Abstract
Background:
The MenAfriNet Consortium supports strategic implementation of case-based meningitis surveillance in key high-risk countries of the African meningitis belt: Burkina Faso, Chad, Mali, Niger, and Togo. We describe bacterial meningitis epidemiology in these 5 countries in 2015–2017.
Methods:
Case-based meningitis surveillance collects case-level demographic and clinical information and cerebrospinal fluid (CSF) laboratory results. Neisseria meningitidis (Nm), Streptococcus pneumoniae (Sp), or Haemophilus influenzae (Hi) cases were confirmed and Nm/Hi were serogrouped/serotyped by real-time PCR, culture, or latex agglutination. We calculated annual incidence in participating districts in each country in cases/100,000 population.
Results:
From 2015–2017, 18,262 suspected meningitis cases were reported; 92% had a CSF specimen available, of which 26% were confirmed as Nm (n=2,433; 56%), Sp (n=1,758; 40%), or Hi (n=180; 4%). Average annual incidences for Nm, Sp, and Hi, respectively, were 7.5, 2.5, and 0.3. Nm incidence was 1.5 in Burkina Faso, 2.7 in Chad, 0.4 in Mali, 14.7 in Niger, and 12.5 in Togo. Several outbreaks occurred: NmC in Niger in 2015–2017, NmC in Mali in 2016, and NmW in Togo in 2016–2017. Of Nm cases, 53% were NmC, 30% NmW, and 13% NmX. Five NmA cases were reported (Burkina Faso, 2015). NmX increased from 0.6% of Nm cases in 2015 to 27% in 2017.
Conclusions:
Though bacterial meningitis epidemiology varied widely by country, NmC and NmW caused several outbreaks, NmX increased though was not associated with outbreaks, and overall NmA incidence remained low. An effective low-cost multivalent meningococcal conjugate vaccine could help further control meningococcal meningitis in the region.
Introduction
For over 100 years, the meningitis belt of sub-Saharan Africa—stretching from Senegal to Ethiopia and including over 400 million people in 26 countries—experienced high endemic rates of meningitis, annual seasonal outbreaks, and explosive epidemics occurring every 5–10 years [1]. Prior to the introduction of the meningococcal serogroup A conjugate vaccine (MACV, MenAfriVac®) in 2010, approximately 90% of all meningitis cases during epidemics in the region were attributable to Neisseria meningitidis serogroup A (NmA) [2]. From 2010–2018, MACV was progressively rolled out using mass vaccination campaigns in 22 of the 26 meningitis belt countries [3]. As a result, a dramatic reduction (≥99%) in both NmA disease and asymptomatic nasopharyngeal carriage was observed [4, 5]. To sustain this reduction and protect more recent birth cohorts, beginning in 2016, MACV has been introduced into the routine childhood immunization programs in 8 countries to date, accompanied by catch-up campaigns for older children [3].
MenAfriNet is an international consortium led and implemented by African Ministries of Health, Agence de Médecine Préventive, the U.S. Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO), with support and collaboration from other international and non-governmental organizations [6]. MenAfriNet was established in 2014 to support strategic implementation of case-based meningitis surveillance in 5 key high-risk countries of the African meningitis belt: Burkina Faso, Chad, Mali, Niger, and Togo. The primary aims of MenAfriNet were to (1) develop a high-quality, sustainable, case-based meningitis surveillance network using standardized tools, (2) assess changes in meningitis epidemiology and vaccine impact, and (3) inform vaccine policy decisions and new vaccine development.
By 2015 all 5 MenAfriNet countries had completed MACV mass vaccination campaigns targeting persons aged 1–29 years, and all but Togo introduced MACV into the routine immunization program for children aged 9–18 months (Supplemental Table 1) [3]. In addition, all 5 countries introduced Haemophilus influenzae serotype b (Hib) conjugate vaccine into the routine immunization program during 2005–2008, and 4 out of 5 countries introduced pneumococcal conjugate vaccine (PCV) for infants in 2011–2014.
In this context, we aimed to describe the epidemiology of bacterial meningitis in the 5 key high-risk MenAfriNet countries using the first 3 years (2015–2017) of data collected via strengthened case-based meningitis surveillance.
Methods
MenAfriNet countries
Burkina Faso, Niger, and Togo began MenAfriNet-supported data collection in January 2015; Mali began in May 2015 and Chad began in April 2016. All districts in Burkina Faso and selected districts in the other 4 countries were included in MenAfriNet [6]. By 2017, 115 (33%) of 347 districts in these countries were included in MenAfriNet and ~32.7 million people were under case-based meningitis surveillance.
Case-based meningitis surveillance
In each of the 5 countries, case-level demographic and clinical information, as well as cerebrospinal fluid (CSF) specimens, were collected from all suspected meningitis cases in MenAfriNet districts using WHO and MenAfriNet guidelines and surveillance tools [7–9]. A paper case notification form was completed at the health facility for each suspected case, and district surveillance officers entered the data into a surveillance database. If a CSF specimen was collected, a copy of the case notification form accompanied the specimen sequentially to district, regional, and national reference laboratories; though in some settings, some intermediate laboratory levels were skipped. At each level, laboratory results were entered into a laboratory database. The surveillance office merged the surveillance database with the laboratory database to generate a master database, which included cases with and without CSF specimens and laboratory results. This master database was sent to the data management team at WHO-AFRO, and the databases from each country were merged into a central MenAfriNet database by the MenAfriNet data management team. Each country that participated in the MenAfriNet Consortium made substantial efforts to maintain high-quality cased-based surveillance and aimed to have a CSF specimen collected and tested for ≥80% of suspected cases. Key performance indicators and data completeness and quality are described elsewhere [10].
Case definitions
According to WHO case definitions for enhanced meningitis surveillance in the meningitis belt [11], a suspected meningitis case was defined as a sudden onset of fever ≥38.5°C with neck stiffness, altered consciousness, or other meningeal signs (including flaccid neck, bulging fontanel, or convulsions in young children). Probable bacterial meningitis was a suspected case with turbid, cloudy, purulent, or xanthochromic CSF; presence of gram-negative diplococci, gram-positive diplococci, or gram-negative bacilli on microscopic examination of CSF; or a CSF white cell count >10/mm3. A confirmed case of meningitis was a suspected or probable case with N. meningitidis (Nm), Streptococcus pneumoniae (Sp), or H. influenzae (Hi) isolated from CSF by culture or detected in CSF by real-time polymerase chain reaction (rt-PCR) or latex agglutination [12]. Cases did not have to meet the probable case definition in order to be confirmed.
Laboratory methods
CSF specimens were transported from local healthcare facilities to district laboratories, which conducted preliminary lab testing such as cytology, Gram staining, or latex agglutination, depending on laboratory capacity. CSF specimens were then sent to a national reference laboratory in both trans-isolate medium at room temperature for culture and a cryotube via a cold chain for rt-PCR targeting the sodC gene for Nm, lytA for Sp, and hpd for Hi [13, 14]. All national reference laboratories in MenAfriNet countries were trained on rt-PCR using standard assays [15]. In Chad, all laboratory testing was done at the national level. In the case of discrepant results for case confirmation, methods were considered confirmatory in the following order: rt-PCR, culture, latex agglutination. Six meningococcal serogroups (A, B, C, W, X, or Y) and Hi serotypes (b or non-b) were determined using latex agglutination or rt-PCR, with rt-PCR considered definitive in cases of discrepancy [14]. Any meningococcal specimens that were not identified as one of the six assessed serogroups by rt-PCR or latex agglutination were considered indeterminate (NmInd), a category which includes both non-ABCWXY serogroups and nongroupable (unencapsulated) strains. Pneumococcal serotyping data from Burkina Faso are presented elsewhere [16].
Statistical methods
We analyzed data from meningitis cases reported in MenAfriNet districts from January 1, 2015 to December 31, 2017. For the purposes of this analysis, a district was included as a MenAfriNet district starting January 1 of the first full calendar year of surveillance implementation; districts that implemented case-based surveillance mid-year were not included until the beginning of the next calendar year. Annual incidences (cases per 100,000 persons) were calculated for MenAfriNet-participating districts in each country using projected age-stratified population census estimates provided by each country. For suspected and probable cases, crude incidences were calculated. For laboratory-confirmed cases, annual incidence was adjusted for the age-stratified proportion of cases with a CSF specimen tested at a national laboratory, where culture and rt-PCR were performed. Within each age stratum (<1 year, 1–4 years, 5–9 years, 10–14 years, 15–29 years and ≥30 years), the number of cases confirmed by culture or rt-PCR at a national laboratory for a specific pathogen was divided by the number of cases with CSF tested via culture or rt-PCR at a national laboratory. This confirmation proportion was then applied to the number of suspected meningitis cases within that age stratum for which no diagnostic testing was performed; this number was then added to confirmed cases to calculate the adjusted incidence. For MenAfriNet-wide incidences, adjusted annual incidences from participating districts in each country for each year of their participation in MenAfriNet were averaged. CFRs were calculated by dividing the number of reported deaths by the total number of cases with a reported outcome.
Data were analyzed using SAS v9.4. Analysis of data collected through routine MenAfriNet surveillance was determined by the CDC’s Human Research Protection Office to be public health non-research, and Institutional Review Board review was not required by any participating institution.
Results
Suspected and probable meningitis cases
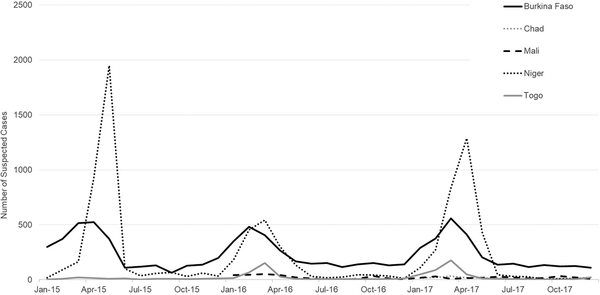
From 2015–2017, 18,262 suspected meningitis cases were reported in MenAfriNet districts: 8,362 in Burkina Faso, 166 in Chad (2017 only), 495 in Mali (2016–2017 only), 8,413 in Niger, and 826 in Togo (Fig 1, Table 1); of 12,920 (71%) with a reported outcome, 1,095 (8%) were fatal. The majority of suspected meningitis cases (92%) had a CSF specimen available for analysis. Seventy-nine percent of specimens were tested at either district, regional, or national reference laboratories by at least one confirmatory test: 15% by latex, 19% by culture, and 73% by rt-PCR.
Fig 1. Epidemic curve of suspected meningitis cases reported per month to case-based meningitis surveillance in MenAfriNet districts, by country, 2015–2017.
Note: As Mali began data collection in 2015 and 2016 was the first complete year of surveillance, only 2016–2017 data are included. Similarly, only 2017 data from Chad are included.
Table 1.
Suspected and probable meningitis cases from case-based meningitis surveillance, MenAfriNet, 2015–2017
| Burkina Faso | Chada | Malia | Niger | Togo | Total | |
|---|---|---|---|---|---|---|
| N (%) | ||||||
| Suspected meningitis cases | 8,362 | 166 | 495 | 8,413 | 826 | 18,262 |
| Age groupb | ||||||
| <1 years | 1,427 (17) | 46 (31) | 142 (29) | 633 (8) | 116 (14) | 2,364 (13) |
| 1–4 years | 2,783 (33) | 41 (28) | 164 (34) | 1,606 (20) | 104 (13) | 4,698 (26) |
| 5–9 years | 1,539 (18) | 23 (16) | 64 (13) | 1,619 (20) | 135 (16) | 3,380 (19) |
| 10–14 years | 1,059 (13) | 11 (7) | 52 (11) | 1,561 (19) | 132 (16) | 2,815 (16) |
| 15–29 years | 883 (11) | 12 (8) | 39 (8) | 1,910 (24) | 152 (18) | 2,996 (17) |
| ≥30 years | 652 (8) | 15 (10) | 26 (5) | 785 (10) | 184 (22) | 1,662 (9) |
| Sexc | ||||||
| Female | 3,753 (45) | 69 (43) | 215 (43) | 3,731 (45) | 399 (48) | 8,167 (45) |
| Male | 4,605 (55) | 91 (57) | 280 (57) | 4,625 (55) | 424 (52) | 10,025 (55) |
| Reported deathsd | 772 (9) | NR | 23 (5) | 269 (8) | 31 (4) | 1,095 (8) |
| Probable bacterial meningitis casese | 3,598 (44) | 71 (43) | 202 (41) | 1,945 (27) | 374 (47) | 6,190 (37) |
| Population under surveillance (2017) | 19,632,147 | 811,414 | 4,027,829 | 7,347,840 | 963,411 | 32,782,641 |
Abbreviations: NR, not reported.
As Mali began data collection in 2015 and 2016 was the first complete year of surveillance, only 2016–2017 data are included. Similarly, only 2017 data from Chad are included.
347 cases are missing age: 19 in Burkina Faso, 18 in Chad, 8 in Mali, 299 in Niger, and 3 in Togo.
70 cases are missing sex: 4 in Burkina Faso, 6 in Chad, 57 in Niger, and 3 in Togo.
Percentage calculated using the number of cases with a reported outcome (n=12,920, 71%) as the denominator.
Percentage calculated using the number of suspected cases with a CSF specimen (n=16,885, 92%) as the denominator.
An eighth (13%, n=2,364) of suspected meningitis cases detected by this case-based surveillance system occurred among children aged <1 year and 39% (n=7,062) occurred in children aged <5 years (Table 1). Thirty-seven percent (n=6,190) of all suspected cases met the probable case definition. The annual average incidences of suspected and probable meningitis were 31.3 and 11.2 cases per 100,000, respectively (Table 3).
Table 3.
Average annual incidence (cases per 100,000 persons) of suspected, probable and laboratory-confirmed meningitis, MenAfriNet, 2015–2017
| Burkina Faso | Chada | Malia | Niger | Togo | Average annual incidenceb | |
|---|---|---|---|---|---|---|
| Suspected meningitis casesc | 14.7 | 20.5 | 6.3 | 52.5 | 47.2 | 31.3 |
| Probable meningitis casesc | 6.3 | 8.8 | 2.6 | 11.9 | 22.1 | 11.2 |
| Laboratory-confirmed meningitis casesd | 5.0 | 7.9 | 1.6 | 16.7 | 15.9 | 10.3 |
| N. meningitidis | 1.5 | 2.7 | 0.4 | 14.7 | 12.5 | 7.5 |
| Serogroup | ||||||
| A | 0.01 | 0 | 0 | 0 | 0 | 0.003e |
| C | 0.1 | 0 | 0.3 | 12.1f | 0 | 3.1 |
| W | 1.1 | 0.2 | 0.03 | 1.4 | 8.9 | 2.9 |
| X | 0.2 | 2.5 | 0.1 | 0.9 | 2.8 | 1.2 |
| Y | 0.01 | 0 | 0.03 | 0 | 0 | 0.01 |
| NmInd | 0.1 | 0 | 0.01 | 0.3 | 0.8 | 0.3 |
| S. pneumoniae | 3.2 | 4.1 | 0.8 | 1.8 | 3.2 | 2.5 |
| H. influenzae | 0.2 | 1.1 | 0.4 | 0.2 | 0.1 | 0.3 |
Abbreviation: NmInd, Neisseria meningitidis with indeterminate serogroup.
Note: Calculated incidences are only for districts participating in MenAfriNet in each country. Incidences by country, year, and pathogen are shown in the Supplemental Figure.
As Mali began data collection in 2015 and 2016 was the first complete year of surveillance, only 2016–2017 data are included. Similarly, only 2017 data from Chad are included.
MenAfriNet-wide average annual incidences were calculated by averaging the adjusted annual incidences from participating districts in each country for each year of their participation in MenAfriNet.
Crude incidence.
Confirmed via latex agglutination, culture, or real-time polymerase chain reaction as N. meningitidis, S. pneumoniae, or H. influenzae. Incidence adjusted for age and the proportion of cases with cerebrospinal fluid tested at a national reference laboratory. An incidence of 0 indicates no confirmed cases with that specific pathogen in that country. It is possible for the crude incidence of probable meningitis to be lower than the adjusted incidence of laboratory-confirmed meningitis, which was adjusted to account for cases with no confirmatory laboratory results available.
Only 5 NmA cases reported in Burkina Faso in the 2015 meningitis season.
Average annual NmC incidence in Niger is driven by the 2015 NmC epidemic: 2015 NmC incidence: 21.5, as compared to 4.1 in 2016 and 10.8 in 2017.
Laboratory-confirmed meningitis
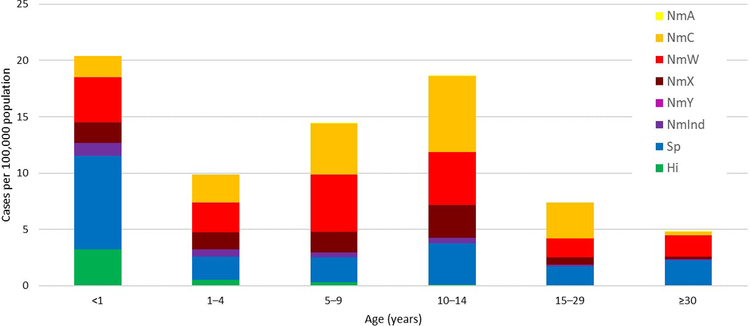
A quarter (n=4,371, 26%) of suspected cases that had a CSF specimen obtained were laboratory-confirmed via latex, culture, or rt-PCR as either Nm (56%; n=2,433), Sp (40%; n=1,758), or Hi (4%; n=180) (Table 2). The CFR for Sp was 17%, compared to 6% for Nm and 9% for Hi. Median age was 9 years among persons with Nm meningitis (interquartile range [IQR]: 5–14 years), 10 years (IQR: 5–17) for Sp, and 1 year (IQR: 0–5) for Hi. Overall, average annual incidences for Nm, Sp, and Hi, respectively, were 7.5, 2.5, and 0.3 per 100,000 (Table 3). The highest incidence of laboratory-confirmed meningitis was among children aged <1 year (20.4), with the lowest incidence observed among persons aged ≥30 years (4.8) (Fig 3). The epidemiology of laboratory-confirmed meningitis varied widely by country and by year (Supplemental Figure).
Table 2.
Laboratory-confirmed meningitis cases from case-based meningitis surveillance, MenAfriNet, 2015–2017
| Burkina Faso | Chada | Malia | Niger | Togo | Total | |
|---|---|---|---|---|---|---|
| N (%) | ||||||
| Laboratory-confirmed meningitis casesb | 2,224 (27) | 64 (39) | 118 (24) | 1,701 (24) | 264 (33) | 4,371 (26) |
| N. meningitidis | 671 (30) | 22 (34) | 32 (27) | 1,494 (88) | 214 (81) | 2,433 (56) |
| Serogroup | ||||||
| A | 5 (1) | 0 | 0 | 0 | 0 | 5 (0.2) |
| C | 40 (6) | 0 | 19 (59) | 1,236 (83) | 0 | 1,295 (53) |
| W | 478 (71) | 2 (9) | 2 (6) | 109 (7) | 128 (60) | 719 (30) |
| X | 91 (14) | 20 (91) | 8 (25) | 127 (9) | 69 (32) | 315 (13) |
| Y | 4 (1) | 0 | 2 (6) | 0 | 0 | 6 (0.2) |
| NmIndc | 53 (8) | 0 | 1 (3) | 22 (1) | 17 (8) | 93 (4) |
| Reported deathsd | 47 (7) | NR | 1 (3) | 59 (6) | 6 (3) | 113 (6) |
| S. pneumoniae | 1,442 (65) | 33 (52) | 58 (49) | 177 (10) | 48 (18) | 1,758 (40) |
| Reported deathsd | 245 (17) | NR | 6 (10) | 14 (16) | 7 (22) | 272 (17) |
| H. influenzae | 111 (5) | 9 (14) | 28 (24) | 30 (2) | 2 (1) | 180 (4) |
| Serotype | ||||||
| Serotype b | 82 (74) | 5 (56) | 18 (64) | 13 (43) | 2 (100) | 120 (67) |
| Non-b serotypes | 29 (26) | 4 (44) | 10 (36) | 17 (57) | 0 | 60 (33) |
| Reported deathsd | 12 (11) | NR | 1 (4) | 1 (7) | 0 | 14 (9) |
Abbreviations: NmInd, Neisseria meningitidis with indeterminate serogroup; NR, not reported.
Note: Case counts stratified by country, pathogen, age group, and year are presented in Supplemental Table 2.
As Mali began data collection in 2015 and 2016 was the first complete year of surveillance, only 2016–2017 data are included. Similarly, only 2017 data from Chad are included.
Confirmed via latex agglutination, culture, or real-time polymerase chain reaction as N. meningitidis, S. pneumoniae, or H. influenzae. Percentage calculated using the number of suspected cases with a CSF specimen (n=16,885, 92%) as the denominator.
Of the 93 NmInd cases, 34 (37%) were determined to be NmInd via real-time polymerase chain reaction, 5 (5%) were determined to be NmInd via culture, and 54 (58%) were determined to be NmInd as they only had latex agglutination results available, which were NmW/Y, precluding identification of a specific serogroup.
Percentage calculated using the number of cases with a reported outcome as the denominator.
Fig 3. Average adjusted annual incidence of laboratory-confirmed meningitis by pathogen and age group, MenAfriNet, 2015–2017.
Abbreviations: Hi, H. influenzae; NmA, N. meningitidis serogroup A; NmC, N. meningitidis serogroup C; NmInd, N. meningitidis with indeterminate serogroup; NmW, N. meningitidis serogroup W; NmX, N. meningitidis serogroup X; NmY, N. meningitidis serogroup Y; Sp, S. pneumoniae.
Meningococcal meningitis
Though one-quarter (23%) of meningococcal meningitis cases occurred among children aged <5 years, the highest incidence was among adolescents aged 10–14 years (14.9) (Table 4). Average annual Nm incidence varied by country: 14.7 in Niger, 12.5 in Togo, 2.7 in Chad, 1.5 in Burkina Faso, and 0.4 in Mali (Table 3).
Table 4.
Average annual incidence (cases per 100,000 persons) of laboratory-confirmed meningitis by pathogen and age group, MenAfriNet, 2015–2017
| Burkina Faso | Chada | Malia | Niger | Togo | Average annual incidenceb | |
|---|---|---|---|---|---|---|
| N. meningitidis | ||||||
| <1 year | 2.3 | 3.4 | 1.4 | 10.8 | 20.1 | 8.8 |
| 1–4 years | 2.6 | 3.9 | 0.7 | 14.2 | 10.8 | 7.3 |
| 5–9 years | 2.9 | 3.6 | 0.2 | 21.4 | 21.8 | 11.8 |
| 10–14 years | 2.4 | 3.6 | 1.2 | 31.2 | 24.0 | 14.9 |
| 15–29 years | 0.6 | 1.9 | 0.3 | 13.6 | 7.5 | 5.6 |
| ≥30 years | 0.3 | 0 | 0.1 | 1.5 | 8.4 | 2.6 |
| S. pneumoniae | ||||||
| <1 year | 8.4 | 27.4 | 4.4 | 8.5 | 4.3 | 8.3 |
| 1–4 years | 2.7 | 6.3 | 1.3 | 1.5 | 0.9 | 2.0 |
| 5–9 years | 4.8 | 2.9 | 0.6 | 0.7 | 2.1 | 2.2 |
| 10–14 years | 5.6 | 2.7 | 0.8 | 2.5 | 5.3 | 3.7 |
| 15–29 years | 2.3 | 0.5 | 0.7 | 1.6 | 2.3 | 1.7 |
| ≥30 years | 1.5 | 2.5 | 0.1 | 1.2 | 5.1 | 2.2 |
| H. influenzae | ||||||
| <1 year | 2.9 | 13.7 | 5.6 | 1.8 | 0 | 3.2 |
| 1–4 years | 0.5 | 0.8 | 0.6 | 0.2 | 0.9 | 0.6 |
| ≥5 years | 0.1 | 0.5 | 0.1 | 0.2 | 0 | 0.1 |
Note: Cases were confirmed via latex agglutination, culture, or real-time polymerase chain reaction as N. meningitidis, S. pneumoniae, or H. influenzae. Calculated incidences are only for districts participating in MenAfriNet in each country. Incidence adjusted for age and the proportion of cases with cerebrospinal fluid tested at a national reference laboratory. An incidence of 0 indicates no confirmed cases with that specific pathogen in MenAfriNet districts in that country.
As Mali began data collection in 2015 and 2016 was the first complete year of surveillance, only 2016–2017 data are included. Similarly, only 2017 data from Chad are included.
MenAfriNet-wide average annual incidences were calculated by averaging the adjusted annual incidences from participating districts in each country for each year of their participation in MenAfriNet.
Of the 2,433 reported meningococcal meningitis cases, 1,295 (53%) were NmC, 719 (30%) NmW, 315 (13%) NmX, 6 (0.2%) NmY, and 93 (4%) NmInd (Table 2). Five confirmed cases of NmA were reported, all in Burkina Faso during the 2014–2015 meningitis season, as previously described [17]; one of these cases was the first documented MACV vaccine failure. CFR did not significantly vary by serogroup: 6% for NmC, 6% for NmW, and 4% for NmX (P=0.2).
Seven notable meningococcal meningitis outbreaks occurred during this time period [18]: NmC in Niger in 2015–2017 (>9,000 suspected cases in 3 outbreaks) [19], NmC in Mali in 2016 (39 suspected cases) [20], and NmW in Togo in 2016–2017 (>1,800 suspected cases in 3 outbreaks) [21]. NmW also predominated in Burkina Faso, and Chad detected mainly NmX. NmC and NmW accounted for the highest average annual incidences among meningococcal serogroups, 3.1 and 2.9 cases per 100,000, respectively (Table 3). NmX increased from 0.6% of Nm cases in 2015 to become the predominant meningococcal serogroup in 2017 (27% of Nm cases). Despite this increase and the wide distribution of NmX cases, NmX was not associated with any outbreaks during this time period. Only 6 serogroup Y cases were reported in MenAfriNet districts: 4 in Burkina Faso in 2015 and 2 in Mali in 2017.
Pneumococcal meningitis
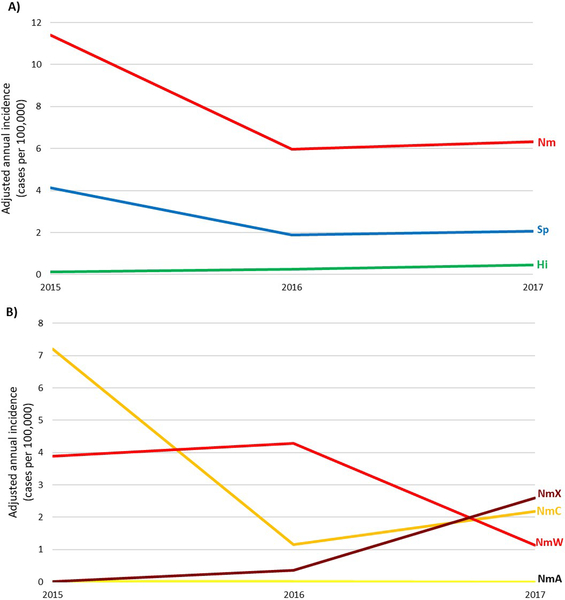
From 2015–2017, 1,758 meningitis cases were laboratory-confirmed as Sp, with a CFR of 17% (Table 2). Incidence was highest among children aged <1 year (8.3 cases per 100,000), and was 1.7–3.7 in older age groups (Table 4). Sp was the predominant bacterial meningitis pathogen in Burkina Faso, Chad, and Mali (Table 3), causing 64% of laboratory-confirmed meningitis in those 3 countries. Overall, the incidence of pneumococcal meningitis in MenAfriNet districts declined from 4.1 per 100,000 in 2015 to 2.1 in 2017 (Fig 2), though incidence remained somewhat steady in children aged <5 years (3.7 in 2015, 2.4 in 2016, 3.9 in 2017).
Fig 2. Adjusted annual incidence of bacterial meningitis by pathogen, MenAfriNet, 2015–2017.
A) 3 main bacterial meningitis pathogens: N. meningitidis (Nm), S. pneumoniae (Sp), and H. influenzae (Hi). B) Meningococcal serogroups: serogroup X (NmX), serogroup C (NmC), serogroup W (NmW), and serogroup A (NmA). Note: As Mali began data collection in 2015 and 2016 was the first complete year of surveillance, only 2016–2017 data are included. Similarly, only 2017 data from Chad are included.
H. influenzae meningitis
A total of 180 Hi cases were reported in MenAfriNet districts (average annual incidence: 0.3 cases per 100,000) (Tables 2 & 3). Incidence was highest in Chad (1.1) and lowest in Togo (0.1). Though there was a low reported incidence of Hi, it increased from 0.1 in 2015 to 0.5 in 2017 (Fig 2). The highest incidence was among infants aged <1 year (3.2). Two-thirds (67%) of Hi cases were reported to be Hib: 71% occurred among children aged <5 years, and 11% were fatal; the remaining Hi cases were reported as non-b.
Discussion
This epidemiologic analysis showed that NmA incidence remains very low following MACV introduction, demonstrating continued positive vaccine impact in the eight years since introduction [17]. Despite the dramatic reduction in NmA disease following MACV introduction, bacterial meningitis epidemiology in this region continues to evolve and can vary widely by country and over time in the same country. Meningococcal meningitis due to non-A serogroups remains an endemic and epidemic threat. The overall epidemiology of meningococcal meningitis in MenAfriNet districts was driven primarily by several large outbreaks that occurred in this region during 2015–2017 [18]: NmC in Niger and Nigeria in 2015–2017 following the emergence of a new strain (ST-10217) [20, 22–24], and NmW in Togo in 2016–2017 [21]. Reactive vaccination to these outbreaks may also have influenced local bacterial meningitis epidemiology in following years. Several countries reported increases in NmX, which became the predominant serogroup in 2017, though no NmX outbreaks were observed. The increased reporting of NmX may be partially attributed to a shift in case confirmation over time from latex agglutination to rt-PCR, as the commercially-available latex agglutination test (Pastorex™) does not detect NmX whereas rt-PCR can confirm NmX cases. Regardless, this increase in reported NmX is concerning as there is no currently licensed vaccine against NmX. An effective and affordable multivalent meningococcal conjugate vaccine targeting serogroups A, C, W, X, and Y could help further control meningococcal disease and epidemics in the region [25].
It is difficult to directly compare these presented incidences with incidences reported in this region prior to MACV introduction, due to differences such as laboratory methods used, sentinel-site vs. population-based surveillance, aggregate vs. case-based surveillance, annual incidence vs. incidence during outbreaks or peak weeks/months, and crude vs. adjusted incidences. However, some comparisons are possible and illustrative. For example, nearly all of these 2015–2017 incidences are lower than reported pre-MACV incidences in MenAfriNet countries. The incidence of suspected meningitis in Burkina Faso was 61 cases per 100,000 in 1997–2010 [2], compared to 14.7 in 2015–2017, and similarly 73 in Niamey, Niger in 2006 [26] vs. 52.5 in Niger in 2015–2017. Pre-MACV NmA incidence was 30.5 in Niamey in 1997 [27], 110 in western Burkina Faso in 2006 [28], 0.3 in Niamey in 2006 [26], and 70 in Tone, Togo in 2007 [28], compared to 0.01 in Burkina Faso and 0 in the other MenAfriNet countries in 2015–2017. In this analysis, the highest NmX incidence was in Togo in 2017 (7.7), though this was lower than previously published NmX incidences in Niamey in 1997 (15.1) [27] and 2006 (27.5) [26], and in Kozah, Togo in 2007 (33) [28]. However, pneumococcal meningitis incidence in Burkina Faso in 2015–2017 (32) was twice that reported in 3 districts in 2002–2005 (14) [29], and in Chad, suspected meningitis incidence in 2017 (20.5) was in between that reported in districts vaccinated (2.48) and unvaccinated (43.8) with MACV in 2012 [30].
The experience of the five key high-risk MenAfriNet countries in conducting high-quality meningitis surveillance demonstrates that long-term investment in case-based surveillance is a valuable platform both for monitoring evolving meningitis epidemiology and for evaluating the effectiveness of bacterial meningitis vaccines, especially MACV integration into routine immunization programs, pneumococcal conjugate vaccines, Hib conjugate vaccines, and a potential future multivalent meningococcal conjugate vaccine [25]. This network collects high-quality, case-based meningitis data in a region where the collection of high-quality surveillance data is a challenge, but is extremely important due to the high disease burden. These case-based data are a critical supplement to the conventional aggregate surveillance reporting via the Integrated Disease Surveillance and Response framework [8], as well as to enhanced meningitis surveillance being conducted elsewhere in the meningitis belt [31]. Therefore, maintenance of case-based surveillance in key high-performing meningitis belt countries beyond the completion of the MenAfriNet project will help ensure continued availability of high-quality data to inform vaccine policy [32].
Only five cases of NmA were reported in MenAfriNet districts [17], providing further evidence of the ongoing effectiveness of MACV. These results support other analyses demonstrating the continued remarkable impact of MACV on both NmA carriage and disease throughout the meningitis belt [4, 5]. These findings, along with the high community acceptance [33], low cost of the vaccine, and long duration of protective immunity [34] (though shorter in children aged <5 years [35]) provide compelling evidence for continued MACV rollout in high-risk country areas inside or bordering the meningitis belt, via mass campaigns, catch-up campaigns, and introduction into the routine immunization system for children aged 9–18 months [3, 36].
This analysis indicates that regional meningitis epidemiology continues to change following MACV introduction. S. pneumoniae was the predominant bacterial meningitis pathogen in Burkina Faso, Chad, and Mali during this time period, causing 64% of laboratory-confirmed meningitis in those 3 countries. In the meningitis belt, pneumococcal meningitis has a distinct seasonality similar to that of meningococcal meningitis, high CFR, and predominance of serotype 1 disease in persons aged >5 years [7, 29, 37–40]. In Burkina Faso, where pneumococcal serotype data have been analyzed, the introduction of 13-valent PCV in 2013 has resulted in a substantial (62%) reduction in meningitis due to PCV13-serotypes, both among children aged <1 targeted for vaccination (77% reduction) and among older age groups benefitting from herd protection (~60% reduction) [7, 16, 39]. However, these declines were more pronounced among other PCV13 serotypes than for serotype 1, which continues to cause outbreaks in the region [40], indicating a potential need to reevaluate pneumococcal meningitis prevention and response strategies in the belt.
Despite the high-quality surveillance conducted in the five MenAfriNet countries, challenges to case-based meningitis surveillance in these countries remain. A perennial challenge is specimen transport. However, creative approaches to tracking specimens using barcodes and a cloud-based system in Burkina Faso may provide insights on how to improve this process and may be scaled up to other countries [41]. Ensuring that all suspected meningitis cases are reported, nearly all suspected meningitis cases have a CSF specimen collected, and every CSF specimen is received and tested at a national reference laboratory via culture or rt-PCR is key to high-quality case-based surveillance. While the MenAfriNet countries have worked towards increasing laboratory confirmation to support surveillance over time, 20% of CSF specimens still did not undergo laboratory confirmation at the national level, indicating remaining room for improvement [10]. Additionally, this case-based surveillance network only focuses on the clinical presentation of meningitis, and does not capture cases with non-meningitis presentations such as meningococcemia [42], therefore presented incidences are minimal estimates of the full burden of invasive disease due to these pathogens. Despite these challenges, our reported incidence rates adjust for changes in culture and real-time PCR testing capacity over time and likely reflect true trends in pathogen-specific incidences for meningitis.
An immense effort has been invested in MACV vaccination campaigns in MenAfriNet countries and elsewhere in the belt, resulting in major successes in mobilizing international and local communities and encouraging high vaccine uptake, and in an extraordinary and unprecedented reduction in meningitis burden and NmA transmission. However, the data emphasize the importance of non-A serogroups and pneumococcal meningitis, and suggest that intensive meningitis surveillance with high levels of laboratory confirmation in a number of countries is needed to provide a representative picture of the dynamic regional epidemiology. Continued thoughtful and successful integration of MACV and PCV into childhood routine immunization programs, along with long-term investments in surveillance and a future multivalent meningococcal conjugate vaccine, could help to ensure that a goal of defeating meningitis by 2030 is met [32, 43].
Supplementary Material
Acknowledgements
The MenAfriNet Consortium (www.menafrinet.org) is an international consortium led and implemented by Ministère de la Santé du Burkina Faso, Ministère de la Santé et de l’Hygiène Publique du Mali, Ministère de la Santé Publique du Niger, Ministère de la Santé Publique du Tchad, Ministère de la Santé et de la Protection Sociale du Togo, Agence de Médecine Préventive, CDC, and WHO, with support and collaboration from other international and non-governmental organizations. The authors thank all MenAfriNet partners, including participating national health systems, health centers, and laboratories. We also thank Pam Srivastava and Denise Hughes for their assistance with MenAfriNet data assembly and cleaning. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of CDC or WHO.
Funding Source
This work was supported by the MenAfriNet Consortium through a grant from the Bill and Melinda Gates Foundation (OPP1084298).
Footnotes
Declaration of interests
J. C. M. participated in the MenAfriNet Consortium while working at Agence de Médecine Préventive but is now an employee of Pfizer, Inc. All other authors: No reported conflicts of interest.
References
- 1.Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg 1999; 93:341–53. [DOI] [PubMed] [Google Scholar]
- 2.Novak RT, Kambou JL, Diomande FV, et al. Serogroup A meningococcal conjugate vaccination in Burkina Faso: analysis of national surveillance data. Lancet Infect Dis 2012; 12:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bwaka A. Status of the rollout of the meningococcal serogroup A conjugate vaccine in African meningitis belt countries in 2018. MenAfriNet Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trotter CL, Lingani C, Fernandez K, et al. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis 2017. [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen PA, Diomande F, Ba AK, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis 2013; 56:354–63. [DOI] [PubMed] [Google Scholar]
- 6.Patel JC. MenAfriNet: a network supporting case-based meningitis surveillance and vaccine evaluation in the meningitis belt of Africa. MenAfriNet Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambire D, Soeters HM, Ouedraogo-Traore R, et al. Nationwide Trends in Bacterial Meningitis before the Introduction of 13-Valent Pneumococcal Conjugate Vaccine-Burkina Faso, 2011–2013. PloS one 2016; 11:e0166384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Technical Guidelines for Integrated Disease Surveillance and Response in the African Region, 2nd Edition Brazzaville, 2010. [Google Scholar]
- 9.WHO-AFRO. Standard operating procedures for case-based surveillance of meningitis, preparedness and response to meningitis epidemics: World Health Organization Regional Office for Africa, 2017. [Google Scholar]
- 10.Mbaeyi SA. Improving case-based meningitis surveillance in 5 countries in the meningitis belt of sub-Saharan Africa, 2015–2017. MenAfriNet Supplement. [DOI] [PubMed] [Google Scholar]
- 11.WHO-AFRO. Standard operating procedures for enhanced meningitis surveillance in Africa: World Health Organization Regional Office for Africa, 2009. [Google Scholar]
- 12.World Health Organization. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae, 2nd edn Geneva: World Health Organization, 2011. [Google Scholar]
- 13.Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Micro 2007; 45:2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Theodore MJ, Mair R, et al. Clinical validation of multiplex real-time PCR assays for detection of bacterial meningitis pathogens. J Clin Micro 2012; 50:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feagins AR, Vuong J. Strengthening Laboratory Systems in the Meningitis Belt to improve Meningitis Surveillance, 2008–2018: A Partners’ Perspective. MenAfriNet Supplement. [DOI] [PubMed] [Google Scholar]
- 16.Soeters HM. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis—Burkina Faso, 2016–2017. MenAfriNet Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diallo AO, Soeters HM, Yameogo I, et al. Bacterial meningitis epidemiology and return of Neisseria meningitidis serogroup A cases in Burkina Faso in the five years following MenAfriVac mass vaccination campaign. PloS one 2017; 12:e0187466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez K. Meningococcal meningitis outbreaks in the African belt after the MenAfriVac introduction, 2011–2017. MenAfriNet Supplement. [Google Scholar]
- 19.Sidikou F, Zaneidou M, Alkassoum I, et al. Emergence of epidemic Neisseria meningitidis serogroup C in Niger, 2015: an analysis of national surveillance data. Lancet Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanogo YO. A new sequence type of Neisseria meningitis serogroup C associated with a meningitis outbreak in Mali. MenAfriNet Supplement. [Google Scholar]
- 21.Mounkoro D. Neisseria meningitidis serogroup W outbreak in Togo, 2016. MenAfriNet Supplement. [Google Scholar]
- 22.Funk A, Uadiale K, Kamau C, Caugant DA, Ango U, Greig J. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013–14. PLoS currents 2014; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper L. Can spatial information improve reactive vaccination for NmC outbreaks?. MenAfriNet Supplement. [Google Scholar]
- 24.Kretz CB, Retchless AC, Sidikou F, et al. Whole-Genome Characterization of Epidemic Neisseria meningitidis Serogroup C and Resurgence of Serogroup W, Niger, 2015. Emerg Infect Dis 2016; 22:1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alderson MR, Laforce FM, Sobanjo-ter Meulen A, Hwang A, Preziosi MP, Klugman K. Eliminating meningococcal epidemics from the African meningitis belt: the case for advanced prevention and control using next generation meningococcal conjugate vaccines. MenAfriNet Supplement 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boisier P, Nicolas P, Djibo S, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis 2007; 44:657–63. [DOI] [PubMed] [Google Scholar]
- 27.Djibo S, Nicolas P, Alonso JM, et al. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop Med Int Health 2003; 8:1118–23. [DOI] [PubMed] [Google Scholar]
- 28.Delrieu I, Yaro S, Tamekloe TA, et al. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PloS one 2011; 6:e19513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaro S, Lourd M, Traore Y, et al. Epidemiological and Molecular Characteristics of a Highly Lethal Pneumococcal Meningitis Epidemic in Burkina Faso. Clin Infect Dis 2006; 43:693–700. [DOI] [PubMed] [Google Scholar]
- 30.Daugla DM, Gami JP, Gamougam K, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 2014; 383:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Standard Operating Procedures for Surveillance of Meningitis, Preparedness and Response to Epidemics in Africa. 2018.
- 32.Novak R. What’s next? Future directions for meningitis surveillance and vaccine evaluation in the meningitis belt. MenAfriNet Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idoko OT, Diallo A, Sow SO, et al. Community Perspectives Associated With the African PsA-TT (MenAfriVac) Vaccine Trials. Clin Infect Dis 2015; 61 Suppl 5:S416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White M, Idoko O, Sow S, et al. Antibody kinetics following vaccination with MenAfriVac: an analysis of serological data from randomised trials. Lancet Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 35.Yaro S, Njanpop Lafourcade BM, Ouangraoua S, et al. Antibody Persistence at the Population Level 5 Years After Mass Vaccination With Meningococcal Serogroup A Conjugate Vaccine (PsA-TT) in Burkina Faso: Need for a Booster Campaign? Clin Infect Dis 2019; 68:435–43. [DOI] [PubMed] [Google Scholar]
- 36.Sambo L, Chan M, Davis S, et al. A Vaccine Meets Its Promise: Success in Controlling Epidemic Meningitis in Sub-Saharan Africa. Clin Infect Dis 2015; 61 Suppl 5:S387–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gessner BD, Sanou O, Drabo A, et al. Pneumococcal serotype distribution among meningitis cases from Togo and Burkina Faso during 2007–2009. Vaccine 2012; 30 Suppl 6:G41–5. [DOI] [PubMed] [Google Scholar]
- 38.Traore Y, Tameklo TA, Njanpop-Lafourcade BM, et al. Incidence, seasonality, age distribution, and mortality of pneumococcal meningitis in Burkina Faso and Togo. Clin Infect Dis 2009; 48 Suppl 2:S181–9. [DOI] [PubMed] [Google Scholar]
- 39.Kambire D, Soeters HM, Ouedraogo-Traore R, et al. Early impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis-Burkina Faso, 2014–2015. J Infect 2018; 76:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozio CH, Abdul-Karim A, Abenyeri J, et al. Continued occurrence of serotype 1 pneumococcal meningitis in two regions located in the meningitis belt in Ghana five years after introduction of 13-valent pneumococcal conjugate vaccine. PloS one 2018; 13:e0203205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diallo AO. Development and implementation of a cloud-based meningitis surveillance and specimen tracking system in Burkina Faso, 2018. MenAfriNet Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese H. Invasive meningococcal disease in Africa’s meningitis belt: more than just meningitis? MenAfriNet Supplement. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Epidemic meningitis control in countries in the African meningitis belt, 2018. Weekly Epidemiological Record 2019; 94:179–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.