Abstract
We evaluated the prostate-specific antigen (PSA) responses to subsequent therapy in patients previously treated with galeterone. Twenty-seven patients were included in the analysis. Modest PSA responses were seen in patients receiving first-line and second-line subsequent therapies. The response to abiraterone was comparable with historic PSA response rates in patients with no prior exposure to second-generation hormonal therapy or chemotherapy.
Background:
Galeterone is a multi-targeted agent with activity as a CYP17 inhibitor, androgen receptor antagonist, and also causes androgen receptor degradation. It has shown meaningful anti-tumor activity with a well-tolerated safety profile in patients with castration-resistant prostate cancer (CRPC) in phase I and II studies; however, the efficacy of currently approved CRPC therapies after treatment with galeterone is unknown. In this study, we evaluate prostate specific antigen (PSA) response of non-protocol therapies following galeterone in a subset of patients treated on the Androgen Receptor Modulation Optimized for Response (ARMOR) 2 study.
Patients and Methods:
Patients who received any subsequent treatment were included. PSA response and treatment duration were summarized by line and type of subsequent therapy.
Results:
Overall, 27 of 40 patients received ≥ 1 post-galeterone treatment, of whom 18 (67%) discontinued galeterone for progression, 14 (52%) received ≥ 2 treatments, and 6 (22%) received ≥ 3 treatments. PSA changed by a median of −36%, −35%, and +60% in patients receiving first-line, second-line, and third-line therapy, respectively. Overall, 18 (67%) received subsequent enzalutamide, 12 (44%) received docetaxel, 9 (33%) received abiraterone, and 5 (19%) received cabazitaxel. PSA changed by a median of −27%, −34%, −39%, and 17% for patients receiving subsequent enzalutamide, docetaxel, abiraterone, and cabazitaxel, respectively, at any line.
Conclusion:
We demonstrate that CRPC therapies exhibit differential anti-tumor activity following galeterone. In this small cohort, abiraterone demonstrates the highest PSA response post-galeterone, whereas enzalutamide and chemotherapy have more modest activity. Larger clinical studies are warranted to fully evaluate the efficacy and safety of second-generation hormonal agents and chemotherapy post-galeterone. Predictive biomarkers will be critical to optimizing patient selection for sequential therapies.
Keywords: Abiraterone, Androgen receptor degradation, Chemotherapy, CYP17 inhibition, Enzalutamide, Resistance
Introduction
Androgen deprivation therapy is the backbone of systemic treatment for patients with recurrent or metastatic prostate cancer. Though androgen deprivation therapy induces clinical responses for most men, castration resistance ultimately develops. It is now understood that the androgen receptor (AR) remains an important driver of disease progression before and after the development of castration-resistant prostate cancer (CRPC). Despite castrate levels of serum testosterone, prostate tumors adapt by increasing androgen synthesis, either by conversion of weak adrenal androgens to more potent androgens or de novo androgen synthesis.1,2 Additionally, AR amplification leading to hypersensitivity and AR mutations leading to receptor promiscuity by nonphysiologic ligands are mechanisms by which resistance develops and AR signaling persists.3-7 More recently, the presence of constitutively active AR splice variants lacking the C-terminal ligand-binding domain have been linked to the development of resistance in CRPC.8
Galeterone is an oral, selective, multi-targeted steroidal agent. Preclinical studies have demonstrated that galeterone is a potent selective CYP17 inhibitor and potent AR antagonist.9 Additionally, galeterone has a unique mechanism of action of disrupting AR signaling via a proteosomal-dependent pathway, leading to AR degradation. This action has been documented in preclinical models of both full-length AR and truncated AR splice variants (ARV).10 The safety and clinical activity of galeterone were evaluated in a phase I study, Androgen Receptor Modulation Optimized for Response (ARMOR1), and the dose-escalation component of the phase II study (ARMOR2 part 1).11 These studies demonstrated that galeterone was well-tolerated and supported dosing of 2550 mg/day for further clinical study.11 Additionally, in ARMOR1, across all doses, 49% of patients achieved a ≥ 30% decline in prostate-specific antigen (PSA) and in ARMOR2 part 1, across all doses, 64% of patients achieved a ≥ 30% decline in PSA, and at the recommended dose of 2550 mg/day, 73% of patients achieved a ≥ 30% decline in PSA.11 It should be noted that the ARMOR2 trial design included a 12-week assessment, and treatment beyond 12 weeks was at the discretion of the treating physician, thus limiting the assessment of duration of response to galeterone. ARMOR2 part 2 continues to accrue participants in the post-enzalutamide cohort. A phase III trial (ARMOR3-SV, ) was accruing men with CRPC who were positive for AR-V7 with a 1-to-1 randomization to galeterone or enzalutamide. The trial was discontinued following review by the independent Data Monitoring Committee, though no safety concerns were cited regarding this recommendation. AR-V7 is the most common AR splice variant and lacks the C-terminal ligand binding site required for abiraterone and enzalutamide to be clinically effective. In patients with AR-V7, recent publications demonstrate poor response to second-generation hormonal therapies.8,12-14
Despite encouraging results in these phase I and II trials of galeterone, it is expected that patients with CRPC will require additional systemic therapy after treatment with galeterone. Subsequent therapies will include already approved agents for CRPC. These include abiraterone, an irreversible potent CYP17 inhibitor; enzalutamide, a potent second-generation anti-androgen; and docetaxel, cabazitaxel, and radium-223, which have all demonstrated efficacy and improved overall survival (OS) in patients with CRPC. The value of these agents after treatment with galeterone and possible cross-resistance between galeterone and currently approved CRPC therapies has not been evaluated. The objective of this analysis is to describe the PSA response to CRPC therapies following treatment with galeterone in a subset of patients treated on the ARMOR2 study.
Methods
Study Population
Patients enrolled on ARMOR2 (part 2) at the following institutions were included in the analysis: Dana-Farber Cancer Institute (Boston, MA; n = 10), Karmanos Cancer Institute (Detroit, MI; n = 10), San Bernadino Urological Association (San Bernadino, CA; n = 5), Tulane Cancer Center (New Orleans, LA; n = 4), Urology Clinics of North Texas (Dallas, TX; n = 4), Beth Israel Deaconess Medical Center (Boston, MA; n = 3), Carolina Urology Partners (Concord, NC; n = 3), and Massachusetts General Hospital (Boston, MA; n = 1). The ARMOR2 cohorts on which these patients were enrolled were comprised of treatment-naive patients with both metastatic and non-metastatic CRPC not having received prior chemotherapy, radium-223, CYP17 inhibitors, or next-generation anti-androgens including enzalutamide. Baseline patient and disease characteristics were collected from the ARMOR2 study database. Duration of galeterone treatment, nadir PSA on treatment with galeterone, and reason for galeterone discontinuation were also collected from the ARMOR2 study database. Information regarding subsequent treatments, including treatment start and stop dates, and PSA data were retrospectively collected from the longitudinal medical record. Uniform data templates were used to ensure consistent data collection at each institution. All sites obtained local Institutional Review Board approval prior to data collection.
Statistical Analysis
Baseline patient and disease characteristics were summarized as median, interquartile range or range for continuous variables, and as number and percentage for categorical variables. The primary objective was to characterize PSA response of patients receiving subsequent CRPC therapies after treatment with galeterone. PSA nadir was reported, and time-to-nadir from baseline was calculated. The percentage of patients with > 30%, > 50%, and > 90% PSA decline was calculated for the total cohort and for patients discontinuing ARMOR2 for progression. Additionally, treatment duration was summarized. The duration of each post-galeterone treatment was defined from the time a patient initiated a subsequent therapy to the time they discontinued the therapy or was censored on the date of last follow-up. The Kaplan-Meier method was used to estimate median duration of subsequent treatment. For patients receiving subsequent sipuleucel-T, the start of the next subsequent treatment was used as the date of sipuleucel-T discontinuation. The statistical analyses were performed using SAS version 9 (SAS Institute, Cary NC).
Results
Patient and Disease Characteristics
At the time of this analysis (May 2016), a total of 27 (67.5%) of 40 ARMOR2 patients had initiated subsequent therapy post-treatment with galeterone. The median age at diagnosis of this cohort was 63 years (interquartile range, 59-75 years), and the majority of patients had a Gleason score ≥ 8 (51%) (Table 1). At baseline on the ARMOR2 study, most patients had metastases (81%), and bone was the most common site of metastasis (56%). AR-V7 status was not routinely assessed, though circulating tumor cells (CTCs) were collected for enumeration and characterization. While on treatment with galeterone, the median PSA declined from 18.7 ng/mL to a nadir of 9.6 ng/mL at a median time of 28 days. In the patients who continued galeterone beyond the mandatory 12 weeks, the median duration of galeterone was 5.5 months (interquartile range, 0.5-19.6 months), and 18 patients (67%) discontinued treatment for disease progression as assessed by clinical, radiologic, and tumor marker (PSA) progression.
Table 1.
Patient and Disease Characteristics
| Characteristic | Total (N = 27) | |
|---|---|---|
| N | % or Median (q1-q3) | |
| Age at diagnosis, y | 26 | 63 (59-75) |
| Gleason score | ||
| ≤6 | 2 | 7 |
| 7 | 10 | 37 |
| ≥8 | 14 | 51 |
| Unknown | 1 | 4 |
| Prior radical prostatectomy | 7 | 26 |
| Prior radiation therapy | 16 | 59 |
| Prior chemotherapy | 1 | 4 |
| Baseline ARMOR2 | ||
| Age, y | 27 | 70 (65-78) |
| Presence of metastases | ||
| No | 5 | 19 |
| Yes | 22 | 81 |
| Sites of metastasis | ||
| Bone | 15 | 56 |
| Bone only | 9 | 33 |
| Lymph node | 12 | 44 |
| Other | 2 | 7 |
| Laboratory data | ||
| PSA, ng/mL | 27 | 18.7 (9.1-45.7) |
| Alkaline phosphatase, U/L | 25 | 84 (69-100) |
| Calcium, mg/dL | 25 | 9.4 (9.2-9.6) |
| Hemoglobin, g/dL | 24 | 12.7 (12.0-13.6) |
| Platelet count, k/uL | 27 | 224 (188-265) |
| On ARMOR2 | ||
| Nadir PSA, ng/mL | 27 | 9.6 (3.0-40.7) |
| Time to nadir PSA from baseline, d | 27 | 28 (14-42) |
| Percent PSA drop | 27 | −49% (−74% to −3%) |
| End of ARMOR2 | ||
| End of treatment PSA, ng/mL | 27 | 24.8 (6.4-86.9) |
| Reason for treatment discontinuation | ||
| Progression | 18 | 67 |
| Toxicity | 6 | 22 |
| Other/unknown | 3 | 11 |
| Progression type | ||
| Clinical progression only | 1 | 4 |
| PSA progression only | 10 | 37 |
| Radiographic progression only | 3 | 11 |
| Clinical and PSA progression | 1 | 4 |
| PSA and radiographic progression | 3 | 11 |
Abbreviations: ARMOR = Androgen Receptor Modulation Optimized for Response; PSA = prostate-specific antigen.
Outcomes of Subsequent Therapies Post-galeterone
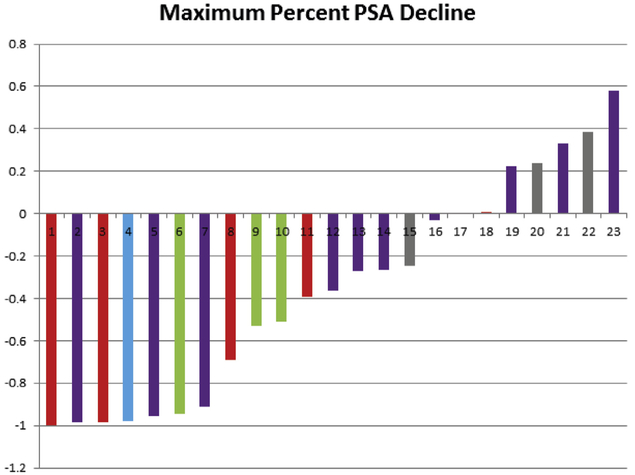
A total of 27 patients received at least 1 subsequent post-galeterone treatment, 14 (52%) patients received at least 2, 6 (22%) received at least 3, 4 (15%) received at least 4, and 2 (7%) received 5 subsequent post-galeterone treatments. Of the 22 patients with metastatic disease, 13 (59%) received at least 2 subsequent therapies, 6 (27%) received at least 3, 4 (18%) received at least 4, and 2 (18%) received at least 5 subsequent therapies. For the total cohort, the median baseline PSA increased with each additional subsequent therapy (Table 2). Overall, the PSA declined by a median of 36% (range, −100% to 58%) for the first subsequent line of therapy, with 43% (n = 10) of patients experiencing a > 50% decline in PSA (Table 2). Figure 1 displays the maximum percent PSA change for each patient during the first subsequent line of therapy for the total cohort. Median duration of the first subsequent therapy was 3.8 months (range, 1.1-31.9+months). PSA declined by a median of −35% (range, −95% to −8%) for the second subsequent therapy with 33% (n = 4) experiencing a > 50% decline in PSA. The median duration of the second subsequent therapy was 2.3 months (range, < 1-11.0 months).
Table 2.
Summary of PSA Kinetics of Subsequent Treatments Post-galeterone in the Total Cohort (n = 27)
| N | N (With PSA Data) |
Median (Range) | N (%) >30% PSA Decline |
N (%) >50% PSA Decline |
N (%) >90% PSA Decline |
|
|---|---|---|---|---|---|---|
| First subsequent therapy | ||||||
| Baseline PSA, ng/mL | 27 | 25 | 53.0 (0.4-606.0) | |||
| Maximum PSA change | 27 | 23 | −36% (−100% to 58%) | 12 (52) | 10 (43) | 7 (30) |
| Enzalutamide | 11 | 10 | −11% (−99% to 58%) | 4 (40) | 3 (30) | 3 (30) |
| Abiraterone | 7 | 6 | −54% (−100% to 1%) | 4 (67) | 3 (50) | 2 (33) |
| Docetaxel | 4 | 3 | −53% (−94% to −51%) | 3 (100) | 3 (100) | 1 (33) |
| Sipuleucel-T | 4 | 3 | 24% (−25% to 38%) | 0 (0) | 0 (0) | 0 (0) |
| Nilutamide | 1 | 1 | −98% | 1 (100) | 1 (100) | 1 (100) |
| Second subsequent therapy | ||||||
| Baseline PSA, ng/mL | 14 | 13 | 205.5 (0.2-1267.3) | |||
| Maximum PSA change | 14 | 12 | −35% (−95% to −8%) | 7 (58) | 4 (33) | 1 (8) |
| Docetaxel | 6 | 5 | −34% (−95% to −8%) | 3 (60) | 1 (20) | 1 (20) |
| Enzalutamide | 5 | 4 | −54%, (61% to −26%) | 3 (75) | 3 (75) | 0 (0) |
| Cabazitaxel | 2 | 2 | −22% to −35% | 1 (50) | 0 (0) | 0 (0) |
| Abiraterone | 1 | 1 | −29% | 0 (0) | 0 (0) | 0 (0) |
| Third subsequent therapy | ||||||
| Baseline PSA, ng/mL | 6 | 6 | 299.7 (42.3-570.0) | |||
| Maximum PSA change | 6 | 5 | 60% (−99% to 110%) | 1 (20) | 1 (20) | 1 (20) |
| Cabazitaxel | 3 | 2 | 17% to 60% | 0 (0) | 0 (0) | 0 (0) |
| Docetaxel | 1 | 1 | 110% | 0 (0) | 0 (0) | 0 (0) |
| Enzalutamide | 2 | 2 | −99% to 73% | 1 (50) | 1 (50) | 1 (50) |
| Any subsequent enzalutamide | ||||||
| Baseline PSA, ng/mL | 18 | 18 | 66.1 (0.2-350.6) | |||
| Maximum PSA change | 18 | 17 | −27% (−99% to 74%) | 8 (47) | 7 (41) | 4 (24) |
| Any subsequent docetaxel | ||||||
| Baseline PSA, ng/mL | 12 | 12 | 211.6 (1.0-606.6) | |||
| Maximum PSA change | 12 | 11 | −34% (−95% to 110%) | 5 (45) | 4 (36) | 2 (18) |
| Any subsequent abiraterone | ||||||
| Baseline PSA, ng/mL | 9 | 7 | 11.3 (0.4-299.8) | |||
| Maximum PSA change | 9 | 7 | −39% (−100% to 1%) | 5 (71) | 4 (57) | 4 (57) |
| Any subsequent cabazitaxel | ||||||
| Baseline PSA, ng/mL | 5 | 5 | 222.8 (42.3-1267.3) | |||
| Maximum PSA change | 5 | 5 | +17% (−35% to 120%) | 1 (20) | 0 (0) | 0 (0) |
Abbreviation: PSA = prostate-specific antigen.
Figure 1. Waterfall Plot of the Maximum Percent PSA Decline During First-line Treatment Post-galeterone in the Total Cohort. Red = Abiraterone; Purple = Enzalutamide; Blue = Nilutamide; Green = Docetaxel; Grey = Sipuleucel-T.
Abbreviation: PSA = prostate-specific antigen.
Of the 18 patients who discontinued galeterone for disease progression, 9 (50%) patients received at least 2, and 5 (28%) received at least 3 subsequent post-galeterone treatments. As seen in the total cohort, the median baseline PSA increased with each additional subsequent therapy (Table 3). Overall, PSA in this group declined by a median of 52% (range, −99% to 58%) for the first subsequent line of therapy, with 57% (n = 8) of patients experiencing a > 50% decline in PSA (Table 3). Figure 2 displays the maximum percent PSA change for each patient who discontinued ARMOR2 for progression during the first subsequent line of therapy.
Table 3.
Summary of PSA Kinetics of Subsequent Treatments Post-galeterone in Patients Progressing on Galeterone (n = 18)
| N (With PSA Data) |
Median (Range) | N (%) >30% PSA Decline |
N (%) >50% PSA Decline |
N (%) >90% PSA Decline |
|
|---|---|---|---|---|---|
| First subsequent therapy | |||||
| Baseline PSA, ng/mL | 14 | 39.6 (0.3-595.1) | 8 (57) | 8 (57) | 5 (34) |
| Maximum PSA change | 14 | −52% (−99% to 58%) | |||
| Second subsequent therapy | |||||
| Baseline PSA, ng/mL | 9 | 208.7 (0.24-1267.3) | 5 (56) | 3 (33) | 1 (11) |
| Maximum PSA change | 9 | −35% (−95% to −8%) | |||
| Third subsequent therapy | |||||
| Baseline PSA, ng/mL | 5 | 335.5 (42.3-570) | 1 (25) | 1 (25) | 1 (25) |
| Maximum PSA change | 4 | 39% (−99% to 110%) | |||
| Any subsequent enzalutamide | |||||
| Baseline PSA, ng/mL | 12 | 75.0 (0.24-350.6) | 5 (42) | 5 (42) | 3 (25) |
| Maximum PSA change | 12 | −27% (−99% to 58%) | |||
| Any subsequent docetaxel | |||||
| Baseline PSA, ng/mL | 11 | 205.5 (1.04-606.6) | 5 (45) | 4 (36) | 2 (18) |
| Maximum PSA change | 10 | 35% (95% to 110%) | |||
| Any subsequent abiraterone | |||||
| Baseline PSA, ng/mL | 3 | 2.5, 11.3, and 299.8 | 2 (67) | 2 (67) | 2 (67) |
| Maximum PSA change | 3 | −99%, −92%, and 1% | |||
| Any subsequent cabazitaxel | |||||
| Baseline PSA, ng/mL | 5 | 222.8 (42.3-1267.3) | |||
| Maximum PSA change | 5 | 17% (−35% to 120%) | 1 (20) | 0 (0) | 0 (0) |
Abbreviation: PSA = prostate-specific antigen.
Figure 2. Waterfall Plot of the Maximum Percent PSA Decline During First-line Treatment Post-galeterone in Patients Progressing on Galeterone. Red = Abiraterone; Purple = Enzalutamide; Blue = Nilutamide; Green = Docetaxel; Grey = Sipuleucel-T.
Abbreviation: PSA = prostate-specific antigen.
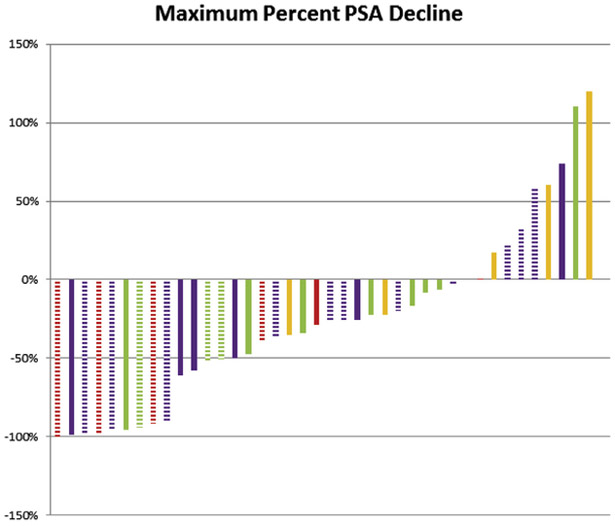
Overall, 18 patients (67%) received any subsequent enzalutamide, 12 (44%) received docetaxel, 9 (33%) received abiraterone, and 5 (19%) received cabazitaxel. In patients progressing on ARMOR2, 67% (n = 12) received any subsequent enzalutamide, 11 (61%) received docetaxel, 5 (28%) received abiraterone, and 5 (28%) received cabazitaxel. Figure 3 displays the maximum percent PSA change for each patient by type of therapy for the total cohort. The median baseline PSA was 11.3 ng/mL at initiation of subsequent abiraterone compared with 66.1 to 222.8 ng/mL for patients initiating treatment with subsequent enzalutamide, docetaxel, or cabazitaxel. With regard to abiraterone, all but 1 patient had metastatic disease (n = 8; 89%), and 78% (n = 7) received abiraterone as the first subsequent line therapy. Of patients receiving subsequent enzalutamide, 15 (83%) had metastatic disease, and 61% (n = 11) received enzalutamide as the first subsequent line of therapy. Of the patients treated with any subsequent abiraterone, the median maximum decline in PSA was 39%, with 57% (n = 4) of patients experiencing a > 50% decline in PSA. The median duration of subsequent abiraterone was 8.6 months. The median maximum decline in PSA was 27% for patients receiving any subsequent enzalutamide, with 41% (n = 7) experiencing a > 50% decline in PSA. The median duration of subsequent enzalutamide was 3.3 months. For patients receiving docetaxel, the median maximum decline in PSA was −34%, whereas for patients receiving subsequent cabazitaxel, the median PSA increased from baseline. The median duration of subsequent docetaxel was 2.8 months and of subsequent cabazitaxel, 1.0 months. Nearly all patients received cabazitaxel following docetaxel (4 of 5; 80%).
Figure 3. Waterfall Plot of the Maximum Percent PSA Decline by Type of Treatment Post-galeterone. Red = Abiraterone; Purple = Enzalutamide; Green = Docetaxel; Yellow = Cabazitaxel. The Hashed Bars Designate Receipt of Agent in the First-line Post-galeterone.
Abbreviation: PSA = prostate-specific antigen.
Discussion
The treatment landscape for patients with metastatic CRPC is rapidly evolving. Over the past 6 years, 5 new agents have demonstrated improvements in OS for men with metastatic CRPC, and many new agents are currently in development. Galeterone is a multi-targeted hormonal agent differentiated by AR degrading properties: galeterone has demonstrated antitumor activity in patients with CRPC.11 A phase III trial of galeterone in patients with ARV7 metastatic CRPC was recently halted because of a Data Safety Monitoring Board conclusion that the primary end point of radiographic progression-free survival was unlikely to be reached. Plans for further development of galeterone are under consideration. Currently approved therapies for patients with metastatic CRPC have distinct mechanisms of action and include abiraterone, cabazitaxel, docetaxel, enzalutamide, radium-223, and sipuleucel-T. Despite expansion of the treatment armamentarium for metastatic CRPC, data from prospective studies informing the optimal sequence of these agents are lacking.
Predictive biomarkers are needed to guide therapy selection. AR-V7 can be measured from circulating tumor cells and appears to be a marker of resistance to abiraterone and enzalutamide.8 The AR-V7 blood biomarker was being utilized in the ARMOR3-SV trial and others and may be incorporated into future routine clinical practice. In this exploratory analysis, we sought to evaluate the PSA response of currently approved metastatic CRPC therapies following treatment with galeterone. Although our analysis is limited by its small sample size and lack of knowledge regarding AR-V7 status, our findings reveal that therapies for metastatic CRPC demonstrate activity following treatment with galeterone. With small numbers, the PSA50 response rate (≥ 50% decline in PSA) and duration of response to abiraterone post-galeterone was 57% and 8.6 months. Enzalutamide post-galeterone had a PSA response of 41% but of short treatment duration. Responses to chemotherapy in these patients were modest. PSA response rates were similar for patients who discontinued galeterone for progression.
Investigators in the field have described primary (no initial response) and secondary (initial response) resistance to second-generation hormone therapies.15 It has been hypothesized that the mechanisms of resistance may differ in the primary and secondary setting, although a full understanding of resistance mechanisms has not been established. Nearly all patients in this analysis had some PSA response to galeterone (median PSA decline, 49%) and therefore would not have demonstrated primary resistance to galeterone. Because the ARMOR2 study design only required 12 weeks of therapy, there is a mix of patients who came off galeterone without progression (33%) and for progression (67%) as assessed by clinical, radiologic, and tumor marker (PSA) progression, which could influence the choice of subsequent therapy. Given partial overlapping mechanisms of action of galeterone, abiraterone, and enzalutamide, there is the potential for cross-resistance between agents. Abiraterone is a potent CYP17 inhibitor with activity against 17-alpha hydroxylase and 17, 20-lyase resulting in androgen synthesis suppression. Unlike galeterone, abiraterone does not degrade the AR and requires administration of concomitant steroids to prevent a syndrome of mineralocorticoid excess. Galeterone is a potent CYP17 inhibitor with greater activity against the lyase function of CYP17 and does not require the co-administration of steroids. Enzalutamide is a potent antiandrogen, like galeterone, that also inhibits AR nuclear translocation, DNA binding, and coactivator recruitment, but like abiraterone is not known to degrade AR.16
In the current analysis, responses to abiraterone post-galeterone (PSA50, 57% in our study) were comparable with expected responses to abiraterone demonstrated in large phase III studies of abiraterone pre-chemotherapy (PSA50, 62%) and post-chemotherapy (PSA50, 29%) (Table 4).17,18 Both of these studies excluded prior CYP17 inhibition and next-generation anti-androgens. Our results highlight the potential persistent activity of abiraterone post-galeterone and the lack of complete cross-resistance between abiraterone and galeterone. A possible explanation for the favorable response to abiraterone could be related to the degree of reduction in median serum hormone concentrations observed with galeterone (ARMOR1) compared with abiraterone. Data from the phase I study of abiraterone demonstrates that 28-day serum testosterone and dehydroepiandrosterone sulfate (DHEAS) levels declined to below the assay lower limit (1 ng/dL for testosterone and 15 μg/dL for DHEAS) and remained at this level until progression,23 whereas reductions in testosterone and DHEAS with galeterone were slightly less than those observed with abiraterone (2 ng/dL for testosterone and 18 μg/dL for DHEAS at week 12 with galeterone).11 The potential responsiveness to abiraterone post-galeterone suggests that persistent AR signaling remains an important mechanism of resistance in CRPC, and further suppression of the AR axis is a key step to overcoming resistance.
Table 4.
Summary of Outcomes of CRPC Therapies From Phase III Clinical Trials
| Therapy | Na | PSA Responseb (%) | Radiographic PFS, mos | OS, mos |
|---|---|---|---|---|
| Abiraterone: pre-chemotherapy17 | 546 | 62 | 16.5 | NR |
| Abiraterone: post-chemotherapy18 | 797 | 29 | 5.6 | 14.8 |
| Enzalutamide: pre-chemotherapy19 | 872 | 78 | NR | 32.4 |
| Enzalutamide: post-chemotherapy20 | 800 | 54 | 8.3 | 18.4 |
| Docetaxel (every 3 weeks)21 | 335 | 45 | NR | 18.9 |
| Docetaxel (weekly)21 | 334 | 48 | NR | 17.4 |
| Cabazitaxel22 | 378 | 17.8 | 2.8 | 15.1 |
Abbreviations: CPRC = castration-resistant prostate cancer; NR = not reached; OS = overall survival; PFS = progression-free survival; PSA = prostate specific antigen.
Denotes the number of patients in the treatment arm on each respective phase III trial.
Defined as a ≥ 50% maximum decline in PSA.
We demonstrate that responses to enzalutamide post-galeterone were slightly lower (PSA50, 41% in our study) than expected responses to enzalutamide demonstrated in large phase III studies of enzalutamide pre-chemotherapy (PSA50, 78%) and post-chemotherapy (PSA50, 54%) (Table 4).19,20 This observation suggests some degree of potential cross-resistance between enzalutamide and galeterone. However, given the small numbers in our dataset, these data require further validation in larger clinical studies.
Several retrospective analyses have demonstrated cross-resistance between AR-targeted therapies (Table 4). Though both abiraterone and enzalutamide have shown improvements in OS for patients with metastatic CRPC,17-20 no prospective study to date has evaluated the appropriate sequence or combination. Several studies have demonstrated modest clinical activity of abiraterone following treatment with enzalutamide.24,25 In a cohort of 38 patients progressing on docetaxel and enzalutamide, abiraterone resulted in ≥ 50% PSA declines in only 3 patients.24 Additionally, several studies report blunted clinical activity of enzalutamide following abiraterone.26-29 In an analysis of 61 patients receiving prior docetaxel and abiraterone, ≥ 50% PSA declines were seen in 21% of patients, and the median duration of enzalutamide treatment was 14.9 weeks.26 Furthermore, several mechanisms of resistance have been reported following treatment with abiraterone and/or enzalutamide, and we hypothesize that these mechanisms are potentially at play following galeterone treatment. These potential mechanisms of resistance include increased intratumoral androgen biosynthesis, AR mutations and amplifications, glucocorticoid receptor overexpression, and activation of alternate pathways.15 Whereas galeterone has demonstrated AR degradation properties, potentially conferring activity in the presence of constitutively active AR splice variants, mechanisms of resistance to galeterone have not been fully characterized, and whether AR splice variants play an ongoing role on resistance to this agent is unknown.
Additionally, in our analysis (limited by small numbers), we demonstrated less responsiveness to chemotherapy post-galeterone. PSA50 response rates to docetaxel were 36% and cabazitaxel 0% compared with historic rates from large phase III studies, which were conducted in the era pre-abiraterone and pre-enzalutamide, of 45% to 48% for docetaxel and 17.8% for cabazitaxel (Table 4).21,22 Prior studies have demonstrated similar efficacy results for docetaxel following abiraterone. In a comparative analysis of 119 patients with CRPC, individuals having received abiraterone prior to docetaxel had a shorter progression-free survival compared with those without prior abiraterone (4.4 vs. 7.6 months; P = .003).30 Though cross-resistance between agents may in part explain our findings, men receiving subsequent chemotherapy may have a greater tumor burden, more aggressive disease, or may be farther along the disease natural history.
Though this is the first study exploring the anti-tumor activity of CRPC therapies post-galeterone, several limitations exist. Our analysis is largely limited by its retrospective design, which has the potential for selection bias and incomplete or variable assessments. All patients in our cohort had received galeterone on a clinical trial, thereby potentially limiting our ability to generalize results to a non-clinical trial population of patients. The total cohort of patients in the analysis is heterogeneous including patients with and without metastatic disease and those discontinuing treatment with galeterone for reasons other than progression. The small sample size of our cohort limits our ability to draw any specific conclusions regarding treatment selection and sequencing. Additionally, we were unable to adjust for baseline prognostic factors that could impact the type of subsequent therapy received. We used the metric “treatment duration” as a surrogate for time-to-progression. Treatment duration reflects “real-world” clinical practice patterns, given that the determination to discontinue a treatment is based on a physician’s discretion regarding patient benefit. Lastly, radiographic data is lacking in part, given that the cohort includes patients without measurable disease. Despite these acknowledged limitations, this analysis is valuable to the field given the active development of AR-targeted therapy in metastatic CRPC.
Conclusions
To our knowledge, this is the first report to evaluate the efficacy of subsequent therapies after galeterone in men with CRPC. In a selected population of clinical trial patients who received galeterone, we demonstrate differential responses to subsequent CRPC therapies. Though galeterone is not approved for metastatic CRPC treatment, our data are meaningful in considering sequential and combinatorial treatment strategies with AR-targeted therapies and, more specifically, CP17 inhibition. The specific mechanisms of resistance to galeterone and other second-generation AR-targeted therapies need to be characterized to inform subsequent treatment selection. Lastly, this work underscores the need to identify and validate predictive biomarkers to guide clinical decision-making and inform patient treatment selections for men with CRPC. Our data are hypothesis-generating, and, if galeterone enters the treatment landscape for patients with CRPC, warrant further validation prospectively.
Clinical Practice Points
Galeterone inhibits prostate cancer tumor growth by CYP17 inhibition, AR antagonism, and AR degradation, thus decreasing the sensitivity of ARs to androgen activity, based on pre-clinical data.
Galeterone has shown anti-tumor activity and a well-tolerated safety profile in patients with CRPC in phase I and II studies.
The efficacy of currently approved CRPC therapies after treatment with galeterone is unknown.
We demonstrate that CRPC therapies exhibit differential activity following exposure to galeterone.
We demonstrate that therapy with abiraterone following treatment with galeterone results in PSA responses comparable with historic responses to abiraterone in patients with no prior exposure to second-generation hormonal therapy or to chemotherapy. In contrast, enzalutamide and chemotherapy post-galeterone result in modest PSA responsiveness.
Our data are hypothesis-generating, and, if galeterone enters the treatment landscape for patients with CRPC, warrant further validation prospectively.
Acknowledgments
This study had research support from the Fairweather Family Fund and Fat Boys Slim Sister’s PMC Prostate Cancer Fund.
Footnotes
Disclosure
This study was funded by Tokai Pharmaceuticals, Inc. The funding source had no role in the design of this study or manuscript preparation. The funding source provided minor manuscript edits following completion of manuscript preparation. Dr McKay reports research funding from Bayer and Pfizer. Dr Taplin reports research funding and advisory board honorarium from Tokai Pharmaceuticals. J. Roberts is an employee at Tokai Pharmaceuticals. The remaining authors have stated that they have no conflicts of interest.
References
- 1.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 2008; 68:6407–15. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008; 68:4447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen EJ, Sowalsky AG, Gao S, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res 2015; 21:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer 2003; 89:552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton MA, Shuster TD, Fertig AM, et al. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin Cancer Res 1997; 3:1383–8. [PubMed] [Google Scholar]
- 6.Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov 2013; 3:1020–9. [DOI] [PubMed] [Google Scholar]
- 7.Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov 2013; 3:1030–43. [DOI] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther 2008; 7:2348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwegyir-Afful AK, Ramalingam S, Purushottamachar P, Ramamurthy VP, Njar VC. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget 2015; 6:27440–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery B, Eisenberger MA, Rettig MB, et al. Androgen Receptor Modulation Optimized for Response (ARMOR) Phase I and II studies: galeterone for the treatment of castration-resistant prostate cancer. Clin Cancer Res 2016; 22:1356–63. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015; 67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu Y, Dai B, Ye D, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep 2015; 5:7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi T, Okuno Y, Hattori-Kato M, Zaitsu M, Mikami K. Detection of AR-V7 mRNA in whole blood may not predict the effectiveness of novel endocrine drugs for castration-resistant prostate cancer. Res Rep Urol 2016; 8:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buttigliero C, Tucci M, Bertaglia V, et al. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat Rev 2015; 41:884–92. [DOI] [PubMed] [Google Scholar]
- 16.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324:787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367:1187–97. [DOI] [PubMed] [Google Scholar]
- 20.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351:1502–12. [DOI] [PubMed] [Google Scholar]
- 22.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376:1147–54. [DOI] [PubMed] [Google Scholar]
- 23.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol 2010; 28:1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 2013; 24:1807–12. [DOI] [PubMed] [Google Scholar]
- 25.Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 2013; 24:1802–7. [DOI] [PubMed] [Google Scholar]
- 26.Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 2014; 120:968–75. [DOI] [PubMed] [Google Scholar]
- 27.Cheng HH, Gulati R, Azad A, et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis 2015; 18:122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadal R, Zhang Z, Rahman H, et al. Clinical activity of enzalutamide in docetaxel-naive and docetaxel-pretreated patients with metastatic castration-resistant prostate cancer. Prostate 2014; 74:1560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 2014; 65:30–6. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer MT, Zhou XC, Wang H, et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur Urol 2014; 66:646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]