Abstract
Introduction
Recent studies have reported a significant increase in the prevalence of colorectal cancer (CRC) in Sub-Saharan Africa (SSA). Further, several studies employing disparate modelling algorithms have projected a significant rise in the frequency of CRC cases in the region. However, lack of good quality data on multiple themes related to CRC including incidence, among others, continues to be a problem in the region. Therefore, this study was designed to collect data on the incidence of CRC in Eritrea.
Methods
We conducted a retrospective analysis using data captured between 2011 and 2017 at the National Health Laboratory (NHL) in Asmara, Eritrea.
Results
241 colorectal cases were identified in the Eritrean National Health Laboratory (NHL) database between 2011 and 2017. In the final analysis we included 94 patients confirmed cases giving an average of 18.8 patients per annum. The average age ± Standard deviation (SD) was 57.62 ± 17.14 with a male: female ratio of 58/36 (1: 1.61). The minimum and maximum age of the patients was 19 and 90 years, respectively. The rectum to colon ratio was 47/47 (1:1). The proportion of patients < 50 years in this cohort was significant. The age-standardized incidence rate (ASIR) in the study period was between 0.97 per 100 000 to 2.21 per 100 000. Similarly, the cumulative ASIR was 9.97 per 100 000. Analysis of trends did not reveal shifts over the study period (P<0.05). However, a strong correlation between incidence and age was established.
Conclusion
This study suggests that the incidence of CRC in Eritrea is relatively low. A significant number of patients were less than 50 years of age. Even then it’s our opinion that this study may underestimate the incidence of CRC.
Introduction
According to a recent Global Burden of Cancer Study (GLOBOCAN) estimate, colorectal cancer (CRC) is the 3rd most commonly diagnosed gastrointestinal malignancy worldwide [1, 2]. Reports indicate that the highest pooled incidence rates for CRC are in Western countries with Low and Medium income Countries (LMIC) reporting relatively low rates. For instance, the crude incidence rate in the US and United Kingdom (UK) are 25/100,000 and 30.2/100 000, respectively. In contrast, a recent meta-analysis reported that the crude incidence of CRC in SSA is 4.04/100,000 (4.38/100,000 for men and 3.69/100,000 for women) [3]. In particular, wide regional variation in the geographical distribution of CRC has been reported in the continent with low crude incidence rates in West Africa (Men: 4.5/100,000 and Women: 3.8/100,000) and East Africa (6.5/100,000) [1]. Similarly, higher incidence rates have been reported in North Africa (7.1/100,000) and Southern Africa (10.9/100,000) [1]. Altogether, the incidence of CRC in SSA appears to correlate strongly with a country’s level of socio-economic development [1]. To illustrate, Mauritius and South Africa (SA), the countries with some of the highest Human Development Index (HDI) in the region; have an incidence of 18.6/100,000 and 11.9/100,000, respectively [1, 4]. Altogether, the data confirms the observation that the variations in incidence and prevalence of CRC correlates strongly with socioeconomic levels.
Although the incidence of CRC in LMIC in SSA is comparatively low, the region has a higher CRC–associated mortality and morbidity. For instance, a past report indicated that of the 600, 000 deaths associated with CRC in 2008, 70% occurred in LMIC [2, 5]. In the meantime, the prevalence, incidence and resultant relevant costs such as disability-adjusted life years (DALY) due to premature death from CRC or from individuals living with CRC are projected to increase [4]. The increasing incidence and prevalence of CRC is part of the on-going epidemiological transition in SSA characterized by a reducing burden of infectious diseases coupled with a rising burden of non-communicable diseases (NCD) [2, 6]. In turn, this outcome has been linked to multiple factors including increasing longevity, rapid societal and economic changes and the presence of associated risk factors [1, 7]. These risks include alcohol consumption, hereditary factors such as familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC), diabetes mellitus, chronic inflammatory diseases of the colon (ulcerative colitis or Crohn’s colitis, among others), poor nutrition (low consumption of fruits and vegetables, high consumption of red processed meat or high—glycemic load carbohydrates), physical inactivity, prior ovarian/endometrial cancer before age 50 and smoking, among others [2, 8, 9]. The interactions and relative contribution of each of these factors to increased risk of CRC are complex and, at times, difficult to unravel. Altogether, a substantial amount of evidence has demonstrated that the epidemiology of CRC is shifting towards increased incidence and prevalence in individuals <50 years of age [1, 4]. The earlier age at onset, apart from being more aggressive disease, has also been associated with poor prognosis.
Considering the relatively high burden of infectious diseases in SSA, the increasing burden of CRC; a chronic condition requiring long—term medical expenditures, can only exacerbate the prevailing vicious cycle of health and poverty. The social and economic impact on individuals, families, community and/or countries or regions will therefore be substantial. A recent report from a group of experts noted that in the 21st century, cancer associated mortality will rank as the single most important barrier to increasing life expectancy worldwide [1]. Although, researchers generally agree that the burden of CRC in SSA will increase, the true incidence, prevalence and burden of CRC remain uncertain [2, 3]. This uncertainty has largely been attributed to the dearth of high-quality cancer registries and the limited number of studies [1, 4, 10]. Additional concern includes the possibility that data from existing registries is often suboptimal/partial or conflicting, hence, incomprehensive [4, 10]. Corroborating this conclusion, trace-back of cancer deaths to obtain the corresponding hospital records has demonstrated that quality of diagnostic information on cancer cases is generally poor [10]. Limited diagnostic infrastructure and late presentation of cases to health care providers also undermine the validity of CRC data from the region. In Eritrea, data on the incidence of CRC is lacking in international literature. Given the paucity of data on CRC and projections of an increase in SSA, understanding the incidence of CRC in Eritrea is imperative. Therefore, this study was designed to collect data on the incidence of CRC in the country.
Method and materials
Study design and setting
We conducted a retrospective analysis using data captured between 2011–2017 at the National Health Laboratory (NHL) in Asmara, Eritrea. NHL serves as a referral facility for all hospitals in Eritrea and has the only pathology laboratory in the country. Therefore, all cancer specimens in the country are processed at the facility. In this regard, the NHL database (Polytech 8.37.C) captures relevant data on all malignancies countrywide. Between 2011–2017, 9 403 cancer cases were recorded among these 241 were Colon (C18) and Rectum (C20) cases. The information captured in the system includes demographic characteristics (sex and age), histopathology (morphology), primary site (topography), behavior, date of diagnosis, differentiation status and stage (number staging), among others. Tumor classification is based on International Classification of Diseases (ICD) for Oncology, 3rd ed. (ICD-10 and ICD-O) [11]. All the cases were also confirmed by a resident pathologist or pathologists in specific reference institutions.
Completeness and data quality
In evaluating cancer incidence, ensuring that incident cases in the population are included is critical. In this study, we evaluated completeness using a number of strategies including stability of incidence rates over time. The use of mortality-to-incidence ratios was not possible due to lack of data on mortalities.
Data analysis
Data extracted from NHL Polytech data base (Polytech 8.37.C) was cross-checked against a hard copy maintained at the facility. Duplicate entries were eliminated based on demographic data and patient phone number. Chi-squire (χ2) test was used to evaluate differences between proportions. A one-sided P value > 0.05 was considered significant. Further, the crude average annual incidence rate (CIR), was calculated by dividing the total number of cases by the corresponding population at risk, expressed per 100 000 person-years. The ASIR was calculated by the direct method, using 10-year age bands (≤ 40–49, 50–59, 60–69 and ≥ 70). ASIR conversions were based on world population net 2014. Annual trends in CRC were evaluated by the annual percentage change (APC) over time period as: [exp (β) -1] × 100). The regression coefficient (β) was estimated from a linear regression between logarithmic-transformed age-standardized cancer rates per calendar year [12]. Data was entered in Microsoft excel 2010 and analyzed using IBM, SPSS software version 20.
Ethical approval and consent
The study was approved by the Health Research Ethics and Protocol Review Committee of the Eritrean Ministry of Health (MOH). Consent to participate was not obtained from the patients. This waiver was premised on the consideration that the study was based on anonymized patient’s records.
Results
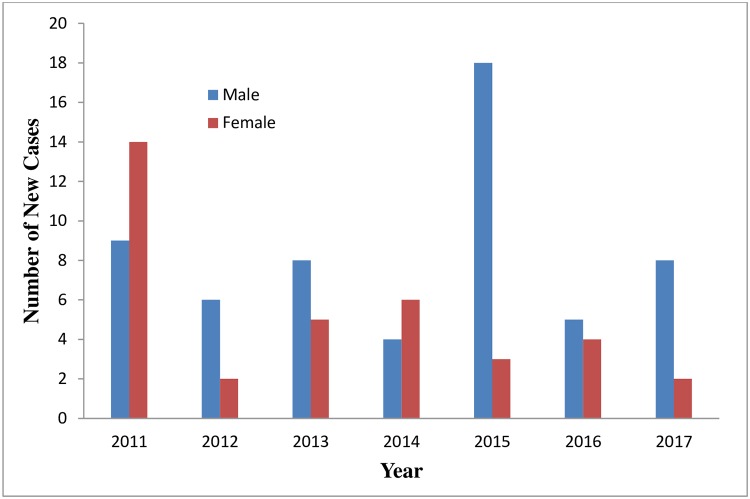
We studied 94 confirmed cases of CRC over the study period (2011–2017). The average age ± standard deviation (SD) was 57.62 ± 17.14 with a male: female ratio of 58/36 (1: 1.61). The minimum and maximum age of the patients was 19 and 90 years. The annualised distribution of cancer cases is shown in Fig 1.
Fig 1. Annualised distribution of new cancer cases.
Information on tumour behaviour, morphology, grade and stage was abstracted from the database. In terms of behavior, 94 (39%) of the patients had malignant tumour and 147 (61%) had benign tumour. Disaggregation of malignant tumor in terms of morphology showed that 84 (89.4%) of the tumours were adenocarcinomas, 9 (9.6%) were squamous cell carcinomas, and 1 (1.1%) were anaplastic carcinomas. Number staging system (Stages 1 and 2 as early colorectal cancer and stages 3 and 4 as progressive colorectal cancer) was used to stage the tumours. The proportions of tumour in the different stages were as follows: 13 (13.93%) of the tumours were in stage 1; 23 (24.5%) were in stage 2; 48(51.1%) were in stage 3 and 10 (10.6%) were in stage 4. Information on cancer grades are shown in Table 1. Altogether, we have to note that data on tumour grades was only available for 50% of the cases. Analysis of the distribution of the different grades and stages of cancers across the disparate age groups did not show any significant difference.
Table 1. Tumour characteristic.
| Tumour characteristic | Patient No% |
|---|---|
| Types of Tumors | |
| Malignant | 94 (39) |
| Benign | 147 (61) |
| Morphology | |
| Adenocarcinoma | 84 (89.4) |
| Squamous Carcinoma | 9 (9.6) |
| Anaplastic Carcinoma | 1 (1.1) |
| Grading | |
| Grade1(Well-differentiated) | 23 (48.9) |
| Grade 2 (Moderately differentiated) | 21 (44.7) |
| Grade 3 (Poorly differentiated | 3 (6.38) |
| Stage (Number Staging) | |
| Stage 1 | 13 (13.83) |
| Stage 2 | 23 (24.5) |
| Stage 3 | 48 (51.1) |
| Stage 4 | 10 (10.6) |
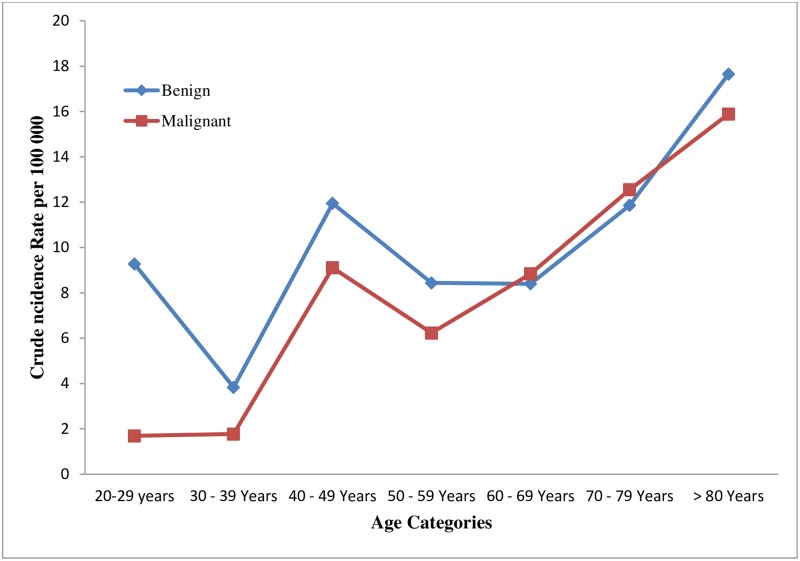
The proportion of patients < 50 years in this cohort was sizable. The data showed that (8/94) 8.5% of the patients were < 29 years (male: female ratio was 6:2 (3:1)); 6/94 (6.38%) were in the 30–39 year age range (male: female ratio was 3:3 (1:1)) and patients in the 49–50 years age range were 16/94 (17.02%) (Male: female ratio was 10:6 (1.67). Most of the colon cancer cases were in the 60–69 age groups. The highest cumulative CIR was observed in patients in the >80 years age band(Fig 2).
Fig 2. Crude incidence rate of colorectal cancers across different age categories.
Disaggregation by sex showed that males were more likely to present with CRC compared to women. In males, the highest CIR and ASIR was in individuals > 80 years of age. In contrast, the highest rate in females was observed in the 60–69 years age band. Overall, the highest ASIR was observed in patients in the 70–79 years age band. The cumulative ASIR over the study period for males and females were 15.75 and 6.23 per 100 000, respectively. Similarly, the cumulative ASIR for both males and females in the study period was 9.92 per 100 000 (Table 2).
Table 2. Seven year age standardised incidence rates and crude incidence rates of colorectal cancer by age groups for both sexes (per 100 000 population).
| Year Bands | Male | Females | Both | |||
|---|---|---|---|---|---|---|
| CIR | ASIR | CIR | ASIR | CIR | ASR | |
| <40 Years | 2.08 | 0.63 | 1.14 | 0.32 | 1.61 | 0.47 |
| 40–49 Years | 7.57 | 2.02 | 4.27 | 1.17 | 5.87 | 1.59 |
| 50–59 Years | 6.42 | 1.31 | 8.53 | 1.69 | 7.51 | 1.51 |
| 60–69 Years | 15.39 | 2.06 | 12.76 | 1.75 | 13.95 | 1.89 |
| 70–79 Years | 63.65 | 4.30 | 11.54 | 0.90 | 31.77 | 2.31 |
| >80 Years | 226.50 | 5.43 | 11.39 | 0.40 | 73.10 | 2.15 |
| 2011–2017 | 15.75 | 6.23 | 9.97 | |||
ASIR: Age standardised Incidence Rate; CIR: Crude incidence rate
Rates per 100 000 for colon and rectal cancers
There were 47 colon and 47 rectal cancer cases giving a rectum to colon ratio of 47/47 (1:1). Trend analysis did not demonstrate significant changes (P value > 0.05) in the magnitude of colonic or rectal tumours during the study period. Additional information on the distribution of colonic and rectal cancers is shown in Table 3.
Table 3. Annualised crude incidence rate per 100,000.
| Years | Colon | CIR | ASIR | Rectum | CIR | ASIR | Both | CIR | ASIR |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | 11 | 0.70 | 1.19 | 12 | 0.76 | 1.27 | 23 | 1.45 | 2.21 |
| 2012 | 4 | 0.25 | 0.42 | 4 | 0.25 | 0.55 | 8 | 0.51 | 0.97 |
| 2013 | 5 | 0.32 | 0.65 | 8 | 0.51 | 0.67 | 13 | 0.82 | 1.32 |
| 2014 | 5 | 0.32 | 0.48 | 5 | 0.32 | 0.63 | 10 | 0.63 | 1.12 |
| 2015 | 9 | 0.57 | 1.00 | 12 | 0.76 | 1.14 | 21 | 1.33 | 2.13 |
| 2016 | 6 | 0.38 | 0.56 | 3 | 0.19 | 0.24 | 9 | 0.57 | 0.93 |
| 2017 | 7 | 0.44 | 0.76 | 3 | 0.19 | 0.5 | 10 | 0.63 | 1.30 |
| 2011–2017 | 47 | 5.06 | 47 | 5.0 | 94 | 9.98 |
ASIR: Age standardised incidence rate; CIR: Crude incidence rate
Annualised ASIR across different age bands for the study duration are shown in Table 4. Analysis of trends did not exhibit year to year changes in the magnitude of CRC.
Table 4. Annualised age standardised rates for each age group per year.
| Colorectal Cancer |
Age Group (Years) | ||||||
|---|---|---|---|---|---|---|---|
| <40 | 40–49 | 50–59 | 60–69 | 70–79 | > 80 | All | |
| Overall | |||||||
| 2011 | 0.13 | 0.4 | 0.36 | 0.57 | 0.51 | 0.24 | 2.21 |
| 2012 | - | 0.20 | 0.18 | 0.09 | 0.26 | 0.24 | 0.97 |
| 2013 | 0.07 | 0.20 | 0.27 | 0.28 | 0.26 | 0.24 | 1.32 |
| 2014 | 0.03 | 0.40 | - | 0.19 | 0.26 | 0.24 | 1.12 |
| 2015 | 0.13 | 0.20 | 0.53 | 0.28 | 0.51 | 0.48 | 2.13 |
| 2016 | 0.03 | 0.1 | 0.18 | 0.38 | - | 0.24 | 0.93 |
| 2017 | 0.03 | 0.1 | 0.09 | 0.09 | 0.51 | 0.48 | 1.30 |
Discussion
CRC incidence and associated mortality are growing in SSA. This phenomenon has been traced to a complex interrelationship between multiple factors associated with the rapidly evolving socioeconomic transition in the region [1]. In this regard, increasing incidence of CRC in SSA is an important marker of the public health challenges associated with the on-going epidemiological transition in SSA. Without doubt, it serves to underscore the importance of continued surveillance of CRC in the region. Against this backdrop, this article provides a status report on the incidence of CRC in Eritrea in 2011–2017. The data indicates that the CIR ranged between 0.97 per 100 000 to 2.21 per 100 000. The low incidence rate reported in this study parallels findings from specific countries in SSA (Gambia and Mozambique (both 1.5 per 100 000)) [2]. Our results are also consistent with the findings by previous investigators in the country [13] and with the assertion that colon cancers are rare in native African (NA) population’s [1]. Instructively, the estimates are lower compared to GLOBOCAN averages for the region [1]. Importantly, we have to note that GLOBOCAN reports on Eritrea are modelled using data from cancer registries from neighbouring countries (Ethiopia in particular). The ratio of CRC between sexes also reflected established patterns indicating a high prevalence in males compared to women 58/36 (1: 1.61). Underscoring this disparity, a recent GLOBOCAN report noted that the age-standardized incidence rate is about 1.4 times higher in men than in women. In contrast, Graham et al. estimated that the male: female ratio of CRC in SSA is 1.17 [4]. Additionally, analysis of trends did not reveal shifts over the study period (p<0.05). This result might be due, in part, to limited statistical power.
A salient consideration that should inform the evaluation of the representativeness of our estimates is that it is largely based on diagnostic and not on screening evaluations. Similar to other countries in SSA, Eritrea lacks a system-wide, organised approach to screening—programmatic and opportunistic screenings are marginal. The complete absence or marginal use of screening tools such as faecal immunochemistry testing (FIT); faecal occult blood testing (FOBT); fecal DNA, capsule endoscopy, colonoscopy, flexible sigmoidoscopy and CT colonography is particularly noteworthy. Lack of access to medical services, low level of awareness, dearth of specialists (gastroenterologists, surgeons, oncologists, and radiation oncologists) and limited utilization of existing, albeit, limited services are important barriers.
Late presentation of CRC cases also appears to be a common feature in Eritrea. A fact reflected in the low ratio between benign and malignant cases 147/94 (1.56) reported in this study and the high proportion of patients (61.7%) with advanced disease (Stage 2 and above). For this reason, it’s our opinion that this result may underestimate the incidence of CRC in the country. Reflecting on this phenomenon, several commentators have noted that the greatest concern related to cancer incidence reports from SSA relates to the systematic under-reporting of cases due to lack of high quality population-based cancer registries (PBCRs) [1]. The low incidence may also be attributed to the low level of screening in the continent.
Overall, this study highlights the need for screening services in the country. The introduction of low cost screening modalities such as guaiac faecal occult blood tests (gFOBT) and faecal immunochemical test (FIT) should be explored. The fact that such programs should target the elderly population in the country is suggested by the relatively high ASR observed in patients > 50 years (Table 1). Multiple reports indicate that systematic application of these techniques either within the framework of a programmatic or opportunistic screening program may enhance detection of undiagnosed CRC [1, 4]. Subsequent removal of pre-neoplastic lesions (polypectomies) may ultimately lower the incidence of CRC in these populations. Regrettably, cancer screening services rank low in the hierarchy of public health priorities in most countries in SSA.
Another important finding in our study is the significant number of patients < 50 years (the breakpoint employed by the Amsterdam criteria for identifying patients with increased odds of developing Lynch Syndrome (LS)—a highly penetrant hereditary cancer syndrome) [14] presenting with CRC. The changing pattern of CRC in SSA including the increasing number of cases in individuals < 50 years has been reported [14,15,16]. This finding may have a dual interpretation. First, it may be a consequence of the prevailing socio-economic transition in the Country. Much has been written about the link between global variation in CRC and westernisation of lifestyles and diets [4, 14]. In line with this thinking, the increasing prevalence of CRC in SSA including the rising incidence among individual’s ≤ 50 years may be an outcome of the dynamics associated with the changing lifestyles [12, 17]. Second, the data suggests the possibility that a proportion of CRC cases in Eritrea are inherited. Reports indicate that up to one-third of the CRC risk may be attributable to hereditary factors and approximately 3~5% of all CRCs are due to hereditary syndromes [14, 18, 19]. Hereditary non-polyposis colorectal cancer (HNPCC); Lynch syndrome (mutations in DNA mismatch repair (MMR) genes, MLH1, MSH2, PMS1, PMS2, MSH6 and EPCAM) [20,21,22,23,24]; familial adenomatous polyposis coli (FAP) (specific germline mutations in APC genes–constitutive activation of Wnt pathway), Cowden syndrome, among others have been implicated in the latter [14, 20, 25, 26]. However, the connection between family history or known syndromes and de novo occurrence of CRC in SSA is generally under-investigated. Emphasising this fact, a study involving a small cohort of CRC patients in Nigeria and South Africa (SA) suggested that HNPCC may be more frequent than hitherto reported [24, 27]. Recognizing the controversy associated with the contribution of hereditary factors to CRC cases in SSA, future research should focus on molecular profiles of CRC in young patients in Eritrea. Investigating the pharmacogenomics profiles of CRC in Eritrea may also inform therapeutic option.
Strength and limitation
In this study, we have attempted to provide a snapshot of the incidence and trend of CRC using the most reliable data source in Eritrea. In a general sense, the data from NHL captures information from all CRC patients in all the national and regional referal hospitals in the country. Given that a majority of these cases were referred by doctors based on the presentation of specific symptoms, the data may underestimate incidence of CRC in the country. Another underlying assumption in this study is that all specimens from CRC cases in Eritrea are inevitably referred to the facility. This may not be true especially for patients from remote parts of the country. However, it’s our opinion that the data from NHL is superior to the alternative option of data collection through hospital–based registries. Another weakness stems from the lack of granularity in our analysis. Importantly, a separate assessment of variations in proximal (right-sided) cancers of the descending sigmoid colon and distal (left-sided) cancers of the rectosigmoid junction among others was not attempted. The sourcing of population denominators used in this study from population pyramid net of 2014 may also be limiting.
Conclusions
This study suggests that the crude incidence rate of CRC in Eritrea is relatively low. Moreover, analysis of trends did not reveal shifts over the study period (2011–2017). However, it’s our opinion that this study may underestimate the incidence of CRC in Eritrea. Even then, the study provides critical baseline information that may help in creating research platforms for risks assessment or identification of putative targets for intervention. Further, a majority of the patients had advanced disease. Altogether, the study suggests that research on cases involving patients who are <50 years is vital. The possibility of establishing aggressive screening programs even for patients < 50 years should be considered. The overwhelming need to expand and integrate primary prevention (public awareness and physicians sensitisation, among others) and early detection measures(screening modalities) into existing healthcare plans and improve data collection gaps exists.
Supporting information
(SAV)
(XLSX)
(XLSX)
Acknowledgments
We would like to thank Eritrean Ministry of Health and Prof. Hartmut Lobeck. We would also like to thank the Eritrean National Health Laboratory (NHL).
Abbreviations
- APC
Adenomatous polyposis coli
- ASR
Age standardised rates
- CRC
Colorectal cancer
- CT
computer Tomography
- FAP
familial adenomatous polyposis coli
- FIT
Faecal immunochemistry testing
- FOBT
Faecal occult blood testing
- HNPCC
Hereditary non-polyposis colorectal cancer
- LMIC
Low and Medium Income Countries
- NHL
National Health Laboratory
- PBCR
Population-based cancer registry
- SSA
sub-Saharan Africa
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, in press. The online GLOBOCAN 2018 database is accessible at http://gco.iarc.fr [DOI] [PubMed]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal Ahmedin, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016; 0:1–9. 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 3.Graham A, Adeloye D, Grant L, Theodoratou E, Campbell H. Estimating the incidence of colorectal cancer in Sub–Saharan Africa: A systematic analysis. Journal of Global Health. 2012. 2 (2). 020404 10.7189/jogh.02.020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5): E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 5.Meetoo D. Chronic disease: the silent global epidemic. Br J Nurs. 2008;17:1320–5. Medline:19060813. 10.12968/bjon.2008.17.21.31731 [DOI] [PubMed] [Google Scholar]
- 6.Giles WH. Preventing non-communicable diseases in sub-Saharan Africa. Global Health Promotion. 2010; Supp(2): 03–05; 363946. [DOI] [PubMed] [Google Scholar]
- 7.Walker AR, Segal I. Colorectal cancer in an African city population in transition. Eur J Cancer Prev. 2002;11:187–91. Medline:11984138 10.1097/00008469-200204000-00012 [DOI] [PubMed] [Google Scholar]
- 8.Marley AR, Nan H. Epidemiology of colorectal cancer. Int J MolEpidemiol Genet 2016;7(3):105–114. [PMC free article] [PubMed] [Google Scholar]
- 9.Haggar FA. Boudhey RP. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clinics in Colon and Rectal Surgery. 2009. 22(4): 191–197. 10.1055/s-0029-1242458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asombang AW, Madsen R, Simuyandi M, Phiri G, Bechtold M, Ibdah JA et al. Descriptive analysis of colorectal cancer in Zambia, Southern Africa using the National Cancer Disease Hospital Database. Pan African Medical Journal. 2018; 30:248 10.11604/pamj.2018.30.248.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. International classification of diseases for oncology, 3rd ed (ICD-O-3); 2000 [cited 2019 February 11]. http://codes.iarc.fr/codegroup/1.
- 12.Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJ, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg. 2011;213(3):352–61. 10.1016/j.jamcollsurg.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 13.Hagos A, Daniel T, Hawaz W, Mengisteab H, Eman D. Fadlelmola FM et al. , Trends in the Incidence of Cancer in Eritrean Hospitals and Eritrean National Health Laboratory 2000–2010, UK Journal of Pharmaceutical and Biosciences. 2016. 4(5): 47–55. [Google Scholar]
- 14.Ngeow J, Eng C. Rectal Cancer: Age Matters in the Affairs of Stage. JNCI J Natl Cancer Inst (2016) 108(1): djv325. [DOI] [PubMed] [Google Scholar]
- 15.Irador DO, Afuwape OO, Anyandipo OO. The Present Status of the Management of Colon and Rectal Cancer in Nigeria. Journal of Cancer Research, 2014, Article ID 267190, 7, 10.1155/2014/267190. [DOI] [Google Scholar]
- 16.Moolla Z, Madiba TE. Trends in demographics andmanagement of obstructing colorectal cancer. World J Surg.2014; 38(9): 2466–70. 10.1007/s00268-014-2595-y [DOI] [PubMed] [Google Scholar]
- 17.Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJ, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg. 2011;213(3):352–61. 10.1016/j.jamcollsurg.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 18.Cronje L. Becker P. J. Paterson A. C. Ramsay M. Hereditary non-polyposis colorectal cancer is predicted to contribute towards colorectal cancer in young South African blacks,” South African Journal of Science, 2009. 105 (1–2): 8–72. [Google Scholar]
- 19.Kolligs FT. Diagnostics and Epidemiology of Colorectal Cancer. Visc Med 2016;32:158–164 10.1159/000446488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC, Gastroenterology, 1999, vol. 116 (pg. 1453–6). [DOI] [PubMed] [Google Scholar]
- 21.O’Keefe SJD, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J et al. Why Do African Americans Get More ColonCancer than Native Africans? The J. Nutr. 2007. 137: 175S–182S. 10.1093/jn/137.1.175S [DOI] [PubMed] [Google Scholar]
- 22.Kolligs FT. Diagnostics and Epidemiology of Colorectal Cancer. Visc Med 2016;32:158–164 10.1159/000446488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson W. F. Umar Anderson Brawley O. W. “Colorectal carcinoma in black and white race,” Cancer and Metastasis Reviews. 2003. 22 (1): 67–82. [DOI] [PubMed] [Google Scholar]
- 24.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG et al. Colorectal Cancer. Nat Rev Dis Primers.; 1: 15065 10.1038/nrdp.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronje L. Becker P. J. Paterson A. C. Ramsay M. Hereditary non-polyposis colorectal cancer is predicted to contribute towards colorectal cancer in young South African blacks,” South African Journal of Science, 2009. 105 (1–2): 8–72. [Google Scholar]
- 26.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the earlydetection of colorectal cancer and adenomatous polyps, 2008: a joint guidelinefrom the American Cancer Society, the US Multi-Society Task Force on ColorectalCancer, and the American College of Radiology. CA Cancer J Clin.2008;58:130–60 10.3322/CA.2007.0018 [DOI] [PubMed] [Google Scholar]
- 27.Irabor D, Adedeji OA, Colorectal cancer in Nigeria: 40 years on. A review. European Journal of Cancer Care, 2009, 18, 110–115. 10.1111/j.1365-2354.2008.00982.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.