Abstract
Study Objectives:
Scleroderma is associated with abnormal skin thickening, interstitial lung disease, pulmonary hypertension, and abnormalities of the upper airway. These changes can cause cardiopulmonary complications, potentially including sleep-disordered breathing. The objective of this study is to examine the risk of sleep-disordered breathing in patients with scleroderma.
Methods:
We retrospectively identified patients with documented scleroderma. We abstracted data from their electronic health records, including findings from antibody tests, serial pulmonary function tests, transthoracic echocardiography, high-resolution computed tomography, and overnight forehead oximetry.
Results:
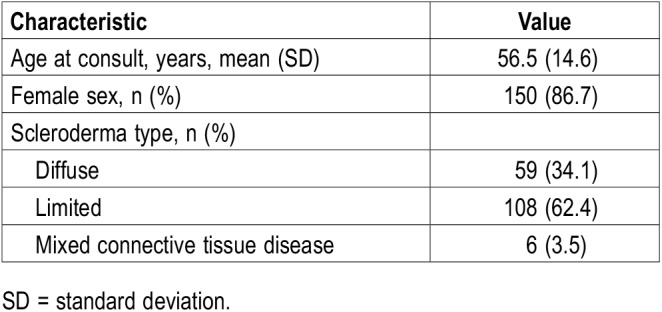
We identified 171 patients with scleroderma. Mean age at the time of initial consult was 56.5 years (range, 18–96 years), and 150 (86.7%) were women. Scleroderma was categorized as limited disease for 108 (62.4%), diffuse disease for 59 (34.1%), and mixed connective tissue disease for 6 (3.5%). Fifty-four patients (31.2%) had abnormal overnight forehead oximetry results, defined as an oxygen desaturation index greater than 5 or a baseline mean arterial oxygen saturation level less than 90%.
Conclusions:
Cardiopulmonary complications are common in patients with scleroderma, one of which may be sleep-disordered breathing. In our cohort, approximately one-third of individuals with scleroderma had evidence of sleep-disordered breathing. Moreover, the rate of sleep-disordered breathing in our population of scleroderma patients was twice the rate of pulmonary hypertension and was approximately the same as the rate of interstitial lung disease. Future prospective studies are needed to further assess the role of sleep-disordered breathing in scleroderma clinical outcomes.
Citation:
Nokes BT, Raza HA, Cartin-Ceba R, Lyng PJ, Krahn LE, Wesselius L, Jokerst CE, Umar SB, Griffing WL, Neville MR, Malhotra A, Parish JM. Individuals with scleroderma may have increased risk of sleep-disordered breathing. J Clin Sleep Med. 2019;15(11):1665–1669.
Keywords: lung, oximetry, scleroderma, sleep apnea syndrome, systemic
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cardiopulmonary complications are a common manifestation of systemic sclerosis. To date, the prevalence for sleep disordered breathing may be underappreciated.
Study Impact: To our knowledge, this is the first study of this size to look at overnight oximetry data and extrapolate the potential prevalence of sleep-disordered breathing in scleroderma.
INTRODUCTION
Scleroderma (also termed systemic sclerosis [SSc]) is a condition associated with abnormal skin thickening, interstitial lung disease (ILD), pulmonary hypertension (PH), esophageal motility disorders, and other complications.1 SSc is a rare disorder, with an incidence rate of 2.3 to 22.8 cases per million persons per year.1 Because of the clinical rarity of SSc, study of the disease course and its effect on sleep has been minimal.1
Scleroderma is broadly categorized as limited or diffuse, as determined by the modified Rodnan skin score,1 which considers the distribution and severity of skin thickening. Diffuse as compared to limited SSc is more likely to be associated with internal organ involvement.1 Moreover, scleroderma, especially the diffuse type, is known to cause oropharyngeal dysfunction,2,3 which may be important in maintaining upper airway patency. The risk of ILD and PH for patients with scleroderma varies with autoantibody subtype.4 Accordingly, the broad array of cardiopulmonary phenotypes in SSc led us to hypothesize that scleroderma might be associated with sleep-disordered breathing (SDB).
One method of screening for SDB is pulse oximetry. This is a valid and cost-effective tool for screening for SDB.5 Although polysomnography (PSG) is the gold standard in screening for SDB, overnight oximetry is a reasonable screening test.6 Evidence of SDB that can be obtained from pulse oximetry include: low pulse oximetry (SpO2) values, elevated oxygen desaturation index (ODI), or the presence of sawtoothing all can suggest evidence of SDB. There is no universally agreed upon ODI cutoff for evidence of SDB, but a cutoff of ≥ 4 events/h is generally used.5,7,8 Notably, the accuracy of pulse oximetry can be affected by peripheral vasoconstriction9 as well as the digital remodeling seen in SSc. For this reason, we chose to use forehead oximetry as a screening tool for SDB in individuals with SSc as part of a comprehensive scleroderma assessment at our tertiary referral center. Forehead, finger, and ears are all reliable sites for oximetry, because of the increased vascular density in these areas (relative to the trunk or other sites).9 Moreover, in routine cardiopulmonary assessments of individuals with scleroderma, forehead oximetry is likely a superior modality for SpO2 assessment.10 In a limited number of agreeable participants, we were also able to obtain dedicated PSG tests. In so doing, we sought to evaluate the prevalence of SDB in SSc.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board.
Data Collection
We retrospectively reviewed records of patients seen at Mayo Clinic (Phoenix, Arizona), a tertiary referral center, from January 1, 2005, through December 31, 2015, for scleroderma evaluation. All patients were seen by one rheumatologist (WLG) and all had a uniform assessment that included clinical evaluation, laboratory tests (including antibody tests), echocardiography, pulmonary function tests, high-resolution computed tomography (CT), and overnight oximetry with a forehead probe. Forehead oximetry was chosen instead of finger pulse oximetry because of the high prevalence of Raynaud phenomenon among patients with scleroderma. Forehead oximetry is a suitable alternative when peripheral oximetry is not an option.11 Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Mayo Clinic.12 REDCap is a secure, Web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
From this database, we reviewed all overnight oximetry studies for evidence of SDB. Overnight oximetry was performed by using a Nonin PalmSat 2500 oximeter (Nonin Medical, Tilburg, the Netherlands) and analyzed with Profox oximetry software (Profox, Coral Springs, Florida). The ODI was calculated by dividing the total number of desaturation events of 4% or greater by the total hours of recording time. An ODI value greater than 5 events/h was considered abnormal. A mean oxygen saturation as measured by SpO2 level below 90% for the entire recording was also considered abnormal. Time below 90% was unfortunately not routinely denoted on oximetry reports unless it was abnormal by the reading sleep physician. Each oximetry tracing was manually inspected by our evidence of SDB, including low SpO2, elevated ODI, or the presence of sawtoothing.
We also obtained demographic data for all patients, including sex, age at consult, and body mass index. The presence of ILD was determined by reviewing pulmonary function tests and reports of high-resolution thoracic CT. A restrictive pattern on pulmonary function studies was determined by a total lung capacity of less than 80% of the predicted normal value and a diffusing capacity of lung for carbon monoxide of less than 80% of the predicted normal. All thoracic CT images were interpreted by a thoracic radiologist, who commented on degree of fibrosis, honeycombing, pulmonary artery size, and whether the esophagus was considered patulous.
PH was defined by a mean pulmonary artery pressure exceeding 25 mm Hg in those who underwent right-sided heart catheterization. Elevated right ventricular systolic pressure on echocardiography was used as a screening parameter for referral for possible right-heart catheterization.
Statistical Analyses
Logistic regression analysis was used to test the predictive capacity of covariates on abnormal oximetry findings, defined as whether a patient had an ODI greater than 5 or a mean oxygen saturation less than 90%.Values of P < .05 were considered significant. Descriptive analyses regarding dedicated sleep studies were extracted directly from REDCap.
RESULTS
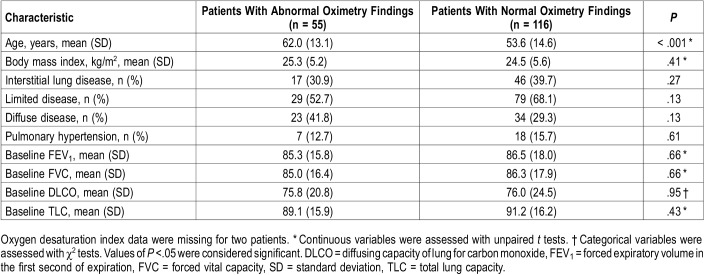
We identified 171 patients with scleroderma. Baseline patient demographic data are detailed in Table 1. None were on supplemental oxygen before or during the oximetry study. In the cohort, 55 patients (32.1%) had abnormal overnight forehead oximetry findings. Fifty-two patients had an elevated ODI (≥ 5). Of those patients with abnormal oximetry readings, the median ODI was 8.2/h (interquartile range, 6.0–11.8) and the mean SpO2 was 92.9%.
Table 1.
Patient characteristics (n = 171).
Of these 53 with an elevated ODI, 47 had a normal mean SpO2, whereas 6 had a low mean SpO2. Two patients had low mean SpO2 without an elevated ODI. Four of the patients with elevated ODI also had evidence of “sawtoothing” on the oximetry tracing. Sawtoothing was not demonstrated in the absence of an elevated ODI.
Of the subgroup with low baseline SpO2 2 had PH without ILD, 1 had both ILD and PH, and 5 had neither ILD nor PH. (Table 2). There were no specific autoantibodies that predicted abnormal oximetry.
Table 2.
Predictors of abnormal oximetry findings.
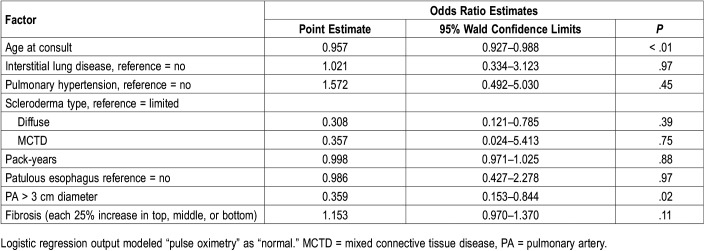
Regression analysis showed that the only predictive variables for abnormal oximetry were age (P < .001) and enlarged pulmonary artery diameter (P = .03); the presence of ILD, PH (as measured by right heart catheterization), smoking history, or scleroderma subtype (diffuse, limited, mixed connective tissue disease) were not predictive factors (Table 3).
Table 3.
Logistic regression for predictive factors of abnormal overnight oximetry findings.
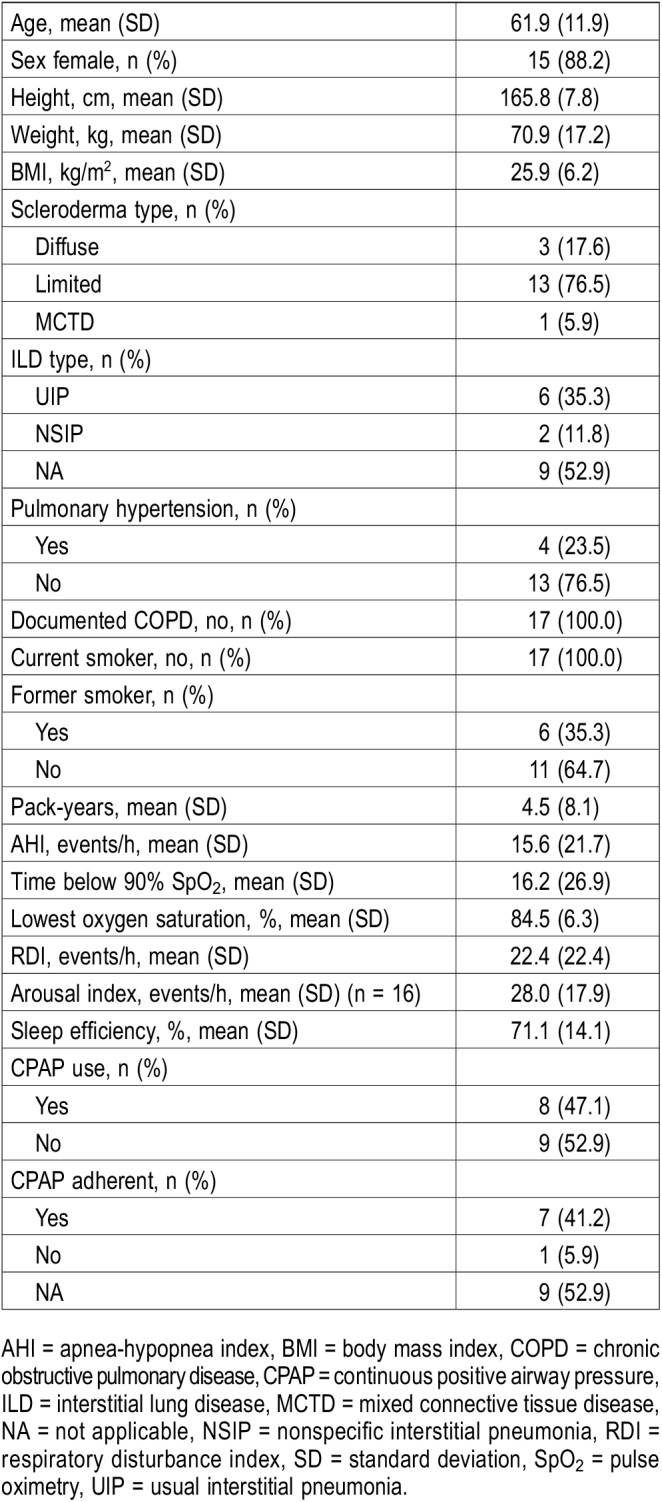
Of those with abnormal oximetry, 17 patients agreed to undergo full polysomnography. Of these, 13 (76.5%) had limited scleroderma, 8 (47.1%) had ILD, and 4 (23.5%) had PH. None were smokers or had chronic obstructive pulmonary disease. The mean apnea-hypopnea index was 15.6 events/h, the mean time below 90% oxygen saturation was 16.2%, the mean respiratory disturbance index was 22.4 events/h. Six of the 17 patients who underwent PSG had concerns about excessive sleepiness; however, they had normal PSG findings and normal overnight oximetry readings. Seven patients had elevated apnea-hypopnea indices that corresponded to abnormal ODIs on oximetry, and four had low baseline oxygen saturation. The sleep study data for these 17 patients are shown in Table 4.
Table 4.
Polysomnography data (n = 17).
DISCUSSION
Our study represents a unique opportunity to examine sleep disturbance associated with scleroderma. Although the study was limited by its retrospective design, we describe a uniquely large cohort of patients with a clinically rare disease that underwent a uniform cardiopulmonary assessment.
As noted, a multitude of cardiopulmonary outcomes, such as PH and ILD, are attributable to scleroderma. PH and ILD are often associated with SDB. In particular, patients with ILD have abnormal sleep architecture, a markedly increased risk of sleep apnea, and nocturnal oxygen desaturation that may manifest as an elevated ODI.13 Further, individuals with World Health Organization group 1 PH have increased risk of SDB.14 Minic et al.15 showed an increased risk of central and obstructive sleep apnea in individuals with World Health Organization group 1 PH. Risk factors for SDB within this population were older age and increased Epworth Sleepiness Scale score.
We believe that, in addition to PH and ILD, scleroderma itself was an independent risk factor for SDB.4,14–16 Scleroderma, especially the diffuse type, is known to cause oropharyngeal dysfunction, which may predispose patients toward SDB.2,3 Scleroderma is characterized by immune dysregulation−associated vascular changes and fibrosis1; these changes affect systemic and pulmonary vascular beds, esophageal mucosal surfaces, and the pharynx.1,17 Scleroderma can cause cricoarytenoid ankyloses, vocal cord nodules, and vocal cord dysfunction, all of which could potentially contribute to SDB.18
Despite the potential for cardiopulmonary pathology in SSc, few studies have evaluated the risk of SDB in patients with scleroderma.19 Small, survey-based studies about quality of life and sleep were conducted by Frech et al19 Prado and colleagues20 assessed the sleep studies of a small group of patients with scleroderma (n = 27) and noted no increased risk for SDB, but they did observe that patients with SSc had reduced sleep efficiency, less rapid-eye movement sleep, and increased arousal index and slow-wave sleep.
In our study, the predictive variables for abnormal overnight oximetry included patient age, which is a risk factor for SDB, irrespective of the presence of underlying lung disease.21 However, the presence of ILD and PH were not predictive of abnormal overnight oximetry findings, even though they are well-defined risk factors for SDB. We speculate that those patients still had risk of SDB because of oropharyngeal and laryngeal fibrosis, though this is not explicitly noted in review of their clinical visits.
The greatest limitation of our study is that it is retrospective and based on chart review. We did not have a sufficient number of dedicated sleep studies because many of these patients came from a broad geographic area and were often not able to stay for formal sleep studies following abnormal oximetry. Forehead oximetry as a modality for assessing presence of SDB may also be problematic, as there is a chance of spuriously low SpO2 with forehead oximetry, especially if the probe is insufficiently secured. However, we were able to manually review each of our tracings for quality assurance.
Despite these limitations, our study is the largest assessment to date of the prevalence of SDB in SSc. Prospective studies evaluating SDB in scleroderma may better determine the effect of this condition on prognosis, and whether therapies such as positive airway pressure are effective.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
ACKNOWLEDGEMENTS
Author contributions: Dr. Nokes and Dr. Raza collected all data and drafted the initial and final versions of the manuscript. Dr. Cartin-Ceba provided extensive oversight in variable selection during database development and edited the manuscript for scientific accuracy. Dr. Lyng and Dr. Krahn helped establish the sleep-related variables for analysis and examined the primary data. Dr. Wesselius provided his clinical insight from caring for a large portion of the patients described in our database and provided guidance in selecting pulmonary physiology-related variables. Dr. Jokerst reviewed all high-resolution computed tomographic images and issued fibrosis scores, honeycombing scores, and pulmonary artery size. Dr. Umar collected the initial patient cohort in conjunction with Dr. Griffing, providing the framework for our database. Dr. Griffing provided patient medical record numbers for our database and extensive insights from his career in managing patients with scleroderma. Matthew Neville performed all statistical analyses and edited the portions describing the statistical analyses. Dr. Malhotra oversaw the discussion of the manuscript and reviewed its content for scientific accuracy. Dr. Parish oversaw the project design and extensively edited the manuscript.
ABBREVIATIONS
- CT
computed tomography
- ILD
interstitial lung disease
- ODI
oxygen desaturation index
- PH
pulmonary hypertension
- PSG
polysomnography
- REDCap
Research Electronic Data Capture
- SDB
sleep-disordered breathing
- SpO2
oxygen saturation
- SSc
systemic sclerosis
REFERENCES
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Crowell MD, Umar SB, Griffing WL, DiBaise JK, Lacy BE, Vela MF. Esophageal motor abnormalities in patients with scleroderma: heterogeneity, risk factors, and effects on quality of life. Clin Gastroenterol Hepatol. 2017;15(2):207–213.e1. doi: 10.1016/j.cgh.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Montesi A, Pesaresi A, Cavalli ML, Ripa G, Candela M, Gabrielli A. Oropharyngeal and esophageal function in scleroderma. Dysphagia. 1991;6(4):219–223. doi: 10.1007/BF02493531. [DOI] [PubMed] [Google Scholar]
- 4.Aithala R, Alex AG, Danda D. Pulmonary hypertension in connective tissue diseases: an update. Int J Rheum Dis. 2017;20(1):5–24. doi: 10.1111/1756-185X.13001. [DOI] [PubMed] [Google Scholar]
- 5.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120(2):625–633. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 6.Yamashiro Y, Kryger MH. Nocturnal oximetry: is it a screening tool for sleep disorders? Sleep. 1995;18(3):167–171. doi: 10.1093/sleep/18.3.167. [DOI] [PubMed] [Google Scholar]
- 7.Lin CL, Yeh C, Yen CW, Hsu WH, Hang LW. Comparison of the indices of oxyhemoglobin saturation by pulse oximetry in obstructive sleep apnea hypopnea syndrome. Chest. 2009;135(1):86–93. doi: 10.1378/chest.08-0057. [DOI] [PubMed] [Google Scholar]
- 8.Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest. 1996;110(6):1489–1492. doi: 10.1378/chest.110.6.1489. [DOI] [PubMed] [Google Scholar]
- 9.Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med. 2013;107(6):789–799. doi: 10.1016/j.rmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Wilsher M, Good N, Hopkins R, et al. The six-minute walk test using forehead oximetry is reliable in the assessment of scleroderma lung disease. Respirology. 2012;17(4):647–652. doi: 10.1111/j.1440-1843.2012.02133.x. [DOI] [PubMed] [Google Scholar]
- 11.Jubran A. Pulse oximetry. Crit Care. 2015;19(1):272. doi: 10.1186/s13054-015-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troy LK, Corte TJ. Sleep disordered breathing in interstitial lung disease: a review. World J Clin Cases. 2014;2(12):828–834. doi: 10.12998/wjcc.v2.i12.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurnheer R, Ulrich S, Bloch KE. Precapillary pulmonary hypertension and sleep-disordered breathing: is there a link? Respiration. 2017;93(1):65–77. doi: 10.1159/000452957. [DOI] [PubMed] [Google Scholar]
- 15.Minic M, Granton JT, Ryan CM. Sleep disordered breathing in group 1 pulmonary arterial hypertension. J Clin Sleep Med. 2014;10(3):277–283. doi: 10.5664/jcsm.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riemekasten G, Philippe A, Nather M, et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70(3):530–536. doi: 10.1136/ard.2010.135772. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Singh J, Kedika R, et al. Role of muscarinic-3 receptor antibody in systemic sclerosis: correlation with disease duration and effects of IVIG. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G1052–G1060. doi: 10.1152/ajpgi.00034.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viner DD, Sabri A, Tucker HM. Bilateral cricoarytenoid joint ankylosis in scleroderma. Otolaryngol Head Neck Surg. 2001;124(6):696–697. doi: 10.1177/019459980112400622. [DOI] [PubMed] [Google Scholar]
- 19.Frech T, Hays RD, Maranian P, Clements PJ, Furst DE, Khanna D. Prevalence and correlates of sleep disturbance in systemic sclerosis–results from the UCLA scleroderma quality of life study. Rheumatology (Oxford) 2011;50(7):1280–1287. doi: 10.1093/rheumatology/ker020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prado GF, Allen RP, Trevisani VM, Toscano VG, Earley CJ. Sleep disruption in systemic sclerosis (scleroderma) patients: clinical and polysomnographic findings. Sleep Med. 2002;3(4):341–345. doi: 10.1016/s1389-9457(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 21.McMillan A, Morrell MJ. Sleep disordered breathing at the extremes of age: the elderly. Breathe (Sheff) 2016;12(1):50–60. doi: 10.1183/20734735.003216. [DOI] [PMC free article] [PubMed] [Google Scholar]