Abstract
Study Objectives:
Short sleep duration contributes to hypertension, yet few behavioral sleep extension interventions have been developed. The goal of our study was to evaluate the feasibility and preliminary efficacy of a technology assisted sleep extension intervention among individuals with prehypertension/stage 1 hypertension on sleep, blood pressure and patient reported outcomes.
Methods:
Adults aged 30–65 with 24h ambulatory blood pressure (ABP) > 120/80 mmHg and average weekday sleep duration < 7 h/night were randomized 2:1 to a 6-week technology assisted intervention versus a self-management control group. The intervention included a wearable sleep tracker, smartphone application, weekly didactic lessons and brief telephone coaching. The control group was instructed to maintain their current sleep schedule. Data were analyzed using descriptive statistics and nonparametric statistics to evaluate differences in between groups as well as prepost changes within each group. We also conducted bivariate correlations to evaluate predictors of change in sleep and ABP.
Results:
A total of 16 adults were randomized into the study (11 intervention, 5 control group, 8 women, mean age 45.8 years, standard deviation 9.8 years.) Results at 6-week follow-up demonstrated greater improvement in the intervention group for total sleep time (P = .027), reductions in 24-hour systolic blood pressure (P = .013) and diastolic blood pressure (P = .026), improvements in sleep disturbance (P = .003) and sleep-related impairment (P = .008). Participants in the intervention group completed 90% of the coaching sessions and rated the enjoyment of the intervention as 4 or 5 out of 5.
Conclusions:
Technology assisted sleep extension intervention is feasible and well liked in this population. Results demonstrate the potential for this intervention to improve sleep duration, quality and 24-hour ABP.
Citation:
Baron KG, Duffecy J, Richardson D, Avery E, Rothschild S, Lane J. Technology assisted behavior intervention to extend sleep among adults with short sleep duration and prehypertension/stage 1 hypertension: a randomized pilot feasibility study. J Clin Sleep Med. 2019;15(11):1587–1597.
Keywords: sleep extension, sleep duration, hypertension, ambulatory blood pressure
BRIEF SUMMARY
Current Knowledge/Study Rationale: Short sleep duration contributes to poorer physical and mental health but few studies have developed interventions to extend sleep. In this study, we conducted a pilot feasibility study of a technology assisted sleep extension intervention that uses a consumer wearable, interactive smartphone app, didactic lessons and brief telephone coaching.
Study Impact: Results of this study demonstrate that this intervention was well received and can extend sleep among individuals with short sleep duration and hypertension. Extending sleep duration with this intervention reduced 24-hour ambulatory blood pressure and improved daytime functioning in this population.
INTRODUCTION
Nearly 1 in 3 American adults have hypertension and over half of individuals with hypertension do not have this condition controlled.1 Given that hypertension is the leading contributor to cardiovascular death and stroke, improving control of hypertension is a major public health priority.2 Lifestyle interventions such as diet and exercise are important components in the prevention and treatment of hypertension.3 Short sleep duration is common and a potentially modifiable contributor to hypertension. There is growing support from experimental and epidemiologic studies that short sleep duration is a causal factor in hypertension.4 It is reported that 70.1 million adults in the United States sleep < 6 hours on weekdays.5 However, few studies have focused on developing interventions to extend sleep duration as a method to reduce blood pressure.
Previous sleep extension interventions have focused on whether extending sleep improves physiological outcomes. As is appropriate in the foundational studies that lead to intervention development, these first studies have used high intensity, one-on-one interventions such as sleep logs, face-to-face educational visits and between session phone calls to encourage patients to extend their time in bed.6 One study of individuals with prehypertension/stage 1 hypertension extended sleep by 35 minutes and demonstrated a blood pressure reduction of 14 mmHg systolic blood pressure (SBP)/8 mmHg diastolic blood pressure (DBP)7 on average. Other studies have demonstrated improvements in metabolic measures,8 appetite and dietary behaviors.9,10 Given these promising results, research is needed to translate these experiments to scalable behavioral interventions that rely less on individual, face to face intervention and also motivate sustained sleep behavior change.
Toward this end, we have focused our intervention development process on using consumer sleep trackers to motivate changes to sleep duration. Consumer sleep trackers provide an opportunity to deliver sleep interventions in an engaging format. Sales of consumer sleep trackers are rapidly increasing and sleep is one of the top behaviors that consumers want to measure.11 We have developed a technology assisted behavioral sleep extension intervention that employs four elements—a wearable sleep tracker, didactic content, interactive smartphone features and brief telephone counseling.12 The intervention is based on principles of cognitive behavioral therapy13 and motivational interviewing14 using techniques such as goal setting and supportive accountability.15 Our initial user testing demonstrated that our intervention was usable and engaging to participants.12 Their feedback was used to hone the intervention and prepare for a longer study among patients with chronic illness.
Therefore, the goal of this study was to conduct a pilot study to evaluate the feasibility and preliminary efficacy of a 6-week sleep extension intervention among individuals with short sleep duration and prehypertension/stage 1 hypertension. The goal of this study was to explore the package intervention composed of elements that have been previously effective in other technology assisted interventions.16,17 If our intervention is effective, the next steps would be to evaluate efficacy of individual components to optimize the intervention. We present details on recruitment of this population, intervention usage, preliminary efficacy of our intervention for sleep and quality of life, and predictors of sleep improvement. Our hypothesis was that our technology assisted sleep extension intervention would be well liked and effective at extending sleep by 30 minutes or more. We also predicted improvements in blood pressure and daytime function.
METHODS
Design Overview
This was a randomized pilot feasibility trial with a 2:1 allocation ratio. The 2:1 allocation ratio was chosen to afford collection of as much data on the intervention as deemed scientifically reasonable.
Participants
Inclusion criteria were age 30–65 years, blood pressure ≤ 120–159/80–99 mmHg on 24-hour ambulatory blood pressure (ABP) monitoring, average workday sleep duration < 7 hours measured on wrist actigraphy, < 8 hours in bed, and a smartphone user. Exclusion criteria included (1) high risk or presence of sleep disorders, including obstructive sleep apnea (OSA), restless legs syndrome (RLS), or insomnia as assessed by history or screening questionnaires; (2) history of cognitive or neurological disorders; (3) presence of any major psychiatric disorder (eg, bipolar I, schizophrenia), current alcohol or substance abuse; (4) unstable or serious medical illness; (5) work hours midnight to 5:00 am > 1 time/mo or travel over 2 time zones in the past 6 months; (6) Use of antihypertensive medications; (7) inability to read and write English; (8) pregnancy or desire to become pregnant during the study period; (9) significant environmental factors disturbing sleep (eg, ≥ 4 awakenings/wk due to caregiving responsibilities); and (10) body mass index ≥ 50 kg/m2 because of difficulty obtaining ABP readings with large arm circumference. This study was approved by the Rush University Medical Center IRB (IRB# 16092701) and all participants provided written informed consent.
Procedure
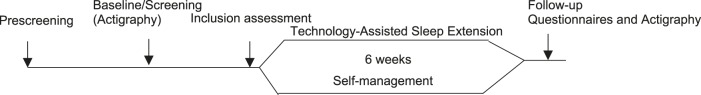
The procedure for this study is illustrated in Figure 1.
Figure 1. Study procedure and visit schedule.
Data Collection
Study data were collected and managed using REDCap electronic data capture tools hosted at Rush University Medical Center.18 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Recruitment
Participants were recruited via flyers posted in and around Rush University Medical Center and through targeted letters to primary care patients at Rush University Medical Center. Letters were sent to primary care patients ages 30–65 who had at least one elevated blood pressure reading (> 120/80 mmHg) in the past 6 months. Records were excluded if the patient was taking antihypertensive medications, had a diagnosis of insomnia or OSA or if they were taking hypnotic medications. We sent lists of patients to their respective primary care physician for review before mailing letters, in order to avoid sending inappropriate letters (eg, patient recently moved, had a major illness or was deceased in the past 6 months). After letters were mailed to potentially eligible patients, study staff followed up with a phone call and/or email to provide study information and conduct further screening if the patient was interested.
Prescreening
We verified inclusion criteria based on a two step screening process. First, participants completed a brief prescreening either online or over the phone to assess for self-reported sleep duration, medications and comorbid medical and psychiatric conditions. We initially only assessed for self-reported sleep disorders at prescreening, but after beginning the trial, we moved the questionnaires to assess for insomnia, OSA and RLS symptoms to the prescreening phase. Second, those who met the initial prescreening criteria were scheduled for a 1-hour inclusion assessment visit in the research office. This visit included consent procedures, pretreatment questionnaires, standardized measurements of height, weight, waist and hip circumference and in-office blood pressure.
Inclusion Assessment
The second step of the screening process was used to verify the inclusion criteria also served as the baseline study visit. Participants completed additional baseline study questionnaires, were trained in the use of the ABP monitor and Actiwatch Spectrum Plus (Philips Respironics, Murrysville, Pennsylvania, United States), and sent home for a 7-day assessment. At the end of the 7-day period, the participant returned to the laboratory for download of the blood pressure and Actiwatch. If eligible and willing to enroll in the study, participants were randomized at this visit and participants in the intervention group were trained on the set up of the sleep tracker (Fitbit Flex 2, Fitbit, Inc., San Francisco, California, United States).
Randomization
Participants were randomized on a 2:1 ratio, intervention to self-management control groups, using a random number generator in random permutated blocks of 4 and 6. Assignments were prepared by the study statistical programmer (EAM), placed in sealed envelopes and provided to the study research assistant.
Intervention
The 6 week intervention involved 4 components—(1) a wearable sleep tracker, (2) interactive smartphone application, (3) weekly didactic content, and (4) brief telephone coaching to facilitate adherence.
-
1.
Wearable sleep tracker: Participants received the Fitbit Flex 2 and were instructed to wear the device both day and night and charge weekly. They were told that their study coach would be able to view their data.
-
2.
Smartphone application: Participants downloaded the Fitbit application on their smartphone at the inclusion assessment visit. They were also provided a study log-in and participant ID to allow secured and confidential study communications. Their assigned study coach was also provided with the participant log-in information, in order to view participants’ data.
-
3.
Didactic content: Participants received weekly didactic information in the form of a study newsletter via email as part of the program. Topics included importance of sleep, techniques to avoid bedtime procrastination, stress and sleep, coping with challenges, optimizing your sleep environment, sleep and the brain and maintaining your gains.
-
4.
Brief telephone coaching: Participants received brief weekly coaching from a study coach (KB). The first coaching session was a 20-minute engagement session, which included introductions, rationale for the program, review of baseline sleep data, clarifying roles of the coach and setting the participants’ goals for the program. Participants were encouraged to increase time in bed by at least 1 hour or up to 8 hours of time in bed. They were also encouraged to increase consistency of their sleep schedule. In the remaining weeks, the coaching consisted of a brief phone call (approximately 5 minutes) to review the weekly data and troubleshoot any barriers toward their goals.
Control Group
Consistent with the objective of a pilot/feasibility trial, we selected a weak comparison group.19 Participants assigned to sleep maintenance control group were instructed to maintain their baseline sleep schedule throughout the study but were not monitored or given specific instructions beyond the verbal request. They were eligible to receive the intervention components at the end of the study (email content, coaching and Fitbit). This control group was selected to determine the degree to which sleep duration changes by enrolling in a research study.
Blinding
Due to the size of this pilot study, participants, coaches and study staff were not blinded.
Sample Size Determination
The sample size was planned to enroll 30 participants, as this was deemed a reasonable number of participants to provide adequate data on feasibility. The study was ended at 16 participants due to the end of the funding period.
Screening Measures
STOP questionnaire20 was administered to screen for high risk of obstructive sleep apnea. In this 4 item question, participants who answer positively to 2 of the 4 yes/no items (snoring, tired, observed apneas or high blood pressure) are considered high risk for OSA.
Insomnia Severity Index21 was administered to screen for insomnia symptoms. In this 7-item questionnaire, participants respond on 5-point items ranging from 0 (no problem) to 4 (very severe problem). Scores range from 0 to 28. Participants with scores ≥ 22–28 were excluded due to severe insomnia symptoms.
International Restless Legs Questionnaire (IRLSQ)22 was administered to screen for symptoms of RLS. On this 10-item questionnaire, participants respond to questions about the presence and severity of RLS symptoms using a Likert scale from 4 (very severe) to 0 (none).
Depressive symptoms were measured via the Patient Health Questionnaire, 8 items (PHQ-8).23 This 8-item measure has the same items as the 9-item questionnaire, but the suicide item is removed. The PHQ-8 scores range from 0–24, with scores ≥ 10 indicating elevated depressive symptoms.23 Participants completed the PHQ-8 at screening/baseline and again at the end of the intervention to assess for change in mood.
Demographics, Health History and Medications
Participants provided their age, sex, race, ethnicity, income, marital status, current medical conditions and medications by self-report.
Sleep Measures
Sleep wake pattern was measured using 7 days of wrist actigraphy using the Actiwatch Spectrum Plus conducted at the inclusion assessment/baseline session and again at the 6-week post intervention visit. Actiwatches were set with default settings for 30-second epochs and scored by study staff using a standardized protocol in Actiware software (version 6.0, Philips Respironics).24 Variables calculated included sleep onset time, sleep offset time, total sleep time (TST), wake after sleep onset (WASO), time in bed (TIB) and sleep efficiency (SE). Participants needed at least 4 valid days to be included in the analyses. The pre and post variables were calculated using matched days to account for a similar number of work and free days in each time period.
Blood Pressure
Participants’ 24-hour ABP was measured using the SpaceLabs OnTrak 90217A Ambulatory Blood Pressure Monitor (ABPM; Spacelabs Healthcare, Hertford, United Kingdom) and scored using the SpaceLabs Sentinal 10 software (Spacelabs Healthcare). ABPM were programmed to take readings at random times every 20 minutes in the daytime 7:01 am to 11:00 pm and every 30 minutes from 11:01 pm to 7:00 am. The software provides summaries of 24-hour SBP, DPB as well as daytime SBP, DBP, night SBP, DBP and % dipping. Participants wore the ABPM for 1 night at the inclusion assessment/baseline and postintervention session (6 weeks). Profiles were considered valid if there were at least 14 daytime readings and 7 reading at night.25
Standardized automated oscillometric blood pressure was also recorded at the baseline and 6-week follow-up visits using an Omron Series 7 Upper Arm Blood Pressure Monitor (Omron, Kyoto, Japan). Participants remained seated for at least 10 minutes. A total of 3 blood pressure recordings were taken and these values were averaged to obtain one value for pre and post in-office blood pressure.
Other Physical Measures
Participants’ height and weight was recorded using a standardized protocol at baseline and 6-week follow-up. Participants’ weight was recorded in light clothing, without shoes. BMI was calculated as weight in kg divided by height in m2.
Patient-Reported Outcomes
Daytime sleepiness was measured by the Epworth Sleepiness Scale.26 In this 8-item questionnaire, participants respond from 0–3 the likelihood of falling asleep in different situations in daily life. Scores range from 0–24 and scores ≥ 10 are considered excessive daytime sleepiness.27
Sleep quality and daytime sleepiness were measured by the PROMIS (Patient-Reported Outcomes Information System) Sleep Disturbance and Sleep Related Impairment scales.28 These scales were developed as part of the National Institute of Health Roadmap initiative. Scores are presented as t scores, with average of 50 and standard deviation of 10. Scores ≥ 60 are considered elevated.
Adherence and Usage
Adherence and usage was measured using attendance to the phone sessions and days with Fitbit usage. Participants were asked to complete free text questions to solicit their feedback on the appearance, layout, reading level, content, helpfulness of lessons and coaching sessions and intervention. They also rated from 1 (not at all) to 5 (very much) how much they liked the intervention and how easy it was to participate in the intervention.
Data Analysis
Data were analyzed in SPSS version 23 (IBM Corp., Armonk, New York, United States) using descriptive statistics and nonparametric statistics to evaluate prepost changes. Mann-Whitney U tests were used to compare average prepost changes between the intervention group and the self-management control group. Wilcoxon signed ranks tests were used to assess within group prepost changes for each group separately. A 30 minute increase in sleep duration was set as the threshold for clinically significant improvement; the % achieving this level of improvement was compared between groups using chi square tests. Finally, bivariate correlations were used to assess change in sleep and ABP variables. Statistical significance was defined as P < .05 based on two-tailed tests.
RESULTS
Recruitment and Screening
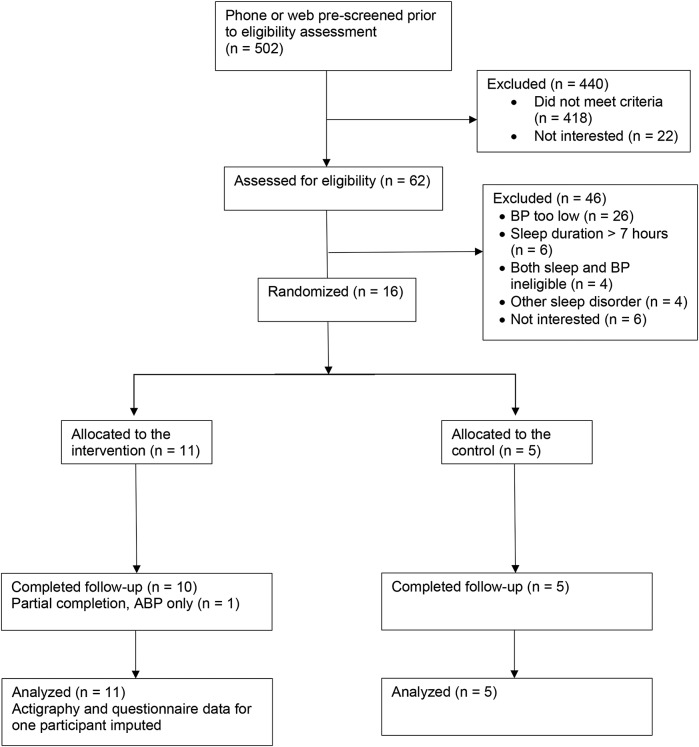
The CONSORT diagram is listed in Figure 2. Participants were recruited from March 2017 to June 2018. We conducted phone or web prescreening among 502 potential participants. Our mailing campaign resulted in the largest number of participants prescreened. We mailed letters to 1,267 potential participants and were able to contact 90% by phone or email. A total of 387 potential participants completed prescreening over the phone or web. An additional 117 participants contacted us via flyers to participant in prescreening. A total of 62 of those prescreened were eligible for in person screening. The most common reason for screening out of the study was failure to meet the ABP criteria. Of the 4 participants who were not interested in continuing, 2 dropped out due to discomfort of the ABP monitors and 2 stated they were too busy to continue.
Figure 2. CONSORT diagram.
BP = blood pressure.
Participant Characteristics
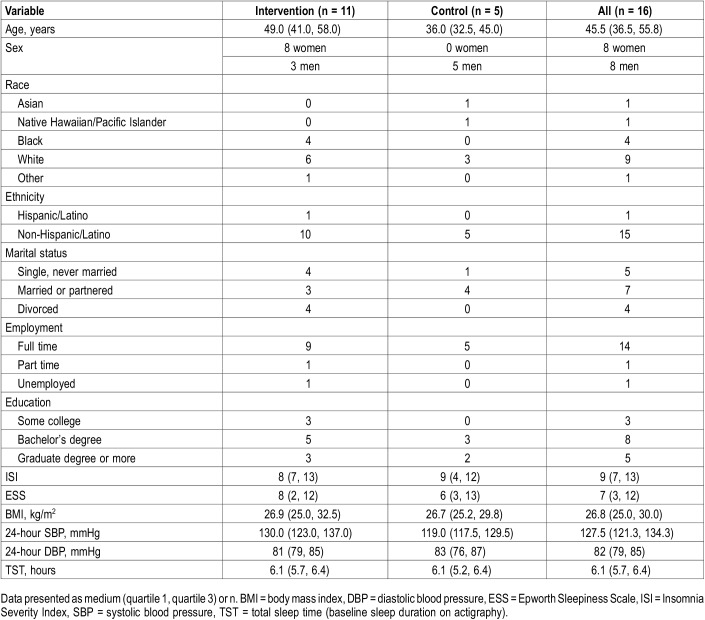
A total of 16 participants were randomized into the study. Participant characteristics are listed in Table 1. Average age of the study participants was 45.8 years. All participants who were randomized completed the study. One participant in the intervention group only completed the ABPM at 6-week follow-up and not actigraphy or questionnaires. Despite use of randomization procedures, age in the intervention group was older and there were fewer women in the self-management group. The majority of participants in this study were working full time and most had a bachelor’s degree or graduate degree education. The average score on the ISI was in the “subthreshold” range for insomnia and the ESS was below the cutoff for excessive sleepiness.
Table 1.
Participant characteristics.
Change in Sleep
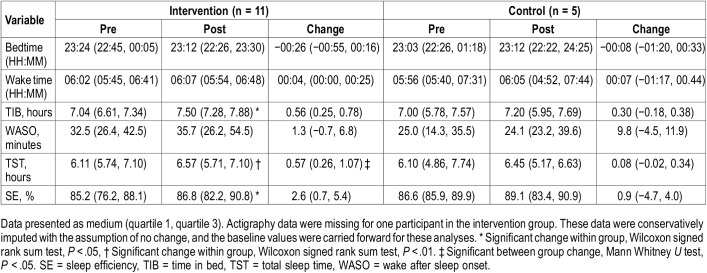
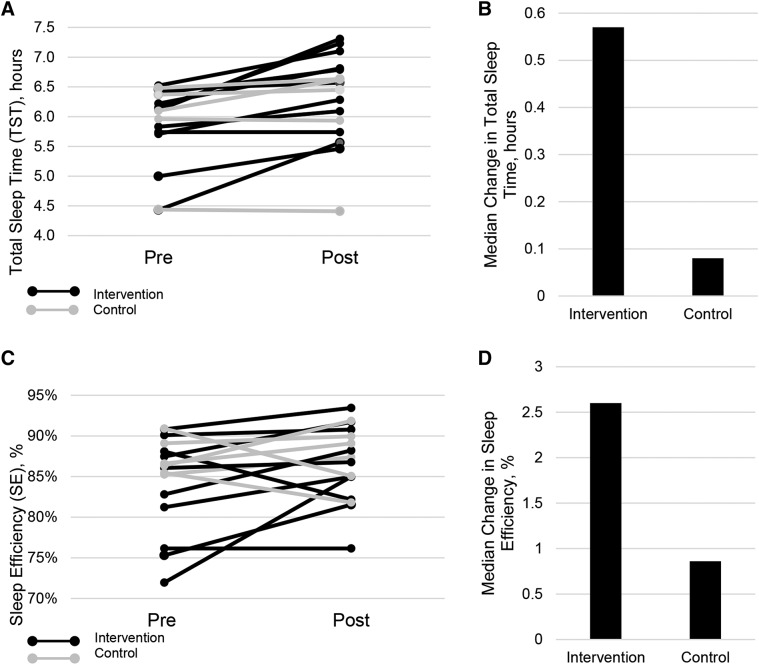
Change in sleep variables for the intervention and self-management groups are listed in Table 2. Approximately half of the participants in the intervention group met criteria for clinically significant improvement (+ TST ≥ 30 minutes, 5 out of 11 or 45.5%) and 1 participant in the self-management group met criteria for improvement (20%). There was significantly greater change in the intervention compared to the self-management group for TST, (P = .027; Figure 3A). When prepost change within each group was evaluated separately, participants in the intervention group demonstrated significant prepost changes for TIB (P = .017), TST (P = .005) and SE (P = .033; Figure 3B). There was a nonsignificant trend for change in bedtime (P = .08) and were no significant changes in wake time or WASO. Participants in the self-management group did not demonstrate significant prepost changes in any of the actigraphy variables.
Table 2.
Change in actigraphy variables.
Figure 3. Change in actigraphy variables.
Participants in the intervention group are depicted in black lines and participants in the control group are depicted in gray lines. The dashed lines indicate the median change in the intervention and control groups. (A) Prepost changes in TST. There was significant between group change in TST (P = .027) as well as within group change in the intervention group (P = .005). (B) Median change score in TST in the intervention versus control groups. (C) Prepost changes in sleep efficiency. There was a significant prepost change for the intervention group in SE (P = .033) and no change in the control group. (D) Median change in sleep efficiency in the intervention versus control groups. TST = total sleep time.
Change in Blood Pressure
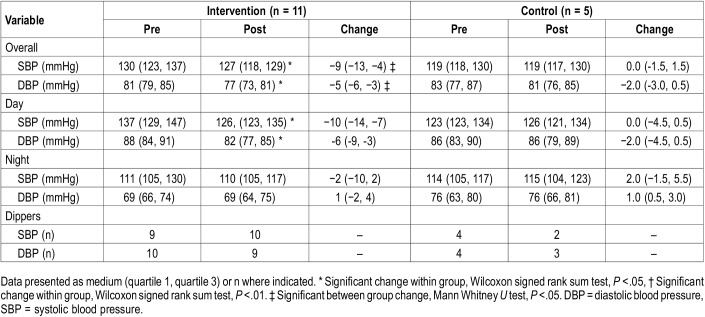
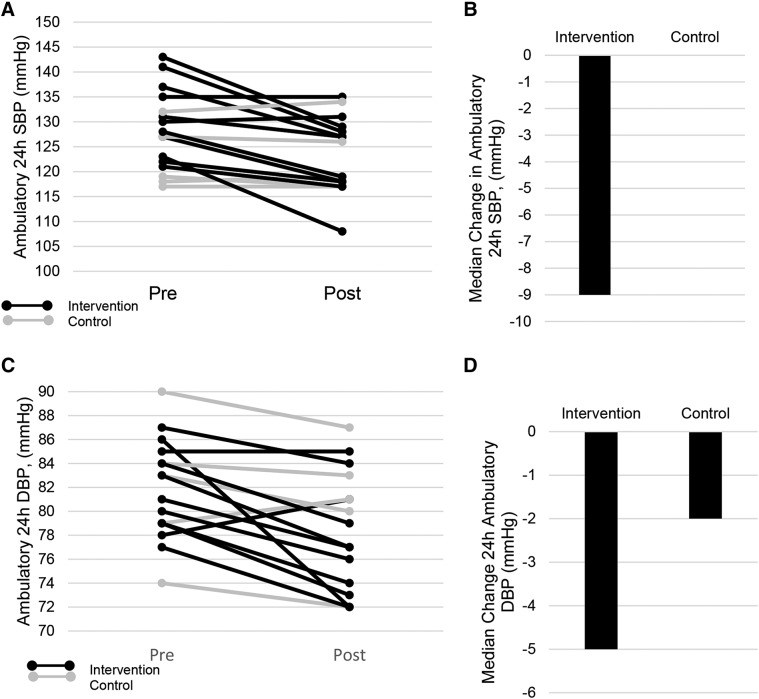
Change in ABP variables for the intervention and self-management groups are listed in Table 3. There were significantly greater changes for 24-hour SBP and DBP in the intervention group compared with the self-management group (P = .026; Figure 4A and Figure 4B). When we evaluated within group prepost changes, the intervention group demonstrated a decrease in 24-hour SBP (P = .007), 24-hour DBP (P = .008), daytime SBP (P = .004), and daytime DBP (P = .007). There were no significant prepost changes in the self-management group.
Table 3.
Ambulatory blood pressure.
Figure 4. Change in ambulatory blood pressure.
Participants in the intervention group are depicted in black lines and participants in the control group are depicted in gray lines. The dashed lines indicate the median change in the intervention and control groups. (A) Change in 24-hour SBP. (B) Median change scores for the intervention and control group in 24-hour SBP. (C) Change in 24-hour DBP in the intervention and control group. (D) Median change score for the intervention and control groups. There were significant between group changes for both SBP and DBP SBP (P = .013, DBP P = .026) as well as within group prepost changes for the intervention group (SBP, P = .007, 24-hour DBP, P = .008). DBP = diastolic blood pressure, SBP = systolic blood pressure.
Patient-Reported Outcomes
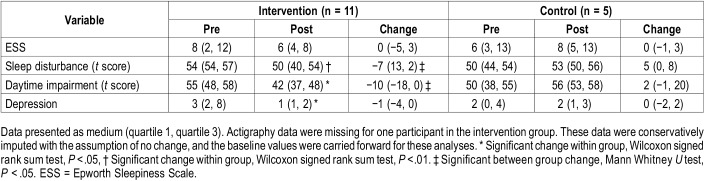
Participants in the intervention group demonstrated greater improvements in sleep quality including decreased scores on sleep disturbance (P = .003) and sleep related impairment compared with the self-management group (P = .008). Participants in the intervention group also demonstrated significant within group prepost changes in the other patient-reported outcome measures including decreased depressive symptoms (P = .024), decreased sleep disturbance (P = .008) and sleep-related impairment (P = .017; Table 4).
Table 4.
Change in patient-reported outcomes.
Other Changes
There were no significant between or within group changes in BMI, in-office SBP or DBP.
Predictors of Improvement in Sleep and Blood Pressure
The predictors of change in TST were change in TIB (r = .66, P = .008) and change in SE (r = .60, P = .014). Change in WASO, bedtime and wake time were not associated with change in TST. None of the actigraphic change variables predicted change in 24-hour blood pressure.
Intervention Adherence, Usage and Participant Feedback
Participants in the intervention group wore the Fitbit for 85% to 100% of study days and completed 90% of coaching sessions. Nine of the 11 participants answered the exit survey questions. Participants reported the layout was appealing (“nice layout”) and there were no reports of problems with reading level or understanding the lessons. Participants were asked if the intervention was too long, too short or just right, all participants responded the intervention length (6 weeks) was “just right.” When asked if the coaching sessions were helpful, all except 1 participant responded “yes” and some added free text such as “very very helpful” and “knowing I was being monitored helped the most.” The participant who responded “no” wrote “She gently led me to consider what I was already doing.” One participant indicated it was hard for her to find the time for phone for coaching, and recommended text-based coaching would be easier. All participants reported they liked the intervention (rated 4 or 5 out of 5) and ease of the intervention was reported as 4 and 5 by 8 out of 9 participants. One participant rated ease of the intervention as a 3.
DISCUSSION
Our overarching goals is to develop the behavioral science of sleep extension. Despite the established role of short sleep duration in hypertension, few studies have tested behavioral sleep extension interventions in this population. The goal of this study was to test the feasibility of a technology assisted sleep extension intervention among participants with short sleep duration and prehypertension/stage 1 hypertension. In contrast to prior sleep extension studies, we are focused on developing a theory-based intervention that can be widely disseminated. Previous sleep extension studies have largely viewed sleep extension as an experimental manipulation, thus focusing on the important mechanistic question of whether extending sleep will improve physiological variables. The details of the techniques used to extend sleep in prior studies are not reported or linked to behavior change theory. For example, Haack and colleagues described their intervention for participants as “given bedtimes that started 30 minutes earlier and ended 30 minutes later than their usual lights out/lights on times.” Participants in both groups were provided with a sleep hygiene handout (contents not reported) and the PI or other staff provided weekly phone calls to enhance adherence. Our technology assisted intervention also used goal setting and weekly telephone check-ins. The innovation is the use of a wearable to increase enjoyment and supportive accountability15 and delivery of structured content based in cognitive behavioral therapy and motivational interviewing. Based on our development testing in this study and others, the wearable sleep tracker was integral to making the intervention enjoyable and engaging.12 This project is the first randomized field test of our intervention and allowed us to evaluate feasibility of our recruitment strategy and screening processes, preliminary efficacy of the intervention in a patient population as well gather important feedback from participants on our intervention.
In comparing our study with prior sleep extension studies, the increases in sleep duration observed in our intervention group were slightly larger than those reported by Haack and colleagues7 (35 minutes) and smaller than those reported by Tasali (1.6 hours).10 The amount of change in sleep duration may be largely driven by baseline sleep duration, and if it is near 7 hours, there could be a ceiling effect. In addition, age-related changes, pain or social factors (eg, commute times) may limit some participants’ ability to extend sleep. We found, not surprisingly, that increases in TIB were the strongest predictor of TST improvements.
In addition to improvements in sleep duration, sleep disturbance and sleep-related impairment also significantly improved in the intervention group. The improvements in patient reported outcomes are critical because participants are more likely maintain a behavior change if they perceive the benefits.29 These improvements in sleep quality are also important to note because our sample included participants with mild to moderate insomnia symptoms who met our sleep duration and time in bed criteria. We included this group with some insomnia symptoms in order to increase the external validity of our study. One concern with sleep extension in insomnia is that increasing time in bed may have the potential to decrease sleep efficiency and WASO. Our findings did not support this concern. We found that our intervention was acceptable in this patient population and increased (rather than decreased) sleep efficiency despite longer time in bed. Therefore, our results would suggest that this intervention is not contraindicated and was in fact beneficial for individuals with mild to moderate insomnia, short sleep duration and < 8 hours in bed.
In terms of blood pressure changes, they are difficult to interpret at this stage of research, given the small sample size. We do report a reduction in ABP in the intervention group and no change in the control group. There is one other prior study that evaluated a 2-hour sleep extension on the weekend, and found no difference with this brief intervention. However, the study was limited by a short intervention duration and single blood pressure recording.30 Our study adds to the results of Haack and colleagues by replicating their findings and also demonstrating that the delivery of the intervention in a more generalizable setting can result in lower ABP readings when collected in the home environment. However, given the imbalance in age and sex between the intervention and control groups, it is not possible to directly compare the two groups or to rule out regression to the mean. The next steps after this study are to evaluate our intervention in a randomized trial in a larger sample.
Finally, in terms of feasibility, adherence to the intervention was excellent and most participants rated the intervention as easy or very easy to use. Although full automation of the intervention would provide the broadest reach, feedback from several participants suggested the use of a human coach was the most motivating and beneficial aspect of this intervention. This is consistent with other areas in mHealth, where use of human support has increased adherence.31 Therefore, future research is needed to optimize the timing and duration of coach contact, in order to minimize staff burden. As a whole, sleep extension interventions are in their infancy and much research is needed to determine how to create lasting changes in sleep behaviors and the impact of these changes on health and wellbeing.
Limitations to this study include the small sample size with baseline differences between the groups in age and sex. Therefore, results of the intervention are not directly comparable between groups, particular for ABP values. In addition, the 6-week time period is a relatively short term intervention, and therefore longer studies are needed to determine the impact of longer term changes in sleep duration. The selection of a sleep maintenance control group allowed us to observe the changes in sleep that occur by enrolling in a research study. However, this did not allow for us to test the effect of attention, which may also be a factor in reducing blood pressure. On the other hand, current recommendations support use of a weak comparator group in early behavioral trials, given that the objective is testing feasibility rather than comparing to a standard treatment (and in this case, there are no existing treatments).19 Use of an attention control in future studies (eg, health education) will be able to delineate whether blood pressure change is due to therapist contact versus change in sleep.
It is also notable that we did not reach our goal of enrolling 30 participants, which speaks to the feasibility of enrollment and also generalizability of this highly selected sample. The biggest limiting factor in enrollment was that we only enrolled participants who had elevated 24-hour blood pressure but were not yet taking antihypertensive medications. Given that patients with short sleep duration may be more difficult to manage their hypertension, enrolling those on medications who still have elevated 24-hour blood pressure who are already taking antihypertension medication may enhance the feasibility of future sleep duration and hypertension studies. In fact, prior studies did include those taking medications who still met sleep and blood pressure criteria.7 Despite these limitations, the strength of this study is that that this pilot study demonstrates that our sleep extension intervention can be delivered in the home to a chronic illness population, was well liked and has the potential for greater scalability compared to prior sleep extension studies.
CONCLUSIONS
Our technology assisted sleep extension intervention was well liked by participants and has the possibility of extending sleep duration, lowering blood pressure and improving sleep quality and daytime function. Recruiting participants with prehypertension/unmedicated hypertension limited the enrollment to our study but participants who did enroll in this pilot trial demonstrated high completion rates and the potential for this intervention to improve blood pressure, sleep and mood.
DISCLOSURE STATEMENT
Work for this study was performed at Rush University Medical School. This project was funded internally by the Department of Behavioral Sciences, Rush University Medical Center. The sponsor was not involved in the study design, data collection, analysis or writing of this manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Olivia DeYonker, MS and Bethlehem Markos for their assistance in data collection and Dr. Kim Williams, Jr., MD, for reviewing our manuscript in progress.
ABBREVIATIONS
- ABP
ambulatory blood pressure
- ABPM
ambulatory blood pressure monitoring
- DBP
diastolic blood pressure
- ESS
Epworth Sleepiness Scale
- ISI
Insomnia Severity Index
- OSA
obstructive sleep apnea
- REDCap
Research Electronic Data Capture
- RLS
restless legs syndrome
- SBP
systolic blood pressure
- SE
sleep efficiency
- TIB
time in bed
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services Healthy People 2020. http://www.healthypeople.gov/hp2020/. Accessed October 1, 2018. [DOI] [PubMed]
- 3.Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24(2):215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 4.Guo X, Zheng L, Wang J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14(4):324–332. doi: 10.1016/j.sleep.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Cunningham TJ, Croft JB. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep. 2015;38(5):829–832. doi: 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning clinical science: unifying the discipline to improve the public health. Clin Psychol Sci. 2014;2(1):22–34. doi: 10.1177/2167702613497932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: a pilot study. J Sleep Res. 2013;22(3):295–304. doi: 10.1111/jsr.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. 2015;38(5):707–715. doi: 10.5665/sleep.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Khatib HK, Hall WL, Creedon A, et al. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. Am J Clin Nutr. 2018;107(1):43–53. doi: 10.1093/ajcn/nqx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tasali E, Chapotot F, Wroblewski K, Schoeller D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite. 2014;80:220–224. doi: 10.1016/j.appet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Year-over-year wearables spending doubles, according to NPD [press release]. Port Washington, NY: The NPD Group, Inc.; February 1, 2016. https://connected-intelligence.com/about-us/press-releases/year-over-year-wearables-spending-doubles-according-npd. Accessed September 24, 2019.
- 12.Baron KG, Duffecy J, Reid K, Begale M, Caccamo L. Technology-assisted behavioral intervention to extend sleep duration: development and design of the sleep bunny mobile app. JMIR Ment Health. 2018;5(1):e3. doi: 10.2196/mental.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck AT. Cognitive Therapy: Basics and Beyond. New York, NY: Guilford Press; 1995. [Google Scholar]
- 14.Rollnick S, Miller W, Butler C. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2007. [Google Scholar]
- 15.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. doi: 10.2196/jmir.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Zeev D, Schueller SM, Begale M, Duffecy J, Kane JM, Mohr DC. Strategies for mHealth research: lessons from 3 mobile intervention studies. Adm Policy Ment Health. 2015;42(2):157–167. doi: 10.1007/s10488-014-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohr DC, Duffecy J, Ho J, et al. A randomized controlled trial evaluating a manualized TeleCoaching protocol for improving adherence to a web-based intervention for the treatment of depression. PLoS One. 2013;8(8):e70086. doi: 10.1371/journal.pone.0070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – aa metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedland KE, King AC, Ambrosius WT, et al. The selection of comparators for randomized controlled trials of health-related behavioral interventions: recommendations of an NIH expert panel. J Clin Epidemiol. 2019;110:74–81. doi: 10.1016/j.jclinepi.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- 21.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien E, Coats A, Owens P, et al. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ. 2000;320(7242):1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9(1):5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31(1):399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- 30.Kubo T, Takahashi M, Sato T, Sasaki T, Oka T, Iwasaki K. Weekend sleep intervention for workers with habitually short sleep periods. Scand J Work Environ Health. 2011;37(5):418–426. doi: 10.5271/sjweh.3162. [DOI] [PubMed] [Google Scholar]
- 31.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]