Abstract
The incentives provided under the Orphan Drug Act (ODA) have been credited for catalyzing the marketing approval of drugs for the treatment of rare diseases by the US Food and Drug Administration. Orphan drug designation, the granting of special status to drugs or biologics (“drugs”) for the treatment of rare diseases, one of the ODA's key incentive programs, has seen major increases in volume over recent years. The new era of precision medicine and the development of therapies directed toward smaller “orphan” subsets of common diseases have been suggested as being a major driver. We evaluated the basis for orphan drug designations and orphan subsets in relation to the impact of precision medicines. We found that the increasing numbers of orphan drug designation determinations were not driven by precision medicines separating common diseases into orphan subsets and that orphan subsets overall also represented a relatively small proportion of designations.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ It has been speculated that the increasing number of new drug approvals for precision medicines targeting rare subpopulations of otherwise common diseases may be impacting the use of the orphan drug designation incentive of the Orphan Drug Act.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This analysis suggests that the increasing number of orphan drug designations over recent years is not driven by precision medicines nor subsetting for otherwise common diseases.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The promise of precision medicine will remain critical to the rare disease community as many of the estimated 7,000 rare diseases still lack any treatment and many are genetically heterogeneous. With advances in genomic medicine, a better understanding of rare diseases, strong advocacy, and continued incentives to drive a sponsor's interest in orphan drug development, the current trends for increased product approvals and orphan drug designations seem likely to continue.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The continued development of innovative precision medicines is crucial to optimize and provide new treatments for patients with rare diseases.
The development of treatments for patients with rare diseases, such as sickle cell anemia, amyotrophic lateral sclerosis, and neuroblastoma, has historically been challenging based on the small number of patients available to study the safety and effectiveness of potential treatments and limited commercial viability. Because development of drugs for these populations were either not pursued or were ultimately abandoned, these drugs eventually became known as “orphan drugs.” The Orphan Drug Act (ODA), which was passed in 1983 in the United States to address this important public health challenge, has been lauded as one of the most successful pieces of US public health legislation.1, 2, 3 The fiscal and regulatory incentives contained in the ODA have shepherded over 700 additional orphan drug indications to market, compared with just 10 that were developed solely by industry prior to its enactment.4
Orphan drug designation provides key incentives of the ODA, including significant tax credits for qualified clinical testing; waiver of the marketing application fee required of sponsors at the time of submission to the US Food and Drug Administration (FDA), which is currently over US $2 million; and may provide for 7 years of marketing exclusivity upon approval of the product. In order to receive these financial incentives, a sponsor must submit a request for orphan drug designation to the FDA before submitting a marketing application. If the sponsor meets the designation criteria (e.g., demonstrates that the disease for which the drug is intended to diagnose, prevent, or treat is rare), which is defined, in part, as a disease or condition affecting <200,000 people in the United States, and provides scientific rationale to show promise for using the drug in that rare disease or condition, the FDA will grant orphan drug designation. In certain limited circumstances, a drug for a common disease (e.g., where the US prevalence is over 200,000) may be eligible for orphan drug designation if the drug specifically treats a disease or condition that meets the regulatory criteria of an “orphan subset.” An orphan subset of a common disease or condition is defined by some characteristic of the drug that would limit its use to a subset of the disease population because the drug is either ineffective or too toxic to use in the remainder of the disease. Examples of such characteristics include the mechanism of action, toxicity profile, or previous clinical experience with the drug.5, 6
The mapping of the human genome in 2003 set the stage for accelerating drug development through precision medicine, with the potential to optimize the safety and effectiveness of treatments by targeting the right drug to the right patient based on an understanding of different molecular characteristics of patients with a similar clinical presentation. Subsequently, as the knowledge of the biological, behavioral, and environmental factors and technologies to characterize individual diseases have progressed over the last decade, the ability to more accurately diagnose diseases and follow their progression and outcomes has begun to greatly improve. Thus, developing drugs with substantial benefits in smaller, molecularly defined, pharmacologically relevant subpopulations of patients with the same clinically recognized disease is increasingly being viewed as a viable pathway for bringing drugs to market.7, 8 Two well‐known examples of FDA‐approved precision medicines are trastuzumab, a humanized monoclonal antibody treatment for a subpopulation of patients with breast cancer, a common disease, whose tumors overexpress the HER2 oncogene, and the orphan designated drug ivacaftor, a potentiator of the CFTR protein for a subpopulation of cystic fibrosis, a rare disease. Thus, precision medicines where the drug's mechanism of action is only relevant in a small subpopulation of patients within a more common disease may qualify for orphan drug incentives as an orphan subset.
The number of requests for orphan drug designation and the number being granted have risen dramatically in the last 8 years leading to speculation as to what is driving this increase. Improvements in genomic technologies allowing for a better understanding of the molecular basis of many rare genetic diseases has been put forward as a reason. However, it has also been speculated that the development of precision medicines for molecularly targeted subpopulations of common diseases has been a driver of these increases.9, 10, 11 To better understand the potential regulatory impacts of precision medicine on the orphan drug designation program, we analyzed the orphan drug designations granted in 2 calendar years based on whether they were a precision medicine and whether they were for use in an orphan subset of a common disease. We also made further classifications of precision medicines based on therapeutic disease class; whether molecular targets were somatic, germline, or pathogen related; and whether the proposed product was a drug or biologic.
Methods
Overview
The overall objective of the analysis was to provide descriptive data evaluating the total number of orphan drug designations granted per calendar year 2010 and 2015 relative to the number granted for orphan subsets and precision medicines in each of those years.
Definitions
Precision medicine, personalized medicine, and targeted therapy are terms that are sometimes used interchangeably, and, in the case of targeted therapies, has also been defined quite broadly to simply mean a drug with a mechanism of action “targeted” toward a particular molecular pathway. For the purpose of our analysis, we use the term “precision medicine,” which we defined as a drug or biologic with a mechanism that acts specifically to treat a genetically or molecularly defined subpopulation of a disease (i.e., for rare or common diseases). Aligning with the ODA framework, we have defined a “common disease” as a disease that occurs in >200,000 persons in the United States. As per the orphan drug regulation, we defined “orphan subset” as a subset of a common disease, such that use of the drug for the subset is appropriate, but that use of the drug outside of that subset (in the remaining persons with the disease) would be inappropriate owing to some property(ies) of the product, for example, toxicity, mechanism of action, or previous clinical experience with the product.5, 6
Data source and methodology
We used internal FDA data to generate a list of the FDA orphan designated drugs for the calendar years 2010 and 2015. We individually analyzed the FDA written review of each orphan drug that was granted designation and categorized whether (i) the product was a precision medicine and (ii) whether the designation was based on an orphan subset of a common disease. Each product was also further categorized into therapeutic disease class (e.g., hematology, oncology, gastrointestinal, neurologic, pulmonary, ophthalmology, immunology, infectious disease, metabolic, and endocrine) and product type (i.e., small molecule drug or biologic). If determined to be a precision medicine, we noted whether the molecular target type was somatic, germline, or pathogen related. Statistical comparisons were done using the χ2 test, and associations with a P value of < 0.05 were considered statistically significant.
Results
Figure 1 shows that of the 195 orphan drug designations in 2010, 26 were for precision medicines. Of the 354 orphan drug designations in 2015, 47 were for precision medicines. Thus, the proportion of orphan drug designations for precision medicines was the same (13%) each year. Figure 2 shows the total number of orphan drug designations based on orphan subsets by calendar year. In 2010, there were 195 orphan drug designations granted, with 17 (9%) being based on an orphan subset. The total number of orphan drug designations in 2015, which was 354, was higher than that of 2010, as expected by the increasing trend in orphan designation requests. Twenty‐eight (8%) of the orphan drug designations granted in 2015 were based on an orphan subset. The difference in the overall proportion of orphan drug designations based on orphan subsets by calendar year was not statistically significant.
Figure 1.
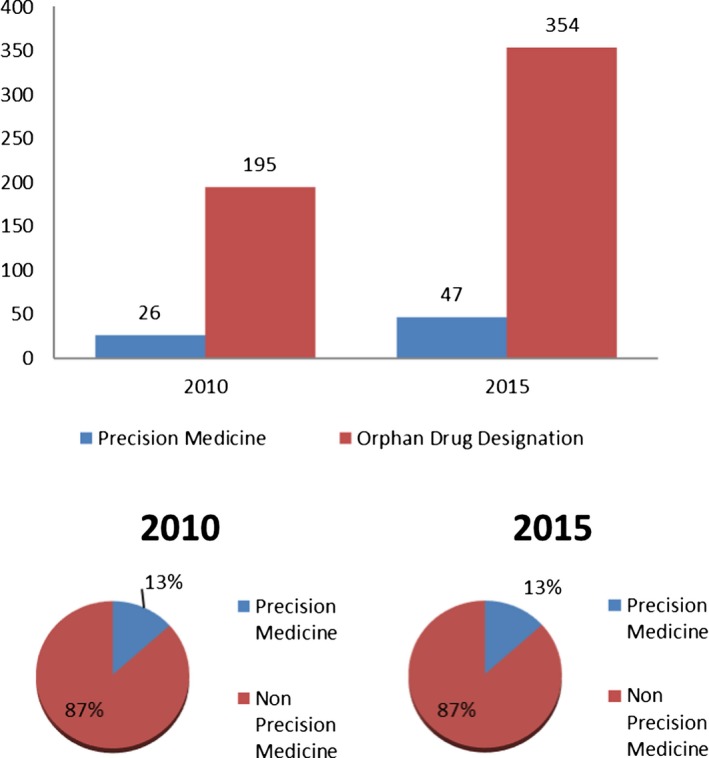
Orphan drug designations for a precision medicine, P value = 0.99.
Figure 2.
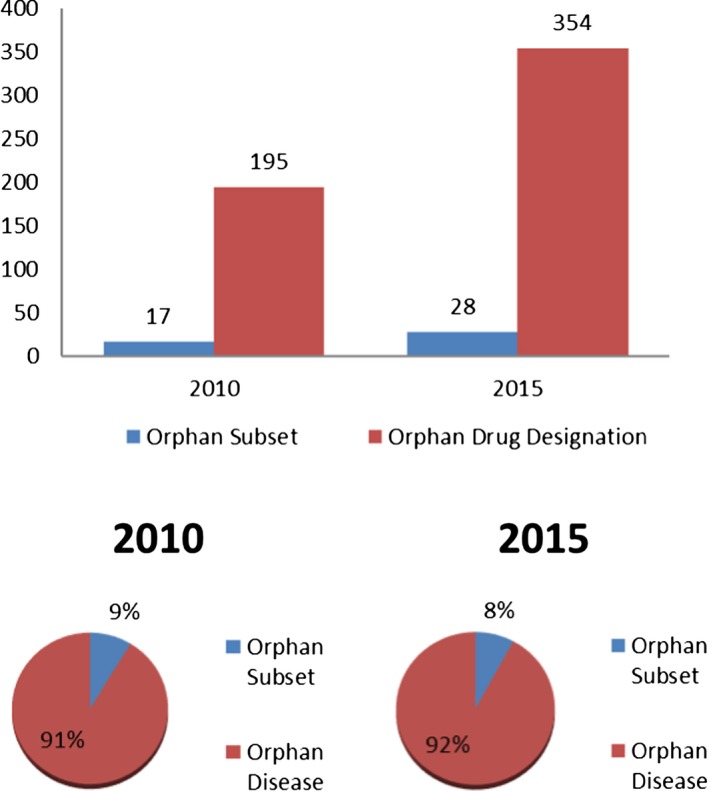
Orphan drug designations for an orphan subset, P value = 0.74.
In 2010, 6 of the 17 orphan subsets were based on a precision medicine, and in 2015, 8 of the 28 orphan subsets were based on a precision medicine, 35% and 29% (Figure 3). Note there was a decrease in the overall percentage in 2015 compared with 2010, although this was not statistically significant. Figure 4 shows the number of orphan subsets based on a precision medicine of all orphan drug designations by calendar year. In 2010, the 6 orphan subsets based on a precision medicine amounted to 3% of the total number of the 195 total orphan drug designations and, in 2015, the 8 orphan subsets based on a precision medicine amounted to 2% of the total number of the 354 total orphan drug designations. The decrease in the overall percentage between the 2 years was not statistically significant. Not shown, but of note as well, the percentage of orphan designations for precision medicines for orphan diseases that were not based on an orphan subset in 2010 and 2015 were 10% (20/195) and 11% (39/354), respectively.
Figure 3.
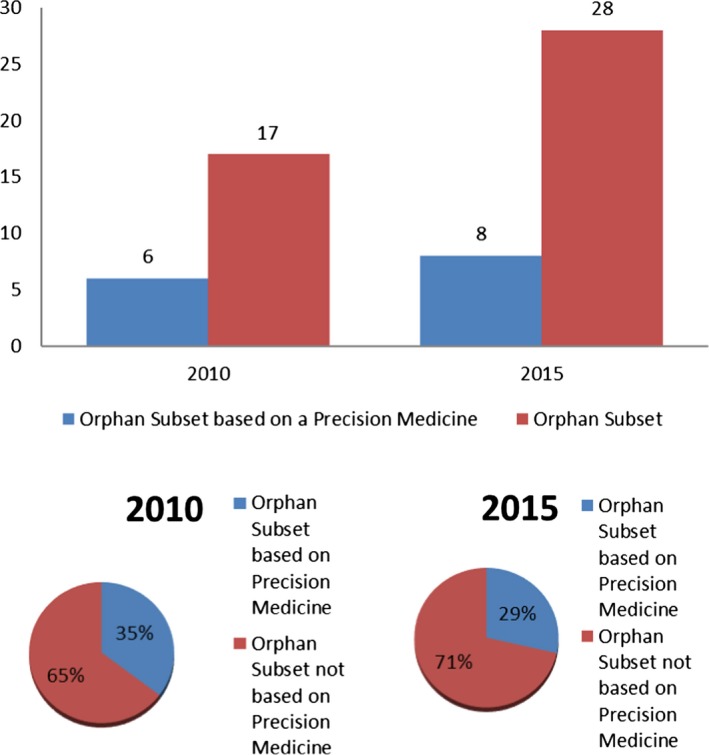
Orphan subsets based on a precision medicine, P value = 0.53.
Figure 4.
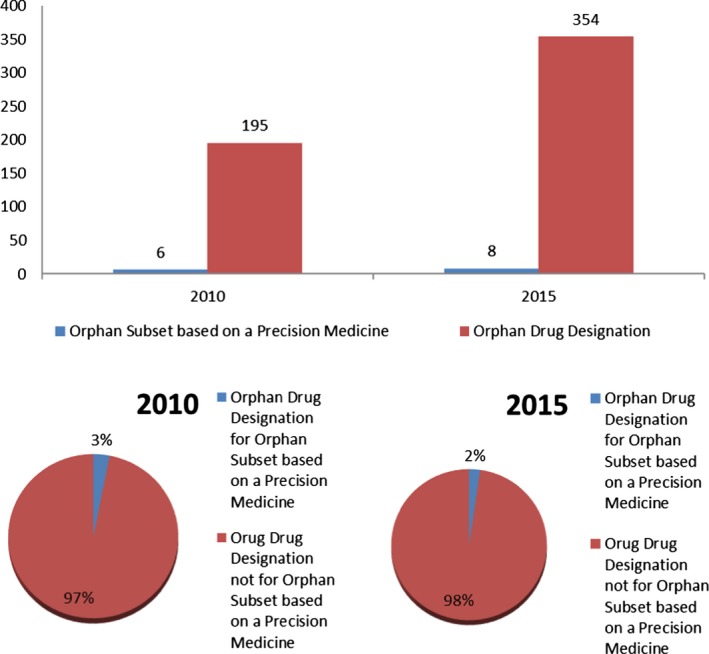
Orphan drug designations for orphan subsets based on a precision medicine, P value = 0.56.
When evaluating by therapeutic disease class, most orphan drug designations for precision medicines for both 2010 (14/26) and 2015 (41/47) were for oncology products (Figure 5). In addition, the percentage of orphan drug designations for precision medicines in oncology increased from 54% in 2010 to 87% in 2015 (Figure 5). Much smaller percentages were seen for ophthalmology, endocrinology, infectious diseases, neurology, and pulmonary diseases. The total number of orphan drug designations for oncology products also increased from 2010 to 2015, to 33% and 44%, respectively. The majority of orphan drug designations for precision medicines for both 2010 and 2015 were for somatic target types (Figure 6), increasing from 54% (14/26) in 2010 to 85% (40/47) in 2015. As shown in Figure 7, the majority of orphan drug designations for precision medicines for both 2010 and 2015 were for biologics, 58% (15/26) and 66% (31/47), respectively. The total number of orphan drug designations for biologics was not the majority for both calendar years, at just 43% (83/195) in 2010 and 39% (137/354) in 2015.
Figure 5.
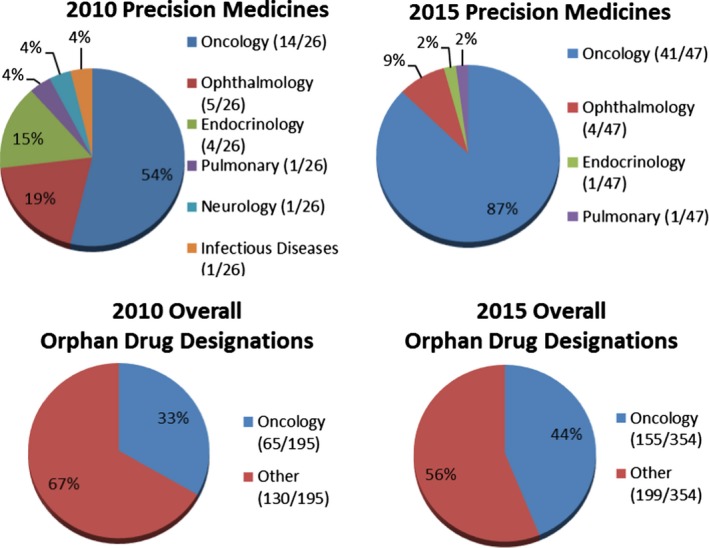
Orphan drug designations by therapeutic disease class.
Figure 6.
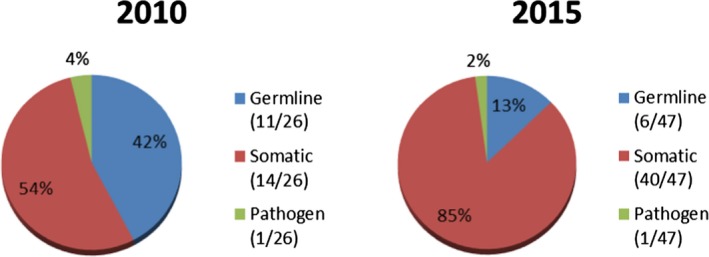
Precision medicines by target type.
Figure 7.
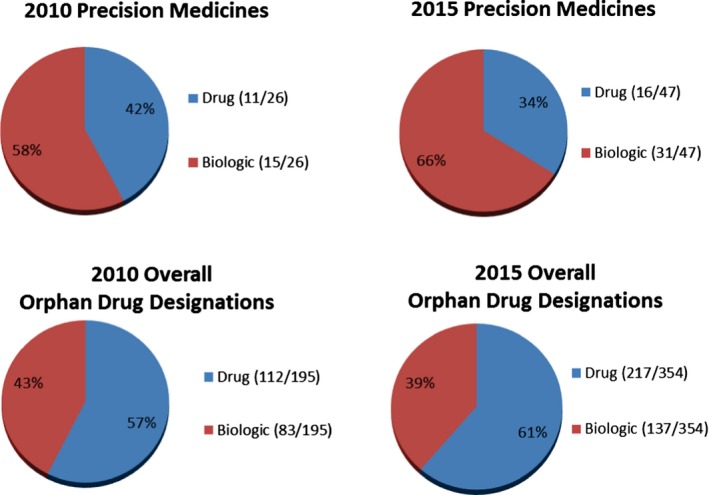
Orphan drug designations by product type.
Discussion
Our study provides the largest, most comprehensive descriptive analysis of orphan drug designation for precision medicines and orphan subsets. Although the total number of orphan drug designations was greater in 2015 than in 2010, which would be expected based on previously reported trends,12 the proportion of orphan drug designations for precision medicines overall is low based on our evaluation of those that were granted in 2010 and 2015 and was stable, at just 13% in both years. Notably, the proportion of orphan drug designations for precision medicines that were based on an orphan subset, the subsetting of an otherwise common disease into a rare disease, is even lower at <5% in both years, and also was stable in 2010 and 2015. The overall proportion of orphan drug designations based on orphan subsets in general was also low at <10% in both years. Additionally, the proportion of orphan subsets that were based on precision medicines, which was based in total on small numbers, was not the majority for either 2010 or 2015 and actually decreased in 2015.
We further categorized all orphan designated precision medicines by therapeutic disease class. In both years, oncology drugs comprised the majority of precision medicines, which increased by 30% from 2010 to 2015. The percentage of the total number of orphan drug designations for oncology drugs in both years also increased, but just by 10%, and did not account for the majority of orphan designated drugs in either year. In line with the majority of precision medicines being oncology drugs, the target type of the majority of precision medicines was somatic, which also increased by over 30% from 2010 to 2015. This is perhaps due to our better understanding to date of the molecular pathogenesis of cancer compared with that of the underlying heterogeneity of rare germline diseases.12, 13, 14
The current upward trend in designations may be the result of an overall increase in industry interest in the development of drugs for rare diseases due to advances in science and the incentives for rare disease product development.1, 2, 3, 11, 12 The number of approvals overall for orphan designated drugs increased from 15 in 2010 to 49 in 2015.15 An analysis of the characteristics of the orphan drugs approved by the Center of Drug Evaluation and Research as new molecular entities (NMEs) from 1983 to 2014 showed that these drugs were highly innovative and provided substantial gains in reducing unmet medical needs for patients with orphan diseases.13 Trends in the most recent 5 years of the study period, 2010−2014, showed increases in orphan drugs for the treatment of cancer, biologics for orphan diseases, more large companies developing orphan drugs, and orphan drugs occupying a larger proportion of the overall NME drug development pipeline. This also supports our results that orphan designations for innovative precision medicines, although small in number overall, were largely for oncology products and biologics and the proportion of each has increased over time.
It has been suggested that an increasing number of new drug approvals for precision medicines targeting rare subpopulations of otherwise common diseases may be impacting the use of incentives of the ODA.10, 11 In comparing this rise in new drug approvals for rare diseases to the steady rise in orphan drug designations, it has been postulated that precision medicines for common diseases have been driving orphan drug designations.9, 10, 11, 16, 17 However, the results of our study indicate that, although the number of orphan drug designations has increased, this increase is not due to the subsetting of common diseases into rare diseases nor by precision medicines.
Our analysis had several potential limitations. We limited our manual analysis to 2 calendar years. We chose 2010 as a baseline as there was a 1.3‐fold increase in orphan drug designations over the previous year and the first year the FDA received >300 orphan drug designation requests, perhaps indicative of an increasing trend toward precision drug development approaches. Also, 2010 was the year prior to a marked increase in the proportion of NMEs receiving marketing approval that had also received orphan drug designation as well as the number of NMEs for smaller, molecularly defined, pharmacologically relevant subsets, primarily in oncology.13, 18 We chose 2015 because we continued to see increases in both the requests for and the granting of orphan drug designations through 2015, and, at the time the analysis was initiated, it was our most recent year with complete data. We acknowledge that a noteworthy year for orphan drug designations based on precision medicines or perhaps a trend could have been seen by including the years between 2010 and 2015 as well as more recent years. However, we believe that based on the progression of scientific knowledge of molecular pathways as well as the steady increase in orphan drug designations and NME approvals for orphan drugs that remarkable increases compared with what we observed are unlikely. Second, at the time of the FDA's review of an orphan drug designation request, the determination of the disease or condition (or orphan subset) that the product is proposed to diagnose, prevent, or treat must be determined based on the available science and scientific consultation at that time. As such, we acknowledge that the scientific understanding and classification of diseases and subsets may evolve and be defined differently over time. Third, orphan drug designation is dependent on whether the request provides information to show that there is sufficient scientific rationale that the drug demonstrates “promise” to diagnose, prevent, or treat the disease or condition. This is best supported by human clinical data with the drug in the disease or condition but may be accepted with data from the drug in an appropriate animal model for the rare disease or condition. In the absence of human data and if no appropriate animal model exists, the application for orphan drug designation may be satisfactorily supported with compelling preclinical in vitro data and supportive information. We acknowledge the lack of human data may be a limitation and upon further clinical development the targeted nature of a drug toward a subpopulation of a disease may change.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
C.M.M., G.R., and K.N. wrote the manuscript. C.M.M. and G.R. designed the research. C.M.M. and G.R. performed the research. C.M.M., G.R., and K.N. analyzed data.
Disclaimer
The views expressed in this article are those of the authors and are not intended to represent the opinions of the US Food and Drug Administration. This manuscript was in preparation during the time that Gayatri Rao was Director of the Office of Orphan Products Development at the FDA.
Acknowledgments
The authors thank Nina Hunter, Debra Lewis, Michael Pacanowski, and Julienne Vaillancourt for their thoughtful comments on the manuscript.
Data Accessibility
The data sets generated and analyzed during the current study are not publicly available as they contain confidential commercial information. Limited information may be available from the corresponding author upon request but will be reviewed to protect proprietary information.
References
- 1. Institute of Medicine (IOM) . Rare diseases and orphan products: accelerating research and development. (The National Academies Press, Washington, DC, 2010). [PubMed] [Google Scholar]
- 2. Melnikova, I. Rare diseases and orphan drugs. Nat. Rev. Drug Discov. 11, 267–268 (2012). [DOI] [PubMed] [Google Scholar]
- 3. Haffner, M.E. History of orphan drug regulation—United States and beyond. Clin. Pharmacol. Ther. 100, 342 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Asbury, C.H. Orphan drugs medical versus market value. (D.C. Health and Company, Lexington, MA, 1985). [Google Scholar]
- 5. US Food and Drug Administration (FDA) . Orphan Drug Act, Public Law No. 97‐414. <https://www.fda.gov/downloads/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphanProductDesignation/UCM517741.pdf> (1983). Accessed May 29, 2019.
- 6. US Food and Drug Administration (FDA) . Code of Federal Regulations. Title 21 Food and Drugs. Chapter I Food and Drug Administration Dept of Health and Human Services. Supchapter D Drugs for Human Use. Part 316 Orphan Drugs. <http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr%26SID=51cf70689d51f0ea4147c0a8ac649321%26rgn=div5%26view=text%26node=21:5.0.1.1.6%26idno=21>. Accessed May 29, 2019.
- 7. Collins, F.S. & Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 372, 793–795 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamburg, M. A. Paving the Way for Personalized Medicine FDA's Role in a New Era of Medical Product Development. US Food and Drug Administration/FY 2012 Innovative Drug Approvals, October 2013. <https://www.fdanews.com/ext/resources/files/10/10-28-13-Personalized-Medicine.pdf>. Accessed May 29, 2019.
- 9. Woodcock, J. The future of orphan drug development. Clin. Pharmacol. Ther. 92, 146–148 (2012). [DOI] [PubMed] [Google Scholar]
- 10. Daniel, M.G. , Pawlik, T.M. , Fader, A.N. , Escanola, N.F. & Makary, M.A. The orphan drug act restoring the mission to rare diseases. Am. J. Clin. Oncol. 39, 210–213 (2015). [DOI] [PubMed] [Google Scholar]
- 11. Kesselheim, A.S. , Treasure, C.L. & Joffe, S. Biomarker‐defined subsets of common diseases: policy and economic implications of orphan drug act coverage. PLoS Med. 14, e1002190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bashaw, E.D. A clinical pharmacology‐regulatory perspective on the approval of drugs for rare diseases. Clin. Pharmacol. Ther. 100, 327–329 (2016). [DOI] [PubMed] [Google Scholar]
- 13. Miller, K.L. & Lanthier, M. Trends in orphan new molecular entities, 1983‐2014: half were first in class, and rare cancers were the most frequent target. Health Aff. 35, 464–470 (2016). [DOI] [PubMed] [Google Scholar]
- 14. Swinney, D.C. Challenges and hurdles to business as usual in drug development for treatment of rare diseases. Clin. Pharmacol. Ther. 100, 339–341 (2016). [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration (FDA) . Orphan Drug Designations and Approvals. <https://www.accessdata.fda.gov/scripts/opdlisting/oopd/>. Accessed May 29, 2019.
- 16. Herder, M. What is the purpose of the orphan drug act? PLoS Med. 14, e1002191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pregelj, L. et al Precision medicines have faster approvals based on fewer and smaller trials than other medicines. Health Aff. 37, 724–731 (2018). [DOI] [PubMed] [Google Scholar]
- 18. Roscoe, D.M. , Hu, Y. & Philip, R. Companion diagnostics: a regulatory perspective from the last 5 years of molecular companion diagnostic approvals. Expert Rev. Mol. Diagn. 15, 869–880 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during the current study are not publicly available as they contain confidential commercial information. Limited information may be available from the corresponding author upon request but will be reviewed to protect proprietary information.


