Abstract
Objective:
Osteoarthritis (OA) is a disease with a substantial public health burden. Quantitative assessments of peri-articular bone may be a biomarker capable of monitoring early disease progression. The purpose of this study was to evaluate whether measures of peri-articular bone associate with longitudinal structural progression.
Methods:
We conducted a 12–18 month longitudinal study using the Osteoarthritis Initiative. Participants received knee dual-energy x-ray absorptiometry (DXA), trabecular magnetic resonance (MR) imaging, and X-Rays. Knee DXAs generated proximal tibial medial:lateral peri-articular bone mineral density (paBMD) measures. Proximal tibial trabecular MR images were assessed for trabecular morphometry: apparent bone volume fraction (BVF), trabecular number, thickness and spacing. Weight-bearing x-rays were assessed for medial tibio-femoral joint space narrowing (JSN). Chi-squared analyses assessed whether peri-articular bone measures were predictive of worsening medial tibiofemoral JSN, adjusted for age, sex, and BMI.
Results:
444 participants, mean age 64.2 ± 9.2 years, BMI 29.5 ± 4.6 kg/m2, and 52% male at baseline. Medial JSN (radiographic progression) occurred in 40 participants (9%). Higher baseline medial:lateral paBMD, apparent BVF, trabecular number and thickness, and lower baseline and decreased trabecular spacing were all associated with more progression ofJSN in the medial compartment. From lowest to highest baseline medial:lateral paBMD quartile groups, 2%, 5%, 11%, and 18% had medial JSN progression respectively between the 36 to the 48 month visits, p-values = 0.001 and 0.002 unadjusted and adjusted. The rate of change in medial:lateral paBMD, apparent BVF, and spacing were associated with more medial JSN. For rate of medial:lateral paBMD change from lowest to highest quartile, the proportion of each group that experienced medial JSN progression were 5%, 5%, 11%, and 18%, with an unadjusted and adjusted p-value of 0.005.
Conclusion:
Baseline and most rates of peri-articular bone change associate with knee OA structural progression, highlighting the close relationship between subchondral bone and JSN. Future studies should focus on developing these measures as predictive and pathophysiological biomarkers, and evaluating their deployment in clinical trials testing bone-targeted therapeutics.
Keywords: Bone Density, DXA, Magnetic Resonance Imaging, MRI, Morphometry, Knee
Knee osteoarthritis (OA) is a major public health problem. Symptomatic knee OA affects almost 10% of the United States population by age 601. Its prevalence is increasing in the aging population2 and it is a frequent cause of dependency in lower limb tasks3,4. The total health care expenditures of this condition have been estimated at $189 billion annually5. Despite this there are no known interventions that ameliorate structural progression of this disorder.
Pathological changes are evident in all structures of a joint with OA. And, hyaline articular cartilage has historically been the primary target for intervention and measurement because cartilage was thought to provide the majority of force dissipation across a joint. However, biomechanical studies have subsequently suggested that periarticular bone dissipates a much greater proportion of loading forces across the joint 6–9. This attenuation of loading forces by periarticular bone is likely critical in protecting against articular damage and is related to its intricate trabecular geometry, including size and structure10. In OA, thickening and disruption of periarticular trabecular architecture occurs early in the disease, and may even antedate cartilage damage 11. These changes, together with the later development of gross pathological damage 12, are likely to further impair periarticular bone’s ability to attenuate compressive forces and predispose to progression 13–16. Clinical studies corroborate the notion that processes in periarticular bone influence the course of OA. OA knees with abnormal periarticular scintigraphic appearances14,15 or subchondral bone marrow lesions on MRI16, have greater risk for progression. Perhaps periarticular bone response in OA predicates stabilization or progression of OA.
Technical modifications to dual x-ray absorptiometry (DXA) and magnetic resonance imaging (MRI) protocols now offer appealing approaches to measurement of periarticular bone. Therefore, the goal of this study was to test the hypothesis that these measures of bone predict structural progression of knee OA in a subsample of the OAI cohort.
PATIENTS AND METHODS
Study Design
This is a 12–18 month longitudinal observational cohort study of enrollees into the Osteoarthritis Initiative (OAI) Bone Ancillary Study with complete longitudinal follow-up of knee dual-energy x-ray absorptiometry (DXA), trabecular MR imaging, and knee X-rays read for radiographic medial joint space narrowing (JSN) scores.
Sample Selection / Setting
The OAI is a multi-center observational study of knee OA with a progression subcohort selected to have symptomatic radiographic knee OA17. The four OAI clinical sites were Memorial Hospital of Rhode Island (Pawtucket, RI,) The Ohio State University (Columbus, Ohio), University of Pittsburgh (Pittsburgh, PA), and University of Maryland / Johns Hopkins University (Baltimore, MD).
This ancillary study was nested within the OAI’s progression subcohort (Figure 1) with inclusion criteria of age 45 – 79 years and at least one knee with both radiographic evidence of knee OA (Osteoarthritis Research Society International [OARSI] atlas 18 osteophyte grade 1–3) and symptoms (“pain, aching or stiffness on most days of the month in the last year”) at their OAI baseline visit. This study was approved by the Institutional Review Boards at the 4 clinical sites, Baylor College of Medicine, and Tufts Medical Center.
Figure 1. Trabecular magnetic resonance image of a knee with medial tibiofemoral osteoarthritis (joint space narrowing score = 2).
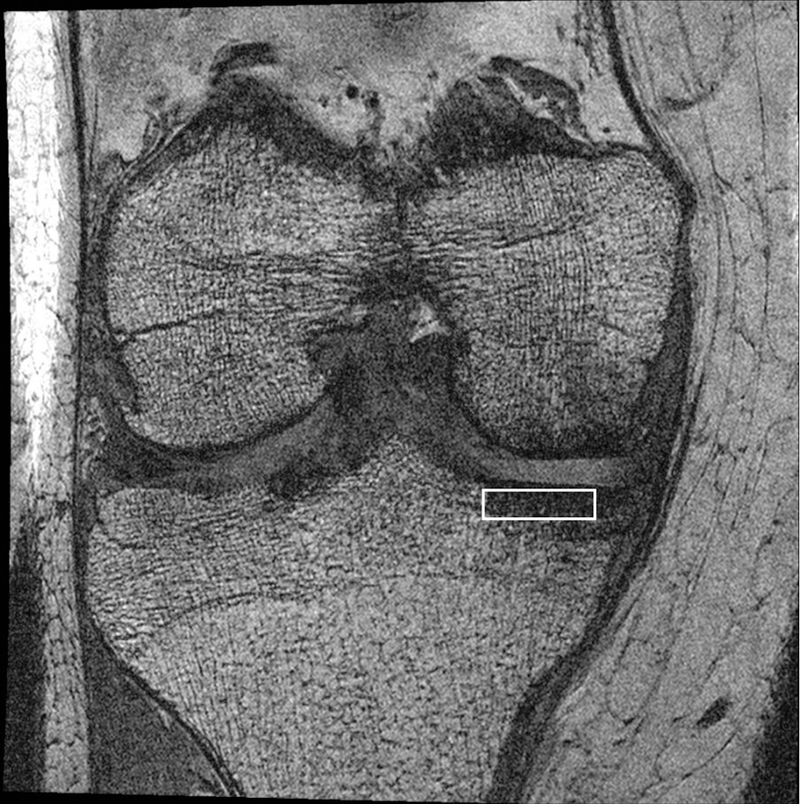
Marrow fat appears white (high intensity signal) while bone appears black (no signal). The white rectangle is the region of interest (ROI).
Inclusion criteria for this ancillary study were willingness to have knee DXA exams and an additional trabecular knee MR imaging sequence at the OAI 30- or 36-month visit and again at the OAI 48-month visit. Those with bilateral knee replacements were excluded. Because recruitment for this ancillary study began later than originally anticipated, the OAI 30- or 36-month visits were thus used as the ancillary baseline visit. Participants were recruited from August 8, 2007 to April 3, 2009. The main outcome of interest was any medial joint space narrowing (JSN) progression between the OAI 36- and 48-month visits
Magnetic Resonance Imaging
Participants had unilateral knee trabecular MR imaging exams on one of 4 identical Siemens Trio 3 Tesla MR systems. The right knee usually had the trabecular imaging acquisition unless this knee had a prior joint replacement, in which case, the left knee had the additional sequence.
Coronal-oblique 3D fast imaging with steady state precision (FISP) was used to visualize the peri-articular trabecular bone 19. Images were obtained in 10.5 minutes using 72 slices, 1 mm slice thickness, 0.23 mm × 0.23 mm in-plane spatial resolution, 12 cm field of view, 512 × 512 matrix (interpolated to 1024 × 1024), 4.92 ms echo time (fat-water in-phase), 20 ms recovery time, 50° flip angle, 180 Hz/pixel readout bandwidth, and phase encode right/left. The chemical shift artifact (signal void in the distal femur) was 2.4 pixels, intentionally shifted superiorly so that it was outside the tibial region of interest.
Trabecular Magnetic Resonance Image Analysis
We performed tibial trabecular morphometry (apparent bone volume fraction, trabecular number, trabecular thickness, and trabecular spacing) on the trabecular MR images using software previously validated in osteoporosis and OA studies 20–25. A rectangular region of interest (ROI) was placed in the proximal medial tibia immediately adjacent to the articular cartilage 26. We focused on the medial compartment as medial tibiofemoral OA is more common than lateral 27,28. The ROI had a height of 3.75 mm and a width that varied from 14 – 17 mm depending on the size of the knee, and was placed on each of 20 consecutive MR slices central to the joint (Figure 1). Details of the software algorithms used to derive these measures have been reported 25. In brief, apparent bone volume fraction is the percentage of the number of pixels contributing to the bone signal void normalized to the total number of pixels in the ROI. The apparent trabecular thickness is determined using the mean value of the mean intercept length for all angles through a given image, measured in millimeters. The apparent trabecular number is calculated by dividing the apparent bone volume fraction by trabecular thickness. And the apparent trabecular spacing is calculated using the equation (1 / ”trabecular number”) – (“trabecular thickness”). An average of each of the measures was taken across the 20 ROIs placed within one knee. Intra-rater (measurement-remeasurement) reliability was good with intraclass correlations: 0.97 (95%CI 0.91 – 0.99) for apparent bone volume fraction, 0.98 (95%CI 0.92 – 0.99) for apparent trabecular thickness, 0.92 (95%CI 0.73 – 0.98) for apparent trabecular number, and 0.77 (95%CI 0.38 – 0.93) for apparent trabecular spacing 26.
Tibial Plateau Dual-Energy X-ray Absorptiometry
DXA systems (Lunar Prodigy Advance, GE Lunar Corp., Madison WI, USA) with investigational knee analysis software (enCORE 2007 Version 11.20.068) were deployed at each OAI clinical site. The lower extremity was positioned with the long axis of the tibia perpendicular to the x-ray beam, and neutrally rotated. A foam knee-positioner, which was posterior to the popliteal fossa, placed the knee in mild flexion. The foot-positioner was then applied to the ipsilateral foot using a Velcro strap around the perimeter of the foot and the positioner. This resulted in the ipsilateral toes oriented perpendicular to the scanning bed and the plantar surface of the ipsilateral foot adjacent to the perpendicular edge of the positioner, parallel to the lower border of the scanner bed. A positioning laser centered the scanner arm 5 centimeters below the inferior pole of the patella. The knee was then imaged using DXA (Figure 2).
Figure 2. DXA of the same knee as in figure 2 with medial tibiofemoral osteoarthritis.
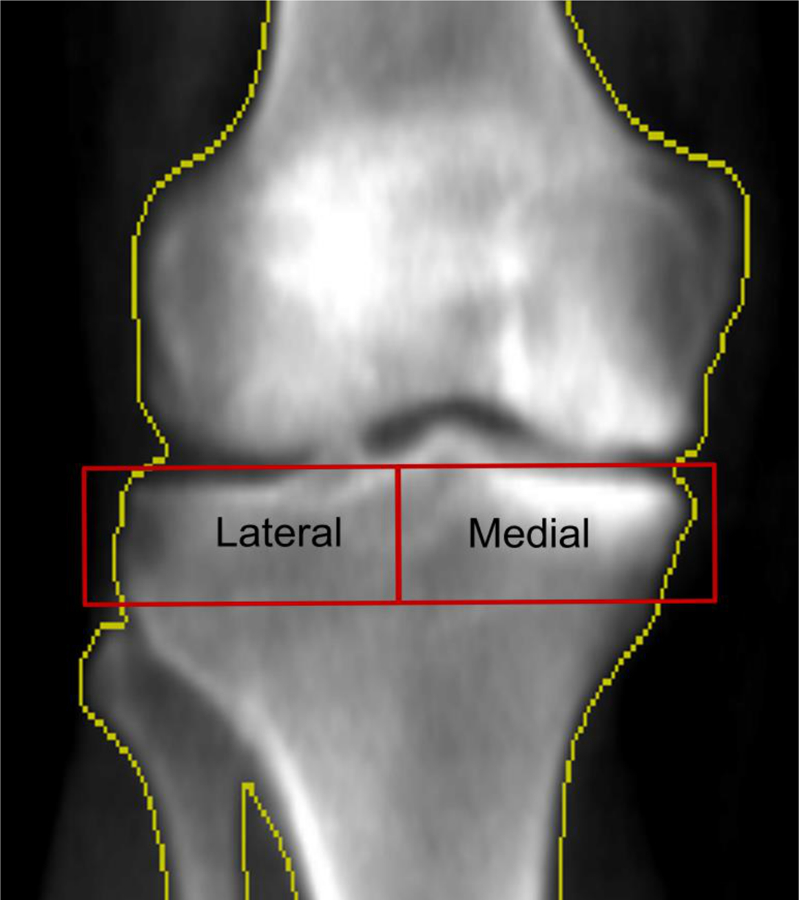
The two red rectangles bounded by the yellow bone border, labeled “Lateral” and “Medial” respectively, are the regions of interest (ROIs) used to measure the medial to lateral paBMD.
Tibial Plateau Dual-Energy X-ray Absorptiometry Image Analysis
Two analysts performed the DXA-based tibial plateau bone mineral density measurements using a customizable ROI. The height of the medial and lateral tibial ROIs was fixed at 20 mm while the ROI width in the medio-lateral direction was set as ½ the distance between the medial and lateral bone edges along a line midway between the far medial and lateral points of the tibial plateau (Figure 2) 29. The bone edges (yellow outline) served as the outer border of the ROis. The ROIs were positioned so that their top edges were just superior and parallel to the medial joint surfaces of the tibia 29. The medial and lateral tibial bone mineral densities were divided to generate medial:lateral paBMD scores. The scan-rescan (with repositioning) intraclass correlation was 0.99 26. Intra-rater reliability (measurement-remeasurement) was excellent at 0.98 – 0.99. Inter-rater reliability (measurement-remeasurement) was excellent at 0.98 – 0.99.
Femoral Neck Dual-Energy X-ray Absorptiometry
The right hip was scanned unless the patient had a history of arthroplasty of this joint. In those cases, the left hip was scanned instead, unless that joint had a history of arthroplasty. This region was scanned using the standard clinical care procedures recommended by Lunar.
Knee Radiographs
Weight-bearing, bilateral, fixed-flexion, posterior-anterior radiographs of the knees were obtained at the OAI 24-, 36- and 48-month visits. These images were centrally scored for overall radiographic severity using Kellgren-Lawrence grades (0 – 4) and medial and lateral JSN grades (0 – 3) using the OARSI Atlas 18 in a paired manner, blinded to time sequence. JSN change was scored both by a whole OARSI grade as well as within OARSI grade medial JSN 30. Within grade narrowing identified worsening in medial JSN over the follow-up period in knees where the JSN OARSI grade was the same at baseline and follow-up. (OAI website (http://oai.epi-ucsf.org/datarelease/), filenames kXR_SQ_BU03_SAS (Version 3.4), kXR_SQ_BU05_SAS (Version 5.4) and kXR_SQ_BU06_SAS (Version 6.2)). The reliability for these readings (read-reread) had weighted kappa [intra-rater reliability] = 0.88 [95%CI 0.80 – 0.95] 31.
Statistical Analysis
For baseline demographics, we provided data from the OAI 24 month visit to assure that we were preceding the baseline bone measures. We included participants with complete radiographic, knee DXA and MR imaging-based trabecular morphometry data at the ancillary study baseline and follow-up time point, focusing on the knee with MR trabecular morphometry images.
Using quartiles of the predictors of bone, we performed chi-squared analyses with the outcome being any medial JSN progression between the OAI 36- and 48-month visits, including whole and within OARSI grade worsening 30. There were 2 groups of predictors: 1) quartiles of baseline bone measures including, medial:lateral paBMD and trabecular morphometry measures and 2) quartiles of rate of change [defined as (follow up – baseline measure) / (duration of follow-up)] for the same measures. We also included multivariable analyses adjusting for covariates of age, sex, and body mass index (BMI) from the OAI 36 month visit. To remove the possibility of a ceiling effect, knees with a medial JSN score of 3, the maximal score, at baseline were excluded. We repeated the analyses with lateral JSN progression as the outcome of interest.
All analyses were performed using SAS version 9.4 except the intraclass correlation coefficient calculations which were performed using SPSS version 19. P-values <0.05 were considered statistically significant.
RESULTS
In total, 629 participants were originally enrolled into the bone study. Of these, 144 had missing or insufficient quality trabecular morphometry MRIs, 34 had missing radiographs, 4 had missing DXAs, one had an artifact on DXA, one had knee arthroplasty by month 48, and 31 had a medial JSN score of 3 by the 36 month visit. This resulted in a final sample comprised 444 participants, among whom there was a slight predominance of men and Caucasian race (Table 1). Comparing those that were included and excluded into this study, the baseline demographics were similar except that the excluded group had a greater number of knees with the extreme of the medial JSN score (because they were not eligible for worsening of the medial JSN score) which likely also likely contributed to a greater prevalence of frequent knee pain at the baseline visit
Table 1.
Participant demographics from the 24 month OAI visit.
| Included n = 444 |
Excluded n = 185 |
|
|---|---|---|
| Age | 63.1 (9.2) | 64.2 (8.6) |
| Body Mass Index | 29.4 (4.5) | 30.6 (5.1) |
| Sex (% male) | 52% | 46% |
| Race | ||
| Caucasian | 75% | 63% |
| Black | 22% | 32% |
| Other | 3% | 5% |
| Frequent knee pain | 44% | 56% |
| Kellgren Lawrence Grade | ||
| 0 | 15% | 7% |
| 1 | 17% | 6% |
| 2 | 36% | 30% |
| 3 | 29% | 30% |
| 4 | 3% | 27% |
| Medial JSN | ||
| 0 | 50% | 30% |
| 1 | 29% | 24% |
| 2 | 21% | 22% |
| 3 | 0% | 25% |
| Lateral JSN | ||
| 0 | 84% | 80% |
| 1 | 6% | 7% |
| 2 | 8% | 11% |
| 3 | 3% | 3% |
The logistic regression results adjusted for age, sex, and BMI, evaluating the baseline values for all the subchondral bone measures were significantly associated with radiographic JSN (or OA status) (Table 2). For baseline medial:lateral paBMD, apparent bone volume fraction, trabecular number and trabecular thickness, progressively higher quartiles more commonly experienced medial JSN progression. From lowest to highest baseline medial:lateral paBMD quartile groups, 2%, 5%, 11%, and 18% had medial JSN progression respectively between the 36 to the 48 month visits, p-values = 0.001 and 0.002 unadjusted and adjusted. For trabecular spacing, the higher the quartile, the less frequent medial JSN progression occurred, from lowest to highest trabecular spacing quartile had 15%, 9%, 5%, and 6% with medial joint space progression, p –values = 0.06 and 0.005 unadjusted and adjusted respectively.
Table 2.
Proportion of medial joint space narrowing (JSN) progression in each quartile of baseline peri-articular bone measure and femoral neck BMD.
| Predictors: Baseline Measures of Peri-articular Bone | Quartile of the Predictor | Proportion of medial JSN progression | χ2 test p-value |
p-value after adjustment for age, sex, and BMI |
|---|---|---|---|---|
| Baseline medial:lateral paBMD | lowest (0.58 – 1.03) | 2/111 (2%) | p = 0.001 | p = 0.002 |
| (1.03 – 1.11) | 6/111 (5%) | |||
| (1.11 – 1.18) | 12/111 (11%) | |||
| highest (1.19 – 1.71) | 20/111 (18%) | |||
| Baseline apparent bone volume fraction | lowest (0.008 – 0.054) | 7/111 (6%) | p = 0.03 | p = 0.003 |
| (0.056 – 0.084) | 6/112 (5%) | |||
| (0.086 – 0.123) | 9/110 (8%) | |||
| highest (0.124 – 0.537) | 18/111 (16%) | |||
| Baseline apparent trabecular number (1/mm) | lowest (0.09 – 0.50) | 8/112 (7%) | p = 0.05 | p = 0.004 |
| (0.50 – 0.72) | 4/110 (4%) | |||
| (0.72 – 0.96) | 12/111 (11%) | |||
| highest (0.97 – 1.88) | 16/111 (14%) | |||
| Baseline apparent trabecular thickness (mm) | lowest (0.096 – 0.110) | 8/115 (7%) | p = 0.0002 | p < 0.0001 |
| (0.111 – 0.117) | 5/109 (5%) | |||
| (0.118 – 0.129) | 5/110 (5%) | |||
| highest (0.130 – 0.285) | 22/110 (20%) | |||
| Baseline apparent trabecular spacing (mm) | lowest (0.25 – 0.97) | 17/112 (15%) | p = 0.06 | p = 0.005 |
| (0.97 – 1.37) | 10/110 (9%) | |||
| (1.38 – 2.06) | 6/111 (5%) | |||
| highest (2.10 – 13.98) | 7/111 (6%) | |||
| Baseline femoral neck BMD (g/cm2) | lowest (0.61 – 0.85) | 11/109 (10%) | p = 0.4 | p = 0.13 |
| (0.85 – 0.95) | 7/110 (6%) | |||
| (0.95 – 1.06) | 8/110 (7%) | |||
| highest (1.06 – 1.47) | 14/110 (12%) | |||
Greater increases in medial:lateral paBMD, apparent bone volume fraction, trabecular number and trabecular thickness, were associated with more medial JSN progression (Table 3) and for trabecular spacing, greater decrease was associated with less medial JSN progression. The relationships of change in the subchondral bone measures as they related to medial JSN progression were similar to that of the baseline measures though the results were not statistically significant for the trabecular number and thickness (Table 3). For rate of medial:lateral paBMD change from lowest to highest quartile, the proportion of each group that experienced medial JSN progression were 5%, 5%, 11%, and 18%, with an unadjusted and adjusted p-value of 0.005. The medial:lateral paBMD ratio performed most consistently when evaluating the baseline and the rate of change results (Tables 2 and 3).
Table 3.
Proportion of medial joint space narrowing (JSN) progression in each rate of change quartile of peri-articular bone measure and femoral neck BMD.
| Predictors: Measures of Peri-articular Bone | Quartile of the Predictor | Proportion of medial JSN progression | χ2 test p-value |
p-value after adjustment for age, sex, and BMI |
|---|---|---|---|---|
| Rate of medial:lateral paBMD change (per yr) | lowest (−0.11 – −0.02) | 5/111 (5%) | p = 0.005 | p = 0.005 |
| (−0.02 – 0) | 5/111 (5%) | |||
| (0 – 0.02) | 11/111 (11%) | |||
| highest (0.02 – 0.43) | 19/111 (18%) | |||
| Rate of apparent bone volume fraction change (per yr) | lowest ( −0.225 – −0.013) | 4/111 (4%) | p = 0.01 | p = 0.009 |
| ( −0.012 – 0 ) | 11/113 (10%) | |||
| (0.001 – 0.014) | 7/109 (7%) | |||
| highest (0.015 – 0.185) | 18/111 (16%) | |||
| Rate of apparent trabecular number change (per yr) | lowest (−0.80 – −0.10) | 5/111 (5%) | p = 0.2 | p = 0.07 |
| (−0.10 – −0.01) | 10/111 (9%) | |||
| (−0.01 – 0.08) | 11/111 (10%) | |||
| highest ( 0.08 – 0.83) | 14/111 (13%) | |||
| Rate of apparent trabecular thickness change (per yr) | lowest (−0.071 – −0.003) | 5/111 (5%) | p = 0.1 | p = 0.08 |
| (−0.003 – 0) | 8/112 (7%) | |||
| (0.001 – 0.004) | 12/110 (11%) | |||
| highest (0.004 – 0.064) | 15/111 (14%) | |||
| Rate of apparent trabecular spacing change (per yr) | lowest (−4.25 – −0.19) | 12/111 (11%) | p = 0.03 | p = 0.03 |
| (−0.19 – 0.02) | 10/111 (9%) | |||
| (0.02 – 0.31) | 16/111 (14%) | |||
| highest (0.31 – 10.11) | 2/111 (2%) | |||
| Rate of femoral neck BMD change (per yr) | lowest (−0.10 – −0.02) | 13/109 (15%) | p = 0.10 | p = 0.32 |
| (−0.02 – 0.00) | 11/110 (9%) | |||
| (0.00 – 0.02) | 10/110 (5%) | |||
| highest (0.02 – 0.24) | 6/110 (6%) | |||
When evaluating lateral JSN progression as the outcome of interest, the only measure of bone that was associated was baseline medial:lateral paBMD (Supplemental tables 2 and 3).
DISCUSSION
This study shows that cross-sectional and most longitudinal measures of periarticular bone, with a higher medial:lateral ratio, greater bone volume fracture, trabecular thickness and number, and lower trabecular spacingin knees of people with OA are associated with loss of medial tibiofemoral radiographic joint space. Interestingly, femoral neck BMD, a measure of systemic bone, both cross-sectionally and longitudingally are not associated with loss of medial tibiofemoral radiographic joint space. These observations support the idea that periarticular bone participates in knee OA progression and is a potential target for therapeutic intervention.
The lateral compartment was not our primary focus and therefore, we did not have trabecular morphometry measures of the lateral compartment. However, we did have measures of lateral joint space. Therefore, we evaluated associations of bone in the medial compartment that were available as they related to lateral JSN progression. As we expected, we found that medial trabecular morphometry measures, cross-sectional and longitudinal, were not predictive of lateral JSN progression. We did find that the ratio of the medial:lateral paBMD ratio at baseline was strongly associated with lateral JSN progression, where a low ratio was associated with more lateral JSN progression. This is consonant with the finding that a high ratio was associated with more medial JSN progression. As there is substantial evidence supporting a relationship between static alignment and the medial:lateral paBMD ratio where varus alignment is associated with high ratios and valgus is associated with low ratios, these findings are congruent with the analyses with medial JSN progression as the outcome. It was a little surprising that rate of medial:lateral paBMD ratio change was not associated with lateral JSN progression. Perhaps this is because it may take a longer observation period to detect this change. Future studies focused on the lateral compartment with longer follow up would be required to further investigate these associations.
The measures of bone deployed in this study were chosen based on their feasibility and prior validation. The DXA measures were obtained using a commercial scanner, have been used in previous epidemiologic studies of knee OA, and were shown to be associated with features of OA cross-sectionally 27,29,32–35. Additionally, a systematic review of studies evaluating periarticular DXA has found that though protocols for obtaining the scans vary between studies, the reliability of these measures is high36. Deployment of MR imaging- trabecular morphometry required us to add a clinically achievable acquisition, optimized to visualize trabecular bone19, to the OAI MR assessments and to operationalize software that had previously been validated for morphometry in osteoporosis and OA 22–25,37. The reproducibility of these DXA and MRI bone morphometry measures were excellent. When validated against cadaveric specimens, we found bone volume fraction the be the most reliable of the MR morphometric measures38.
Taken together, the directionality of the associations of the bone measures with OA progression give a sense of increasing subchondral areal density accompanied by thickening and compression of trabecular structure. These results are consistent with a previous study that found tibial paBMD to be predictive of medial tibial cartilage defects39, and with other studies associating OA severity with measures of subchondral and peri-articular bone pathology, including bone marrow lesions, attrition, and fractal signature analysis 6,16,28,40–46. This may be a reflection of sclerosis seen on radiographs given the prior associations between sclerosis and paBMD27. Histologic and computer simulation studies support that adaptation to high load may contribute to OA bone structural changes6,47. However, and somewhat counter-intuitively, studies using tissue specimens of osteoarthritic periarticular bone have found that the volumetric density is in fact reduced 48,49. While this might appear at odds with the radiographic and DXA appearances, these observations are all compatible with a subchondral milieu in which there is increased turnover, increased volume of bone that favors osteoid, is less mineralized and softer, leading to thickening and compression of trabeculae.
Our study does not resolve the conundrum of whether changes in the peri-articular bone can initiate the sequence of events that leads to subsequent cartilage damage, as initially contended by Radin 6. However, it is salient that our measures were associated with JSN, a measure of OA structural progression. These findings thus highlight the close inter-relationship between changes in bone and cartilage loss, and demonstrate the construct validity of these measurements as predictive and process biomarkers of knee OA progression. It is postulated that there are multiple phenotypes of OA50, and perhaps the measures from knee DXAs may offer prediction in the context of identifying those who will respond to anti-resorptive agents51 and ultimately serve as a target for intervention.
As previously noted, our sample comprised individuals with prevalent symptomatic OA in at least one knee although the more symptomatic knee was not targeted for study. Due to limited resources, we preferentially studied the right knee. Furthermore, we eliminated baseline JSN 3 scored knees to enable progression to be measured. Thus, our results are not necessarily applicable to people without OA, however we have studied knees over a range of radiographic JSN. It would be appropriate to repeat this study among patients with two normal knees and those at risk of OA before generalizing to this group.
Limitations to our study include that measurement of MR imaging-based trabecular morphometry does not directly measure the trabecular bone (no signal on conventional MR imaging), but rather is based on a signal void in the hyperintense marrow fat. Therefore, these are apparent trabecular bone measures. Nevertheless, these MR imaging-based trabecular bone measures have been validated against histology in vertebral, femoral and calcaneal specimens24. Also, for reasons of feasibility we measured trabecular morphometry only on the medial tibia. This issue might explain why the trabecular measures appeared to be less strongly associated with progression than DXA, which was obtained from both femoral and tibial sides of the joint. The computation of a ratio from the medial and lateral tibial subchondral bone density measures introduces an internal standardization to this approach and may add information. For these reasons, we do not view our analysis as an equal test of the two measures. Future studies should consider performing bilateral morphometry to generate an integrated metric analogous to medial:lateral paBMD.
Within the limits of this study, we have shown that a diverse panel of bone measurements reflect the state of the periarticular bone, and strongly associate with longitudinal structural progression of knee OA. These data were derived from a study of substantial sample size with high quality evaluations of participant characteristics as well as excellent MRI and DXA exams.
Our results indicate that clinically evaluable processes in peri-articular bone relate strongly to knee OA progression over a relatively short timeframe (12 months). These observations highlight the close participation of the peri-articular bone in the pathogenesis of OA. Future studies should focus on the development of these measures as predictive and OA disease process biomarkers, and evaluate their deployment in clinical trials.
Supplementary Material
Significance and Innovation.
This study shows that periarticular health in knees in people with OA associate with loss of radiographic joint space over a relatively short time frame.
The close participation of the peri-articular bone with joint space loss in the pathogenesis of OA suggests that bone could be a target for therapeutic intervention.
ACKNOWLEDGEMENT
Sources of Funding
The Role of Bone in Knee Osteoarthritis Progression was sponsored by NIH/NIAMS (grant R01 AR054938). Periarticular Bone Density as a Biomarker for Early Knee OA was sponsored by NIH/NIAMS (grant R01 AR060718). Dr. Lo is supported by K23 AR062127, an NIH/NIAMS funded mentored award. This work was supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Disclosure of Potential Competing Interests
None of the authors have a conflict of interest that could influence this work.
The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
REFERENCES
- 1.Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Lifetime Risk and Age at Diagnosis of Symptomatic Knee Osteoarthritis in the US. Arthritis care & research 2013;65:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 1987;30:914–8. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 1994;84:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum 2009;60:3546–53. [DOI] [PubMed] [Google Scholar]
- 6.Radin EL, Martin RB, Burr DB, Caterson B, Boyd RD, Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res 1984;2:221–34. [DOI] [PubMed] [Google Scholar]
- 7.Hayes WC, Swenson LW Jr., Schurman DJ. Axisymmetric finite element analysis of the lateral tibial plateau. J Biomech 1978;11:21–33. [DOI] [PubMed] [Google Scholar]
- 8.Clark JM, Huber JD. The structure of the human subchondral plate. J Bone Joint Surg Br 1990;72:866–73. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino A, Wallace WA. Impact-absorbing properties of the human knee. J Bone Joint Surg Br 1987;69:807–11. [DOI] [PubMed] [Google Scholar]
- 10.Simkin PA. Hydraulically loaded trabeculae may serve as springs within the normal femoral head. Arthritis Rheum 2004;50:3068–75. [DOI] [PubMed] [Google Scholar]
- 11.Burr DB. Bone In: Brandt KD, Doherty M, Lohmander LS, eds. Osteoarthritis. 2nd ed. Oxford: Oxford University Press; 2003:125–33. [Google Scholar]
- 12.Kellgren J, Lawrence JS. The epidemiology of chronic rheumatism: atlas of standard radiographs. Vol 2 Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 13.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 1986:34–40. [PubMed]
- 14.Mazzuca SA, Brandt KD, Schauwecker DS, Buckwalter KA, Katz BP, Meyer JM, et al. Bone scintigraphy is not a better predictor of progression of knee osteoarthritis than Kellgren and Lawrence grade. J Rheumatol 2004;31:329–32. [PubMed] [Google Scholar]
- 15.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis 1993;52:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 2003;139:330–6. [DOI] [PubMed] [Google Scholar]
- 17.The Osteoarthritis Initiative: Protocol for the Cohort Study. (Accessed January 24, 2012, at http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.)
- 18.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 2007;15 Suppl A:A1–56. [DOI] [PubMed] [Google Scholar]
- 19.Schneider E, Lo GH, Sloane G, Fanella L, Hunter DJ, Eaton CB, et al. Magnetic resonance imaging evaluation of weight-bearing subchondral trabecular bone in the knee. Skeletal Radiol 2011;40:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beuf O, Ghosh S, Newitt DC, Link TM, Steinbach L, Ries M, et al. Magnetic resonance imaging of normal and osteoarthritic trabecular bone structure in the human knee. Arthritis Rheum 2002;46:385–93. [DOI] [PubMed] [Google Scholar]
- 21.Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, et al. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage 2004;12:997–1005. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar S, Genant HK. Magnetic resonance imaging in osteoporosis. Eur J Radiol 1995;20:193–7. [DOI] [PubMed] [Google Scholar]
- 23.Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, et al. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 1997;12:111–8. [DOI] [PubMed] [Google Scholar]
- 24.Majumdar S, Kothari M, Augat P, Newitt DC, Link TM, Lin JC, et al. High-resolution magnetic resonance imaging: three-dimensional trabecular bone architecture and biomechanical properties. Bone 1998;22:445–54. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar S, Newitt D, Jergas M, Gies A, Chiu E, Osman D, et al. Evaluation of technical factors affecting the quantification of trabecular bone structure using magnetic resonance imaging. Bone 1995;17:417–30. [DOI] [PubMed] [Google Scholar]
- 26.Lo GH, Tassinari AM, Driban JB, Price LL, Schneider E, Majumdar S, et al. Cross-sectional DXA and MR measures of tibial periarticular bone associate with radiographic knee osteoarthritis severity. Osteoarthritis Cartilage 2012;20:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo GH, Zhang Y, McLennan C, Niu J, Kiel DP, McLean RR, et al. The ratio of medial to lateral tibial plateau bone mineral density and compartment-specific tibiofemoral osteoarthritis. Osteoarthritis Cartilage 2006;14:984–90. [DOI] [PubMed] [Google Scholar]
- 28.Reichenbach S, Guermazi A, Niu J, Neogi T, Hunter DJ, Roemer FW, et al. Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage 2008;16:1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech 1998;31:423–30. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 31.Central reading of knee X-rays for Kellgren and Lawrence grade and individual radiographic features of tibiofemoral knee OA. (Accessed May 21, 2013, at http://oai.epi-ucsf.org/datarelease/SASDocs/kXR_SQ_BU_descrip.pdf.)
- 32.Wada M, Maezawa Y, Baba H, Shimada S, Sasaki S, Nose Y. Relationships among bone mineral densities, static alignment and dynamic load in patients with medial compartment knee osteoarthritis. Rheumatology (Oxford) 2001;40:499–505. [DOI] [PubMed] [Google Scholar]
- 33.Madsen OR, Schaadt O, Bliddal H, Egmose C, Sylvest J. Bone mineral distribution of the proximal tibia in gonarthrosis assessed in vivo by photon absorption. Osteoarthritis Cartilage 1994;2:141–7. [DOI] [PubMed] [Google Scholar]
- 34.Lo GH, Hunter DJ, Zhang Y, McLennan CE, Lavalley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum 2005;52:2814–21. [DOI] [PubMed] [Google Scholar]
- 35.Akamatsu Y, Mitsugi N, Taki N, Kobayashi H, Saito T. Medial versus lateral condyle bone mineral density ratios in a cross-sectional study: A potential marker for medial knee osteoarthritis severity. Arthritis Care Res (Hoboken) 2012;64:1036–45. [DOI] [PubMed] [Google Scholar]
- 36.Sepriano A, Roman-Blas JA, Little RD, Pimentel-Santos F, Arribas JM, Largo R, et al. DXA in the assessment of subchondral bone mineral density in knee osteoarthritis--A semi-standardized protocol after systematic review. Semin Arthritis Rheum 2015;45:275–83. [DOI] [PubMed] [Google Scholar]
- 37.Majumdar S, Link TM, Augat P, Lin JC, Newitt D, Lane NE, et al. Trabecular bone architecture in the distal radius using magnetic resonance imaging in subjects with fractures of the proximal femur. Magnetic Resonance Science Center and Osteoporosis and Arthritis Research Group. Osteoporos Int 1999;10:231–9. [DOI] [PubMed] [Google Scholar]
- 38.Driban JB, Barbe MF, Amin M, Kalariya NS, Zhang M, Lo GH, et al. Validation of quantitative magnetic resonance imaging-based apparent bone volume fraction in peri-articular tibial bone of cadaveric knees. BMC Musculoskelet Disord 2014;15:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dore D, Quinn S, Ding C, Winzenberg T, Cicuttini F, Jones G. Subchondral bone and cartilage damage: a prospective study in older adults. Arthritis Rheum 2010;62:1967–73. [DOI] [PubMed] [Google Scholar]
- 40.Neogi T, Felson D, Niu J, Lynch J, Nevitt M, Guermazi A, et al. Cartilage loss occurs in the same subregions as subchondral bone attrition: a within-knee subregion-matched approach from the Multicenter Osteoarthritis Study. Arthritis Rheum 2009;61:1539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, Lavalley MP, et al. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum 2006;54:1529–35. [DOI] [PubMed] [Google Scholar]
- 42.Kothari A, Guermazi A, Chmiel J, Dunlop D, Song J, Almagor O, et al. Within-Subregion Relationship Between Bone Marrow Lesions and Subsequent Cartilage Loss in Knee Osteoarthritis. Arthritis Care Res 2010;62:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis 2008;67:683–8. [DOI] [PubMed] [Google Scholar]
- 44.Kraus VB, Feng S, Wang S, White S, Ainslie M, Brett A, et al. Trabecular morphometry by fractal signature analysis is a novel marker of osteoarthritis progression. Arthritis Rheum 2009;60:3711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraus VB, Feng S, Wang S, White S, Ainslie M, Le Graverand MP, et al. Subchondral bone trabecular integrity predicts and changes concurrently with radiographic and MRI determined knee osteoarthritis progression. Arthritis Rheum 2013. [DOI] [PMC free article] [PubMed]
- 46.Hunter DJ, Gerstenfeld L, Bishop G, Davis AD, Mason ZD, Einhorn TA, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther 2009;11:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox LG, van Donkelaar CC, van Rietbergen B, Emans PJ, Ito K. Alterations to the subchondral bone architecture during osteoarthritis: bone adaptation vs endochondral bone formation. Osteoarthritis Cartilage 2012. [DOI] [PubMed]
- 48.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage 2004;12 Suppl A:S20–30. [DOI] [PubMed] [Google Scholar]
- 49.Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res 1997;12:641–51. [DOI] [PubMed] [Google Scholar]
- 50.Herrero-Beaumont G, Roman-Blas JA, Bruyere O, Cooper C, Kanis J, Maggi S, et al. Clinical settings in knee osteoarthritis: Pathophysiology guides treatment. Maturitas 2017;96:54–7. [DOI] [PubMed] [Google Scholar]
- 51.Bruyere O, Cooper C, Arden N, Branco J, Brandi ML, Herrero-Beaumont G, et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015;32:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


