Abstract
Objective
Rheumatoid arthritis (RA) and periodontitis share several pathological features including bone and soft tissue destruction and high levels of circulating inflammatory proteins. Studies related to cytokines in the periodontal inflammatory exudate (gingivocrevicular fluid, GCF) of RA patients might provide insight into the association between periodontitis and RA. The aim of our study was to review the literature on cytokines in GCF of RA patients including the effect of anti‐rheumatic treatment with biological disease‐modifying anti‐rheumatic drugs (DMARDs) and periodontal treatment on these cytokines.
Materials and methods
MedLine/PubMed searches with different combinations of keywords “rheumatoid arthritis or RA” and “crevicular fluid or GCF” until June 2019 revealed 64 articles. Ten cross‐sectional observational studies and nine treatment studies fulfilled the inclusion criteria.
Results
Rheumatoid arthritis patients have increased circulating and GCF levels of pro‐inflammatory cytokines and proteins, despite anti‐rheumatic treatment with biological DMARDs. Presence of periodontitis was accompanied by higher cytokine and protein levels. Treatment of periodontitis resulted in a decrease of these levels.
Conclusion
Analysis of GCF of RA patients reveals that the relationship between periodontitis and RA is bidirectional, probably caused by a non‐specific inflammatory burden. Data for a specific relationship are barely present in GCF.
Keywords: gingivocrevicular fluid, periodontitis, rheumatoid arthritis, treatment
1. INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by synovitis leading to irreversible joint destruction. Autoantibody production and immune dysregulation precede clinical onset (Paul, Kandy, & Krishnan, 2017). The etiology of autoimmunity in RA remains unclear, but is presumed to be initiated at inflamed mucosal surfaces of the lungs and oral cavity (i.e., periodontal disease) in combination with genetic and environmental factors (Mikuls, Payne, Deane, & Thiele, 2016). Periodontal disease (periodontitis) is primarily triggered by bacterial infection and leads to inflammation and destruction of the supporting soft and hard tissues of the teeth (the periodontium; Potempa, Mydel, & Koziel, 2017).
Rheumatoid arthritis and periodontitis share several pathological features, including bone and soft tissue destruction and high levels of circulating inflammatory markers (Culshaw, McInnes, & Liew, 2011). Epidemiologic, clinical, and serologic studies generally claim a link between periodontitis and RA (Potempa et al., 2017). This bidirectional association between RA and periodontitis can, among others, be explained by shared risk factors such as genetic predisposition, smoking, and a higher local and systemic inflammatory burden as well as by induction of autoimmunity by chronic infection with certain periodontal pathogens such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans (Konig et al., 2016; Rosenstein, Greenwald, Kushner, & Weissmann, 2004; Wegner et al., 2010).
In RA, ongoing research is focused on finding biomarkers for diagnosis, prognosis, treatment selection, and optimized therapy (Jog & James, 2017). Two of the most common autoantibodies in patients with RA are rheumatoid factor (RF), directed against the Fc portion of the IgG class of antibodies, and antibodies against citrullinated proteins (ACPAs). In addition, antibodies against carbamylated proteins (anti‐CarP) can precede clinical diagnosis of RA (Gan et al., 2015). All these autoantibodies are considered to be potentially pathogenic (Derksen, Huizinga, & van der Woude, 2017). In addition, many cytokines and chemokines are active in the joints of RA patients. These cytokines are critical in inflammation, joint damage, and RA‐associated comorbidities (Brennan & McInnes, 2008). The number of elevated cytokines and chemokines in preclinical seropositive RA, as measured in serum, predicts time to diagnosis in an age‐dependent manner (Deane et al., 2010).
Traditional approaches to control RA rely on conventional synthetic disease‐modifying anti‐rheumatic drugs (csDMARDS). Advances in understanding key events in the pathogenesis of RA have led to additional biological DMARDs. When RA is clinically apparent, a number of cytokines, for example, tumor necrosis factor‐α (TNF‐α), interleukin (IL)‐6, and IL‐1 receptor antagonist, are successfully targeted in RA treatment with biological DMARDs (Jog & James, 2017). It is not yet well known whether treatment with biological DMARDS, besides its effect on the periodontal inflammatory burden, also results in changes in saliva and gingival crevicular fluid (GCF). For example, Äyräväinen et al., (2018) recently showed that treatment of RA with synthetic or biologic DMARDs did not affect salivary MMP‐8 levels (Äyräväinen et al., 2018).
Gingival crevicular fluid is the exudate of the periodontium that can be collected from the gingival crevice around the teeth. Leakage of GCF out of the periodontal pocket increases when the gingival crevice becomes inflamed and reflects severity of periodontal inflammation. GCF is composed of serum and locally generated components such as tissue breakdown products, inflammatory mediators, substances from bacteria in the dental biofilm, and antibodies in response to these bacteria (Champagne et al., 2003). Biochemical analysis of GCF offers a non‐invasive means of assessing the host response in periodontal disease (Lamster, 1997). GCF can be obtained with paper strips and crevicular washes. These methods are very technique sensitive as the quantity and quality of GCF samples are highly affected by the method of collection and analysis (Guentsch et al., 2011).
Because of the lack of uniformity in the methodological design of studies aiming to detect which cytokines are most involved in chronic periodontitis, Tomás et al., (2017) recently developed cytokine‐based predictive models to estimate the probability of presence of chronic periodontitis. Models based on a pro‐inflammatory cytokine profile (granulocyte‐macrophage colony‐stimulating factor (GMCSF), IL‐1α, IL‐1β, IL‐6, IL‐12p40, IL‐17A, IL‐17F, and TNF‐α) and an anti‐inflammatory cytokine profile (interferon gamma [IFNγ], IL‐2, IL‐3, and IL‐4) have a high predictive ability to distinguish patients with chronic periodontitis from periodontally healthy subjects. For example, smoking‐adjusted models with an outstanding predictive accuracy showed that IL‐1α, IL‐1β, and IL‐17A in GCF are good biomarkers for distinguishing patients with chronic periodontitis from periodontally healthy individuals. The predictive ability of these pro‐inflammatory cytokines was further increased by incorporating anti‐inflammatory cytokines IFNγ and IL‐10 in the GCF cytokine model.
Studies related to cytokines in GCF of RA patients can be helpful in explaining the association between periodontitis and RA. Cytokines in RA patients with periodontitis have lastly been reviewed in 2014 (Javed et al., 2014). The aim of this focused review was to update the current knowledge on cytokine expression in GCF of RA patients as well as to assess the effect of anti‐rheumatic treatment with biological DMARDs and periodontal treatment on these cytokines.
2. MATERIALS AND METHODS
Focused research questions for our review were as follows: (a) Which cytokines have been assessed in GCF of RA patients and what is the difference in expression of cytokines between RA patients and individuals without RA? (b) What is the influence of both RA and periodontal treatments on expression of these cytokines in GCF?
MedLine/PubMed searches with different combinations of keywords “rheumatoid arthritis or RA”, “crevicular fluid or GCF,” “cytokines” and “treatment” until June 2019 revealed 64 articles. Eligibility criteria were original, clinical studies, with use of statistical methods, published in English with a reference list of original and review studies. Further inclusion criteria were RA diagnosed according to the ACR 1987 or ACR 2010 revised criteria (Aletaha et al., 2010; Arnett et al., 1988), assessment of cytokines in GCF of RA patients and systemically healthy controls (except for RA treatment studies), and description of periodontal status of the study groups.
Of the 64 potentially eligible papers, 24 papers passed the inclusion criteria (Figure 1). Four studies had to be excluded after in‐depth analysis because no healthy control group was included. One study had to be excluded in addition because of inconsistent or lacking data and no response of the authors on repeated request of additional data (Özçaka, Alpöz, Nalbantsoy, Karabulut, & Kabasakal, 2018). Eight of the remaining 20 studies had been reviewed before by Javed et al., 2014. To allow for comparison of the various studies, when appropriate, only patient groups with or without chronic periodontitis and with or without RA were considered. When available, concentrations instead of amounts of cytokines were used for comparison between the study groups.
Figure 1.
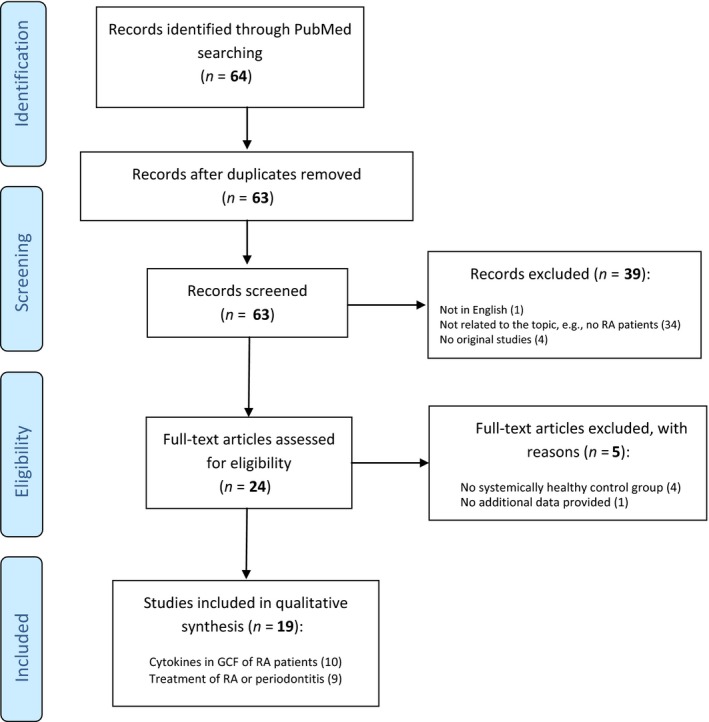
Study identification and selection progress
3. RESULTS
3.1. Cytokines in GCF of RA patients
Most of the studies on biomarkers in GCF (Table 1) assessed pro‐inflammatory cytokines (TNF‐α, IL‐1β, IL‐6, IL‐17A, IL‐17F) and other inflammatory mediators implicated in tissue destruction in periodontitis and RA such as matrix metalloproteinases (MMPs), the pro‐inflammatory mediator prostaglandin E2 (PGE2), neutrophil elastase, and the receptor activator of nuclear factor‐kappa B ligand (RANKL) and its inhibitor osteoprotegerin (OPG). Anti‐inflammatory cytokines studied in GCF of RA patients were IL‐4, IL‐10, IL‐18, and IL‐17E. Four studies compared cytokines in GCF and serum (Cetinkaya, Guzeldemir, Ogus, & Bulut, 2013; Gümüş et al., 2013a, 2013b; Silosi et al., 2015). Some studies had overlapping study populations (Biyikoğlu et al., 2006, 2009; Bozkurt, Berker, Akkuş, & Bulut, 2000; Bozkurt, Yetkin Ay, Berker, Tepe, & Akkuş, 2006; Gümüş et al., 2013a, 2013b).
Table 1.
Studies on cytokines in gingivocrevicular fluid (GCF) of patients with rheumatoid arthritis (RA) and systemically healthy controls (HC) with (+) or without (−) chronic periodontitis (CP)
| Study | Patients (number) | Sex (M/F) | Age (years, mean ± SD) | RA duration (years, mean ± SD) | RA treatment (number of patients) | Cytokine(s) investigated in GCF | Same cytokine(s) investigated in serum | Periodontal assessment | Periodontal classification | Differences in periodontal status between study groups | Differences in concentrations of cytokines in GCF between study groups | Differences in concentrations of cytokines in serum between study groups |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bender et al., (2019) | RA (10) | 2/8 | 63 ± 12 | 11 (range 2–22) | no detailed information provided | IL1‐β, MCP−1, MCP−3 | no | PSR, number of teeth, sites with PD > 5mm | Armitage 1999 |
RA significantly less teeth compared to HC + CP and HC‐CP HC + CP significantly higher PSR and sites with PD > 5 mm compared to RA and HC‐CP |
Significantly higher amount of IL1‐β in RA compared to HC‐CP (no concentration assessed) No difference in amount of MCP−1 and MCP−3 between all study groups |
– |
| HC + CP (10) | 4/6 | 57 ± 11 | – | – | ||||||||
| HC‐CP (10) | 7/3 | 38 ± 8 | – | – | ||||||||
| Kirchner et al., (2017) |
RA (103, +CP in 65%) |
45/58 | 56 ± 11 | 11 ± 6 | MTX (65), leflunomide (18), chloroquine (10), sulfasalazine (5), biological DMARDs (21), steroids (61) | MMP−8 | no | PD, BOP, CAL | Page & Eke, 2007 | RA versus HC: PD, BOP, CAL significantly higher in HC | RA + CP versus HC + CP: significantly higher in RA + CP | – |
|
HC (104, +CP in 79%) |
36/68 | 57 ± 12 | – | – | RA‐CP versus HC‐CP: no significant difference | |||||||
| Silosi et al., (2015) | RA + CP (12) | 3/9 | n.a. (range 38–62) | n.a. | no detailed information provided | MMP−9 | yes | PD, BOP, CAL, PI | ≥4 teeth with PD > 6mm on both maxillaries and radiographic evidenceof bone loss | n.a. | RA + CP versus HC + CP: significantly higher in RA + CP | RA + CP versus HC + CP: significantly higher in RA + CP |
| HC + CP (14) | 6/8 | n.a. (range 39–68) | ‐ | ‐ | ||||||||
| Cetinkaya et al., (2013) | RA (17) | 3/14 | 48 ± 11 | 6 ± 6 | MTX + sulfasalazine (15), leflunomide (2) | IL1‐β, IL−4, IL−10, TNF‐α | yes (only IL1‐β and IL−10) | PD, CAL, GI, PI | Armitage 1999 | RA versus HC + CP: PD, CAL, GI significantly higher in HC + CP | RA versus HC + CP: no significant differences of all assessed cytokines | RA versus HC + CP: significantly higher Il−1β in HC + CP |
| HC + CP (16) | 10/6 | 44 ± 7 | – | – | RA versus HC‐CP: PD, CAL, GI significantly higher in RA | RA versus HC‐CP: all assessed cytokines significantly higher in HC‐CP | RA versus HC‐CP: significantly higher Il−1β in HC‐CP | |||||
| HC‐CP (16) | 8/8 | 28 ± 6 | – | – | HC + CP versus HC‐CP: PD, CAL, GI significantly higher in HC + CP | HC + CP versus HC‐CP: all assessed cytokines significantly higher in HC‐CP | HC + CP versus HC‐CP: no significant differences | |||||
| Gümüş et al., (2013a) and (2013b) | RA + CP (17) | 0/17 | n.a. (range 25–64) | n.a. | no detailed information provided | IL−17, RANKL, OPG, TNF‐α, APRIL, BAFF | yes | PD, BOP, CAL, PI | not specified | RA + CP versus HC + CP: only PD significantly higher in RA + CP | RA + CP versus HC + CP: significantly higher RANKL, Il−17, TNF‐α, APRIL, BAFF in RA + CP | RA + CP versus HC + CP: significantly higher RANKL, Il−17, TNF‐α, APRIL, BAFF in RA + CP, significantly higher OPG in HC + CP |
| HC + CP (13) | 0/17 | n.a. (range 41–66) | – | – | ||||||||
| Biyikoğlu et al., (2009) | RA (25) | 6/19 | 54 ± 10 | 18 ± 10 | MTX + prednisolone | MMP−8, MMP−13, TIMP−1 | no | PD, BOP, CAL, PI | Armitage 1999 | RA versus HC + CP: no differences in PD, BOP, CAL, PI | RA versus HC + CP: no significant differences | ‐ |
| HC + CP (25) | 14/11 | 50 ± 8 | – | – | RA versus HC‐CP: PD, BOP, CAL, PI significantly higher in RA | RA versus HC‐CP: significantly higher concentration of MMP−8 in RA | ||||||
| HC‐CP (24) | 12/12 | 49 ± 7 | – | – | HC + CP versus HC‐CP: PD, BOP, CAL, PI significantly higher in HC + CP | HC + CP versus HC‐CP: significantly higher MMP−8 in HC + CP | ||||||
| Miranda et al., (2007) | RA (17) | 2/15 | 50 ± 11 | 12 ± 10 | NSAIDs, MTX, sulfasalazine, prednisolone | IL1‐β, IL−18, neutrophil elastase | no | PD, BOP, CAL, GI, PI | n.a. | RA versus HC: no significant differences in PD, BOP, CAL, GI, PI | Significantly higher amount (no concentration assessed) of Il−1β in HC | – |
| HC (17) | 2/15 | 49 ± 11 | – | – | ||||||||
| Biyikoğlu et al., (2006) | RA (23) | 5/18 | 53 ± 10 | 16 ± 10 | MTX + prednisolone | IL1‐β, PGE2 | no | PD, BOP, CAL, PI | Armitage 1999 | RA versus HC + CP: no significant differences in PD, BOP, CAL, PI | No significant differences between all study groups | ‐ |
| HC + CP (17) | 9/8 | 49 ± 7 | – | – | RA versus HC‐CP: PD, BOP, CAL, PI significantly higher in RA | |||||||
| HC‐CP (17) | 3/14 | 41 ± 7 | – | – | HC + CP versus HC‐CP: PD, BOP, CAL, PI significantly higher in HC + CP | |||||||
| Bozkurt et al., (2006) | RA + CP (17) | 5/12 | 47 ± 11 | n.a. | prednisolone, indomethacin, chloroquine | IL−4, IL−10 | no | PD, CAL, GI, PI | CAL > 2 mm at > 2 sites at in > 3 teeth per quadrant and radiographic evidence of bone loss | RA + CP versus HC + CP: only PD significantly higher in HC + CP | RA + CP versus HC + CP: Il−4 significantly higher in RA + CP | – |
| HC + CP (17) | 11/6 | 44 ± 10 | – | – | RA + CP versus HC‐CP: PD, CAL, GI, PI significantly higher in RA | RA + CP versus HC‐CP: both cytokines significantly higher in HC‐CP | ||||||
| HC‐CP (17) | 9/8 | 36 ± 4 | – | – | HC + CP versus HC‐CP: PD, CAL, GI, PI significantly higher in HC + CP | HC + CP versus HC‐CP: both cytokines significantly higher in HC‐CP | ||||||
| Bozkurt et al., (2000) | RA + CP (15) | 9/6 | 48 ± 7 | n.a. (range 1–8 years) | prednisolone, indomethacin, chloroquine | IL−6 | no | PD, CAL, GI, PI | not specified | RA + CP versus HC + CP: only PI significantly higher in RA | No significant differences between all study groups | – |
| HC + CP (15) | 11/4 | 47 ± 7 | – | – | RA + CP versus HC‐CP: PD, CAL, GI, PI significantly higher in RA | |||||||
| HC‐CP (15) | 8/7 | 46 ± 7 | – | – | HC + CP versus HC‐CP: PD, CAL, GI, PI significantly higher in HC + CP |
Abbreviations: APRIL, a proliferation inducing ligand; BAFF, B cell activating factor; BOP, bleeding on probing; CAL, clinical attachment level; DMARDs, disease‐modifying anti‐rheumatic drugs; GI, gingivitis index; IL, interleukin; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; MTX, methotrexate; n.a., not assessed; NSAIDs, non‐steroidal anti‐inflammatory drugs; OPG, osteoprotegerin; PD, periodontal pocket depth; PGE2, prostaglandin E2; PI, plaque index; PSR, Periodontal screening and recording index (Lo Frisco et al., 1993) Armitage 1999 (Armitage, 2000), Page and Eke (Page & Eke, 2007); RANKL, receptor activator of nuclear factor‐kappa β ligand; TIMP, tissue inhibitor of MMP; TNF‐α, tumor necrosis factor‐α.
3.1.1. Pro‐inflammatory cytokines
Levels of IL‐1β in GCF of RA patients and periodontitis patients were comparable, but lower than in periodontally healthy controls, however (Cetinkaya et al., 2013). However, Miranda et al., (2007) found higher IL‐1β levels in GCF of healthy controls while Biyikoğlu et al., (2006) showed IL‐1β levels in GCF were comparable in periodontitis patients with or without RA. Recently, Bender et al., (2019) reported significantly higher levels of IL‐1β in GCF of RA patients. Serum IL‐1β was significantly lower in the RA patients than in periodontitis patients and periodontally healthy controls (Cetinkaya et al., 2013).
IL‐6 levels in GCF did not differ between RA patients with periodontitis, periodontitis patients without RA, and periodontally healthy controls (Bozkurt et al., 2000). Bozkurt et al., (2000) presumed, however, that although pockets probing depths and gingival bleeding indices in periodontally diseased individuals were higher compared to periodontally healthy controls, severity of periodontitis might have been too low to detect difference in GCF IL‐6 levels. The IL‐17A/E ratio was significantly higher in female RA patients than in controls (Gümüş et al., 2013b).
3.1.2. Cytokines involved in inflammation and tissue destruction
Miranda et al., (2007) found higher neutrophil elastase levels in GCF of periodontally matched healthy controls compared to RA patients. Biyikoğlu et al., (2006) showed that GCF levels of PGE2, t‐PA and its inhibitor PAI‐2 were comparable in periodontitis patients with or without RA, while these levels were lower in periodontally healthy controls. In another study, with overlapping study populations, Biyikoğlu et al., (2009) showed that total amounts of MMP‐8 in GCF were lower in the healthy controls than in RA‐gingivitis, RA‐periodontitis, and healthy‐periodontitis patients, while MMP‐13 amounts did not differ between the studied groups. Moreover, patients with RA and gingivitis or periodontitis exhibited levels of MMP‐8 and ‐13 and TIMP‐1 that were similar to systemically healthy patients. GCF, but not serum, levels of MMP‐9 were also found to be higher in RA patients with periodontitis compared to periodontitis patients without RA (Silosi et al., 2015). With increasing severity of periodontal disease, increasing levels of MMP‐8 levels were observed in GCF of both RA patients and persons without RA (Kirchner et al., 2017). When the periodontal status was healthy, no differences in MMP‐8 levels were found between RA patients and controls, but in case of periodontitis, MMP‐8 levels in GCF of RA patients with periodontitis were higher. In addition, Kirchner et al., (2017) assessed the presence of 11 periodontal pathogens in extracted DNA of the GCF samples (A. actinomycetemcomitans, P. gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, Parvimonas micra, Fusobacterium nucleatum, Campylobacter rectus, Eubacterium nodatum, Eikenella corrodens, and Capnocytophaga species). Prevalence of the selected periodontal pathogens and distribution of them according to periodontal disease severity was comparable between patients with and without RA.
Gümüş et al., (2013a) hypothesized that B cells are involved in an altered balance of pro‐ and anti‐inflammatory cytokines in periodontitis and RA. Therefore, these authors evaluated GCF and serum levels of TNF‐α, APRIL, BAFF, IL‐17A, and IL‐17B in female patients with and without RA with periodontal disease. Although bleeding on probing scores were high, pocket probing depths were relatively low in the various groups, which questions the severity of periodontitis. Despite long‐term use of anti‐rheumatic treatment (no detailed information provided), they reported an increase of all assessed TNF family cytokines in GCF and in serum of the RA patients. GCF concentrations of APRIL and BAFF were very low in patients without RA. Thus, compared to patients without RA, RA patients have locally and systemically increased levels of the assessed pro‐inflammatory cytokines of the TNF family. Also Balci Yuce et al., (2017) in their treatment study (Table 3) showed that TNF‐α, RANKL, and OPG were higher in GCF and serum of patients with periodontitis, irrespective of their RA status, than in healthy controls.
Table 3.
Studies on influence of non‐surgical periodontal therapy on cytokines in gingivocrevicular fluid (GCF) of patients with rheumatoid arthritis (RA)
| Study | Patients (number) | Sex (M/F) | Age (years, mean ± SD) | RA duration (years, mean ± SD | DAS28 (baseline) | Follow‐up | RA treatment (number of patients) | Cytokine(s) investigated in GCF | Same cytokine(s) investigated in serum | Periodontal assessment | Periodontal classification | Difference in periodontal status between study groups | Difference in concentrations of cytokines in GCF between study groups | Difference in concentrations of cytokines in serum between study groups | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Pretreatment versus post‐treatment | Baseline | Pretreatment versus post‐treatment | Baseline | Pretreatment versus post‐treatment | ||||||||||||
| Cosgarea et al., (2019) | RA + CP (18) | 4/14 | 52 ± 11 | 15 ± 6 | median 4.8 (IQR 3.9–5.7 |
6 months (15 RA + CP, 18 HC + CP) |
NSAIDs (13), DMARDS (18), sulfasalasin (2), steroids (6), anti‐cytokine therapy (3) | MMP−8, IL−1β, IL−10, | no | PD, BOP, CAL, PI | Armitage 1999 |
Similar values in BOP, CAL, PI. PD higher in HC + CP |
Significant improvements in PD, BOP, CAL. No difference in PI for RA + CP |
Significantly higher amount MMP−8 and IL−1β in RA + CP (no concentrations assessed) | RA + CP: no significant differences | ‐ | ‐ |
| HC + CP (18) | 8/10 | 44 ± 11 | – | – | – | HC + CP: significantly higher Il−10 post‐treatment | |||||||||||
| Balci Yuce et al., (2017) | RA + CP (17) | 6/11 | 51 ± 8 | n.a. | – | 6 weeks | Maintenance therapy, not specified |
TNF‐α, RANKL, OPG, 25‐hydroxyvitamin D |
TNF‐α, RANKL, OPG, 25‐hydroxyvitamin D |
CAL, GI, PI | Armitage 1999 | Similar values in CAL, GI, PI between RA + CP and HC + CP, but higher than in HC | Significant improvements in CAL, PI, GI in both CP groups, but still higher than in HC |
25‐hydroxyvitamin D levels higher in RA + CP and CP No significant differences in TNF‐α, RANKL, levels Higher OPG levels in HC |
Reduction of 25‐hydroxyvitamin D and TNF‐α levels in RA + CP only Reduce |
Similar values of 25‐hydroxyvitamin D levels Higher OPG levels in RA + CP |
Increase in OPG in CP, decrease in RANKL in RA + CP, but no significant increase in OPG/RANKL |
| HC + CP 18) | 9/9 | 50 ± 9 | – | ||||||||||||||
| HC (18) | 9/9 | 49 ± 10 | – | ||||||||||||||
| Kurgan et al., (2017) | RA + CP (15) | 6/9 | 49 ± 13 | n.a. | mean 3.0 SD 1.4 | 3 months | Maintenance therapy, not specified | t‐PA, PAI−2 | no | PD, BOP, CAL, GI, PI | Armitage 1999 | Similar values in PD, BOP, GI, PI, but higher than in HC | Significant improvements in PD, BOP, CL, PI, GI in both CP groups, but still higher than in HC | Higher t‐PA and PAI‐levels in RA + CP and HC + CP compared to HC | Reduction of t‐PA levels in RA + CP | – | – |
| HC + CP (15) | 7/8 | 42 ± 7 | |||||||||||||||
| HC (15) | 6/9 | 39 ± 7 | |||||||||||||||
| Kurgan et al., (2016) | RA + CP (13) | 4/9 | 49 ± 14 | n.a. | median 2.6 (IQR 2.4–4.0) | 3 months | NSAIDs (10), MTX (10), sulfasalasin (2), steroids (9) | MMP−8, PGE2, IL−6 | no | PD, BOP, GI, PI | Armitage 1999 |
Similar values in PD, BOP, GI, PI |
Significant improvements in PD, BOP and GI in both groups | Significantly higher amount PGE2 in RA + CP (no concentrations assessed | RA + CP: significantly lower amount of all assessed cytokines post‐treatment | – | – |
| HC + CP (13) | 7/6 | 41 ± 7 | – | – | – | HC + CP: MMP−8 amount significantly lower post‐treatment | |||||||||||
| Bıyıkoğlu et al., (2013) | RA + CP (15) | 6/9 | 47 ± 8 | 6 ± 4 | mean 4.2 SD 1.0 | 6 months (10 RA + CP, 13 HC + CP) | MTX (15), leflunomide (2), prednisolone (14), chloroquine12), sulfasalasin (3), anti‐CD20 (1), anti‐TNF‐α (1) | IL−1β, TNF‐α | yes | PD, BOP, CAL, PI | Armitage 1999 | Similar values in PD, BOP, CAL, PI | Significant improvements in PD, CAL, BOP and PI in both groups | No significant differences | RA + CP: TNF‐α significantly higher post‐treatment |
Higher TNF‐α in RA + CP |
No significant differences in both groups |
| HC + CP (15) | 9/6 | 47 ± 7 | – | – | – | HC + CP: TNF‐α significantly higher post‐treatment, IL1‐β significantly lower post‐treatment | |||||||||||
Abbreviations: Armitage 1999 (Armitage, 2000); BOP: bleeding on probing; CAL: clinical attachment level; DAS28: disease activity score 28 joint count; DMARDs: disease‐modifying anti‐rheumatic drugs; GI: gingivitis index; HC + CP: healthy controls with CP; IL: interleukin; MMP: matrix metalloproteinase; MTX: methotrexate; n.a.: not assessed; NSAIDs: non‐steroidal anti‐inflammatory drugs; OPG: osteoprotegerin; PAI‐2: plasminogen activator inhibitor‐2; PD: periodontal pocket depth; PGE2: prostaglandin E2; PI: plaque index; RA + CP: RA patients with chronic periodontitis (CP); RANKL; receptor activator of nuclear factor‐kappa β ligand; TNF‐α: tumor necrosis factor‐α; t‐PA: tissue/blood vessel‐type plasminogen activator.
3.1.3. Anti‐inflammatory cytokines
Concentration of IL‐4 and IL‐10 in GCF of RA patients and periodontitis patients was comparable, but lower than in periodontally healthy controls (Bozkurt et al., 2006; Cetinkaya et al., 2013). Serum IL‐10 was comparable between RA patients, periodontitis patients, and periodontally healthy controls (Cetinkaya et al., 2013). The IL‐17A/E ratio was significantly higher in female RA patients than in controls (Gümüş et al., 2013b).
3.2. Effect of treatment
3.2.1. Periodontal condition in RA patients
Overall, RA patients have significantly less teeth and a worse periodontal condition than controls with or without chronic periodontitis (Bender et al., 2019; Biyikoğlu et al., 2006, 2009; Bozkurt et al., 2006; Cetinkaya et al., 2013; Gümüş et al., 2013a, 2013b; Kirchner et al., 2017), but some studies reported no obvious differences in periodontal condition between RA patients and controls (Bozkurt et al., 2000; Miranda et al., 2007).
3.2.2. Anti‐rheumatic treatment with biological DMARDs
With regard to anti‐rheumatic treatment and their effect on the composition of GCF, only the effect of anti‐TNF‐α treatment on cytokine levels has been reported (Table 2). The studies of Mayer, Balbir‐Gurman, and Machtei (2009), and Mayer, Elimelech, Balbir‐Gurman, Braun‐Moscovici, and Machtei (2013) used overlapping study groups. The other two eligible studies lack a control group of RA patients not receiving anti‐TNF‐α therapy (Kadkhoda, Amirzargar, Esmaili, Vojdanian, & Akbari, 2016; Üstün et al., 2013). None of the patients had previous anti‐TNF‐α treatment. Next to anti‐TNF‐α treatment, all patients were allowed to continue their conventional anti‐rheumatic treatment consisting of non‐steroidal anti‐inflammatory drugs (NSAIDs), steroids, and/or csDMARDs. Periodontal status was recorded before and after anti‐TNF‐α treatment and classified according to the 1999 periodontal disease classification criteria (Armitage, 2000).
Table 2.
Studies on influence of anti‐rheumatic treatment with biological DMARDs on cytokines in gingivocrevicular fluid (GCF) of patients with rheumatoid arthritis (RA)
| Study | Patients (number) | Sex (M/F) |
Age (years, mean ± SD) |
RA duration (years, mean ± SD | DAS28 (mean ± SD, baseline) | Anti‐rheumatic treatment with biological DMARDs | Duration of treatment (follow‐up) |
Other RA treatment |
Cytokine(s) investigated in GCF | Same cytokine(s) investigated in serum | Periodontal assessment | Periodontal classification | Difference in periodontal status between study groups | Difference in concentrations of cytokines in GCF between study groups or pre–post‐treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kadkhoda et al., (2016) | RA+ (36) | 10/26 | 41 ± 12 | n.a. | n.a. | anti‐TNF‐α (etanercept 25 mg 2/week) | 6 weeks | no detailed information provided | TNF‐α | no | PD, BOP, GI, PI | All patients had generalized gingival inflammation and redness concomitant with BOP, with or without PD ≥ 5 mm. | Pre‐ versus post‐treatment: significantly lower BOP and GI post‐treatment | Pre‐ versus post‐treatment: TNF‐α significantly lower post‐treatment |
| Mayer et al., (2013) | RA+ (10) | 3/7 | 54 ± 6 | 16 ± 14 | n.a. | anti‐TNF‐α (infliximab 3 mg/kg every 8 weeks) | 26 ± 8 months | no detailed information provided | TNF‐α | no | PD, BOP, GI, PI | Armitage 1999 | RA + versus RA‐: PD, BOP, GI significantly lower in RA+ | RA + versus RA‐: TNF‐α significantly higher in RA‐ |
| RA‐ (12) | 5/7 | 48 ± 12 | 5 ± 2 | n.a. | – | – | ||||||||
| Üstün et al., (2013) | RA+ (16) | 9/7 | 35 ± 8 | 4 ± 2 | 5.1 ± 0.7 | anti‐TNF‐α: adalimumab 40 mg on days 0 and 14 (7 patients) orinfliximab 3 mg/kg on days 0 and 14 (9 patients). | 30 days | NSAIDs, MTX, sulfasalazine, hydroxychloroquin, prednisolone (max. 5 mg/day) | Il−1β, Il−8 | no | PD, BOP, CAL, GI, PI | Armitage 1999 (with CP: n = 10, without CP: n = 6) | Pre‐ versus post‐treatment: significantly higher BOP and GI post‐treatment (10 with CP, 6 without CP) | Pre‐ versus post‐treatment: significantly lower Il−1β, Il−8 post‐treatment |
| Mayer et al., (2009) | RA+ (10) | 3/7 | 54 ± 9 | 16 ± 13 | 4.8 ± 0.9 | anti‐TNF‐α (infliximab 200 mg every 8 weeks) | 26 ± 8 months | NSAIDs, MTX, sulfasalazine, hydroxychloroquine | TNF‐α | no | PD, BOP, CAL, GI, PI | Armitage 1999 | RA + versus RA‐: CAL, BOP, GI significantly higher in RA+ | RA + versus RA‐: TNF‐α amount (no concentration assessed) significantly higher in RA‐ |
| RA‐ (10) | 5/5 | 47 ± 16 | 5 ± 2 | 5.1 ± 1.1 | – | – |
Abbreviations: Armitage 1999 (Armitage, 2000); BOP, bleeding on probing; CAL, clinical attachment level; DAS28, disease activity score 28 joint count; GI, gingivitis index; MTX, methotrexate; n.a., not assessed; NSAIDs, non‐steroidal anti‐inflammatory drugs; PD, periodontal pocket depth; PI, plaque index; RA‐, RA patients receiving no treatment with biological DMARDS; RA+, RA patients receiving treatment with biological disease‐modifying anti‐rheumatic drugs (DMARDs); TNF‐α, tumor necrosis factor‐α.
GCF TNF‐α levels significantly decreased after starting anti‐TNF‐α therapy compared to pretreatment levels (Kadkhoda et al., 2016) as well as to GCF TNF‐α levels in RA patients not receiving anti‐TNF‐α therapy (Mayer et al., 2009, 2013). In addition, compared to pretreatment, GCF IL‐1β and IL‐8 levels had decreased significantly after 4 weeks of anti‐TNF‐α therapy in RA patients with periodontitis (Üstün et al., 2013). Furthermore, anti‐TNF‐α therapy led to significant improvement in periodontal indices of inflammation, independent of oral hygiene status of the patients (Kadkhoda et al., 2016; Mayer et al., 2013; Üstün et al., 2013) as well as that RA disease activity decreased significantly in RA patients with and without chronic periodontitis (Üstün et al., 2013). The other studies (Kadkhoda et al., 2016; Mayer et al., 2009, 2013) did not report whether RA disease activity changed. In summary, anti‐TNF‐α treatment in RA patients apparently lowers levels of pro‐inflammatory cytokines in GCF (TNF‐α, IL‐1β, and IL‐8), which, besides the beneficial effect on RA, may result in decreased inflammation of the periodontium.
3.2.3. Periodontal treatment
Five studies assessed the influence of periodontal treatment on biomarkers in GCF of RA patients (Table 3). Non‐surgical periodontal therapy consisted of oral hygiene instructions and scaling and rootplaning without use of antibiotics. RA patients were allowed to continue their anti‐rheumatic treatment consisting of NSAIDs, steroids, csDMARDs, and incidentally biological DMARDs (anti‐TNF‐α or anti‐CD20; Bıyıkoğlu et al., 2013). All studies had a control group consisting of periodontitis patients without RA receiving periodontal treatment. Follow‐up ranged from 6 weeks to 6 months. Kurgan et al. (Kurgan et al., 2016, 2017) used overlapping study groups.
Two studies compared GCF cytokine levels with those of serum before and after non‐surgical periodontal treatment (Balci Yuce et al., 2017; Bıyıkoğlu et al., 2013). Bıyıkoğlu et al., (2013) followed their patients up to 6 months after treatment. IL‐1β levels in GCF decreased in periodontitis patients without RA only, whereas no significant change was observed in serum levels of this cytokine. Also Cosgarea et al., (2019) did not observe a decrease of IL‐1β levels in RA patients with periodontitis. Furthermore, while no changes over time in serum TNF‐α concentrations were seen, GCF TNF‐α levels increased in both groups. Bıyıkoğlu et al., (2013) had no good explanation for this result. On the contrary, and in line with what was expected, Balci Yuce et al., (2017) showed that GCF TNF‐α levels decreased in patients with and without RA, while serum TNFa levels did not change. Balci Yuce et al., (2017) also showed that periodontal therapy resulted in a decrease of GCF vitamin D levels in RA patients, while again no changes of vitamin D levels were found in serum. GCF and serum RANKL/OPG ratios did not change either. Furthermore, periodontal treatment in RA patients with periodontitis did not result in a significant decrease of IL‐1β, MMP‐8, and IL‐10 levels (Cosgarea et al., 2019).
Kurgan et al., (2016) reported that MMP‐8 levels decreased significantly in GCF in patients with or without RA after non‐surgical periodontal treatment, whereas for IL‐6 and PGE2 GCF levels a significant decrease was observed only in RA patients. In a follow‐up study, Kurgan et al., (2017) additionally assessed main components of the plasminogen activating system (t‐PA and PAI‐2). They showed that t‐PA in GCF significantly decreased in RA patients after periodontal therapy. Its inhibitor PAI‐2 did not change significantly, however. Thus, non‐surgical periodontal therapy in RA patients seems to have limited effects on the local action of the plasminogen activating system.
Of note, Bıyıkoğlu et al., (2013) reported a significant decrease and Cosgarea et al., (2019) a tendency of decrease of RA disease activity 6 months after periodontal therapy, while Kurgan et al., (2016) did not observe a lower RA disease activity 3 months after periodontal therapy. The higher DAS 28 joint count scores at baseline (4.2 ± 1.0) in the study of Bıyıkoğlu et al., (2013) compared to those (3.0 ± 1.4) reported by Kurgan et al., (2016) may account for this different finding. Also, in the study of Cosgarea et al., (2019) the baseline DAS score was higher (4.8). RA disease activity was not mentioned in the study of Balci Yuce et al., (2017).
In summary, periodontal treatment results in a local anti‐inflammatory effect as the pro‐inflammatory cytokines (IL‐1β, IL‐6, TNF‐α) and mediators implicated in tissue destruction (MMP‐8, PGE2) decrease in GCF, but not in serum. This effect seems to be more pronounced in RA patients with periodontitis than in periodontitis patients without RA. Together with the reported improvement of RA disease activity, RA patients can benefit from periodontal treatment.
4. DISCUSSION
Overall, RA patients have increased GCF and serum levels of pro‐inflammatory cytokines and proteins, despite anti‐rheumatic treatment. Presence of periodontitis increases these levels. The interaction of RA and periodontitis is also reflected in the effect of treatment. Anti‐TNF‐α therapy decreases pro‐inflammatory cytokines in GCF especially when RA and periodontitis coexist. Besides the beneficial effect on RA, anti‐TNF‐α therapy also lowers clinical periodontal inflammation. Although it has been shown that RA treatment lowers levels of pro‐inflammatory cytokines in GCF, clinical periodontal inflammation does not return levels in healthy subjects without chronic periodontitis. This is in line with a recent study of Ziebolz et al., (2018) who reported that also in patients on immunosuppressive medications, RA remains to be associated with periodontal inflammation. Similarly, periodontal treatment exerts a local anti‐inflammatory effect from which RA patients benefit too as this local effect was often more pronounced in periodontitis patients with RA compared to periodontitis patients without RA. Although periodontal treatment alone also might not be sufficient to lower systemic cytokine levels, a systemic effect of periodontal therapy has been reflected in a decrease of RA disease activity.
Rheumatoid arthritis and periodontitis have in fact different etiologies, but pathology in both diseases results from an imbalance in pro‐ and anti‐inflammatory cytokines. Coexistence of RA and periodontitis may aggravate both of them, which is reflected by the phenomenon that an increased severity of periodontal disease is accompanied by higher RA disease activity scores (de Smit et al., 2012). For the same reason, treatment of one disease may impact the other disease.
Methodological concerns related to the collection and analysis of GCF are important factors that need to be considered when studying GCF (Lamster & Ahlo, 2007). Contradictory results could be due to the lack of uniformity in the methodological design of the studies. For example, quantity and quality of GCF samples are highly affected by the method of collection and analysis. GCF collection with filter paper strips is used in most studies and is the method of choice for most biomarkers in immunologic studies (Guentsch et al., 2011). Standardized collection of GCF is almost impossible in observational studies, as different patient groups are investigated and ideal sampling conditions to avoid contamination of GCF samples with blood are mostly absent (patients with good oral hygiene and low gingival inflammation). As a result, contradictory data may be obtained, underlining the lack of understanding of the complexity of the association between periodontitis and RA (Schmalz et al., 2017).
Although it is hypothesized that inflamed mucosal surfaces are likely initiation sites for RA, the most common RA‐associated autoantibodies, that is, RF and ACPAs, have not yet been studied in GCF of RA patients. Up to now, only Harvey et al., (2013) reported on ACPAs in GCF of periodontally inflamed sites in individuals without RA. Activity of human and bacterial citrullinating enzymes (PAD and PPAD), however, has been studied in GCF of RA patients. The enzyme activity was clearly associated with periodontitis but to a lesser extent with RA and the presence of P. gingivalis (Laugisch et al., 2016). Regarding expression of inflammatory proteins in GCF, a specific role for certain periodontal pathogens was not found (Kirchner et al., 2017; Laugisch et al., 2016).
Keeping in mind the methodological concerns related to collection and analysis of GCF, this focused review points toward a bidirectional relationship between periodontitis and RA, which is probably caused by non‐specific inflammatory burden. RA patients could benefit from periodontal screening, as periodontal treatment in RA patients lowers local periodontal inflammatory burden and improves RA disease activity scores. Data for a more specific relationship are barely present in GCF. To determine potential causality between periodontitis and RA, presence and production of RA‐associated antibodies in the periodontium should be investigated, preferably with assessment of the microbial composition of the dental biofilm. In this respect, Eriksson et al., (2019) very recently showed that most RA patients with moderate or severe periodontitis were seropositive for ACPA (86%) as well as that these patients had a subgingival microbial profile that differed from RA patients with no or mild periodontitis and had higher levels of oral and systemic inflammatory mediators.
ACKNOWLEDGEMENTS
This research was partly funded by the Dutch Arthritis Foundation (ReumaNederland) as well as by a Abel Tasman Talent Program sandwich PhD grant from the Graduate School of Medical Sciences of the University of Groningen.
Rahajoe PS, Smit MJ, Kertia N, Westra J, Vissink A. Cytokines in gingivocrevicular fluid of rheumatoid arthritis patients: A review of the literature. Oral Dis. 2019;25:1423–1434. 10.1111/odi.13145
REFERENCES
- Aletaha, D. , Neogi, T. , Silman, A. J. , Funovits, J. , Felson, D. T. , Bingham, C. O. , … Hawker, G. (2010). 2010 Rheumatoid arthritis classification criteria: An American College of rheumatology/European league against rheumatism collaborative initiative. Arthritis and Rheumatism, 62(9), 2569–2581. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- Armitage, G. C. (2000). Development of a classification system for periodontal diseases and conditions. Northwest Dentistry, 79(6), 31–35. [PubMed] [Google Scholar]
- Arnett, F. C. , Edworthy, S. M. , Bloch, D. A. , Mcshane, D. J. , Fries, J. F. , Cooper, N. S. , … Hunder, G. G. (1988). The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism, 31(3), 315–324. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- Äyräväinen, L. , Heikkinen, A. M. , Kuuliala, A. , Ahola, K. , Koivuniemi, R. , Moilanen, E. , … Sorsa, T. (2018). Anti‐rheumatic medication and salivary MMP‐8, a biomarker for periodontal disease. Oral Diseases, 24(8), 1562–1571. 10.1111/odi.12930 [DOI] [PubMed] [Google Scholar]
- Balci Yuce, H. , Gokturk, O. , Aydemir Turkal, H. , Inanir, A. , Benli, I. , & Demir, O. (2017). Assessment of local and systemic 25‐hydroxy‐vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. Journal of Oral Science, 59(3), 397–404. 10.2334/josnusd.16-0677 [DOI] [PubMed] [Google Scholar]
- Bender, P. , Egger, A. , Westermann, M. , Taudte, N. , Sculean, A. , Potempa, J. , … Eick, S. (2019). Expression of human and Porphyromonas gingivalis glutaminyl cyclases in periodontitis and rheumatoid arthritis‐A pilot study. Archives of Oral Biology, 97(1), 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bıyıkoğlu, B. , Buduneli, N. , Aksu, K. , Nalbantsoy, A. , Lappin, D. F. , Evrenosoğlu, E. , & Kinane, D. F. (2013). Periodontal therapy in chronic periodontitis lowers gingival crevicular fluid interleukin‐1beta and DAS28 in rheumatoid arthritis patients. Rheumatology International, 33(10), 2607–2616. 10.1007/s00296-013-2781-5 [DOI] [PubMed] [Google Scholar]
- Biyikoğlu, B. , Buduneli, N. , Kardeşler, L. , Aksu, K. , Oder, G. , & Kütükçüler, N. (2006). Evaluation of t‐PA, PAI‐2, IL‐1beta and PGE(2) in gingival crevicular fluid of rheumatoid arthritis patients with periodontal disease. Journal of Clinical Periodontology, 33(9), 605–611. 10.1111/j.1600-051X.2006.00961.x [DOI] [PubMed] [Google Scholar]
- Biyikoğlu, B. , Buduneli, N. , Kardeşler, L. , Aksu, K. , Pitkala, M. , & Sorsa, T. (2009). Gingival crevicular fluid MMP‐8 and ‐13 and TIMP‐1 levels in patients with rheumatoid arthritis and inflammatory periodontal disease. Journal of Periodontology, 80(8), 1307–1314. 10.1902/jop.2009.090130 [DOI] [PubMed] [Google Scholar]
- Bozkurt, F. Y. , Berker, E. , Akkuş, S. , & Bulut, S. (2000). Relationship between interleukin‐6 levels in gingival crevicular fluid and periodontal status in patients with rheumatoid arthritis and adult periodontitis. Journal of Periodontology, 71(11), 1756–1760. 10.1902/jop.2000.71.11.1756 [DOI] [PubMed] [Google Scholar]
- Bozkurt, F. Y. , Yetkin Ay, Z. , Berker, E. , Tepe, E. , & Akkuş, S. (2006). Anti‐inflammatory cytokines in gingival crevicular fluid in patients with periodontitis and rheumatoid arthritis: A preliminary report. Cytokine, 35(3–4), 180–185. 10.1016/j.cyto.2006.07.020 [DOI] [PubMed] [Google Scholar]
- Brennan, F. M. , & McInnes, I. B. (2008). Evidence that cytokines play a role in rheumatoid arthritis. The Journal of Clinical Investigation, 118(11), 3537–3545. 10.1172/JCI36389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinkaya, B. , Guzeldemir, E. , Ogus, E. , & Bulut, S. (2013). Proinflammatory and anti‐inflammatory cytokines in gingival crevicular fluid and serum of patients with rheumatoid arthritis and patients with chronic periodontitis. Journal of Periodontology, 84(1), 84–93. 10.1902/jop.2012.110467 [DOI] [PubMed] [Google Scholar]
- Champagne, C. M. E. , Buchanan, W. , Reddy, M. S. , Preisser, J. S. , Beck, J. D. , & Offenbacher, S. (2003). Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontology, 2000(31), 167–180. 10.1034/j.1600-0757.2003.03110.x [DOI] [PubMed] [Google Scholar]
- Cosgarea, R. , Tristiu, R. , Dumitru, R. B. , Arweiler, N. B. , Rednic, S. , Sirbu, C. I. , … Eick, S. (2019). Effects of non‐surgical periodontal therapy on periodontal laboratory and clinical data as well as on disease activity in patients with rheumatoid arthritis. Clinical Oral Investigation, 23(1), 141–151. 10.1007/s00784-018-2420-3 [DOI] [PubMed] [Google Scholar]
- Culshaw, S. , McInnes, I. B. , & Liew, F. Y. (2011). What can the periodontal community learn from the pathophysiology of rheumatoid arthritis? Journal of Clinical Periodontology, 106–113, 10.1111/j.1600-051X.2010.01669.x [DOI] [PubMed] [Google Scholar]
- Deane, K. D. , O'Donnell, C. I. , Hueber, W. , Majka, D. S. , Lazar, A. A. , Derber, L. A. , … Holers, V. M. (2010). The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age‐dependent manner. Arthritis and Rheumatism, 62(11), 3161–3172. 10.1002/art.27638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen, V. F. A. M. , Huizinga, T. W. J. , & van der Woude, D. (2017). The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Seminars in Immunopathology, 39(4), 437–446. 10.1007/s00281-017-0627-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, K. , Fei, G. , Lundmark, A. , Benchimol, D. , Lee, L. , Hu, Y. O. O. , … Yucel‐Lindberg, T. (2019). Periodontal health and oral microbiota in patients with rheumatoid arthritis. Journal of Clinical Medicine, 8, E630 10.3390/jcm8050630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, R. W. , Trouw, L. A. , Shi, J. , Toes, R. E. M. , Huizinga, T. W. J. , Demoruelle, M. K. , … Holers, V. M. (2015). Anti‐carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. The Journal of Rheumatology, 42(4), 572–579. 10.3899/jrheum.140767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentsch, A. , Kramesberger, M. , Sroka, A. , Pfister, W. , Potempa, J. , & Eick, S. (2011). Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. Journal of Periodontology, 82(7), 1051–1060. 10.1902/jop.2011.100565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gümüş, P. , Buduneli, E. , Bıyıkoğlu, B. , Aksu, K. , Saraç, F. , Buduneli, N. , & Lappin, D. F. (2013a). Gingival crevicular fluid and serum levels of APRIL, BAFF and TNF‐alpha in rheumatoid arthritis and osteoporosis patients with periodontal disease. Archives of Oral Biology, 58(10), 1302–1308. 10.1016/j.archoralbio.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Gümüş, P. , Buduneli, E. , Bıyıkoğlu, B. , Aksu, K. , Saraç, F. , Nile, C. , … Buduneli, N. (2013b). Gingival crevicular fluid, serum levels of receptor activator of nuclear factor‐kappa B ligand, osteoprotegerin, interleukin‐17 in rheumatoid arthritis and osteoporosis patients with periodontal disease. Journal of Periodontology, 84(11), 1–13. 10.1902/jop.2013.120595 [DOI] [PubMed] [Google Scholar]
- Harvey, G. P. , Fitzsimmons, T. R. , Dhamarpatni, A. A. S. S. K. , Marchant, C. , Haynes, D. R. , & Bartold, P. M. (2013). Expression of peptidylarginine deiminase‐2 and ‐4, citrullinated proteins and anti‐citrullinated protein antibodies in human gingiva. Journal of Periodontal Research, 48(2), 252–261. 10.1111/jre.12002 [DOI] [PubMed] [Google Scholar]
- Javed, F. , Ahmed, H. B. , Mikami, T. , Almas, K. , Romanos, G. E. , & Al‐Hezaimi, K. (2014). Cytokine profile in the gingival crevicular fluid of rheumatoid arthritis patients with chronic periodontitis. Journal of Investigative and Clinical Dentistry, 5(1), 1–8. 10.1111/jicd.12066 [DOI] [PubMed] [Google Scholar]
- Jog, N. R. , & James, J. A. (2017). Biomarkers in connective tissue diseases. Journal of Allergy and Clinical Immunology, 140(6), 1473–1483. 10.1016/j.jaci.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadkhoda, Z. , Amirzargar, A. , Esmaili, Z. , Vojdanian, M. , & Akbari, S. (2016). Effect of TNF‐α blockade in gingival crevicular fluid on periodontal condition of patients with rheumatoid arthritis. Iranian Journal of Immunology, 13(3), 197–203. [PubMed] [Google Scholar]
- Kirchner, A. , Jäger, J. , Krohn‐Grimberghe, B. , Patschan, S. , Kottmann, T. , Schmalz, G. , … Ziebolz, D. (2017). Active matrix metalloproteinase‐8 and periodontal bacteria depending on periodontal status in patients with rheumatoid arthritis. Journal of Periodontal Research, 52(4), 745–754. 10.1111/jre.12443 [DOI] [PubMed] [Google Scholar]
- Konig, M. F. , Abusleme, L. , Reinholdt, J. , Palmer, R. J. , Teles, R. P. , Sampson, K. , … Andrade, F. (2016). Aggregatibacter actinomycetemcomitans‐induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Science Translational Medicine, 8(369), 369ra176 10.1126/scitranslmed.aaj1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurgan, Ş. , Fentoğlu, Ö. , Önder, C. , Serdar, M. , Eser, F. , Tatakis, D. N. , & Günhan, M. (2016). The effects of periodontal therapy on gingival crevicular fluid matrix metalloproteinase‐8, interleukin‐6 and prostaglandin E2 levels in patients with rheumatoid arthritis. Journal of Periodontal Research, 51(5), 586–595. 10.1111/jre.12337 [DOI] [PubMed] [Google Scholar]
- Kurgan, Ş. , Önder, C. , Balcı, N. , Fentoğlu, Ö. , Eser, F. , Balseven, M. , … Günhan, M. (2017). Gingival crevicular fluid tissue/blood vessel‐type plasminogen activator and plasminogen activator inhibitor‐2 levels in patients with rheumatoid arthritis: Effects of nonsurgical periodontal therapy. Journal of Periodontal Research, 52(3), 574–581. 10.1111/jre.12425 [DOI] [PubMed] [Google Scholar]
- Lamster, I. B. (1997). Evaluation of components of gingival crevicular fluid as diagnostic tests. Annals of Periodontology, 2(1), 123–137. 10.1902/annals.1997.2.1.123 [DOI] [PubMed] [Google Scholar]
- Lamster, I. B. , & Ahlo, J. K. (2007). Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Annals of the New York Academy of Sciences, 1098(1), 216–229. 10.1196/annals.1384.027 [DOI] [PubMed] [Google Scholar]
- Laugisch, O. , Wong, A. , Sroka, A. , Kantyka, T. , Koziel, J. , Neuhaus, K. , … Eick, S. (2016). Citrullination in the periodontium—a possible link between periodontitis and rheumatoid arthritis. Clinical Oral Investigations, 20(4), 675–683. 10.1007/s00784-015-1556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, Y. , Balbir‐Gurman, A. , & Machtei, E. E. (2009). Anti‐tumor necrosis factor‐alpha therapy and periodontal parameters in patients with rheumatoid arthritis. Journal of Periodontology, 80(9), 1414–1420. 10.1902/jop.2009.090015 [DOI] [PubMed] [Google Scholar]
- Mayer, Y. , Elimelech, R. , Balbir‐Gurman, A. , Braun‐Moscovici, Y. , & Machtei, E. E. (2013). Periodontal condition of patients with autoimmune diseases and the effect of anti‐tumor necrosis factor‐α therapy. Journal of Periodontology, 84(2), 136–142. 10.1902/jop.2012.120009 [DOI] [PubMed] [Google Scholar]
- Mikuls, T. R. , Payne, J. B. , Deane, K. D. , & Thiele, G. M. (2016). Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? The Journal of Allergy and Clinical Immunology, 137(1), 28–34. 10.1016/j.jaci.2015.10.024 [DOI] [PubMed] [Google Scholar]
- Miranda, L. A. , Islabão, A. G. , Fischer, R. G. , Figueredo, C. M. S. , Oppermann, R. V. , & Gustafsson, A. (2007). Decreased interleukin‐1β and elastase in the gingival crevicular fluid of individuals undergoing anti‐inflammatory treatment for rheumatoid arthritis. Journal of Periodontology, 78(8), 1612–1619. 10.1902/jop.2007.060520 [DOI] [PubMed] [Google Scholar]
- Özçaka, Ö. , Alpöz, E. , Nalbantsoy, A. , Karabulut, G. , & Kabasakal, Y. (2018). Clinical periodontal status and inflammatory cytokines in primary Sjögren syndrome and rheumatoid arthritis. Journal of Periodontology, 89(8), 959–965. 10.1002/JPER.17-0730 [DOI] [PubMed] [Google Scholar]
- Page, R. C. , & Eke, P. I. (2007). Case definitions for use in population‐based surveillance of periodontitis. Journal of Periodontology, 78(Suppl. 7), 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Paul, B. J. , Kandy, H. I. , & Krishnan, V. (2017). Pre‐rheumatoid arthritis and its prevention. European Journal of Rheumatology, 4(2), 161–165. 10.5152/eurjrheum.2017.16006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa, J. , Mydel, P. , & Koziel, J. (2017). The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nature Reviews Rheumatology, 13(10), 606–620. 10.1038/nrrheum.2017.132 [DOI] [PubMed] [Google Scholar]
- Rosenstein, E. D. , Greenwald, R. A. , Kushner, L. J. , & Weissmann, G. (2004). Hypothesis: The humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation, 28(6), 311–318. 10.1007/s10753-004-6641-z [DOI] [PubMed] [Google Scholar]
- Schmalz, G. , Davarpanah, I. , Jäger, J. , Mausberg, R. F. , Krohn‐Grimberghe, B. , Schmidt, J. , … Ziebolz, D. (2017). MMP‐8 and TIMP‐1 are associated to periodontal inflammation in patients with rheumatoid arthritis under methotrexate immunosuppression – First results of a cross‐sectional study. Journal of Microbiology, Immunology and Infection, 52(3), 386–394. 10.1016/j.jmii.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Silosi, I. , Cojocaru, M. , Foia, L. , Boldeanu, M. V. , Petrescu, F. , Surlin, P. , & Biciusca, V. (2015). Significance of circulating and crevicular matrix metalloproteinase‐9 in rheumatoid arthritis‐chronic periodontitis association. Journal of Immunology Research, 2015, 1–6. 10.1155/2015/218060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit, M. , Westra, J. , Vissink, A. , Doornbos‐van der Meer, B. , Brouwer, E. , van Winkelhoff, A. J. , … van Winkelhoff, A. J. (2012). Periodontitis in established rheumatoid arthritis patients: A cross‐sectional clinical, microbiological and serological study. Arthritis Research & Therapy, 14(5), R222 10.1186/ar4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás, I. , Arias‐Bujanda, N. , Alonso‐Sampedro, M. , Casares‐de‐Cal, M. A. , Sánchez‐Sellero, C. , Suárez‐Quintanilla, D. , & Balsa‐Castro, C. (2017). Cytokine‐based predictive models to estimate the probability of chronic periodontitis: Development of diagnostic nomograms. Scientific Reports, 7(1), 11580 10.1038/s41598-017-06674-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün, K. , Erciyas, K. , Kısacık, B. , Sezer, U. , Pehlivan, Y. , Öztuzcu, S. , … Onat, A. M. (2013). Host modulation in rheumatoid arthritis patients with TNF blockers significantly decreases biochemical parameters in periodontitis. Inflammation, 36(5), 1171–1177. 10.1007/s10753-013-9652-9 [DOI] [PubMed] [Google Scholar]
- Wegner, N. , Wait, R. , Sroka, A. , Eick, S. , Nguyen, K.‐A. , Lundberg, K. , … Venables, P. J. (2010). Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α‐enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis & Rheumatism, 62(9), 2662–2672. 10.1002/art.27552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebolz, D. , Rupprecht, A. , Schmickler, J. , Bothmann, L. , Krämer, J. , Patschan, D. , … Patschan, S. (2018). Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: Results from a cross‐sectional study. Journal of Periodontology, 89(11), 1310–1317. 10.1002/JPER.17-0616 [DOI] [PubMed] [Google Scholar]


