Abstract
Study Objectives:
The aim of this study was to investigate the association of bedtime with the prevalence of diabetes mellitus (DM) based on a large community-based population.
Methods:
In total, 5,420 participants (2,574 males and 2,846 females; aged 63.5 ± 11.0 years) from the Sleep Heart Health Study database were selected in this study. Sleep habit was recorded based on a questionnaire administered to patients upon recruitment. Bedtime was categorized as 11:00 pm and before, 11:00 pm to 12:00 am, and 12:00 am and later in the current study. Multivariate logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) to determine the relationship between bedtime and the prevalence of DM.
Results:
The distribution of weekday bedtime at 11:00 pm and before, 11:00 pm to 12:00 am, 12:00 am and later was observed in 3,316 participants (61.2%), 991 participants (18.3%), and 1,113 participants (20.5%), respectively. Meanwhile, individuals with weekday bedtime of 12:00 am and later had a higher prevalence of DM than those with bedtime at 11:00 pm to 12:00 am, and 11:00 pm and before (10.6% versus 5.7% versus 6.6%, respectively; P < .001). In the adjusted multivariate logistic regression model, bedtime at 12:00 am and later on a weekday was significantly associated DM prevalence (OR 1.446, 95% CI 1.107–1.888, P = .007). No significant association was found between weekend bedtime and DM.
Conclusion:
Late bedtime at 12:00 am and later on a weekday may be a risk factor for the prevalence of DM. Stable sleep timing leads to lower risk of DM deserves future exploration.
Citation:
Yan B, Fan Y, Zhao B, He X, Yang J, Chen C, Ma X. Association between late bedtime and diabetes mellitus: A large community-based study. J Clin Sleep Med. 2019;15(11):1621–1627.
Keywords: bedtime, diabetes mellitus, sleep habits, Sleep Heart Health Study
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study was performed to investigate the role of late bedtime in the prevalence of diabetes mellitus. It is the first study to explore the association between bedtime and diabetes mellitus in a community-based population.
Study Impact: The current study found a high prevalence of diabetes mellitus among participants who slept after 12:00 am on weekdays. Our results showed that appropriate sleep timing before 12:00 am on weekdays may be a useful way to decrease the risk of diabetes mellitus.
INTRODUCTION
Diabetes mellitus (DM), characterized by high blood sugar levels, is one of the most common chronic diseases in the world. As a critical public health disease with huge social and economic effects on medical expenditure and services, the incidence of diabetes is constantly on the increase.1 Unhealthy lifestyle, including poor dietary habit, alcohol use, tobacco smoking, and lack of physical activity are usually closely related to the risk of DM.2–4 Sleep is a biobehavioral phenomenon that can affect the hormones and inflammation involved in regulating blood glucose concentration.5,6 During sleep deprivation, alterations in glucose tolerance, insulin resistance, and melatonin secretion occur with a decline in islet cell sensitivity, which may promote the development of DM.7–9 Cappuccio et al and Gottlieb et al have shown that poor sleep quality, and short and long sleep duration increased the risk of DM.10,11 People with short sleep duration tend to have a late bedtime. A longitudinal study revealed that late bedtime increased salivary glucose levels in children.12 Reutrakul et al also found that delaying sleep on the weekend was associated with poorer glycemic control in patients with DM.13 These findings indicated that late bedtime might correlate with the incidence of DM. However, the appropriate sleep timing associated with a decreased risk of DM remains unclear. We therefore conducted the current study to investigate the relationship between bedtime and DM based on a large community-based population.
METHODS
Study Population
The Sleep Heart Health Study (SHHS) is a community-based, multicenter cohort study investigating the cardiovascular consequences of sleep-disordered breathing (ClinicalTrials.gov identifier: NCT00005275). Details of the study design have been previously reported.14 Between 1995 and 1998, participants were recruited from prospective cohort studies including the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, the Framingham Offspring and Omni Study, the Strong Heart Study, the Tucson Epidemiological Study of Obstructive Lung Disease, cohort studies of respiratory disease in Tucson, and cohort studies of hypertension in New York. Written consent was provided by all the participants and the study protocol was approved by the institutional review board of each participating institution. Access to the SHHS database was granted after acquiring a signed agreement with Brigham and Women’s Hospital. Participants in our study were excluded if (1) there were missing data regarding DM and bedtime (364 individuals), or (2) they worked on night shifts (20 individuals). Finally, 5,420 individuals were included in our analyses.
Data Collection
Bedtime was assessed using questions such as “At what time do you usually fall asleep on weekdays or workdays (hour, minute, am or pm)?” and “At what time do you usually fall asleep on weekends or your non-work days (hour, minute, am or pm)?” In this study, bedtime was classified as 11:00 pm and before, 11:00 pm to 12:00 am, and 12:00 am and later. Sleep duration was defined as the length of sleep time between bedtime and wake-up time. The apnea-hypopnea index (AHI) was calculated as all apnea and hypopnea episodes per hour of sleep accompanied by at least a 4% drop in oxygen saturation based on the baseline polysomnography records.
DM was defined based on data on self-reported DM status and use of oral hypoglycemic medications and insulin that were collected during the SHHS interview. Participants’ data including age, sex, body mass index (BMI), education level, smoking status, alcohol use, triglycerides and cholesterol levels, and history of hypertension were obtained from the baseline examination of SHHS.
Statistical Analysis
Continuous and categorical characteristic variables among the three groups were presented as mean (± standard deviation) and number (percentage), and were compared by analysis of variance and chi-square tests. Logistic regression was used to estimate the association of bedtime with the risk of DM during weekdays and weekends. All potential confounders were chosen based on the existing literature and their relationships with bedtime and DM. Logistic regression analysis, following the Harrell guideline, was performed to identify risk factors, estimate odds ratios (ORs), and determine 95% confidence intervals (CIs). The final multivariate logistic regression model was adjusted for age, sex, education, BMI, smoking status, alcohol use, AHI, history of hypertension, sleep duration, triglyceride, total cholesterol, and high-density lipoprotein. Subgroup analysis was further conducted to explore the relationship between bedtime and DM. The analyses were implemented in SPSS version 24.0 (SPSS Inc., Chicago, Illinois, USA). All statistical tests were two-sided, and a value of P < .05 was considered statistically significant.
RESULTS
Participants’ Characteristics
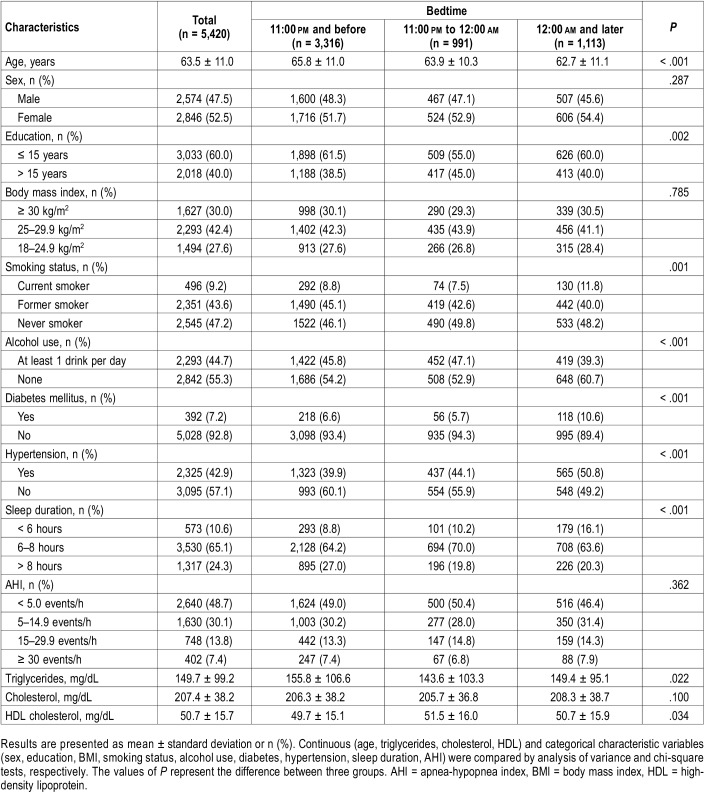
The current study included 2,574 males and 2,846 females aged 63.5 ± 11.0 years. The distribution of bedtime of 11:00 pm and before, 11:00 pm to 12:00 am, and 12:00 am and later was observed in 3,316 participants (61.2%), 991 participants (18.3%), and 1,113 participants (20.5%), respectively. Participants with a weekday bedtime of 12:00 am and later tended to comprise more females, current smokers, DM, and hypertension. Participants who reported bedtimes of 11:00 pm and before were older and more likely to have a drink every day (Table 1).
Table 1.
Characteristics by bedtime categories.
Weekday or Weekend Bedtime and DM
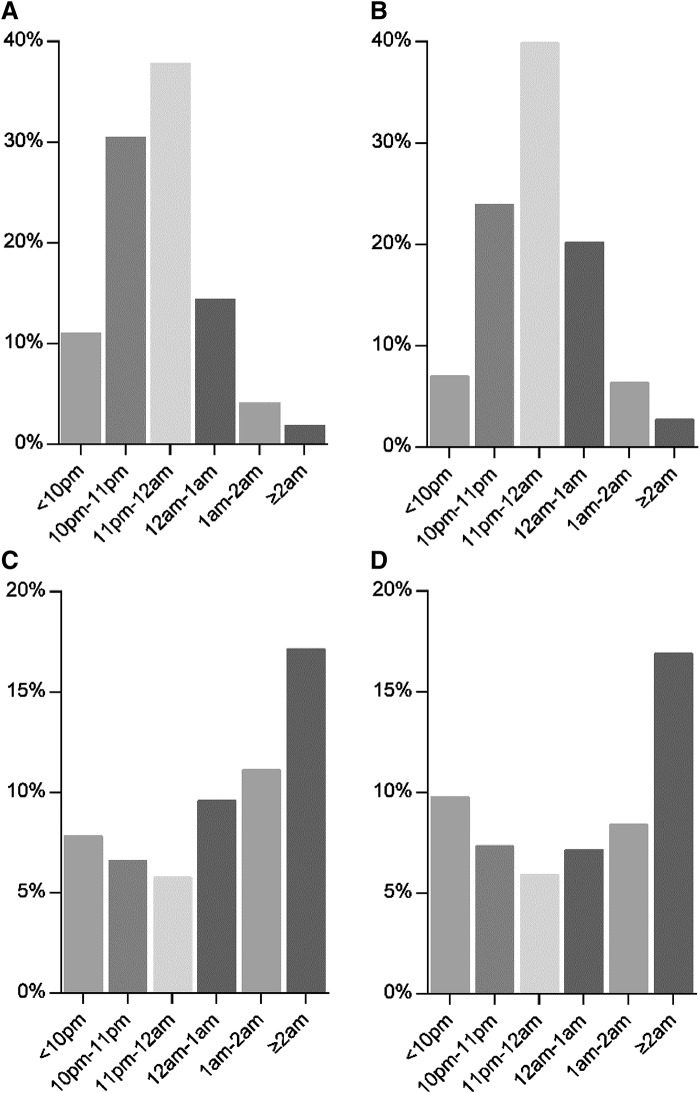
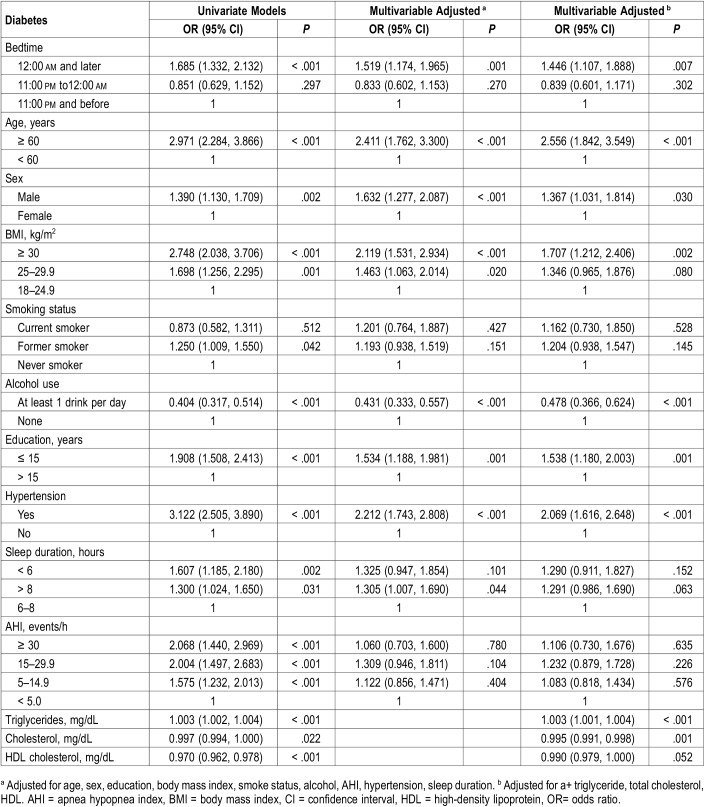
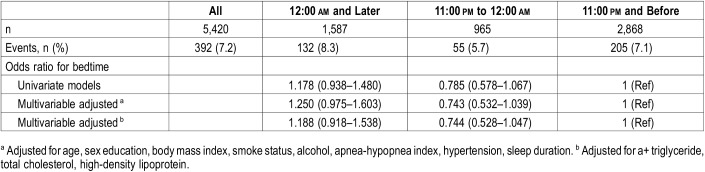
Figure 1 shows the distribution of all participants and DM prevalence at different bedtimes on weekdays and weekends. A higher proportion of individuals with late bedtime at 12:00 am and later on weekdays tended to have DM than those with bedtime at 11:00 pm to 12:00 am, and 11:00 pm and before (10.6% versus 5.7% versus 6.6%, respectively, P < .001). Univariate logistic regression analysis showed that a bedtime of 12:00 am and later on weekdays increased the risk of DM (OR 1.685, 95% CI 1.332–2.132, P < .001). After multivariate adjustment for age, sex, education, BMI, smoking status, alcohol use, AHI, history of hypertension, sleep duration, triglyceride, total cholesterol, and high-density lipoprotein, weekday bedtime at 12:00 am and later was associated with the prevalence of DM (OR 1.446, 95% CI 1.107–1.888, P = .007) (Table 2). However, there was no significant association between weekend bedtime and DM (Table 3).
Figure 1. The distribution of all participants and DM prevalence at different bedtimes on weekdays and weekends.
(A) The distribution of weekday bedtime categories in all participants. (B) The distribution of weekend bedtime categories in all participants. (C) The distribution of DM in different weekday bedtime categories. (D) The distribution of DM in different weekend bedtime categories. DM = diabetes mellitus.
Table 2.
Odds ratios and 95% confidence intervals for bedtime associated with diabetes mellitus on weekdays.
Table 3.
Odds ratios and 95% confidence intervals for bedtime associated with diabetes mellitus on the weekend.
Subgroup Analysis
We also performed subgroup analyses stratified by age (60 years and older versus younger than 60 years), sex (male versus female), education (> 15 years versus ≤ 15 years), BMI (18–24.9 kg/m2 versus 25–29.9 kg/m2 versus ≥ 30 kg/m2), smoking status (current versus former versus no), alcohol use (at least 1 drink per day versus none), hypertension (yes versus no), sleep duration (< 6 hours versus 6 to 8 hours versus > 8 hours), to investigate the correlations between bedtime and DM. Significant interactions were not found in these analyses (data not shown).
DISCUSSION
Sleep plays an important role in the regulation of glucose metabolism and neuroendocrine function in adults.15,16 Sleep deprivation and sleep duration could affect blood glucose utilization and insulin regulation, and intentional disturbance of circadian rhythm resulted in hyperglycemia.10,17,18 However, there is little evidence about the relationship between sleep timing at night and DM. In this large community-based study, we investigated the role of bedtime in the development of DM and found that late bedtime at 12:00 am and later on weekdays was associated with the development of DM.
A growing body of evidence shows that sleep timing at night was closely related to human health. Individuals with late bedtime tend to have a high BMI, and an increased risk of overweight and obesity in both children and adolescents.19,20 In adults, late bedtime was also associated with physical activity, depression, and osteoporosis.21–23 A previous study showed that going to bed late could increase salivary glucose levels in children.12 In addition, patients with DM with late bedtimes during the weekend tended to have poorer glycemic control.13 However, the most appropriate sleep timing at night is still unclear. In our study, we divided weekday and weekend bedtime into three groups (11:00 pm and before, 11:00 pm to 12:00 am, and 12:00 am and later) and found that participants with bedtime at 12:00 am and later had an obviously high prevalence of DM. Our results indicated that sleep before 12:00 am on weekdays may be an efficient way to decrease the risk of DM. The association of weekend bedtime with DM was also investigated, but no significant correlation was found. Individuals tend to go to bed later on weekends than weekdays in daily life. This finding may be caused by the different bedtime habits on weekdays and weekends.
Many studies have shown that sleep duration was associated with the incidence of DM. Yadav et al found that both short and long-term sleep duration contribute to the development of type 2 diabetes.11 A meta-analysis also documented that short (< 6 hours) or long (< 8 hours) sleep duration was significantly associated with the development of diabetes and elevation of glycosylated hemoglobin levels when compared with normal sleep duration.24 In this study, we performed a subgroup analysis stratified by sleep duration to further explore the relationship between bedtime and DM. Weekday bedtime at 12:00 am and later was still associated with DM in participants with 6 to 8 hours’ sleep duration (OR 1.436, 95% CI 1.108–2.026, P = .039). Moreover, no significant interaction was found in these analyses.
Recent studies found that delaying sleep at night is associated with insulin resistance and glucose intolerance due to hormonal disruption such as lower testosterone and melatonin levels, which may lead to an increased caloric intake and weight gain.25–28 In addition, Shechter et al and Baron et al found that people with late bedtime were more likely to have unhealthy dietary habits and lifestyle such as snacking behavior, consuming excess calories at dinner, and lower physical activity.23,29 A previous study showed that individuals with late bedtime tended to have a short sleep duration and a longer duration of light exposure. Light exposure was found to be closely related with circadian disruption, which may lead to the increased risk of obesity, diabetes, and metabolic syndrome.13 Late sleepers, therefore, were prone to increased sympathetic nerve activity, which could inhibit insulin secretion by pancreatic beta cells and further lead to glucose intolerance.30
To our knowledge, this is the first study to explore the association between bedtime and DM based on a large community-based population. Our results found a suitable sleep timing (before 12:00 am) for the general population that would decrease the risk of DM. This study also had several limitations. Bedtime and sleep duration in the current study was self-reported based on the sleep habit questionnaire. Measurement errors and recall bias may be inevitable. Therefore, we cannot fully exclude the possibility of residual confounding from incomplete adjustment for sleep duration as a potential explanation for our findings. Because fasting blood glucose or glycated hemoglobin was not collected in the baseline SHHS examination, we did not analyze the effect of late bedtime on fasting blood glucose or glycated hemoglobin level. This will be investigated in a future study.
CONCLUSIONS
The current study detected a high prevalence of DM among participants who slept at 12:00 am and later on weekdays. Our results showed that late bedtime at 12:00 am and later on weekdays may be a risk factor for the prevalence of DM. Appropriate sleep timing for decreasing the risk of DM is worth further investigation.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the First Affiliated Hospital of Xi'an Jiaotong University. This study was supported by National Heart, Lung, and Blood Institute cooperative agreements U01HL53916 (University of California, Davis), U01HL53931 (New York University), U01HL53934 (University of Minnesota), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53940 (University of Washington), and U01HL53941 (Boston University). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors appreciate the Brigham and Women’s Hospital for sharing the Datasets of Sleep Heart Health Study (SHHS). In addition, SHHS acknowledges the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, the Framingham Offspring and Omni Study, the Strong Heart Study, Tucson Epidemiological Study of Obstructive Lung Disease, the cohort studies of respiratory disease in Tucson, and cohort studies of hypertension in New York. SHHS is particularly grateful to the members of these cohorts who agreed to participate in SHHS as well. SHHS further recognizes all of the investigators and staff who have contributed to its success. A list of SHHS investigators, staff and their participating institutions is available on the SHHS website, www.jhucct.com/shhs. Author responsibility information: B.Y., J.Y., C.C., and X.M. raised the idea for the study. B.Y., J.Y., B.Z., Y.F., and X.H. contributed to the study design, writing, and review of the report. B.Y. and X.M. acquired the data in SHHS and B.Y. and X.M. participated in further data analysis. X.M. handled supervision in our study. All authors approved the final version of the report.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- DM
diabetes mellitus
- OR
odds ratio
- SHHS
Sleep Heart Health Study
REFERENCES
- 1.Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–970. doi: 10.2337/dc17-1962. [DOI] [PubMed] [Google Scholar]
- 2.Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4(2):287–302. doi: 10.1007/s13679-015-0155-x. [DOI] [PubMed] [Google Scholar]
- 4.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 5.Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep duration and diabetes risk: population trends and potential mechanisms. Curr Diab Rep. 2016;16(11):106. doi: 10.1007/s11892-016-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5(2):93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- 7.Peschke E, Bahr I, Muhlbauer E. Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int J Mol Sci. 2013;14(4):6981–7015. doi: 10.3390/ijms14046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LY, Tiong C, Tsai CH, et al. Early-life sleep deprivation persistently depresses melatonin production and bio-energetics of the pineal gland: potential implications for the development of metabolic deficiency. Brain Struct Funct. 2015;220(2):663–676. doi: 10.1007/s00429-014-0716-x. [DOI] [PubMed] [Google Scholar]
- 9.Taub LF, Redeker NS. Sleep disorders, glucose regulation, and type 2 diabetes. Biol Res Nurs. 2008;9(3):231–243. doi: 10.1177/1099800407311016. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 12.Alqaderi H, Redline S, Tavares M, Goodson JM. Effect of late bedtime on salivary glucose and abdominal obesity in children. Sleep Biol Rhythms. 2017;15(3):227–233. [Google Scholar]
- 13.Reutrakul S, Siwasaranond N, Nimitphong H, et al. Relationships among sleep timing, sleep duration and glycemic control in type 2 diabetes in Thailand. Chronobiol Int. 2015;32(10):1469–1476. doi: 10.3109/07420528.2015.1105812. [DOI] [PubMed] [Google Scholar]
- 14.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 15.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 16.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayes JF, Balantekin KN, Altman M, et al. Sleep patterns and quality are associated with severity of obesity and weight-related behaviors in adolescents with overweight and obesity. Child Obes. 2018;14(1):11–17. doi: 10.1089/chi.2017.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olds TS, Maher CA, Matricciani L. Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep. 2011;34(10):1299–1307. doi: 10.5665/SLEEP.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto N, Nanri A, Kochi T, et al. Bedtime and sleep duration in relation to depressive symptoms among Japanese workers. J Occup Health. 2013;55(6):479–486. doi: 10.1539/joh.13-0074-oa. [DOI] [PubMed] [Google Scholar]
- 22.Tian Y, Shen L, Wu J, et al. Sleep duration and timing in relation to osteoporosis in an elderly Chinese population: a cross-sectional analysis in the Dongfeng-Tongji cohort study. Osteoporos Int. 2015;26(11):2641–2648. doi: 10.1007/s00198-015-3172-4. [DOI] [PubMed] [Google Scholar]
- 23.Shechter A, St-Onge MP. Delayed sleep timing is associated with low levels of free-living physical activity in normal sleeping adults. Sleep Med. 2014;15(12):1586–1589. doi: 10.1016/j.sleep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Dashti HS, Follis JL, Smith CE, et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101(1):135–143. doi: 10.3945/ajcn.114.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309(13):1388–1396. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305(21):2173–2174. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerterp-Plantenga MS. Sleep, circadian rhythm and body weight: parallel developments. Proc Nutr Soc. 2016;75(4):431–439. doi: 10.1017/S0029665116000227. [DOI] [PubMed] [Google Scholar]
- 29.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM, Lithell H, Landsberg L. Mechanisms of disease - hypertension and associated metabolic abnormalities - the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334(6):374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]