep eating. Br J Cancer. 2016;115(6):635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 2014;24(8): 472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci USA. 2013;110(22):8882–7.•This study demonstrates that under hypoxia, tumor cells bypass de novo lipo-genesis and resort to scavenging of serum fatty acids for support of growth and survival.
- 67.Peck B, Schug ZT, Zhang Q, Dankworth B, Jones DT, Smethurst E, et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016;4:6.••Important study utilizing functional genomics to identify stearoyl-CoA desaturase (SCD) as desaturating enzyme responsible for survival of breast and prostate cancer cells.
- 68.Peck B, Schulze A. Lipid desaturation—the next step in targeting lipogenesis in cancer? FEBS J. 2016;283(15):2767–78. [DOI] [PubMed] [Google Scholar]
- 69.Lewis CA, Brault C, Peck B, Bensaad K, Griffiths B, Mitter R, et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene. 2015;34(40):5128–40. [DOI] [PubMed] [Google Scholar]
- 70.Tosi F, Sartori F, Guarini P, Olivieri O, Martinelli N. Delta-5 and delta-6 desaturases: crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv Exp Med Biol. 2014;824:61–81. [DOI] [PubMed] [Google Scholar]
- 71.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812(8): 1007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poulsen L, Siersbaek M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23(6):631–9. [DOI] [PubMed] [Google Scholar]
- 73.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12(3):181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–56. [DOI] [PubMed] [Google Scholar]
- 75.Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9(1):1–9. [PubMed] [Google Scholar]
- 76.Garcia-Bates TM, Bernstein SH, Phipps RP. Peroxisome proliferator-activated receptor gamma overexpression suppresses growth and induces apoptosis in human multiple myeloma cells. Clin Cancer Res. 2008;14(20):6414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aouali N, Palissot V, El-Khoury V, Moussay E, Janji B, Pierson S, et al. Peroxisome proliferator-activated receptor gamma agonists potentiate the cytotoxic effect of valproic acid in multiple myeloma cells. Br J Haematol. 2009;147(5):662–71. [DOI] [PubMed] [Google Scholar]
- 78.Aouali N, Broukou A, Bosseler M, Keunen O, Schlesser V, Janji B, et al. Epigenetic activity of peroxisome proliferator-activated receptor gamma agonists increases the anticancer effect of histone deacetylase inhibitors on multiple myeloma cells. PLoS One. 2015;10(6):e0130339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yousefi B, Shafiei-Irannejad V, Azimi A, Samadi N, Zarghami N. PPAR-gamma in overcoming kinase resistance in chronic myeloid leukemia. Cell Mol Biol (Noisy-le-grand). 2016;62(8):52–5. [PubMed] [Google Scholar]
- 80.Lubet RA, Fischer SM, Steele VE, Juliana MM, Desmond R, Grubbs CJ. Rosiglitazone, a PPAR gamma agonist: potent promoter of hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers. Int J Cancer. 2008;123(10):2254–9. [DOI] [PubMed] [Google Scholar]
- 81.Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-gamma ligands for the treatment of breast cancer. Expert Opin Investig Drugs. 2005;14(6):557–68. [DOI] [PubMed] [Google Scholar]
- 82.Forootan FS, Forootan SS, Gou X, Yang J, Liu B, Chen D, et al. Fatty acid activated PPARgamma promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget. 2016;7(8):9322–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galbraith L, Leung HY, Ahmad I. Lipid pathway deregulation in advanced prostate cancer. Pharmacol Res. 2018. [DOI] [PubMed] [Google Scholar]
- 84.Ahmad I, Mui E, Galbraith L, Patel R, Tan EH, Salji M, et al. Sleeping Beauty screen reveals Pparg activation in metastatic prostate cancer. Proc Natl Acad Sci USA. 2016;113(29):8290–5.•This study links PPAR gamma activation with PTEN loss and aggressiveness in prostate cancer.
- 85.Boyd AL, Reid JC, Salci KR, Aslostovar L, Benoit YD, Shapovalova Z, et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017;19(11):1336–47. [DOI] [PubMed] [Google Scholar]
- 86.Benvenuti S, Cellai I, Luciani P, Deledda C, Baglioni S, Giuliani C, et al. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Investig. 2007;30(9):RC26–30. [DOI] [PubMed] [Google Scholar]
- 87.Suchacki KJ, Roberts F, Lovdel A, Farquharson C, Morton NM, MacRae VE, et al. Skeletal energy homeostasis: a paradigm of endocrine discovery. J Endocrinol. 2017;234(1):R67–79. [DOI] [PubMed] [Google Scholar]
- 88.Ayers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgammaby FABP4. Biochemistry. 2007;46(23):6744–52. [DOI] [PubMed] [Google Scholar]
- 89.Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275(24):18527–33. [DOI] [PubMed] [Google Scholar]
- 90.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–59. [DOI] [PubMed] [Google Scholar]
- 91.Jiang M, Jerome WG, Hayward SW. Autophagy in nuclear receptor PPARgamma-deficient mouse prostatic carcinogenesis. Autophagy. 2010;6(1):175–6. [DOI] [PubMed] [Google Scholar]
- 92.Jiang M, Strand DW, Franco OE, Clark PE, Hayward SW. PPARgamma: a molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation. 2011;82(4–5):220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grabacka M, Reiss K. Anticancer properties of PPARalpha-effects on cellular metabolism and inflammation. PPAR Res. 2008;2008: 930705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iroz A, Montagner A, Benhamed F, Levavasseur F, Polizzi A, Anthony E, et al. A specific ChREBP and PPARalpha cross-talk is required for the glucose-mediated FGF21 response. Cell Rep. 2017;21(2):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4(1):61–70. [DOI] [PubMed] [Google Scholar]
- 96.Tung S, Shi Y, Wong K, Zhu F, Gorczynski R, Laister RC, et al. PPARalpha and fatty acid oxidation mediate glucocorticoid resistance in chronic lymphocytic leukemia. Blood. 2013;122(6): 969–80. [DOI] [PubMed] [Google Scholar]
- 97.Messmer D, Lorrain K, Stebbins K, Bravo Y, Stock N, Cabrera G, et al. A selective novel peroxisome proliferator-activated receptor (PPAR)-alpha antagonist induces apoptosis and inhibits proliferation of CLL cells in vitro and in vivo. Mol Med. 2015;21:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huss JM, Levy FH, Kelly DP Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J BiolChem. 2001;276(29):27605–12. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Wang G, Shi Y, Sun L, Gorczynski R, Li YJ, et al. PPAR-delta promotes survival of breast cancer cells in harsh metabolic conditions. Oncogene. 2016;5(6):e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li YJ, Sun L, Shi Y, Wang G, Wang X, Dunn SE, et al. PPAR-delta promotes survival of chronic lymphocytic leukemia cells in energetically unfavorable conditions. Leukemia. 2017;31 (9): 1905–14. [DOI] [PubMed] [Google Scholar]
- 101.Zuo X, Xu W, Xu M, Tian R, Moussalli MJ, Mao F, et al. Metastasis regulation by PPARD expression in cancer cells. JCI Insight. 2017;2(1):e91419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, et al. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004;64(9):3162–70. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan R, Pare GC, Frederiksen LJ, Semenza GL, Graham CH. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008;7(7):1961–73. [DOI] [PubMed] [Google Scholar]
- 104.Doktorova H, Hrabeta J, Khalil MA, Eckschlager T. Hypoxia-induced chemoresistance in cancer cells: the role of not only HIF-1. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(2):166–77. [DOI] [PubMed] [Google Scholar]
- 105.Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, et al. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008;99(1):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diedrich J, Gusky HC, Podgorski I. Adipose tissue dysfunction and its effects on tumor metabolism. Horm Mol Biol Clin Invest. 2015;21(1):17–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–70. [DOI] [PubMed] [Google Scholar]
- 109.Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxidative Med Cell Longev. 2014;2014:908539.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12): 1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sandoval H, Kodali S, Wang J. Regulation of B cell fate, survival, and function by mitochondria and autophagy. Mitochondrion. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zou Z, Chang H, Li H, Wang S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis. 2017;22(11):1321–35. [DOI] [PubMed] [Google Scholar]
- 113.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7(6):e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27(2):156–7. [DOI] [PubMed] [Google Scholar]
- 115.Herroon MK, Rajagurubandara E, Diedrich JD, Heath EI, Podgorski I. Adipocyte-activated oxidative and ER stress pathways promote tumor survival in bone via upregulation of Heme Oxygenase 1 and Survivin. Sci Rep. 2018;8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181(1):346–53. [DOI] [PubMed] [Google Scholar]
- 117.Kamihara Y, Takada K, Sato T, Kawano Y, Murase K, Arihara Y, et al. The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/beta-catenin signaling in human multiple myeloma. Oncotarget. 2016;7(39):64330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47. [DOI] [PubMed] [Google Scholar]
- 119.Irwin ME, Rivera-Del Valle N, Chandra J. Redox control of leukemia: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2013;18(11):1349–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen YF, Liu H, Luo XJ, Zhao Z, Zou ZY, Li J, et al. The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol Hematol. 2017;112:21–30. [DOI] [PubMed] [Google Scholar]
- 121.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–64. [DOI] [PubMed] [Google Scholar]
- 122.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. [DOI] [PubMed] [Google Scholar]
- 123.Jabbour E, Ottmann OG, Deininger M, Hochhaus A. Targeting the phosphoinositide 3-kinase pathway in hematologic malignancies. Haematologica. 2014;99(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiang F, Wu K, Liu Y, Shi L, Wang D, Li G, et al. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int J Biochem Cell Biol. 2017;84:14–21. [DOI] [PubMed] [Google Scholar]
- 125.Hardy S, St-Onge GG, Joly E, Langelier Y, Prentki M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem. 2005;280(14): 13285–91. [DOI] [PubMed] [Google Scholar]
- 126.Kaneko A, Satoh Y, Tokuda Y, Fujiyama C, Udo K, Uozumi J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. Int J Urol. 2010;17(4):369–76. [DOI] [PubMed] [Google Scholar]
- 127.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004;10(3):245–7. [DOI] [PubMed] [Google Scholar]
- 128.Gu Z, Wu J, Wang S, Suburu J, Chen H, Thomas MJ, et al. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis. 2013;34(9):1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fuentes NR, Salinas ML, Kim E, Chapkin RS. Emerging role of chemoprotective agents in the dynamic shaping of plasma membrane organization. Biochim Biophys Acta. 2017;1859(9 Pt B):1668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–96. [DOI] [PubMed] [Google Scholar]
- 131.Mithal P, Allott E, Gerber L, Reid J, Welbourn W, Tikishvili E, et al. PTEN loss in biopsy tissue predicts poor clinical outcomes in prostate cancer. Int JUrol. 2014;21(12):1209–14. [DOI] [PubMed] [Google Scholar]
- 132.Yoshimoto M, Ludkovski O, DeGrace D, Williams JL, Evans A, Sircar K, et al. PTEN genomic deletions that characterize aggressive prostate cancer originate close to segmental duplications. Genes Chromosom Cancer. 2012;51(2):149–60. [DOI] [PubMed] [Google Scholar]
- 133.Choucair K, Ejdelman J, Brimo F, Aprikian A, Chevalier S, Lapointe J. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity BMC Cancer. 2012;12:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang H, Qi XY, Claudio J, Zhuang L, Patterson B, Stewart AK. Analysis of PTEN deletions and mutations in multiple myeloma. LeukRes. 2006;30(3):262–5. [DOI] [PubMed] [Google Scholar]
- 135.Zhang J, Choi Y, Mavromatis B, Lichtenstein A, Li W. Preferential killing of PTEN-null myelomas by PI3K inhibitors through Akt pathway Oncogene. 2003;22(40):6289–95. [DOI] [PubMed] [Google Scholar]
- 136.Singh G, Chan AM. Post-translational modifications of PTEN and their potential therapeutic implications. Curr Cancer Drug Targets. 2011;11(5):536–47. [DOI] [PubMed] [Google Scholar]
- 137.Kitagishi Y, Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging (review). Int J Mol Med. 2013;31(3): 511–5. [DOI] [PubMed] [Google Scholar]
- 138.Hardaway AL, Podgorski I. IL-1beta, RAGE and FABP4: targeting the dynamic trio in metabolic inflammation and related pathologies. Future Med Chem. 2013;5(10):1089–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113(12):1774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guaita-Esteruelas S, Bosquet A, Saavedra P, Guma J, Girona J, Lam EW, et al. Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol Carcinog. 2017;56(1):208–17. [DOI] [PubMed] [Google Scholar]
- 141.Zong WX, Rabinowitz JD, White E. Mitochondria and cancer. Mol Cell. 2016;61(5):667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexo-kinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277(9):7610–8. [DOI] [PubMed] [Google Scholar]
- 144.Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy Cell Death Differ. 2015;22(2):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta. 2002;1555(1–3):14–20. [DOI] [PubMed] [Google Scholar]
- 146.Bustamante E, Pedersen PL. Mitochondrial hexokinase of rat hepatoma cells in culture: solubilization and kinetic properties. Biochemistry. 1980;19(22):4972–7. [DOI] [PubMed] [Google Scholar]
- 147.Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci USA. 1977;74(9):3735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bustamante E, Morris HP, Pedersen PL. Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J BiolChem. 1981;256(16): 8699–704. [PubMed] [Google Scholar]
- 149.Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988;263(33):17422–8. [PubMed] [Google Scholar]
- 150.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mazure NM. VDAC in cancer. Biochim Biophys Acta. 2017;1858(8):665–73. [DOI] [PubMed] [Google Scholar]
- 152.Ahmad A, Ahmad S, Schneider BK, Allen CB, Chang LY, White CW. Elevated expression of hexokinase II protects human lung epithelial-like A549 cells against oxidative injury. Am J Phys Lung Cell Mol Phys. 2002;283(3):L573–84. [DOI] [PubMed] [Google Scholar]
- 153.Bryson JM, Coy PE, Gottlob K, Hay N, Robey RB. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J Biol Chem. 2002;277(13):11392–400. [DOI] [PubMed] [Google Scholar]
- 154.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276(46):43407–12. [DOI] [PubMed] [Google Scholar]
- 155.Shu Y, Lu Y, Pang X, Zheng W, Huang Y, Li J, et al. Phosphorylation of PPARgamma at Ser84 promotes glycolysis and cell proliferation in hepatocellular carcinoma by targeting PFKFB4. Oncotarget. 2016;7(47):76984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Burgermeister E, Seger R. PPARgamma and MEK interactions in cancer. PPAR Res. 2008;2008:309469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakano A, Miki H, Nakamura S, Harada T, Oda A, Amou H, et al. Up-regulation of hexokinaseII in myeloma cells: targeting myeloma cells with 3-bromopyruvate. J Bioenerg Biomembr. 2012;44(1):31–8. [DOI] [PubMed] [Google Scholar]
- 158.Chen Z, Zhang H, Lu W, Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta. 2009;1787(5):553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanchez WY, McGee SL, Connor T, Mottram B, Wilkinson A, Whitehead JP, et al. Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. Br J Cancer. 2013;108(8):1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jitschin R, Braun M, Qorraj M, Saul D, Le Blanc K, Zenz T, et al. Stromal cell-mediated glycolytic switch in CLL cells involves Notch-c-Myc signaling. Blood. 2015;125(22):3432–6. [DOI] [PubMed] [Google Scholar]
- 161.Akers LJ, Fang W, Levy AG, Franklin AR, Huang P, Zweidler-McKay PA. Targeting glycolysis in leukemia: a novel inhibitor 3-BrOP in combination with rapamycin. Leuk Res. 2011;35(6): 814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Christofk HR, Heiden Vander MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3. [DOI] [PubMed] [Google Scholar]
- 163.Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017;27(3):329–51.•• Important study demonstrating nonglycolytic function of PKM2 in regulation of tumor cell survival via Bcl2 stabilization.
- 164.He Y, Wang Y, Liu H, Xu X, He S, Tang J, et al. Pyruvate kinase isoform M2 (PKM2) participates in multiple myeloma cell proliferation, adhesion and chemoresistance. Leuk Res. 2015;39(12): 1428–36. [DOI] [PubMed] [Google Scholar]
- 165.Kwon OH, Kang TW, Kim JH, Kim M, Noh SM, Song KS, et al. Pyruvate kinase M2 promotes the growth of gastric cancer cells via regulation of Bcl-xL expression at transcriptional level. Biochem Biophys Res Commun. 2012;423(1):38–44. [DOI] [PubMed] [Google Scholar]
- 166.Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150(4):685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;478(7375):118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao X, Wang H, Yang JJ, Liu X, Liu ZR Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, et al. PPARgamma contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peery RC, Liu JY, Zhang JT. Targeting survivin for therapeutic discovery: past, present, and future promises. Drug Discov Today. 2017. [DOI] [PubMed] [Google Scholar]
- 172.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114(8):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bai H, Ge S, Lu J, Qian G, Xu R. Hypoxia inducible factor-1alpha-mediated activation of survivin in cervical cancer cells. J Obstet Gynaecol Res. 2013;39(2):555–63. [DOI] [PubMed] [Google Scholar]
- 174.Li W, Chen YQ, Shen YB, Shu HM, Wang XJ, Zhao CL, et al. HIF-1alpha knockdown by miRNA decreases survivin expression and inhibits A549 cell growth in vitro and in vivo. Int J Mol Med. 2013;32(2):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun XP, Dong X, Lin L, Jiang X, Wei Z, Zhai B, et al. Up-regulation of survivin by AKT and hypoxia-inducible factor 1alpha contributes to cisplatin resistance in gastric cancer. FEBS J. 2014;281(1):115–28. [DOI] [PubMed] [Google Scholar]
- 176.Zhang M, Coen JJ, Suzuki Y, Siedow MR, Niemierko A, Khor LY, et al. Survivin is a potential mediator of prostate cancer metastasis. Int J Radiat Oncol Biol Phys. 2010;78(4):1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zohny SF, El-Shinawi M. Significance of survivin and Bcl-2 homologous antagonist/killer mRNA in detection of bone metastasis in patients with breast cancer. Med Oncol. 2011;28(Suppl 1): S108–14. [DOI] [PubMed] [Google Scholar]
- 178.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and −7. Biochemistry. 2001;40(4):1117–23. [DOI] [PubMed] [Google Scholar]
- 179.Tsubaki M, Takeda T, Ogawa N, Sakamoto K, Shimaoka H, Fujita A, et al. Overexpression of survivin via activation of ERK1/2, Akt, and NF-kappaB plays a central role in vincristine resistance in multiple myeloma cells. Leuk Res. 2015;39(4):445–52. [DOI] [PubMed] [Google Scholar]
- 180.Oto OA, Paydas S, Tanriverdi K, Seydaoglu G, Yavuz S, Survivin DU. EPR-1 expression in acute leukemias: prognostic significance and review of the literature. Leuk Res. 2007;31(11):1495–501. [DOI] [PubMed] [Google Scholar]
- 181.Smolewski P, Robak T. Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies. Curr Mol Med. 2011;11(8):633–49. [DOI] [PubMed] [Google Scholar]
- 182.Ju L, Zhang X, Deng Y, Han J, Yang J, Chen S, et al. Enhanced expression of Survivin has distinct roles in adipocyte homeostasis. Cell Death Dis. 2017;8(1):e2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hagenbuchner J, Kuznetsov AV, Obexer P, Ausserlechner MJ. BIRC5/Survivin enhances aerobic glycolysis and drug resistance by altered regulation of the mitochondrial fusion/fission machinery. Oncogene. 2013;32(40):4748–57.• This study demonstrates potential utility of glycolysis inhibitors in targeting anti-apoptotic effects of survivin.
- 184.Rivadeneira DB, Caino MC, Seo JH, Angelin A, Wallace DC, Languino LR, et al. Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion. Sci Signal. 2015;8(389):ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]