Abstract
Repeated quantitative measurement of bacterial DNA on whole blood has been shown to be a promising method for monitoring bloodstream infection (BSI) with selected bacterial species. To enable broad use of this method, we developed a quantitative droplet digital PCR (ddPCR) method for 16S rDNA. It was validated with species-specific ddPCRs for Staphylococcus aureus (nuc), Streptococcus pneumoniae (lytA), and Escherichia coli (uidA) on spiked whole blood samples and on repeated whole blood samples (days 0, 1–2, 3–4, 6–8, and 13–15) from 83 patients with BSI with these pathogens. In these patients, 16S rDNA and species-specific DNA were detected in 60% and 61%, respectively, at least at one time-point. The highest positivity rates were seen in S. aureus BSI, where 92% of the patients were 16S rDNA-positive and 85% nuc-positive. Quantitative 16S rDNA and species-specific DNA showed strong correlations in spiked samples (r = 0.98; p < 0.0001) and clinical samples (r = 0.84; p < 0.0001). Positivity for 16S rDNA was rapidly cleared in patients with S. pneumoniae and E. coli BSI, but more slowly and sometimes persisted, in those with S. aureus BSI. The initial 16S rDNA load was higher in BSI patients with sepsis (Sepsis-3 definition) than without sepsis (median 2.38 vs. 0 lg10 copies/mL; p = 0.031) and in non-survivors than in survivors (median 2.83 vs. 0 lg10 copies/mL; p = 0.006). 16S rDNA ddPCR appears to be a promising method for bacterial DNA monitoring during BSI. The clinical value of such monitoring should be further studied.
Introduction
Bloodstream infections (BSI) frequently lead to sepsis, one of the most common causes of death worldwide [1]. Blood culture (BC) is the gold standard for identifying the pathogen in BSI, but the method is limited by low sensitivity and a long turn-around-time [2]. Molecular methods for identification of BSI pathogens have been developed in the last decades [3–6]. Apart from pathogen identification, some molecular methods such as quantitative PCR enable quantification of bacterial DNA. Our group [7] and others [8–11] have linked a high load of bacterial DNA in the bloodstream to severity of sepsis. A few other studies have shown that the dynamics of bacterial DNA load reflect the disease course and response to treatment [12–14]. Accordingly, it has been proposed that repeated quantitative measurement of bacterial DNA could be used to evaluate the appropriateness of antibiotic treatment [15]. However, these studies of DNA dynamics have focused on DNA from only one bacterial species at a time. To be clinically useful, a diagnostic tool for DNA dynamics should monitor DNA from many different bacterial species.
The gene encoding 16S ribosomal RNA (16S rDNA) is present in all bacteria, which makes it an interesting target for detecting and quantifying bacterial DNA. A potential limitation of quantitative 16S rDNA detection, however, is that the copy number of 16S rDNA genes per genome differ between bacterial species, whereas most species-specific genes occur in only one copy [16, 17]. The 16S rDNA copy number per genome for the three most prevalent pathogens in community-onset BSI, Staphylococcus aureus, Streptococcus pneumoniae and Escherichia coli [18, 19], has been estimated at five to six, four, and seven, respectively [16].
Digital PCR is a PCR technology for absolute DNA quantification without the need of external standard curves, developed from real-time PCR [20]. Droplet digital PCR (ddPCR) [21] is today the most widespread application of digital PCR and has demonstrated a greater quantification precision and a higher reproducibility compared to real-time PCR, with a comparable sensitivity [22].
The primary objective of this study was to develop a tool to consecutively measure bacterial DNA in blood during BSI. For this purpose, we optimized a 16S rDNA ddPCR and validated it against species-specific ddPCRs for S. aureus, S. pneumoniae, and E coli. The secondary objective was to relate the 16S rDNA load to disease severity in BSI patients, hypothesizing that consecutively measuring 16S rDNA load would be clinically valuable.
Materials and methods
Patients
Patients who sought medical care at the Emergency Department at Örebro University Hospital, Örebro, Sweden, from February 2011 to June 2014, with suspected bacterial infection were eligible for enrollment in a prospective study, as described earlier [23]. Exclusion criteria were age under 18 and infection with human immunodeficiency virus (HIV), Hepatitis B, or C. All patients provided written informed consent. Together with BC bottles, EDTA blood samples were collected. When a BC bottle signaled positive and S. aureus, S. pneumoniae, or E.coli was identified (days 1–2), the patient was enrolled. Further blood samples, including EDTA tubes, were then collected on days 1–2, 3–4, 6–8, and 13–15.
Blood cultures
According to routine practice, two pairs of BCs were collected per patient. For each BC a volume of 8–10 mL of venous blood was inoculated in a Bactec Aerobic/F bottle and the same volume in a BactecPlus Anaerobic/F bottle. The BC bottles were then incubated in the Bactec (Becton Dickinson and Company, Franklin Lakes, NJ, USA) system for up to seven days.
Serial dilutions of samples with reference strain bacteria
Reference strain bacteria, S. aureus (CCUG 35601), S. pneumoniae (CCUG 33638), and E. coli (CCUG 7620), were cultivated overnight, and two to three colonies of each were mixed with 1 mL saline solution. Serial dilutions were then performed, first taking 2.5μL of the aliquot suspension into 2.5 mL of whole blood, for a dilution of 1:103, then by diluting another five times down to 1:108. These dilutions were later used in the species-specific ddPCRs, as positive controls in optimization and patient sample experiments and for validation of the ddPCR assays on bacteria-spiked whole blood. DNA concentrations of the different dilutions can be found in the result section.
A reference strain of Haemophilus influenzae (CCUG 33391), was prepared in the same way but only in dilutions of 1:103 (7492 copies/mL) and 1:104, for use as positive controls in all 16S rDNA ddPCR experiments.
DNA extraction
Patient samples were frozen in aliquots of 1 mL EDTA blood at −70°C with 20% glycerol within 3 days of sample collection. Bacterial DNA was extracted using the Select NA Blood Pathogen Kit (Molzym, Bremen, Germany) on an Arrow instrument (Diasorin, Solna, Sweden) following the manufacturer’s instructions, and frozen. DNA extractions of the bacteria-spiked blood samples were performed in the same way, but those samples were not frozen as the PCR was run on the same day.
Droplet digital PCR
In ddPCR, the PCR master mix, including the target template, is divided into about 15,000 water-in-oil droplets, and individual PCR reactions are performed in each of them. Following PCR, each droplet is analyzed to determine the fraction of PCR positive droplets in the original sample, after which the absolute target DNA template concentration can be calculated.
In the study, DNA was detected and quantified using the QX100 Droplet Digital PCR system (Bio-Rad Laboratories Inc., Pleasanton, CA, USA) according to the manufacturer’s instructions [24]. Template DNA (5μL), 1 x Bio-Rad Residual DNA Quantification Supermix, forward primer, reverse primer, probe and UV-radiated water was mixed to a final volume of 20μL, with an estimated DNA concentration range of 1–120000 copies per 20 μL reaction. The PCR mix was put into a droplet generator cartridge, droplet generator oil was added, and the cartridge was placed into a droplet generator. The generated droplet emulsion was transferred to a 96-well PCR plate and amplified in a thermal cycler. After amplification the plates were transferred to, and read, in a droplet reader.
For broad-range ddPCR, we used primers and a probe targeting the pan-bacterial 16S rDNA, as previously described [25]. For species-specific ddPCR, we used primers and probes targeting the nuc gene [26, 27], lytA gene [28], and uidA gene [29], all well-studied single-copy genes, specific for S. aureus, S. pneumoniae, and E. coli, respectively. All primer and probe sequences are presented in Table 1.
Table 1. Primer and probe sequences for 16S rDNA and species-specific Staphylococcus aureus (nuc), Streptococcus pneumoniae (lytA), and Escherichia coli (uidA) droplet digital PCRs.
| Primer/probea | DNA sequence 5´to 3´b |
|---|---|
| 16S rDNA F | AACAGGATTAGATACCCTGGTAG |
| 16S rDNA R | GGTTCTKCGCGTTGCWTC |
| 16S rDNA P | 6-FAM AACACTGCTCCACCGCT- MGBNFQ |
| nuc F | GCGATTGATGGTGATACGGTT |
| nuc R | AGCCAAGCCTTGACGAACTAAAGC |
| nuc P | 6-FAM-ATGGTAGARAATGC-MGBNFQ |
| lytA F | CAGCGGTTGAACTGATTGA |
| lytA R | TGGTTGGTTATTCGTGCAA |
| lytA P | 6-FAM-AGCTGGAATTAAAACGCACGAG-MGBNFQ |
| uidA F | CAGTCTGGATCGCGAAAACTG |
| uidA R | ACCAGACGTTGCCCACATAATT |
| uidA P | 6-FAM-ATTGAKCAGCRTTGG-MGBNFQ |
aF = forward, R = reverse, P = probe
b6-FAM = 6-carboxyfluorescein, MGBNFQ = Minor groove binder non fluorescent quencher. K = G/T, R = A/G, W = A/T (wobble bases)
The design of the ddPCR assays included an optimization process, with protocols for adjustment of different parameters, in order to obtain ddPCR results with coherent clusters of positive droplets, well separated from the negative background. First, a temperature optimization was done where a temperature gradient from 55 to 65 0C was used according to the manufacturer’s recommended thermal cycling protocol. The optimal annealing/extension temperature was set at 59°C for the 16S rDNA and lytA ddPCRs, 58°C for the nuc ddPCR, and 56°C for the uidA ddPCR. Thereafter experiments for primer and probe concentration titration were performed, where different concentrations of primers (0.5, 0.9 and 1.2 μM) and probes (0.1, 0.25, 0.4 μM) were tested. Optimal primer concentrations were set at 1.2 μM for both forward and reverse primers for the 16S rDNA, and 0.9μM for all species-specific genes. Optimal probe concentrations were set at 0.4 μM for the 16S rDNA PCR, 0.1 μM for the nuc and lytA PCRs, and 0.25 μM for the uidA PCR.
Duplicate DNA extractions from the whole blood dilutions of reference strain bacteria (S. aureus/CCUG 35601, S. pneumonia/CCUG 33638, E. coli/CCUG 7620 and H. influenzae/CCUG 33391) were used for the ddPCR optimization, in the dilution of 1:103 in the temperature optimization, and 1:103 and 1:104 in primer and probe concentration experiments. UV-irradiated water was used as negative controls.
After ddPCR optimization, ddPCR was run on the whole blood dilutions (1:103–1:108) of reference strain bacteria and on patient samples. All ddPCR experiments were performed in duplicate and the mean result reported. Duplicate DNA extractions from the whole blood dilutions of reference strain bacteria, in the dilution of 1:103, were used as positive controls in the analyses on patient samples. The negative controls were presumed negative blood samples from 20 patients with negative BC and a laboratory confirmed non-bacterial diagnosis. A cut-off of 200 copies/mL was set for 16S rDNA ddPCR based on the results (mean plus two standard deviations) of the presumed negative samples.
Clinical data and definitions
Clinical assessments were made on sampling days, and data were collected on demographics, co-morbidities, site(s) of infection, antibiotic treatment, length of hospital stay, intensive care unit admission, and mortality. Co-morbidity was evaluated using the Charlson Comorbidity Index [30]. Clinical conditions were classified according to the Sepsis-3 criteria, and patients with an acute change of ≥2 points in SOFA score due to the infection were considered to have sepsis [31].
Statistics
Descriptive statistics are presented as medians with minimum and maximum values for continuous variables and as percentages for categorical variables. The non-parametric Mann-Whitney-U test was used for between-group comparisons, and Fischer’s exact test was used to compare proportions. A p-value of <0.05 was considered significant. The non-parametric Spearman’s rho test was used to assess correlation between the two different methods. Significant correlation was set at 0.01 (two-tailed). The SPSS software package (IBM corp., Armonk; NY, USA), version 22, was used for the statistical analyses. To calculate DNA template concentration in ddPCR, Poisson distribution statistics were performed using the Quantasoft software package (Bio-Rad Laboratories Inc., Pleasanton, CA, USA), version 1.4.
Ethics
The Regional Ethical Review Board in Uppsala, Sweden (ref: 2009/024) approved the study.
Results
Patient characteristics
A total of 83 patients with BSI were enrolled in the study, 27 with S. aureus BSI, 30 with S. pneumonie BSI, and 26 with E. coli BSI. The patients were sampled repeatedly from admission (day 0) to day 13–15 (Table 2).
Table 2. Samples collected and analyzed with 16S rDNA ddPCR and species-specific ddPCR in 83 patients with Staphylococcus aureus, Streptococcus pneumoniae, or Escherichia coli bloodstream infection.
| Results | Numbers (% of analyzed samples) | ||||
|---|---|---|---|---|---|
| Day 0 | Days 1–2 | Days 3–4 | Days 6–8 | Days 13–15 | |
| All patients (n = 83) | |||||
| Samples collected | 30 | 72 | 55 | 65 | 56 |
| Samples analyzed | 30 | 63 | 35 | 21 | 8 |
| 16S rDNA ddPCR + | 18 (60) | 35 (56) | 16 (46) | 8 (38) | 4 (50) |
| Species-specific ddPCR + | 21 (70) | 34 (54) | 16 (46) | 4 (19) | 0 |
| S. aureus BSI patients (n = 27) | |||||
| Samples collected | 7 | 24 | 20 | 24 | 17 |
| Samples analyzed | 7 | 24 | 18 | 13 | 6 |
| 16s rDNA ddPCR + | 7 (100) | 18 (75) | 13 (72) | 8 (62) | 4 (67) |
| nuc ddPCR + | 7 (100) | 19 (79) | 10 (56) | 3 (23) | 0 |
| S. pneumoniae BSI patients (n = 30) | |||||
| Samples collected | 9 | 28 | 15 | 25 | 25 |
| Samples analyzed | 9 | 28 | 7 | 3 | 1 |
| 16S rDNA ddPCR + | 4 (44) | 11 (39) | 2 (29) | 0 | 0 |
| lytA ddPCR + | 5 (56) | 9 (32) | 0 | 1 (33) | 0 |
| E. coli BSI patients (n = 26) | |||||
| Samples collected | 14 | 20 | 20 | 16 | 14 |
| Samples analyzed | 14 | 11 | 10 | 5 | 1 |
| 16S rDNA ddPCR + | 7 (50) | 4 (36) | 2 (20) | 0 | 0 |
| uidA ddPCR + | 9 (64) | 6 (55) | 6 (60) | 0 | 0 |
As shown, not all patients had the full sample series, and not all samples were analyzed with ddPCR. The reason a sample was not analyzed was usually ddPCR negativity in the previous sample, or, in a few cases, lack of enough blood volume.
Demographic and clinical characteristics are given in Table 3.
Table 3. Demographic and clinical characteristics of the study-population.
|
Total cohorta N = 83 |
Bloodstream infection etiologya | |||
|---|---|---|---|---|
|
Staphylococcus aureus n = 27 |
Streptococcus pneumoniae n = 30 |
Escherichia coli n = 26 |
||
| Demographic data | ||||
| Median age, years | 72 (24–93) | 77 (24–93) | 70 (30–89) | 76 (29–93) |
| Female | 39 (47) | 4(15) | 21 (70) | 14 (54) |
| Co-morbidities | ||||
| Ischemic heart disease | 24 (29) | 9 (33) | 6 (20) | 9 (35) |
| COPD | 6 (7) | 1 (4) | 5 (17) | 0 |
| Diabetes mellitus | 18 (22) | 6 (22) | 2 (7) | 10 (39) |
| Renal failure | 7 (8) | 3 (11) | 0 | 3 (12) |
| Active malignancy | 9 (11) | 3 (11) | 3 (10) | 3 (12) |
| Charlson’s score | 1 (0–8) | 1 (0–8) | 1 (0–8) | 2 (0–7) |
| Immuno-suppressive treatmentb | 8 (10) | 1 (4) | 3 (10) | 4 (15) |
| Clinical data | ||||
| Antibiotics before analyses | 6 (7) | 1 (4) | 0 | 5 (19) |
| Adequate initial antibiotic treatment | 80 (96) | 25 (93) | 30 (100) | 25 (96) |
| Body temperature,°C, at admission | 38.7 (35.6–41.0) | 38.6 (35.6–40.9) | 38.6 (35.8–41) | 39.1 (37.1–40.0) |
| SOFA score increase at admission | 1 (0–7) | 1 (0–7) | 2 (0–6) | 1 (0–7) |
| Sepsis (SOFA score increase ≥2) | 41 (49) | 13(48) | 22 (73) | 6 (23) |
| Hospitalization length, days | 8 (2–120) | 14 (3–120) | 5 (2–70) | 4 (2–25) |
| Intensive care unit admission | 16 (19) | 7 (26) | 7 (23) | 2 (8) |
| 90-days mortality | 10 (12) | 7 (26) | 1 (3) | 2 (8) |
| Focus of infection | ||||
| Endovascular | 7 (8) | 6 (22) | 0 | 1 (4) |
| Skin/soft tissue/bone | 16 (19) | 15 (55) | 1 (3) | 0 |
| Respiratory tract | 26 (31) | 1 (4) | 25 (83) | 0 |
| Gastrointestinal | 1 (1) | 0 | 0 | 1 (4) |
| Urinary tract | 21 (25) | 0 | 0 | 21 (81) |
| Central nervous System | 4 (5) | 0 | 4 (13) | 0 |
| Unknown | 8 (10) | 5 (19) | 0 | 3 (12) |
a Data are presented as numbers (percentages) for categorical variables and as median values (ranges) for continuous variables.
bMethotrexate, chemotherapeutics, or cortisol dosing equivalent to prednisolone ≥ 20mg.
The group with S. pneumoniae BSI had the most severe presentation at admission, with 73% meeting the Sepsis-3 criteria versus 48% in the S. aureus BSI group and 23% in the E. coli BSI group. However, the 90-day mortality rate was the lowest (3%) among patients with S. pneumoniae BSI compared with 26% and 8% in the S. aureus and E. coli BSI groups, respectively.
16S rDNA ddPCR results of spiked blood samples
Table 4 shows the results of the 16S rDNA ddPCR analyses compared with species-specific ddPCRs in serial dilutions of whole blood spiked with S. aureus, S. pneumoniae, or E. coli.
Table 4. Results from 16s rDNA and species-specific ddPCR analyses of serial dilutions of whole blood, spiked with Staphylococcus aureus, Streptococcus pneumoniae and Escherichia coli.
|
Dilution factor |
S. aureus |
S. pneumoniae |
E. coli |
|||
|---|---|---|---|---|---|---|
| 16S rDNA copies/mL |
nuc DNA copies/mL |
16S rDNA copies/mL |
lytA DNA copies/mL |
16S rDNA copies/mL |
uidA DNA copies/mL |
|
| 103 | 1,127,502 | 229,000 | 448,500 | 112,150 | 2,075,003 | 314,500 |
| 104 | 115,500 | 22,500 | 42,000 | 9,775 | 190,750 | 23,050 |
| 105 | 26,574 | 3,475 | 6,152 | 1,600 | 38,003 | 4,950 |
| 106 | 4,950 | 235 | 2,024 | 143 | 5,950 | 775 |
| 107 | 1,452 | 180 | 352 | 60 | 1,750 | 300 |
| 108 | 402 | 0 | 452 | 0 | 287 | 0 |
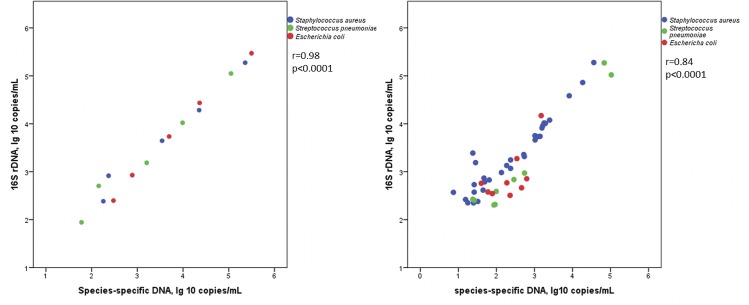
For all bacteria, the last dilution with a positive result for 16S rDNA was 1:108, and for species-specific DNA it was 1:107. 16S rDNA was still detected in the dilution with the lowest concentration, indicating an at least 10-fold lower limit of detection for the 16S rDNA ddPCR than for the species-specific ddPCRs. The correlation between 16S rDNA and species-specific DNA for all spiked samples, positive in both methods, is illustrated in Fig 1A.
Fig 1. 16S rDNA in relation to species-specific DNA (nuc for Staphylococcus aureus, lytA for Streptococcus pneumoniae, and uidA for Escherichia coli), in samples with DNA detected by both methods.
(A) Whole blood samples spiked with bacteria in serial dilutions; (B) Whole blood samples from patients sampled repeatedly during bloodstream infection, one to four samples per patient.
The median ratio between the 16S rDNA and the species-specific load was 6.6 (range, 3.9–21.1), for all samples together, 7.6 (range, 4.9–21.1) for S. aureus, 4.3 (range, 3.9–14.1) for S. pneumoniae, and 7.7 (range, 5.8–8.3) for E. coli. There was no significant difference in median ratios between the three bacteria groups (p = 0.22, 0.22, and 0.84 for S. aureus vs. S. pneumoniae, S. aureus vs. E. coli, and S. pneumoniae vs. E. coli, respectively). In a closer look at the results from the three dilutions with the highest bacteria concentration, the median ratios were nearer to the assumed 16S rDNA copies/genome for each pathogen: 5.1 (range: 4.9–7.7) for S. aureus, 4.0 (range, 3.9–4.3), for S. pneumoniae, and 7.6 (range: 6.6–8.3) for E. coli.
16S rDNA positivity in BSI patients
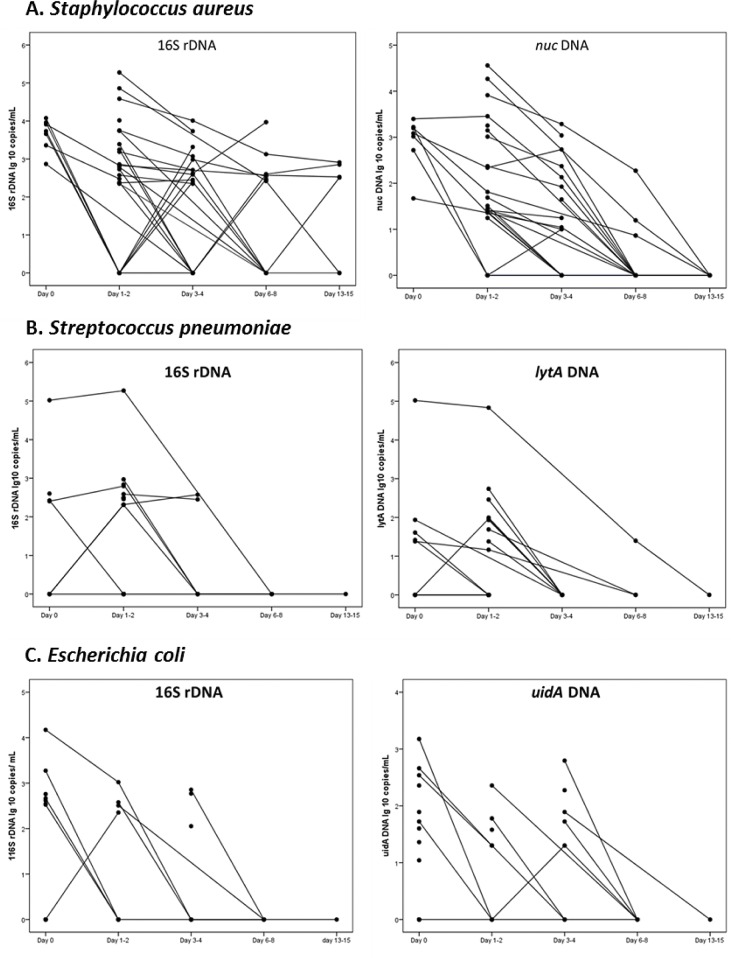
Sample frequency and positivity rates for 16S rDNA compared with species-specific ddPCR in blood from patients with BSI caused by S. aureus, S. pneumoniae, and E. coli are summarized in Table 2. The highest positivity rates were seen in S. aureus BSI, where 92% of the patients were 16S rDNA-positive and 85% nuc-positive, at least at one time point. Corresponding figures for E. coli and S. pneumoniae BSI were 46% (16S rDNA)/62% (uidA), and 43% (16S rDNA)/40% (lytA), respectively. Among patients with S.aureus BSI, both methods had a sensitivity of 100% on admission (day 0), and bacterial DNA was still detected in blood by both methods in about 80% of cases on days 1–2. Thereafter, positivity rates declined, and on days 13–15 no patients had detectable nuc DNA, while four still had detectable 16S rDNA in blood. Initial sensitivity was lower among patients with S. pneumoniae and E. coli BSI, and only a few patients had detectable bacterial DNA with any of the methods on days 3–4.
Dynamics of bacterial DNA loads during the course of BSI
Fig 2 demonstrates the dynamics of the bacterial DNA load in whole blood in the groups with S. aureus (A), S. pneumoniae (B), and E. coli (C) BSI measured with 16S rDNA and species-specific ddPCR.
Fig 2. Quantitative data of 16S rDNA and species-specific DNA.
(A) nuc for Staphylococcus aureus, (B) lytA for Streptococcus pneumoniae, and (C) uidA for Escherichia coli in individual patients with bloodstream infection and detected DNA.
The results from the S. aureus species-specific ddPCR have recently been published in a separate article focusing on nuc DNA loads in S. aureus bacteremia [32].
Results from the 16S rDNA and the species-specific ddPCR for cases shown positive by both methods were significantly correlated for S. aureus (r = 0.92, p < 0.0001) and S. pneumoniae (r = 0.83, p = 0.005), but not for E. coli (r = 0.57, p = 0.11). In the entire study group there was a significant correlation between results for the two methods (Fig 1B). Among patients with positive ddPCR in both methods, the median 16S rDNA/species-specific DNA ratio was 5.4 (range: 1.0–102.1). In all six cases where the ratio was higher than 15, the species-specific DNA load was low, at a median of 25 (range: 7–47) copies/mL. The median 16S rDNA/species-specific DNA ratios for the different BSI species were 6.5 (range: 3.8–102.1) for S. aureus, 2.5 (range: 1.0–11.1) for S. pneumoniae, and 4.5 (range: 1.0–14.4) for E. coli. There was a significant difference in median ratio between the S. aureus and S. pneumoniae groups (p = 0.002) but not between the S. aureus and E.coli groups (p = 0.66) or between the S. pneumoniae and E.coli groups (p = 0.052).
In most cases with repeatedly positive ddPCR, the load gradually fell until it was negative, but in a few cases the load increased between two sampling points (Fig 2). Four S. aureus cases were still positive for 16S rDNA at days 13–15. Three of them had stable 16S rDNA compared to the previous assessment point (days 6–8; Fig 2A) while one patient had negative 16S rDNA (detected but below cut-off) on days 6–8. All four patients with persistent DNAemia at days 13–15 had complicated S.aureus infections or severe illness (two patients with infective endocarditis, one patient with pancreatic cancer and a catheter-related infection and one with an iliopsoas abscess).
16S rDNA load on days 1–2 in relation to sepsis and mortality
BSI patients with sepsis at admission were compared with BSI patients without sepsis for 16S rDNA load at days 1–2 (just after blood culture positivity). Measured in lg10 copies/mL, patients with sepsis had significantly higher 16S rDNA load in blood (median 2.38; range: 0–5.28) than non-septic patients (median 0; range: 0–3.19) (p = 0.031). Fig 3 shows the bacterial DNA load on days 1–2 measured by 16S rDNA ddPCR in BSI patients with and without sepsis.
Fig 3. Bacterial DNA load measured by 16S rDNA droplet digital PCR on days 1–2 in patients with bloodstream infection.
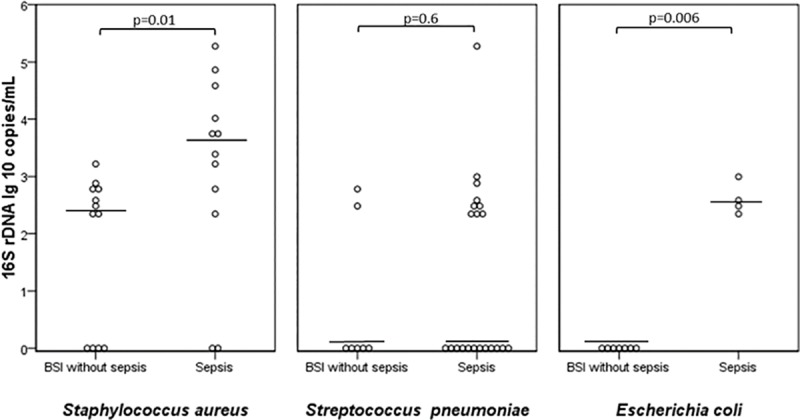
Comparison between patients with and without sepsis.
Patients with S. aureus and E. coli BSI with sepsis had significantly higher 16S rDNA loads than patients without sepsis, although no such association was found among S. pneumoniae BSI patients. Patients who died due to infection within 90 days were compared with survivors, and found to have significantly higher 16S rDNA load on days 1–2 in the entire study-group (median 2.83, range: 0–4.58 vs. median 0, range,:0–5.28 lg10 copies/mL) (p = 0.006). However, when studied separately, the associations did not reach significance level in any of the different bacteria groups.
Five patients had 16S rDNA loads above 10.000 copies/L on days 1–2 (one patient with S. pneumoniae BSI with meningitis, three with S. aureus BSI with endocarditis, and one with prosthetic joint infection).
Discussion
In this study we developed a ddPCR for quantification of 16S rDNA in whole blood and validated it against species-specific (nuc, lytA, and uidA) ddPCRs by analyzing bacterial-spiked blood samples in 10-fold dilutions and blood samples from patients with S. aureus, S. pneumoniae, and E. coli BSI. The results show that 16S rDNA ddPCR generated higher copy numbers, which can be explained by 16S rDNA’s presence in multiple copies in each bacterial genome. The ddPCR results were concordant between 16S rDNA and species-specific ddPCR in bacterial-spiked samples, and, for S. aureus and S. pneumoniae, in BSI patient samples. The inadequate concordance between 16S rDNA and uidA DNA from E. coli BSI patients might be due to few positive samples in the cohort, whereof many had low DNA loads (Fig 1B).
Several previous studies have compared ddPCR with real-time PCR and reported a smaller effect of inhibitors, greater precision, and higher reproducibility [22, 33]. ddPCR has been used for DNA quantification in various fields, such as quantifying HIV viral load [33]. The method has been used only rarely to quantify bacterial DNA in human samples [34] and no previous reports have evaluated this method in BSI assessment.
In virology, the measurement of viral load in blood is well established and used as an important part of diagnostics and treatment-response monitoring in HIV and many other viral infections [35]. Although quantification of pathogenic DNA in BSI assessment has been proposed as a potential prognostic marker [36], research into its role has been sparse. Studies have shown that a high initial DNA load is associated with both sepsis at admission and mortality [7, 10, 12] and that default DNA clearance is associated with treatment failure and mortality [12–14]. Our study found a high initial 16S rDNA load associated with sepsis and mortality, and the few patients with very high DNA loads had severe and complicated infections.
The potential value of pathogen quantification, together with a trend towards broad-range molecular approaches, often targeting rDNA, highlights the importance of correctly interpreting quantitative data from amplified rDNA products. In our study, the concordance between 16S rDNA and species-specific ddPCR was high (Fig 1) without adjusting for species-variation in copy-numbers/bacterial cell. Accordingly, quantitative 16S rDNA ddPCR appears to be a useful marker for bacterial load in BSI patients. However, to determine the true copy number, it is necessary to enable copy number adjustment by identification of the bacteria. Certainly, high-throughput technologies for broad-range species identification is on the march, but in current circumstances a 16S rDNA ddPCR without bacterial identification is considerably more time- and cost-effective. However, knowledge about the etiology is needed for clinical interpretation of the 16S rDNA load, since our results show considerable differences in the dynamics of the DNA load between species (Fig 2). The groups with S. pneumoniae and E. coli BSI had a relatively low initial positivity rate, and only a few cases were still 16S rDNA positive on days 3–4. Probably E. coli and S. pneumoniae DNA persistence is uncommon in BSI, given that the infections are rightly treated. Accordingly, persistent DNA positivity might be an indicator for treatment failure in BSI of these etiologies.
The group with S. aureus BSI had a high initial 16S rDNA positivity rate, most cases were still positive on days 3–4, and even on the last sampling point, cases with positivity were noted. (Table 2). BSI persistence is a well-known feature of S. aureus BSI, linked to complicated infections [37]. This is why routine follow-up BCs are recommended for S. aureus BSI [38]. According to our results, the value of monitoring bacterial DNA during BSI is probably the greatest in S. aureus BSI.
In the present study, results in patients with low loads of bacterial DNA showed more discrepancy between 16S rDNA and species-specific DNA. This can be explained by a diminishing performance of the methods in cases of low concentrations of target DNA. Many of the clinical samples were ddPCR-negative even at the first time point. Studies have shown that 50% of BSI episodes have bacterial bloodstream concentrations of only 0.01–1.0 CFU/mL [2, 39, 40]. Even though the pathogen DNA load is reported to be higher [41], the bacterial concentration in BSI is probably frequently below the limit of detection for most molecular methods, especially when small sample volumes of 1–2 mL are used.
Our study has limitations. First, we only included patients with S. aureus, S. pneumoniae, and E.coli BSI, and thus, extrapolation of the results to BSI of all etiologies is precarious. Second, we did not perform any species identification apart from the three species-specific PCR analyzes. Accordingly, there is a possibility that a fraction of the found 16S rDNA derives from other bacteria, possibly due to contamination, poly-microbial or secondary infection. Third, the number of patients in each subgroup with different bacterial etiologies was small, especially in the S. pneumoniae and E. coli groups, where samples were frequently ddPCR negative. Finally, not all patients had full sample series. Still, the study provided useful information on the relation between 16S rDNA and species-specific DNA and on dynamic variations between the different bacterial etiologies of BSI.
In conclusion, our results suggest that 16S rDNA ddPCR can serve as a method for broad-range quantification of bacterial DNA load during BSI, with a sensitivity comparable to species-specific ddPCRs. We found that a high initial 16S rDNA load was associated with both sepsis at admission and mortality, which indicates a potential clinical value. Further studies are needed to identify the role of 16S rDNA ddPCR within sepsis management.
Supporting information
(XLSX)
Acknowledgments
This work was supported by grants from the Research Committee of Örebro County Council and the Örebro University Hospital Research Foundation.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was supported by grants from the research committee of Örebro County Council, grant numbers OLL-885741 and OLL-652771 and from the Örebro University Hospital Research Foundation, grant number OLL-117771. The funders had no role in the study design, data collection and analysis or decision to publish.
References
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 2.Lamy B, Dargere S, Arendrup MC, Parienti JJ, Tattevin P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Front Microbiol. 2016;7:697 10.3389/fmicb.2016.00697 eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloos F, Sachse S, Kortgen A, Pletz MW, Lehmann M, Straube E, et al. Evaluation of a polymerase chain reaction assay for pathogen detection in septic patients under routine condition: an observational study. PloS one. 2012;7(9):e46003 Epub 2012/10/03. 10.1371/journal.pone.0046003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, et al. A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol. 2008;197(3):313–24. Epub 2007/11/17. 10.1007/s00430-007-0063-0 . [DOI] [PubMed] [Google Scholar]
- 5.Wellinghausen N, Kochem AJ, Disque C, Muhl H, Gebert S, Winter J, et al. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. Journal of clinical microbiology. 2009;47(9):2759–65. Epub 2009/07/03. 10.1128/JCM.00567-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordana-Lluch E, Gimenez M, Quesada MD, Rivaya B, Marco C, Dominguez MJ, et al. Evaluation of the Broad-Range PCR/ESI-MS Technology in Blood Specimens for the Molecular Diagnosis of Bloodstream Infections. PLoS One. 2015;10(10):e0140865 10.1371/journal.pone.0140865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler I, Josefson P, Olcen P, Molling P, Stralin K. Quantitative data from the SeptiFast real-time PCR is associated with disease severity in patients with sepsis. BMC infectious diseases. 2014;14:155 Epub 2014/03/25. 10.1186/1471-2334-14-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackett SJ, Guiver M, Marsh J, Sills JA, Thomson AP, Kaczmarski EB, et al. Meningococcal bacterial DNA load at presentation correlates with disease severity. Arch Dis Child. 2002;86(1):44–6. Epub 2002/01/25. 10.1136/adc.86.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters RP, de Boer RF, Schuurman T, Gierveld S, Kooistra-Smid M, van Agtmael MA, et al. Streptococcus pneumoniae DNA load in blood as a marker of infection in patients with community-acquired pneumonia. Journal of clinical microbiology. 2009;47(10):3308–12. Epub 2009/08/14. 10.1128/JCM.01071-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rello J, Lisboa T, Lujan M, Gallego M, Kee C, Kay I, et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136(3):832–40. Epub 2009/05/13. 10.1378/chest.09-0258 . [DOI] [PubMed] [Google Scholar]
- 11.Øvstebø R, Brandtzaeg P, Brusletto B, Haug KBF, Lande K, Høiby EA, et al. Use of Robotized DNA Isolation and Real-Time PCR To Quantify and Identify Close Correlation between Levels of Neisseria meningitidis DNA and Lipopolysaccharides in Plasma and Cerebrospinal Fluid from Patients with Systemic Meningococcal Disease. J Clin Microbiol. 2004;42(7):2980–7. 10.1128/JCM.42.7.2980-2987.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho YC, Chang SC, Lin SR, Wang WK. High levels of mecA DNA detected by a quantitative real-time PCR assay are associated with mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia. Journal of clinical microbiology. 2009;47(5):1443–51. Epub 2009/03/13. 10.1128/JCM.01197-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang YC, Chang SC, Wang WK. Using the rate of bacterial clearance determined by real-time polymerase chain reaction as a timely surrogate marker to evaluate the appropriateness of antibiotic usage in critical patients with Acinetobacter baumannii bacteremia. Crit Care Med. 2012;40(8):2273–80. Epub 2012/07/20. 10.1097/CCM.0b013e3182515190 . [DOI] [PubMed] [Google Scholar]
- 14.Chuang YC, Chang SC, Wang WK. High and increasing Oxa-51 DNA load predict mortality in Acinetobacter baumannii bacteremia: implication for pathogenesis and evaluation of therapy. PLoS One. 2010;5(11):e14133 10.1371/journal.pone.0014133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Zanten AR. Real-time polymerase chain reaction to evaluate antibiotic appropriateness: should we spread the news to multiply it? Crit Care Med. 2012;40(8):2492–3. 10.1097/CCM.0b013e318258e7f3 [DOI] [PubMed] [Google Scholar]
- 16.Vetrovsky T, Baldrian P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PloS one. 2013;8(2):e57923 10.1371/journal.pone.0057923 Epub 2013 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kembel SW, Wu M, Eisen JA, Green JL. Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance. PLoS Comput Biol. 2012;8(10):e1002743 10.1371/journal.pcbi.1002743 Epub 2012 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–64. 10.1128/CMR.00002-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sogaard M, Norgaard M, Dethlefsen C, Schonheyder HC. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: a population-based cohort study. Clin Infect Dis. 2011;52(1):61–9. 10.1093/cid/ciq069 [DOI] [PubMed] [Google Scholar]
- 20.Morley AA. Digital PCR: A brief history. Biomol Detect Quantif. 2014;1(1):1–2. 10.1016/j.bdq.2014.06.001 eCollection Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–10. 10.1021/ac202028g Epub 2011 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10(10):1003–5. 10.1038/nmeth.2633 Epub 013 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cajander S, Rasmussen G, Tina E, Magnuson A, Soderquist B, Kallman J, et al. Dynamics of monocytic HLA-DR expression differs between bacterial etiologies during the course of bloodstream infection. PLoS One. 2018;13(2):e0192883 10.1371/journal.pone.0192883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Droplet Digital PCR Applications Guide: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf. Bio-Rad Laboratories Inc.
- 25.Kramski M, Gaeguta AJ, Lichtfuss GF, Rajasuriar R, Crowe SM, French MA, et al. Novel sensitive real-time PCR for quantification of bacterial 16S rRNA genes in plasma of HIV-infected patients as a marker for microbial translocation. J Clin Microbiol. 2011;49(10):3691–3. 10.1128/JCM.01018-11 Epub 2011 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berglund C, Molling P, Sjoberg L, Soderquist B. Predominance of staphylococcal cassette chromosome mec (SCCmec) type IV among methicillin-resistant Staphylococcus aureus (MRSA) in a Swedish county and presence of unknown SCCmec types with Panton-Valentine leukocidin genes. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2005;11(6):447–56. Epub 2005/05/11. 10.1111/j.1469-0691.2005.01150.x . [DOI] [PubMed] [Google Scholar]
- 27.Bogestam K, Vondracek M, Karlsson M, Fang H, Giske CG. Introduction of a hydrolysis probe PCR assay for high-throughput screening of methicillin-resistant Staphylococcus aureus with the ability to include or exclude detection of Staphylococcus argenteus. PLoS One. 2018;13(2):e0192782 10.1371/journal.pone.0192782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheppard CL, Harrison TG, Morris R, Hogan A, George RC. Autolysin-targeted LightCycler assay including internal process control for detection of Streptococcus pneumoniae DNA in clinical samples. J Med Microbiol. 2004;53(Pt 3):189–95. 10.1099/jmm.0.05460-0 [DOI] [PubMed] [Google Scholar]
- 29.Yoshitomi KJ, Jinneman KC, Weagant SD. Optimization of a 3'-minor groove binder-DNA probe targeting the uidA gene for rapid identification of Escherichia coli O157:H7 using real-time PCR. Molecular and cellular probes. 2003;17(6):275–80. Epub 2003/11/07. . [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. Epub 1987/01/01. 10.1016/0021-9681(87)90171-8 . [DOI] [PubMed] [Google Scholar]
- 31.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler I, Cajander S, Rasmussen G, Ennefors T, Molling P, Stralin K. High nuc DNA load in whole blood is associated with sepsis, mortality and immune dysregulation in Staphylococcus aureus bacteraemia. Infect Dis (Lond). 2019;51(3):216–26. 10.1080/23744235.2018.1562205 Epub 2019 Jan 24. [DOI] [PubMed] [Google Scholar]
- 33.Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PloS one. 2013;8(4):e55943 10.1371/journal.pone.0055943 Epub 2013 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sze MA, Abbasi M, Hogg JC, Sin DD. A comparison between droplet digital and quantitative PCR in the analysis of bacterial 16S load in lung tissue samples from control and COPD GOLD 2. PLoS One. 2014;9(10):e110351 10.1371/journal.pone.0110351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewin SR, Vesanen M, Kostrikis L, Hurley A, Duran M, Zhang L, et al. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J Virol. 1999;73(7):6099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opota O, Jaton K, Greub G. Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2015;21(4):323–31. Epub 2015/02/18. 10.1016/j.cmi.2015.02.005 . [DOI] [PubMed] [Google Scholar]
- 37.Khatib R, Riederer K, Saeed S, Johnson LB, Fakih MG, Sharma M, et al. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin Infect Dis. 2005;41(5):594–8. Epub 2005/08/05. 10.1086/432472 . [DOI] [PubMed] [Google Scholar]
- 38.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Torok ME, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11(3):208–22. 10.1016/S1473-3099(10)70285-1 [DOI] [PubMed] [Google Scholar]
- 39.Werner AS, Cobbs CG, Kaye D, Hook EW. Studies on the bacteremia of bacterial endocarditis. JAMA. 1967;202(3):199–203. [PubMed] [Google Scholar]
- 40.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36(6):1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacconi A, Richmond GS, Baroldi MA, Laffler TG, Blyn LB, Carolan HE, et al. Improved sensitivity for molecular detection of bacterial and Candida infections in blood. J Clin Microbiol. 2014;52(9):3164–74. 10.1128/JCM.00801-14 Epub 2014 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.